Abstract
Suppression of transgene expression in a conditional transgenic mouse model of spinocerebellar ataxia 1 (SCA1) reverses the Purkinje cell pathology and motor dysfunction that are hallmarks of SCA1. We previously showed that cerebellar neurochemical levels measured by magnetic resonance spectroscopy (MRS) correlate with progression of pathology and clinical status of patients and that abnormal neurochemical levels normalize upon suppression of transgene expression, indicating their potential as robust surrogate markers of treatment effects. Here we investigated the relative sensitivities of MRS, histology, transgene expression and motor behavioral testing to disease reversal in conditional SCA1 mice. Transgene expression was suppressed by doxycycline administration and treated and untreated mice were assessed by MRS at 9.4 tesla before and after treatment and with an accelerating Rotarod, histology and quantitative polymerase chain reaction (qPCR) for ataxin-1 transgene expression following doxycycline treatment. The MRS-measured N-acetylaspartate-to-myo-inositol ratio (NAA/Ins) correlated significantly with the molecular layer (ML) thickness and transgene expression. NAA/Ins, ML thickness and transgene expression were highly significantly different between the treated vs. untreated groups (p<0.0001), while the Rotarod assessment showed a trend for treatment effect. MRS, qPCR and histology had high sensitivity/specificity to distinguish treated from untreated mice, all with areas under the curve (AUC) = 0.97–0.98 in receiver operating characteristic (ROC) analyses, while Rotarod had significantly lower sensitivity and specificity (AUC = 0.72). Therefore, MRS accurately reflects the extent of recovery from neurodegeneration with sensitivity similar to invasive measures, further validating its potential as a surrogate marker in pre-clinical and clinical treatment trials.
Keywords: Spinocerebellar ataxia, magnetic resonance spectroscopy, Rotarod, histology, transgenic, receiver operating characteristic analysis, outcome measures
Introduction
Spinocerebellar ataxia 1 (SCA1) is a fatal hereditary neurodegenerative disorder that results in loss of motor coordination and balance, primarily caused by cerebellar Purkinje cell dysfunction and loss. It is dominantly inherited, typically late onset and is one of nine known neurodegenerative diseases that are caused by an expanded CAG repeat coding for polyglutamine tracts in the affected protein. In addition to the atrophy and loss of Purkinje cells, the pathology involves deep cerebellar nuclei, the inferior olive and the pons (Zoghbi and Orr, 2009).
As in many other neurodegenerative diseases (Taylor et al., 2002), toxic accumulation of the mutant protein is causal for pathology in SCA1 (Zoghbi and Orr, 2009). Consistently, suppressing the expression of the mutant ataxin-1 reverses the pathology and ataxic phenotype in mouse models of SCA1 (Zu et al., 2004). Therefore, therapies designed to reduce the synthesis of the mutant protein, such as gene silencing by RNA interference (RNAi), are some of the most promising therapeutic routes in SCA1 (Keiser et al., 2014; Keiser et al., 2013; Xia et al., 2004), as well as in other polyglutamine diseases (Kordasiewicz et al., 2012). Successful translation of such treatments to the clinic will necessitate robust outcome measures that accurately reflect how well the gene expression is suppressed and that can also be used to monitor treatment efficacy in patients. In this respect, animal models enable validation of such non-invasive outcome measures against invasive measures that are typically used in pre-clinical work, namely gene expression and histology.
Using a mouse model of SCA1 (the SCA1[82Q] line) that overexpresses the mutant human ataxin-1 protein in Purkinje cells and reproduces the cerebellar neuropathological and behavioral features of the human disease (Burright et al., 1995; Clark et al., 1997), we have shown that neurochemical levels measurable by magnetic resonance spectroscopy (MRS) reflect the pathological progression in SCA1 (Öz et al., 2010b). Remarkably, the same neurochemicals, namely N-acetylaspartate (NAA), myo-inositol (Ins) and glutamate, also distinguished patients with SCA1 from controls and correlated with scores on a standardized ataxia rating scale (Öz et al., 2010a). We further showed that abnormal neurochemical levels reverse towards wild-type (WT) levels upon suppression of transgene expression in a conditional SCA1[82Q] line (Öz et al., 2011b) and that early neurochemical changes are detectable even in the absence of overt pathology in a knock-in model of SCA1 (Emir et al., 2013). Together these data demonstrated the potential of MRS-measured neurochemical levels as robust biomarkers of progressive neurodegeneration and its reversal in SCA1.
Two important remaining questions prior to clinical translation of these potential surrogate markers are: 1) how accurately they reflect the suppression of gene expression and 2) how their sensitivity to detect treatment effects compares to other standard non-invasive motor behavioral assessments, which are easier to administer. Therefore we investigated the correlations between and relative sensitivities of two non-invasive (MRS and Rotarod assessment of motor ability) and two invasive outcome measures (histology and qPCR assessment of transgene expression) to detect treatment effects in conditional SCA1[82Q] mice.
