Abstract
Ileal Crohn's disease (CD) arising from the alteration of intestinal homeostasis is characterized by two features, namely a decrease in Paneth cell-produced antimicrobial peptides that play a key role in maintaining this balance and an increase in NOD2, an intracellular sensor. Although mutations in NOD2 are highly correlated with the incidence of CD, the physiological role of NOD2 in intestinal immunity remains elusive. Here, we show that NOD2 can down-regulate the expression of human enteric antimicrobial peptides during differentiation of the Paneth cell lineage. This finding, which links the decrease of human enteric antimicrobial peptides to increased NOD2 in ileal CD patients, provides a new view into the pathogenesis of ileal CD.
Crohn's disease (CD), the main clinical phenotype of inflammatory bowel disease (IBD)1, is a chronic, relapsing inflammatory disorder2. Although CD can occur anywhere in the gastrointestinal tract, it primarily affects the terminal ileum where as many as 75% of CD patients have inflammation3. The terminal ileum is characterized by two relevant features: the greatest number of Paneth cells4 that are generally absent from the colon and rectum, except in IBD5, and the highest microbial density, which is low in healthy proximal small intestine3,6. Human Paneth cells serve as a key arm of innate mucosal immunity to maintain the intestinal homeostasis between a host and its colonizing microbes by secreting antimicrobial peptides7,8. These antimicrobial peptides are composed predominantly of human enteric α-defensin 5 and 6 (HD5 and HD6) as well as lysozyme and secretory phospholipase A2 (sPLA2), to a lesser extent9. These peptides not only have a strong antibacterial function against Gram-positive and Gram-negative bacteria, but they also have activity against viruses, fungi and protozoa7,10,11,12. Their antimicrobial activities contribute to their roles in intestinal innate immunity. In addition, human Paneth cells express NOD213, a member of the nucleotide-binding oligomerization domain-leucine-rich repeat (NOD-LRR) proteins14, and the Paneth cell expression of NOD2 is increased in CD patients13. Although mutations in NOD2 are highly correlated with a diminished expression of human enteric α-defensin15 and the incidence of CD16,17, the physiological role of NOD2 in intestinal immunity remains elusive.
The purpose of this study was to determine whether NOD2 may regulate the expression of human enteric antimicrobial peptides. For this purpose, we should choose a suitable cell line because human Paneth cells do not survive under in vitro culture conditions13,18. Because Caco2 intestinal epithelial cells can display characteristics of small intestinal epithelial differentiation in vitro19,20 and constitutively express the NOD2 gene13, they are suitable for in vitro studies to investigate the physiological role of NOD2 in specialized intestinal epithelial cells such as Paneth cells.
Results
Activation of FGFR-3-mediated signaling induces in vitro differentiation of Caco2 cells along the Paneth cell lineage
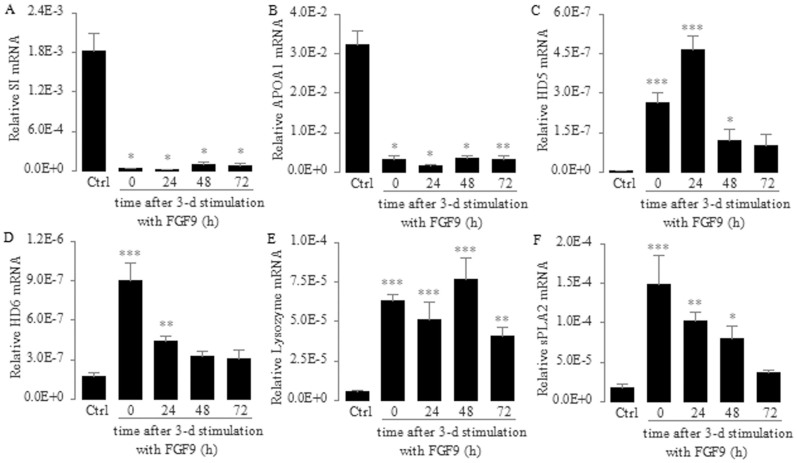