Methods
Study design
All animal experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee and performed according to the National Research Council’s Guide for theCare and Use of Laboratory Animals. A total of 28 male conditional SCA1[82Q] (condSCA1[82Q]) transgenic mice that overexpress the mutant human ataxin-1 with an 82 glutamine stretch were studied. These mice were generated using the regulatory region from the Pcp2/L7 gene to direct expression of the tetracycline-responsive activator, tTA, to cerebellar Purkinje cells (Zu et al., 2004). Therefore the expression of the transgene is under doxycycline control, i.e. doxycycline binds to tTA and inhibits transgene expression. Mice from the background strain (tTA) (N=14, male) were used as WT controls. CondSCA1[82Q] mice were obtained from 6 different litters and tTA mice from 2 different litters to introduce inter-litter variability. Only male mice were studied to obviate the need to control for the phase of estrous cycle in female mice (a requirement difficult to reconcile with scanner time availability) and because gender was not shown to influence neurochemical levels in prior MRS studies (Nagae-Poetscher et al., 2004; Pouwels and Frahm, 1998). In addition, exclusion of female mice from the analysis had not changed the findings in our prior study (Öz et al., 2011b).
Half of the condSCA1[82Q] mice were treated with doxycycline from 12 to 24 weeks of age to suppress transgene expression (Fig. 1). All tTA mice were also treated with doxycycline from 12 to 24 weeks to account for potential effects of the doxycycline treatment on the neurochemical profiles. Note that our prior study indicated that doxycycline treatment alone does not significantly alter the neurochemical profile in WT mice (Öz et al., 2011b). All mice were scanned at 9.4 tesla (T) before treatment began at 12 weeks and after treatment ended at 24 weeks. Motor ability of the mice was assessed at 24 weeks using an accelerating Rotarod apparatus on 4 consecutive days, one of which coincided with the second and last MR scan. On the day of both Rotarod and MR acquisition, the Rotarod assessment was always done before MR scanning to avoid effects of anesthesia on motor performance. After the last MR scan/Rotarod assessment, mice were asphyxiated by CO2, brains were harvested and cerebellum bisected. Half of the cerebellum was used for histology (with hematoxylin-and-eosin staining) and the other half for ataxin-1 transgene expression (with qPCR).
Figure 1.
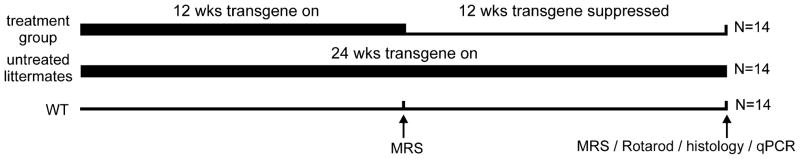
Study design. Transgene expression in the condSCA1[82Q] mice was suppressed with doxycycline administration in drinking water with sucrose from 12–24 weeks in the treatment group. Transgenic, untreated littermates received sucrose water during the same period to match sugar intake and wild type (WT, tTA background) mice received doxycycline in sucrose water. The gene on periods are shown with a bold line.
Doxycycline administration
Doxycycline (Medisca Inc., Plattsburgh, NY) was administered in drinking water (1.5 mg/mL doxycycline in a 4% sucrose solution). With this protocol the mice received approximately 350–400 mg of doxycycline per kg of body weight per day. Untreated SCA1 littermates received 2.4% sucrose water to match sugar intake between groups, which was determined based on the water intake of the first few litters receiving treatment.
MR scanning
For MR scanning, animals were induced with 3–4% isoflurane and a 1:1 mixture of O2:N2O and then maintained anesthetized with 1.5–2% isoflurane while keeping body temperature at 36–37ºC and respiration rate at 80–110 breaths per minute, as described previously (Öz et al., 2010b; Öz et al., 2011b). The scanning time for each animal was ~50 min.
All experiments were performed using a 9.4 T/31 cm Varian/Magnex system and a quadrature surface coil, as described previously (Öz et al., 2010b; Öz et al., 2011b). Briefly, the volume-of-interest (VOI, 1.8 × 2.1 × 1.8 mm3, Fig. 2) was placed centered on the midline in the cerebellum based on coronal and sagittal multi-slice images obtained with a rapid acquisition with relaxation enhancement (RARE) sequence (Hennig et al., 1986) (repetition time TR = 4 s, echo train length = 8, echo time TE = 60 ms, slice thickness = 1 mm, 7 slices). Reproducible VOI positioning between mice and between the two scans of each mouse was accomplished based on cerebellar landmarks (Fig. 2). First- and second-order shims were adjusted for the VOI using FASTMAP with echo-planar readout (Gruetter and Tkáč, 2000). Localized 1H MR spectra were acquired with a short-echo localization by adiabatic selective refocusing (LASER) sequence (TE = 15 ms, TR = 5 s, 256 averages) (Garwood and DelaBarre, 2001) combined with variable power RF pulses with optimized relaxation delays (VAPOR) water suppression (Tkáč et al., 1999). Single shots were frequency and phase corrected prior to summing. Unsuppressed water spectra acquired from the same VOI were used as a metabolite quantification reference.
Figure 2.