Although Caco2 cells can spontaneously differentiate along the enterocyte lineage in vitro21,22, they also express abundant FGFR-323, which is a critical regulator of Paneth cell differentiation during mouse gut development24. Therefore, we treated Caco2 cells with FGF9, a high affinity ligand for FGFR-325, and determined whether the activation of FGFR-3-mediated signaling induces in vitro differentiation of Caco2 cells along the Paneth cell lineage. We found that the mRNA expression of SI and APOA1, which encode two enterocyte differentiation markers25, was greatly decreased. These significant decreases were sustained for at least 72 h after a consecutive 3-day treatment with FGF9 (Fig. 1A and 1B), suggesting that the differentiation of Caco2 cells along the enterocyte lineage is suppressed and that this differential inhibition is stable. In contrast, we found that the mRNA expression of HD5, HD6, lysozyme and sPLA2, which encode four Paneth cell differentiation markers, was greatly increased after a consecutive 3-day treatment with FGF9 (Fig. 1C–1F). In addition, we found that these significant increases were sustained for at least 24 h after a consecutive 3-day treatment with FGF9 (Fig. 1C–1F). These results indicate that the activation of FGFR-3-mediated signaling can induce the in vitro differentiation of Caco2 cells along the Paneth cell lineage and maintain this induction of differentiation for a period of time.
Figure 1. Effect of FGFR-3-mediated signaling on the expression of intestinal lineage differentiation markers in Caco2 cells.
Enterocyte markers: sucrase-isomaltase (SI; A) and apolipoprotein A-1 (APOA1; B). Paneth cell markers: human α-defensin 5 (HD5; C), human α-defensin 6 (HD6; D), Lysozyme (E) and secretory phospholipase A2 (sPLA2; F). Caco2 cells were treated with FGF9 (10 ng/ml) daily for 3 consecutive days. After that, the cells were not treated with FGF9 but their medium was changed every 24 h. Total RNA was isolated and mRNA levels were determined using real-time PCR and normalized to 18 S rRNA at the indicated time points after the 3-day stimulation with FGF9. Control (Ctrl) cells were not treated with FGF9. Data are shown as the mean ± SD of three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001 vs. control groups.
NOD2 signaling down-regulates the expression of human enteric antimicrobial peptides during differentiation of the Paneth cell lineage
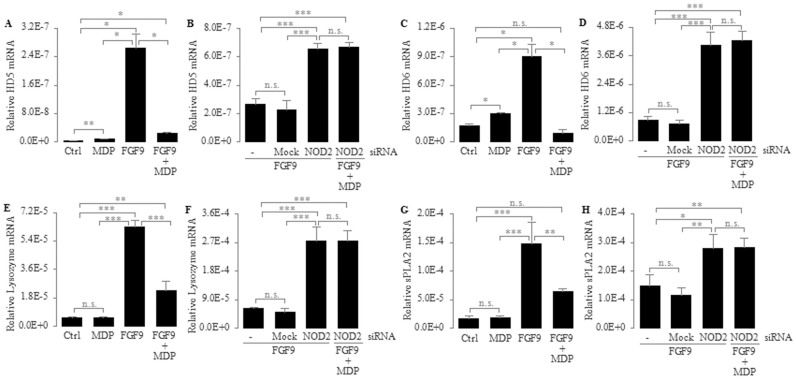
To determine the effect of the NOD2 gene on the expression of human enteric antimicrobial peptides, we first asked whether NOD2 regulates FGF9-induced expression of human enteric antimicrobial peptides during differentiation of the Paneth cell lineage. We utilized FGF9 with or without MDP, an agonist for NOD226, to stimulate Caco2 cells for 3 consecutive days and examined the mRNA expression of HD5, HD6, lysozyme and sPLA2 using real-time PCR. We found that the mRNA expression of HD5, HD6, lysozyme and sPLA2 was decreased approximately 10.6-, 9.6-, 2.7- and 2.3-fold, respectively, in Caco2 cells treated with MDP plus FGF9 compared with FGF9 only (Fig. 2A, 2C, 2E and 2G). This result indicates that MDP-NOD2 signaling can down-regulate the expression of human enteric antimicrobial peptides, especially enteric α-defensin, during differentiation of the Paneth cell lineage.