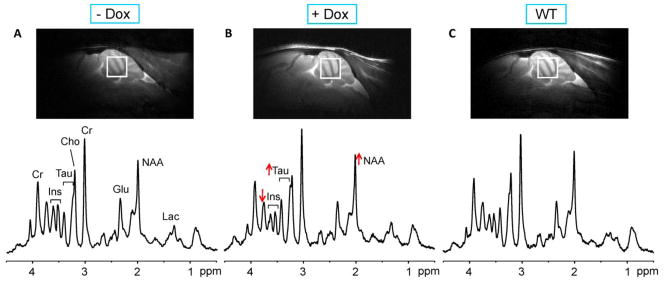
Voxel placement and spectral quality in condSCA1[82Q] and WT mice. Mid-sagittal T2-weighted images and localized proton MR spectra (LASER, TE = 15 ms, TR = 5 s) obtained at 24 weeks of age are shown for an untreated condSCA1[82Q] mouse (A), a doxycycline treated condSCA1[82Q] mouse (B) and a WT mouse (C). The voxel position for the spectra is shown on each image. The spectra were processed identically, weighted with the same Gaussian function prior to Fourier transformation and scaled based on neurochemical concentrations obtained by LCModel. The alterations in N-acetylaspartate (NAA), myo-inositol (Ins) and taurine (Tau) with treatment are shown with arrows. Other prominent peaks in the spectra are marked: creatine (Cr), choline (Cho), glutamate (Glu), lactate (Lac).
Metabolite quantification
The contribution of individual metabolites to the spectra was quantified using LCModel (Provencher, 1993), as described previously (Öz et al., 2010b; Öz et al., 2011b). Reliability criteria for reporting metabolites were identical to our prior studies, namely metabolites quantified with Cramér-Rao lower bounds (CRLB) ≤ 50% in at least 90% of the spectra were reported and the sum of metabolites with high correlations (correlation coefficient < - 0.5) were reported, as in the case of total creatine (tCr, creatine + phosphocreatine) and total choline (tCho, glycerophosphocholine + phosphocholine).
Assessment of motor ability
The motor coordination and balance of the mice were assessed at 24 weeks of age using an accelerating Rotarod apparatus (Ugo Basile North America, Inc., Collegeville, PA) as described previously (Clark et al., 1997; Zu et al., 2004). Assessments were always performed by the same investigator, in the same setting and at the same time of day. Briefly, mice were assessed for four trials per day with 10 min between trials on 4 consecutive days. During a trial, the rod accelerated from 4 to 40 rpm over 5 min and then remained at 40 rpm for an additional 5 min. Trials lasted until the mouse fell from the rod or for a maximum of 10 min. A passive rotation was recorded if the mouse passively rotated twice, where the mouse hangs on to the rod and rides it around one full revolution without walking on the rod. The trial was stopped immediately following the second passive rotation and latency recorded. Means of the latencies on the 4 trials on each day were calculated for statistical analysis. Statistical results did not change after excluding data collected with passive rotations.
Analysis of transgene expression
Following harvesting of the brain, half of the cerebellum was flash frozen in liquid nitrogen and stored at −80º until use for qPCR analysis. Total RNA was isolated using the TRIzol Reagent (Life Technologies) following the manufacturer’s protocol. cDNA was then made using the SuperScript VILO Master Mix kit (Life Technologies) using 1 μg or RNA as per the kit’s directions. Reverse transcriptase qPCR was then conducted on the Roche LightCycler 480 II. Reagents included Roche Probes Master Mix, Roche Universal ProbeLibrary Human Probe #67, hATXN1 2777F: 5′-AGAGATAAGCAACGACCTGAAGA-3′, hATXN1 2888R 5′-CCAAAACTTCAACGCTGACC-3′. Roche reference gene assay mouse βActin was used as a control. Primers and Probe design were all done through www.universalprobelibrary.com, against gene NM000332.3. The hATXN1 2775F and hATXN1 2888R PCR products were run on an agarose gel, which showed one band. The primers and probe were optimized for concentration, and a cDNA dilution series to determine PCR efficiency. Cycling parameters were as follows: pre-incubation 95º C for 10 minutes, amplification 95º C for 10 seconds, 60º C for 10 seconds, single acquisition, 40 cycles.
Histology
Following harvesting of the brain, half of the cerebellum was fixed in 10% formalin. Histology was performed in a blind fashion on paraffin-embedded sections using hematoxylin-and-eosin (H & E) staining. To quantify the pathological involvement, the molecular layer (ML) thickness was measured at the primary fissure (Öz et al., 2010b). In addition, each sample was rated according to the following severity scale: 0, no pathological changes; 0.5, near-normal but ML somewhat thinner; 1, mild changes including heterotopic Purkinje cells, vacuoles in Purkinje cells, thinning of ML, largely confined to the posterior lobules; 2, similar to 1 but more widespread; heterotopic Purkinje cells more numerous and often higher in the ML; 3, widespread ML thinning, numerous heterotopic Purkinje cells involving anterior lobules nearly as frequently as posterior, mild Purkinje cell loss, primarily in the posterior lobules; 4, severe disorganization of cerebellar cortex with generalized severe atrophy of ML, frequent heterotopic Purkinje cells and Purkinje cell loss.