Figure 2. Differential regulation of mRNA expression of HD5 (A, B), HD6 (C, D), Lysozyme (E, F) and sPLA2 (G, H) by different stimuli in Caco2 cells.
(A, C, E, G) Caco2 Cells were treated with MDP (10 μg/ml), FGF9 (10 ng/ml) or FGF9 (10 ng/ml) plus MDP (10 μg/ml) daily for 3 consecutive days. (B, D, F, H) Caco2 cells were transfected with transfection reagent only (mock) or NOD2 siRNA (50 nM) for 6 h, then incubated with normal growth medium for an additional 18 h. Subsequently, these cells were treated with FGF9 (10 ng/ml) or FGF9 (10 ng/ml) plus MDP (10 μg/ml) daily for 3 consecutive days. Total RNA was isolated and mRNA levels were determined using real-time PCR and normalized to 18 S rRNA. Control (Ctrl) cells were not treated. Data are shown as the mean ± SD of three independent experiments; n.s., not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
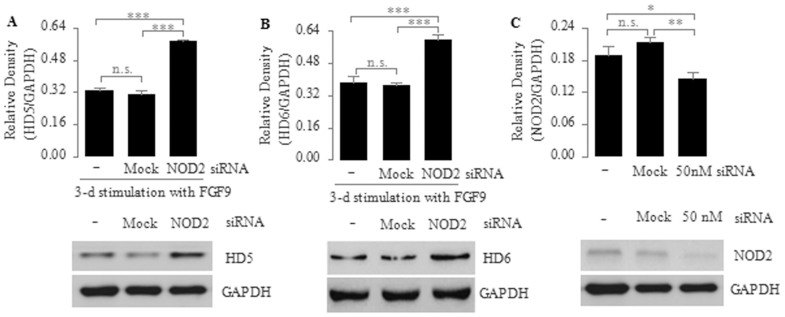
To further substantiate the role of NOD2 in regulating FGF9-induced expression of human enteric antimicrobial peptides, we transfected Caco2 cells with a NOD2-specific siRNA, followed by a consecutive 3-day stimulation with FGF9 or FGF9 plus MDP. We found that the mRNA expression of HD5, HD6, lysozyme and sPLA2 was significantly higher in Caco2 cells transfected with NOD2-siRNA than in untransfected or mock transfected cells (Fig. 2B, 2D, 2F and 2H), suggesting that NOD2 signaling can down-regulate the FGF9-induced expression of human enteric antimicrobial peptides. Consistent with this result, the protein expression of HD5 and HD6 was significantly increased in FGF9-stimulated NOD2-siRNA-transfected cells compared with untransfected or mock transfected cells (Fig. 3A and 3B), thus further confirming that NOD2 signaling can down-regulate the expression of human enteric antimicrobial peptides during differentiation of the Paneth cell lineage; however, we found no significant differences in the mRNA expression of HD5, HD6, lysozyme and sPLA2 between FGF9-stimulated NOD2-siRNA-transfected cells and NOD2-siRNA transfectants stimulated with FGF9 plus MDP (Fig. 2B, 2D, 2F and 2H), suggesting that the down-regulated mRNA expression of human enteric antimicrobial peptides is indeed mediated by MDP via NOD2. In addition, we found that NOD2 protein expression was significantly lower in NOD2-siRNA transfected cells than in mock or untransfected cells (Fig. 3C), thus confirming the efficiency of NOD2-knockdown via siRNA.
Figure 3. Down-regulation of FGF9-mediated protein expression of HD5 (A) and HD6 (B) by NOD2 in Caco2 cells.
(A, B) Caco2 cells were transfected with transfection reagent only (mock) or NOD2 siRNA (50 nM) for 6 h, then incubated with normal growth medium for an additional 18 h. Subsequently, these cells were treated with FGF9 (10 ng/ml) daily for 3 consecutive days. Whole-cell extracts were analyzed for HD5 by immunoblotting. (C) Transfection efficiency was tested after a 72-h transfection with siRNA via immunoblotting. Top, quantitative analysis of proteins; bottom, representative immunoblot images. Data are shown as the mean ± SD of three independent experiments; n.s., not significant, *P < 0.05; **P < 0.01; ***P < 0.001.
NOD2 itself differentially regulates the expression of human enteric antimicrobial peptides
We next determined whether NOD2 itself can affect the expression of human enteric antimicrobial peptides. We treated Caco2 cells with the NOD2 agonist MDP and then determined the mRNA expression of HD5, HD6, lysozyme and sPLA2 using real-time PCR. We found that the mRNA expression of HD5 and HD6 was increased approximately 2.8- and 1.7-fold, respectively, in Caco2 cells after a consecutive 3-day treatment with MDP compared with untreated control (Ctrl) cells (Fig. 2A and 2C). This result demonstrates that NOD2 itself can slightly up-regulate the expression of human enteric α-defensin 5 and 6, which is consistent with the report showing the decreased expression of Paneth cell α-defensins in NOD2-knockout mice27; however, the mRNA expression of lysozyme and sPLA2 was not significantly different between MDP-treated cells and untreated control (Ctrl) cells (Fig. 2E and 2G), suggesting that NOD2 itself does not affect the expression of lysozyme and sPLA2.
FGF9 does not regulate NOD2 expression during differentiation of the Paneth cell lineage
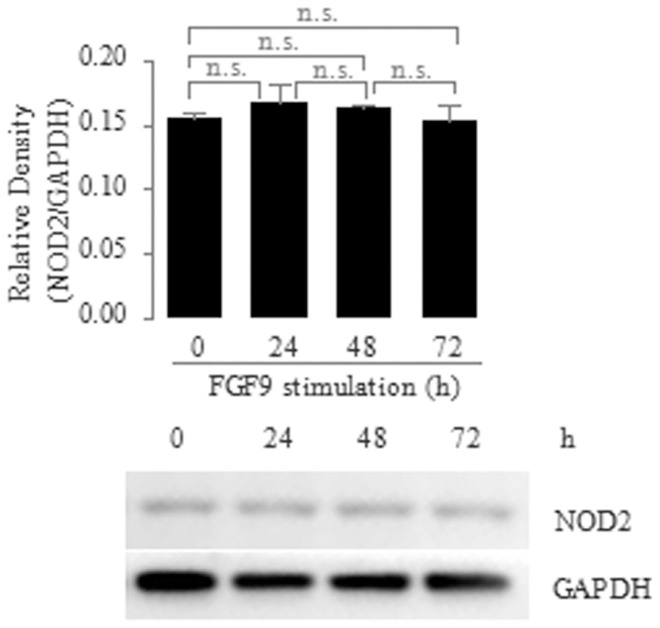
Finally, we determined whether FGF9 treatment affected NOD2 expression during differentiation of the Paneth cell lineage. We treated Caco2 cells with FGF9 and determined the protein expression of NOD2 via immunoblotting at different times of induction of cell differentiation. We found that NOD2 protein expression was not significantly different between FGF9-treated cells and untreated control (Ctrl) cells (Fig. 4). This result indicates that FGF9 does not regulate NOD2 expression during differentiation of the Paneth cell lineage.
Figure 4. No Effect of FGF9 on protein expression of NOD2 in Caco2 cells.
Caco2 cells were treated with FGF9 (10 ng/ml) for the indicated time points, after which whole-cell extracts were prepared and analyzed for NOD2 via immunoblotting. Top, quantitative analysis of proteins; bottom, the representative immunoblotting image from three independent experiments. Data are shown as the mean ± SD of three independent experiments; n.s., not significant.