Statistical Analysis
Each metabolite, ML thickness, pathology severity score, qPCR relative transgene expression, and Rotarod mean latency on day 4 were summarized separately by group and by study week (12 and 24) using means, standard deviations, and histograms. Repeated measures ANOVA models were used to estimate five pre-specified comparisons of interest (Table 1) among groups and across weeks for each metabolite; multiple comparisons adjustment was done with a step-down Bonferroni method (Holm, 1979). Diagnostics were carried out to verify that model assumptions were met. Spearman correlations were computed between each metabolite and each of ML thickness, pathology severity score, transgene expression, and Rotarod latency. Logistic regression was used to estimate ROC curves for predicting untreated vs. treated condSCA1[82Q] mice at week 24 based on each metabolite, ML thickness, pathology severity score, transgene expression, and Rotarod latency. Based on the repeated measures ANOVA models, 3 metabolites (NAA, Ins, taurine) were also used to jointly predict treated vs. untreated condSCA1[82Q] mice at week 24 using a multiple logistic regression. Predictive ability was quantified as AUC of the ROC curve; with the AUC of transgene expression as the reference method, each other AUC was compared to the transgene expression AUC using DeLong's non-parametric test (DeLong et al., 1988).
Table 1.
Statistical significance of comparisons of the treated and untreated conditional SCA1[82Q] and WT mice for neurochemicals that showed significant differences in more than one of the 5 pairwise comparisons. Neurochemicals that showed a difference only at 12 weeks between SCA1 vs. WT (glucose, glutamine, lactate) are marked in Fig. 3. P-values corrected for multiple comparisons within each metabolite or metabolite ratio using the stepdown Bonferroni/Holm approach (Holm, 1979).
| Comparison | Age (wks) | NAA | Ins | NAA / Ins | Tau | tCr |
|---|---|---|---|---|---|---|
| Treated SCA1 vs. untreated SCA1 | 12 | 0.5559 | 0.3194 | 0.9705 | 0.6065 | 0.9530 |
| SCA1 vs. WT | 12 | 0.0008 | <0.0001 | < 0.0001 | 0.15885 | 0.0028 |
| Treated SCA1 vs. untreated SCA1 | 24 | 0.0372 | 0.0008 | < 0.0001 | 0.0135 | 0.9530 |
| Treated SCA1 vs. WT | 24 | 0.6013 | 0.2123 | 0.0812 | <0.0001 | 0.0653 |
| Untreated SCA1 vs. WT | 24 | 0.0126 | <0.0001 | < 0.0001 | 0.0355 | 0.0140 |
NAA: N-acetylaspartate, Ins: myo-inositol, Tau: taurine, tCr: total creatine, creatine + phosphocreatine.
Results
Effect of transgene suppression on cerebellar neurochemical levels in conditional SCA1 mice
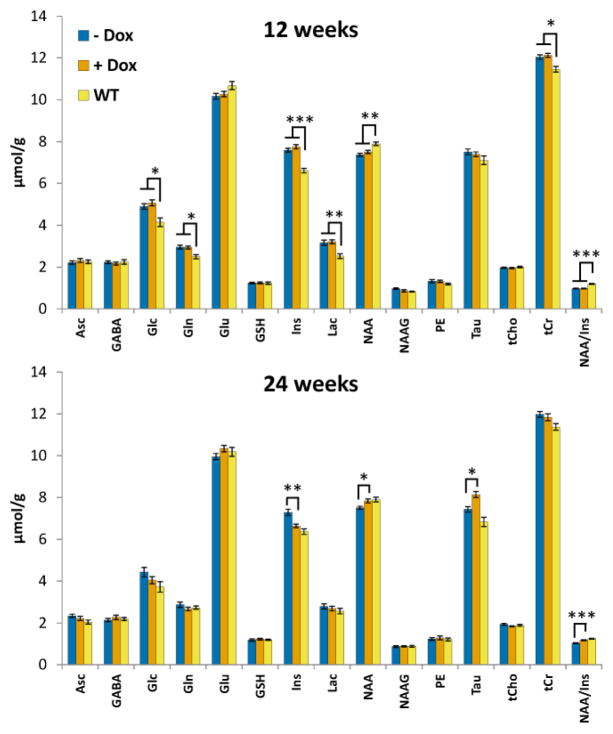
High quality MR spectra (good signal-to-noise ratio, resolution, water/artifact suppression) were obtained from the cerebella of mice in all groups (Fig. 2). The gene-on condSCA1[82Q] mice did not show any indication of atrophy on the conventional T2-weighted images, consistent with prior work (Öz et al., 2011b). On the other hand, a number of neurochemical differences were observed between the condSCA1[82Q] and WT mice starting at 12 weeks, with the strongest differences being the neuronal marker NAA, putative glial marker Ins and lactate at this age (Fig. 3, Table 1). Following treatment with doxycycline, NAA, Ins and their ratio were significantly different between the treated condSCA1[82Q] vs. untreated littermates. Consistently, the levels of these neurochemicals at 24 weeks were no longer different between the treated condSCA1[82Q] mice vs. WT controls while the significant difference remained between the untreated condSCA1[82Q] vs. WT mice (Table 1). Since NAA and Ins were also the neurochemicals that most reliably distinguished patients with SCA1 from controls (Öz et al., 2010a), we focused on their ratio for correlation analyses with the invasive outcome measures (see below).
Figure 3.
Cerebellar neurochemical profiles of the untreated condSCA1[82Q] (− Dox), doxycycline treated condSCA1[82Q] (+ Dox) and WT mice (N=14 per group) at 12 and 24 weeks. Error bars shown are SEM. For simplicity, only the significant differences between the combined (treated + untreated) condSCA1[82Q] mice vs. WT are shown at 12 weeks (since there were no significant differences between the condSCA1[82Q] mice randomly assigned to treatment vs. no treatment before doxycycline administration, see Table 1) and only the significant differences between treated vs. untreated condSCA1[82Q] mice are shown at 24 weeks. * p < 0.05, ** p < 0.001, *** p < 0.0001, p-values corrected for multiple comparisons using the stepdown Bonferroni/Holm approach (Holm, 1979). Asc: ascorbate/vitamin C, GABA: γ-aminobutyric acid, Glc: glucose, Gln: glutamine, Glu: glutamate, GSH: glutathione, Ins: myo-inositol, Lac: lactate, NAA: N-acetylaspartate, NAAG: N-acetylaspartylglutamate, PE: phosphoethanolamine, Tau: taurine, tCho: glycerophosphocholine + phosphocholine, tCr: creatine + phosphocreatine.
In addition to NAA and Ins, taurine was a robust predictor of treatment status in condSCA1[82Q] mice (Fig. 3). Other neurochemicals that were different between condSCA1[82Q] vs. WT mice at 12 weeks, namely glucose, glutamine, lactate and tCr, showed a trend to approach WT values at 24 weeks (Fig. 3). These differences were likely detected at 12 weeks due to the higher statistical power, where 28 condSCA1[82Q] mice were compared with 14 WT mice, while at 24 weeks the comparisons were between 14 mice per group and therefore remained at trend level.
Effect of transgene suppression on motor ability in conditional SCA1 mice
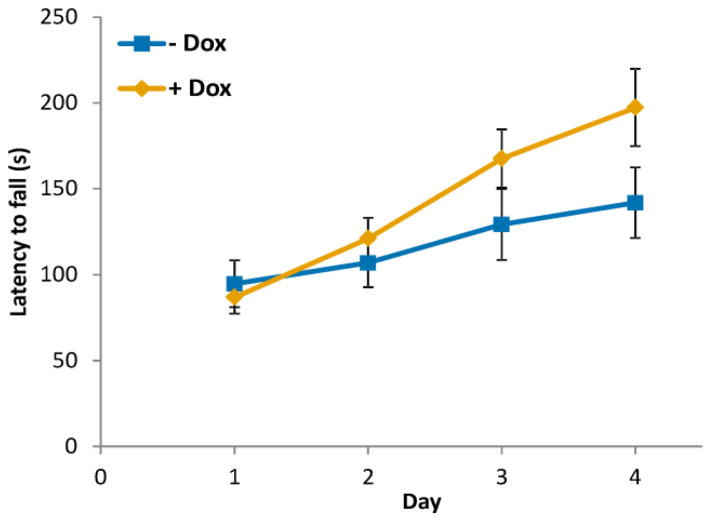
Here we focus only on the treated vs. untreated condSCA1[82Q] mice since the outcome of the Rotarod assessment is sensitive to minor changes in the environment, presenting a confound on comparing groups assessed on different days. In our study design, the treated condSCA1[82Q] mice and their untreated littermates were assessed in parallel on the same days, allowing a robust comparison. The treated condSCA1[82Q] mice performed progressively better than their untreated littermates with each trial day (Fig. 4). However, due to the large variance in the data, the group difference in average latency over all 4 days did not reach significance (p = 0.16, repeated measures ANOVA). A comparison of only the day 4 data revealed the clear trend for the better performance of the doxycycline treated mice (p = 0.08, 2 tailed, 2-sample equal variance t-test), despite the large overlap between the latencies obtained in the two groups (Fig. 5D).
Figure 4.
Effect of transgene suppression on motor behavior in condSCA1[82Q] mice. Rotarod performance progressively improved with each trial day in the treated mice (+ Dox) vs. untreated littermates (− Dox) (N=14 per group), but did not reach statistical significance. Error bars shown are SEM.
Figure 5.