Discussion
This study assessed whether NOD2 can regulate the expression of human enteric antimicrobial peptides. Our data in Caco2 cells show that NOD2 can down-regulate the expression of human enteric antimicrobial peptides during differentiation of the Paneth cell lineage. We found that the Caco2 cells treated with FGF9 can be induced into differentiation along the Paneth cell lineage (Fig. 1). During this induction of cell differentiation, the mRNA expression of human enteric antimicrobial peptides (HD5, HD6, Lysozyme and sPLA2) was decreased in the presence of the stimulation of NOD2 agonist MDP but was increased when NOD2 was knocked down by a NOD2-specific siRNA (Fig. 2). In addition, we found that the protein levels of HD5 and HD6, the main constituents of human enteric antimicrobial peptides, were increased in FGF9-stimulated NOD2-siRNA-transfected cells compared with untransfected cells (Fig. 3), thus further confirming that NOD2 can down-regulate the expression of human enteric antimicrobial peptides during differentiation of the Paneth cell lineage.
Whether the molecular mechanism of NOD2-mediated down-regulation of expression of human enteric antimicrobial peptides is dependent on or independent of the ability of NOD2 to recruit and activate downstream cofactor RICK or others remains unknown; however, previous findings show that NOD2 activated by MDP can bind and activate RICK, a caspase recruitment domain (CARD)-containing serine/threonine kinase28,29. In conjunction with our own findings, we speculate that FGFR-3-NOD2 signaling may recruit a kind of signaling molecules different from RICK to inhibit FGFR-3-induced expression of human enteric antimicrobial peptides. We hypothesize that this signaling pathway may enhance the inhibition efficiency through recruiting more of that kind of signaling molecules when NOD2 is simultaneously activated upon MDP stimulation.
Although NOD2 can down-regulate FGF9-induced expression of HD5, HD6, Lysozyme and sPLA2, NOD2 itself can up-regulate the expression of HD5 and HD6. We found that the mRNA expression of HD5 and HD6 was increased in MDP-stimulated cells compared with untreated control cells (Fig. 2). This result is in line with the previous report showing the decreased expression of Paneth cell α-defensins in NOD2-knockout mice27. Because FGF9 does not affect NOD2 expression during differentiation of the Paneth cell lineage (Fig. 4), it is extremely interesting to explore the mechanism by which NOD2 dually regulates the expression of human enteric α-defensins under the different types of NOD2 stimuli.
In this study, we used Caco2 cells, serving as a functional modal, to investigate whether NOD2 regulates the expression of human enteric antimicrobial peptides in the Paneth cell lineage. This cell line is suitable because primary Paneth cells do not survive in vitro13,18; however, Caco2 cells can display characteristics of small intestinal epithelial differentiation in vitro21,22, suggesting that they maintain intestinal stem cell functions. In addition, they constitutively express the NOD2 gene13 and also express abundant FGFR-325, which is a critical regulator of Paneth cell differentiation during gut development24. Finally, we found that Caco2 cells activated by FGFR-3-mediated signaling for 3 consecutive days express Paneth cell lineage-specific genes. Thus, Caco2 cells are suitable for this in vitro study to investigate the role of the NOD2 protein in the Paneth cell lineage.
In summary, our results indicate that NOD2 can down-regulate the expression of human enteric antimicrobial peptides during differentiation of the Paneth cell lineage. In light of NOD2 over-expression in CD patients13, our data provide a plausible explanation for the diminished levels of human enteric antimicrobial peptides in ileal CD patients. This finding is significant because a strongly advocated view is that the ineffective bacterial clearance that results from the reduced expression of human enteric antimicrobial peptides27 induces and sustains the abnormal adaptive immune responses observed in CD patients30,31. In addition, T helper 1 (Th1) cytokines such as factor α (TNFα) and interferon-γ (IFNγ) can up-regulate NOD2 expression in intestinal epithelial cells32,33,34. Based on these findings, a hypothesis for the role of NOD2 in the pathogenesis of CD is proposed (Fig. 5) in which NOD2 constitutes a critical link between the innate and adaptive immunity in the intestinal tract. Indeed, anti-TNFα, anti-IFNγ or anti-interleukin-12 administration is an effective therapeutic strategy in CD35,36,37,38,39,40,41, although none are a permanent cure for CD. As discussed above, we speculate that if these cytokine-based therapies work by interrupting the over-expression of NOD2, the most effective therapy for CD patients will be directed at antagonism of NOD2-mediated inhibition of human antimicrobial peptides.