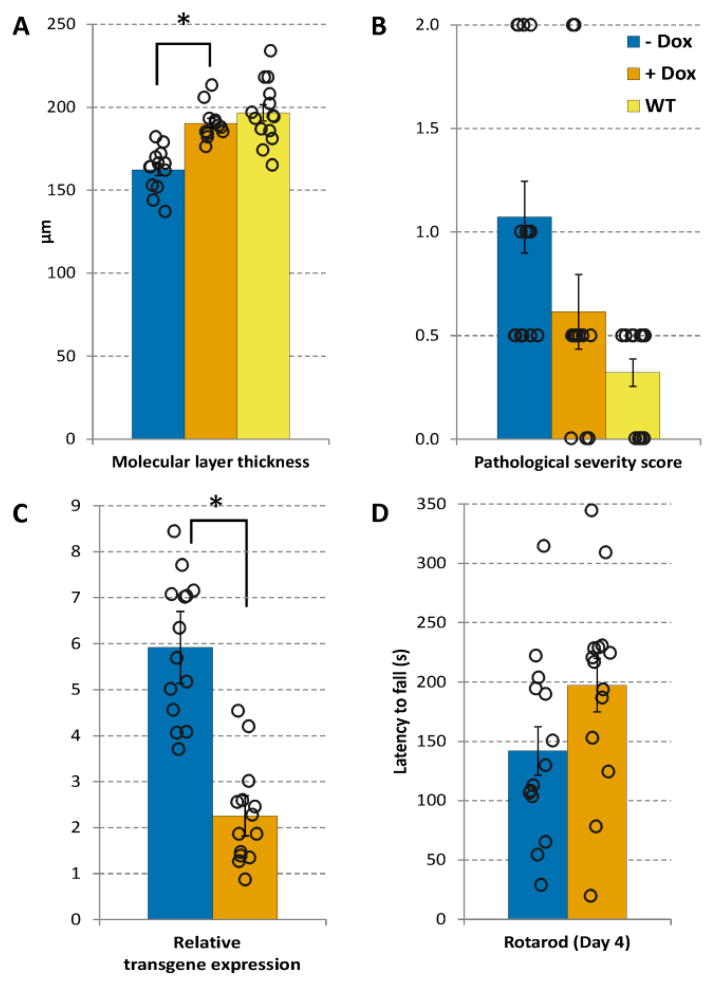
Assessment of transgene suppression in condSCA1[82Q] mice with histology (A, B), qPCR (C) and Rotarod on day 4 (D). Values shown are mean ± SEM in treated (+ Dox) and untreated (− Dox) condSCA1[82Q] and WT mice at 24 weeks. In addition, data from individual mice are overlaid with each bar plot to demonstrate the variability in each measure. The pathological severity score (B) ranges from 0 – 4, with 0 = no pathological changes; 0.5 = near normal but molecular layer (ML) somewhat thinner; 1 = mild changes including heterotopic Purkinje cells and thinning of ML, largely confined to the posterior lobules and 2 = similar to 1 but more widespread; heterotopic PCs more numerous and often higher in the ML (Öz et al., 2010b). * p < 0.0001, unpaired, two-tailed t-test comparison of the treated vs. untreated condSCA1[82Q] mice.
Invasive assessments of transgene suppression in conditional SCA1 mice
Quantitative PCR analysis demonstrated the suppression of transgene expression, with little overlap in relative transgene expression between treated and untreated condSCA1[82Q] mice (Fig. 5C). These data also showed that the doxycycline treatment did not work like an on/off switch and there was residual transgene expression upon doxycycline administration, i.e. a range of efficacies of the treatment, which allowed us to evaluate the correlations of the MRS measures with transgene expression (see below).
As expected, the histology measures of the condSCA1[82Q] mice normalized towards WT levels with treatment. Due to lower variance the ML thickness was more sensitive than the severity score to detect the treatment effect. Namely, the difference between the ML thickness was significantly different between treated vs. untreated condSCA1[82Q] mice (Fig. 5A), while the difference in the severity score remained at trend level (p = 0.08, 2 tailed, 2-sample equal variance t-test).
MRS accurately reflects degree of transgene expression and pathology reversal in SCA1
To investigate the correlations between the invasive and non-invasive outcome measures, data from all 3 groups were pooled. Here we focus only on correlations with p < 0.01 due to the large number of parameters investigated. While both NAA and Ins were individually correlated with ML thickness and transgene expression (e.g. r = −0.43, p = 0.006 for Ins vs. ML thickness and r = 0.42, p = 0.025 for Ins vs. qPCR), the strongest correlations were detected between NAA/Ins vs. ML thickness (r = 0.45, p = 0.004) and between NAA/Ins vs. qPCR (r = −0.59, p < 0.001), indicating that this measure reflects the degree of transgene expression and pathology reversal with doxycycline (Fig. 6).
Figure 6.
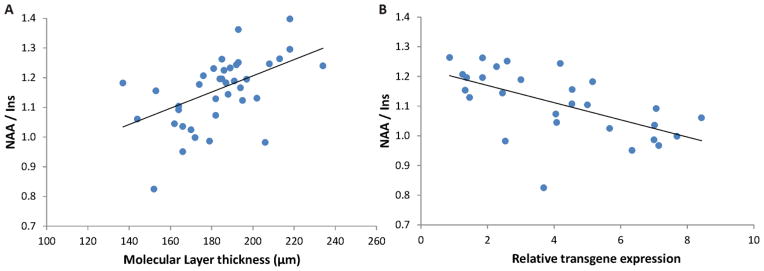
Correlations between A) MRS and histology and B) MRS and qPCR measures. Data from all mice are shown in A), while only data from condSCA1[82Q] mice are shown in B since WT mice have no transgene expression. The NAA-to-myo-inositol ratio is significantly correlated with both molecular layer thickness at the primary fissure and relative transgene expression (p < 0.005).