Figure 5. Schematic diagram of the proposed hypothesis for the role of NOD2 in the pathogenesis of ileal CD.

Th1 cytokines such as TNFα and IFNγ up-regulate NOD2 expression. A large increase in NOD2 levels causes a large decrease in antimicrobial peptides including HD5, HD6, lysozyme and sPLA2, leading to weakened mucosal defenses,and alterations in the luminal microbes. These changes lead to the destruction of intestinal homeostasis, which then induces and/or sustains inflammation in susceptible individuals. This pathogenesis ultimately results in ileal CD. Abbreviations: NOD2, nucleotide-binding oligomerization domain 2; CD, Crohn's disease; TNFα, tumor necrosis factor α; IFNγ, interferon-γ; HD5, human α-defensin 5; HD6, human α-defensin 6; sPLA2, secretory phospholipase A2.
Methods
Cell culture and stimulation
Caco2 cells (ATCC) were cultured in Dulbecco's modified Eagle medium (HyClone) supplemented with 20% fetal calf serum (HyClone), 2 mM L-glutamine, 100 U/ml Penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere with 5% CO2. The cells were used between passages 15 and 30. For all of the experiments, to better mimic the steric conditions existing in the intestine in vivo, the cells were plated at a subconfluent cell density onto 6-well Millicell hanging filter inserts (3 μm pore size, Polyethylene Terephthalate, Millipore) that allow free access of media to their apical and basolateral sides. Media were changed every 24 h. To determine the role of NOD2, fibroblast growth factor 9 (FGF9) (10 ng/ml; R&D Systems), a high affinity ligand for the fibroblast growth factor receptor-3 (FGFR-3); muramyl dipeptide (MDP) (10 μg/ml; InvivoGen), an agonist for intracellular NOD2; and FGF9 (10 ng/ml) plus MDP (10 μg/ml) were added to both sides of the inserts daily starting at 24 h post-plating and ending at 72 h post-plating.
SiRNA
After 24-h culture with antibiotic-free normal growth medium containing 20% fetal calf serum, Caco2 cells that were approximately 60% confluent were transfected with the NOD2 siRNA (50 nM; Santa Cruz Biotechnology) and Transfection Reagent (Santa Cruz Biotechnology) mixture or Transfection Reagent only (Mock) for 6 h, then incubated with normal growth medium for an additional 18 h. Subsequently, these cells were stimulated upon the addition of FGF9 (10 ng/ml) or FGF9 (10 ng/ml) plus MDP (10 μg/ml) daily for 3 days. After that, total RNA was isolated and analyzed using real-time PCR. Whole-cell extracts were prepared and analyzed via immunoblotting. The transfection efficiency was tested after a 72-h transfection with siRNA via immunoblotting.
Real-time quantitative RT-PCR
Total cellular RNA was isolated using the RNAiso Plus Kit (Takara) and then cDNA synthesis was performed using the PrimeScript RT reagent Kit with gDNA Eraser (Takara) to eliminate genomic DNA contamination. Real-time PCR was performed in triplicate using the LightCycler 480 System (Roche). Each 20 μl PCR reaction contained 5 μl of cDNA corresponding to 25 ng of RNA as a template, 0.5 μM of each primer (table 1), and 1 × LightCycler 480 SYBR Green I Master (Roche). The samples were loaded into the LightCycler 480 Multiwell Plate 96 (Roche) and incubated for an initial denaturation at 95°C for 10 min followed by 45 cycles, with each cycle consisting of 95°C for 10 s, a “touchdown” of −1°C/cycle from 65°C 60°C for 20 s, followed by 72°C for 20 s. Relative mRNA levels were calculated according the 2−ΔCT method, using 18 S rRNA as the reference and internal standard.