In addition, taurine levels were significantly correlated with transgene expression (r = − 0.49, p = 0.009) and tCho levels were correlated with latency to fall on Rotarod on day 4 (r = − 0.41, p = 0.008). No other correlations were detected between the outcome measures with a p < 0.01.
MRS has comparable sensitivity to invasive assessments to detect treatment effects in SCA1
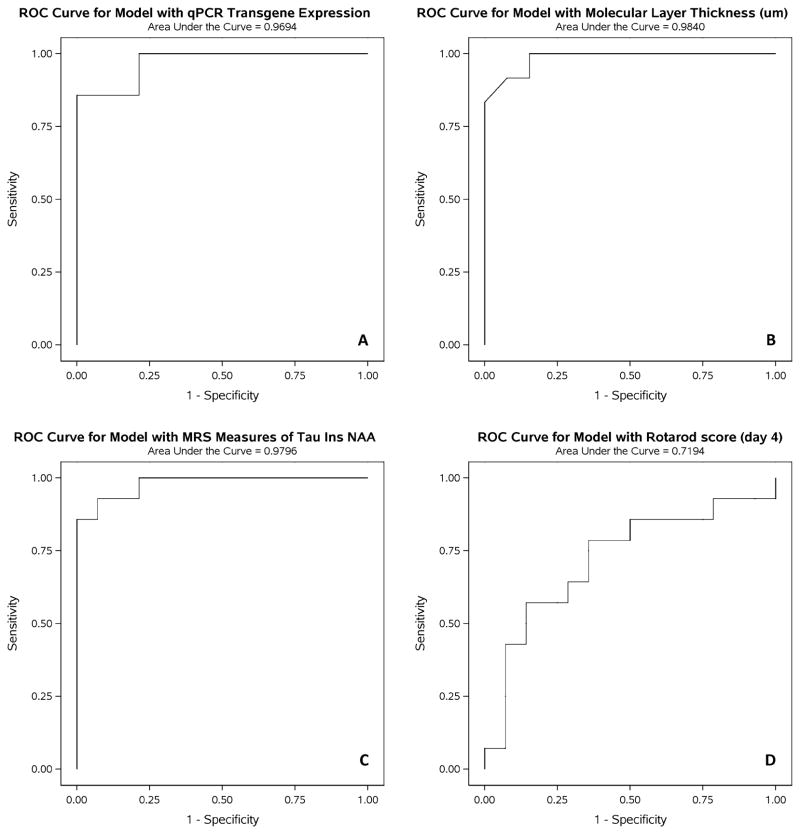
The sensitivity and specificity of the outcome measures to distinguish treated from untreated condSCA1[82Q] mice was investigated using ROC analyses. NAA, Ins and taurine were selected as the MRS measures in this analysis due to the significant differences observed in their levels between treated and untreated condSCA1[82Q] mice (Fig. 3), as well as their correlations with qPCR (Fig. 6), which is the ‘gold standard’ assay to measure treatment efficacy in this model. Since the largest difference between treated and untreated condSCA1[82Q] mice was observed on day 4 of Rotarod testing, these data were used for the ROC analysis. A perfect group separation is indicated with an AUC of 1 in these analyses. The AUCs of the ROC curves for qPCR, ML thickness and MRS (Fig. 7) were all in the 0.97–0.98 range and the ML thickness and MRS curves were statistically not different from the qPCR curve (p > 0.7). The ROC curve for Rotarod had an AUC of 0.72, which was significantly different from the qPCR curve (p = 0.03).
Figure 7.
ROC analyses for the sensitivity and specificity to distinguish the treated from untreated condSCA1[82Q] mice for: A) qPCR, B) histology, C) MRS measures (NAA, myo-inositol, taurine) and D) Rotarod on day 4. The MRS measures have similar sensitivity/specificity to the invasive measures qPCR and histology, all with areas under the curve (AUC) = 0.97 – 0.98, while Rotarod testing has lower sensitivity and specificity.
Discussion
Here we took advantage of a conditional transgenic mouse model to compare the sensitivities of invasive and non-invasive outcome measures to assess treatment effects in SCA1. We had previously shown that MRS is sensitive to disease reversal using the same model (Öz et al., 2011b), however transgene expression was not assessed in that study. Therefore it was not clear how well MRS reflects the level of mutant ataxin-1 expression, a critical question in view of the highly promising therapeutic efforts that involve gene silencing in SCA1 (Keiser et al., 2014; Keiser et al., 2013; Xia et al., 2004). The current study demonstrated that MRS-measured NAA/Ins is strongly correlated with gene expression (Fig. 6) and that the method has comparable sensitivity to histological and gene expression assays to detect treatment effects (Fig. 7). Tremendous effort has been put into developing MRI reporter agents to image gene expression, either during normal brain function or when inhibiting mRNA translation (Ifediba and Moore, 2012; Koretsky, 2012; Vandsburger et al., 2013), however these contrast-based techniques are currently far from clinical translation. Here we are using an MR modality that does not require exogenous contrast agents, is currently available in the clinic and has already been shown to sensitively detect neurochemical alterations in patients with SCAs (Öz et al., 2011a). By quantifying endogenous neurochemicals in selected brain regions, MRS detects the downstream effects of suppressing gene expression, which seem to strongly correlate with the gene expression levels.