Table 1. Real-time quantitative RT-PCR primer sequences.
| Gene | Sense | Antisense |
|---|---|---|
| APOA1 | AGC TTG CTG AAG GTG GAG GT | ATC GAG TGA AGG ACC TGG C |
| SI | ACC CAA TCG TTT CCG GTT CA | GGG TTT TGG GCA ACC TTC AC |
| HD5 | GCC ATC CTT GCT GCC ATT C | AGA TTT CAC ACA CCC CGG AGA |
| HD6 | CCT CAC CAT CCT CAC TGC TGT TC | CCA TGA CAG TGC AGG TCC CAT A |
| Lysozyme | AAA ACC CCA GGA GCA GTT AAT | CAA CCC TCT TTG CAC AAG CT |
| sPLA2 | TGA CGA CAG GAA AGG AAG CCG CAC | AGG GAA GAG GGG ACT CAG CAA CGA G |
| 18 S rRNA | TTT GTT GGT TTT CGG AAC TGA | CGT TTA TGG TCG GAA CTA CGA |
APOA1: apolipoprotein A-1; SI: sucrase-isomaltase; HD5: human α-defensin 5; HD6: human α-defensin 6; sPLA2: secretory phospholipase A2.
Immunoblotting
The cells were lysed for 30 min on ice in RIPA lysis buffer (10 mM Tris (pH 8.0), 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, and 0.5% deoxycholate, supplemented with the protease inhibitor PMSF. After being centrifuged at 14,000 × g for 30 min at 4°C the supernatants were collected. SDS-polyacrylamide gel electrophoresis and western blotting were performed in accordance with standard protocols. Monoclonal mouse anti-HD5 (Millipore), anti-HD6 (Biorbyt) and polyclonal goat anti-NOD2 (Santa Cruz Biotechnology) were diluted at 1:1000, 1:1000 and 1:200, respectively. Monoclonal rabbit anti-GAPDH (Cell Signaling Technology) was diluted at 1:1000. Secondary antibodies were all diluted at 1:4000. Image J software was used to quantify and analyze the density of the protein bands.
Statistical Analysis
The results are shown as the mean ± standard deviation. Statistical significance was determined by one-way analysis of variance with Tukey's multiple comparisons under equal variances or with Dunnett T3's multiple comparisons under unequal variances; a value of P < 0.05 was considered statistically significant.
Author Contributions
G.T. designed the studies, performed the experiments, wrote the manuscript and prepared the table and figures. F.Z. conceived the studies and reviewed the manuscript. R.L., C.L., X.Z., F.W., J.M., S.L. and W.Z. reviewed the manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81370502).
References
- Podolsky D. K. Inflammatory bowel disease. N Engl J Med 347, 417–29 (2002). [DOI] [PubMed] [Google Scholar]
- Benitez J. M. et al. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn's disease monitoring. Gut 62, 1806–16 (2013). [DOI] [PubMed] [Google Scholar]
- Ramasundara M., Leach S. T., Lemberg D. A. & Day A. S. Defensins and inflammation: the role of defensins in inflammatory bowel disease. J Gastroenterol Hepatol 24, 202–8 (2009). [DOI] [PubMed] [Google Scholar]
- Porter E. M., Bevins C. L., Ghosh D. & Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci 59, 156–70 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapamaki M. M. et al. Gene expression of group II phospholipase A2 in intestine in ulcerative colitis. Gut 40, 95–101 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick D. A. & Mahida Y. R. Paneth cells: their role in innate immunity and inflammatory disease. Gut 54, 1802–9 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins C. L. & Salzman N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9, 356–68 (2011). [DOI] [PubMed] [Google Scholar]
- Baumgart D. C. & Sandborn W. J. Crohn's disease. Lancet 380, 1590–605 (2012). [DOI] [PubMed] [Google Scholar]
- Wehkamp J. et al. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett 580, 5344–50 (2006). [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Lichtenstein A. K. & Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol 11, 105–28 (1993). [DOI] [PubMed] [Google Scholar]
- Lehrer R. I. Primate defensins. Nat Rev Microbiol 2, 727–38 (2004). [DOI] [PubMed] [Google Scholar]
- Selsted M. E. & Ouellette A. J. Mammalian defensins in the antimicrobial immune response. Nat Immunol 6, 551–7 (2005). [DOI] [PubMed] [Google Scholar]
- Lala S. et al. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology 125, 47–57 (2003). [DOI] [PubMed] [Google Scholar]
- Inohara N., Chamaillard M.,McDonald C. & Nuñez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74, 355–83 (2005). [DOI] [PubMed] [Google Scholar]
- Wehkamp J. et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut 53, 1658–64 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001). [DOI] [PubMed] [Google Scholar]
- Ogura Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–6 (2001). [DOI] [PubMed] [Google Scholar]
- Gassler N. et al. Expression of calnexin reflects paneth cell differentiation and function. Lab Invest 82, 1647–59 (2002). [DOI] [PubMed] [Google Scholar]
- Molmenti E. P., Perlmutter D. H. & Rubin D. C. Cell-specific expression of alpha 1-antitrypsin in human intestinal epithelium. J Clin Invest 92, 2022–34 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau F., Zhu Y. & Traber P. G. Sucrase-isomaltase gene transcription requires the hepatocyte nuclear factor-1 (HNF-1) regulatory element and is regulated by the ratio of HNF-1 alpha to HNF-1 beta. J Biol Chem 276, 32122–8 (2001). [DOI] [PubMed] [Google Scholar]
- Mariadason J. M. et al. Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res 61, 3465–71 (2001). [PubMed] [Google Scholar]
- Saaf A. M. et al. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal colon and colon cancer. Mol Biol Cell 18, 4245–60 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K. Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol 264, G179–86 (1993). [DOI] [PubMed] [Google Scholar]
- Vidrich A. et al. Fibroblast growth factor receptor-3 regulates Paneth cell lineage allocation and accrual of epithelial stem cells during murine intestinal development. Am J Physiol Gastrointest Liver Physiol 297, G168–78 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodrick B. et al. Fibroblast growth factor receptor-3 (FGFR-3) regulates expression of paneth cell lineage-specific genes in intestinal epithelial cells through both TCF4/beta-catenin-dependent and -independent signaling pathways. J Biol Chem 286, 18515–25 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin S. E. et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278, 8869–72 (2003). [DOI] [PubMed] [Google Scholar]
- Kobayashi K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–4 (2005). [DOI] [PubMed] [Google Scholar]
- Kobayashi K. et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416, 194–9 (2002). [DOI] [PubMed] [Google Scholar]
- Abbott D. W., Wilkins A., Asara J. M. & Cantley L. C. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol 14, 2217–27 (2004). [DOI] [PubMed] [Google Scholar]
- Kelsall B. Getting to the guts of NOD2. Nat Med 11, 383–4 (2005). [DOI] [PubMed] [Google Scholar]
- Strober W., Murray P. J., Kitani A. & Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 6, 9–20 (2006). [DOI] [PubMed] [Google Scholar]
- Gutierrez O. et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem 277, 41701–5 (2002). [DOI] [PubMed] [Google Scholar]
- Hisamatsu T. et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124, 993–1000 (2003). [DOI] [PubMed] [Google Scholar]
- Rosenstiel P. et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 124, 1001–9 (2003). [DOI] [PubMed] [Google Scholar]
- Targan S. R. et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 337, 1029–35 (1997). [DOI] [PubMed] [Google Scholar]
- Mannon P. J. et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med 351, 2069–79 (2004). [DOI] [PubMed] [Google Scholar]
- Bouma G. & Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3, 521–33 (2003). [DOI] [PubMed] [Google Scholar]
- Strober W. & Fuss I. J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140, 1756–67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn W. J. et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med 367, 1519–28 (2012). [DOI] [PubMed] [Google Scholar]
- D'Haens G. et al. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology 116, 1029–34 (1999). [DOI] [PubMed] [Google Scholar]
- Rutgeerts P. et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology 126, 402–13 (2004). [DOI] [PubMed] [Google Scholar]