The current study further demonstrated that MRS is more sensitive to detect treatment effects than standard motor assessment in rodents. The need to use a large number of animals to detect statistically significant differences with behavioral testing is known anecdotally; this study confirmed this quantitatively using ROC analyses.
Characteristics of the conditional transgenic SCA1 model
The condSCA1[82Q] mice were investigated at a relatively early disease stage based on the pathological assessment (Fig. 5A, 5B). In fact, the mice presented with milder pathology at the same ages relative to our prior study (Öz et al., 2011b), because of downward drift in CAG numbers in the transgenic line with generations. The mild disease stage is more relevant to future human applications as neuroprotective therapies will likely be most effective at early stages (prior to substantial cell loss). In addition, validation of non-invasive imaging methods at an early stage and the demonstration of an ability to detect subtle disease effects and their reversal are critical.
The neurochemical abnormalities detected in the condSCA1[82Q] mice were consistent with prior observations. Based on all our MRS studies in SCA1 mice so far, the earliest changes are observed in taurine, glutamine, tCr and tCho, indicative of osmolytic changes and of disturbances in membrane phospholipid and energy metabolism (Emir et al., 2013). These are followed by changes in the neuronal viability marker NAA, the putative gliosis marker Ins and later by the neurotransmitter glutamate (Öz et al., 2010b). In addition, elevations of glucose and lactate are observed at all disease stages (Öz et al., 2010b) and may indicate deficits in mitochondrial metabolism. Consistent with these prior studies, we observed higher Ins, tCr, glutamine, glucose, lactate and lower NAA levels in the condSCA1[82Q] mice vs. controls (Fig. 3). Unlike prior studies (Emir et al., 2013; Öz et al., 2010b; Öz et al., 2011b), taurine levels in the WT group were lower, rather than higher, than the condSCA1[82Q] mice. The reason for this difference is unclear, but may be related to a different osmolytic status of the tTA mice than the FVB strain, which we had used as the background control for the transgenic SCA1 model in the past (Öz et al., 2010b; Öz et al., 2011b). On the other hand, consistent with prior work, the doxycycline treatment increased taurine levels in the condSCA1[82Q] mice (Fig. 3).
The neurochemicals that showed the strongest response to doxycycline administration were NAA, Ins and taurine; namely NAA and taurine increased and Ins decreased with treatment (Fig. 3). These were the same neurochemicals that distinguished the treated from untreated condSCA1[82Q] mice in the early stage group in our prior study, which received doxycycline from 6–12 weeks (Öz et al., 2011b). This is entirely consistent with the fact that the pathological severity at 24 weeks in the current study was comparable to the pathological severity at 12 weeks in the prior study. We had observed a partial normalization of neurochemical levels in the prior study, which could have been due to a lower dose of doxycycline used leading to an incomplete suppression of transgene expression. The rationale in using the low dose doxycycline chow was that high dose doxycycline had to be administered in sucrose solution, the long term administration of which may cause diabetes. Upon recent demonstration that doxycycline in sucrose solution can be administered for up to 14 weeks without causing diabetes, the current study used the higher doxycycline dose. While the pathological and neurochemical reversal was almost complete in the current study (no statistical differences between the neurochemical levels except taurine and pathology measures of treated vs. WT mice, Table 1, Fig. 5A, 5B), we also observed residual transgene expression with the high dose doxycycline treatment.
Characteristics of invasive and non-invasive outcome measures tested for SCA1
The outcome measures assessed in this investigation reflect different aspects of disease and therefore ideally should be utilized in conjunction in treatment trials. Thus, qPCR measures the expression of the mutant gene and the other modalities measure the downstream effects of this gene modulation, with histology detecting primarily the dendritic atrophy of Purkinje cells (as cell loss only occurs at late stages in these models), MRS measuring alterations in cellular physiology and Rotarod assessing the ultimate symptomatic outcome. While all of these measures are available to pre-clinical investigations of potential treatments in rodents, only imaging and motor assessment are available to assess effects of treatments on the brain for human trials. In addition, invasive measures will also be challenging to use in primate investigations as treatments move through the pipeline towards human testing.
We observed little overlap between treated and untreated condSCA1[82Q] mice in the qPCR, ML thickness and MRS measures, which translated to very high AUC values in ROC analyses for these measures. A perfect classifier is indicated by an AUC of 1 in this type of analysis. Interestingly, our prior pilot investigation demonstrated separation of patients with SCA1 from healthy controls without overlap, i.e. with 100% specificity and sensitivity, when using NAA/Ins (Öz et al., 2010a). Note however that any potential treatments in humans will likely have a smaller effect size, i.e. not completely normalize neurochemical levels to healthy control values, thereby reducing the sensitivity/specificity to detect treatment effects.
There was more variability and greater group overlap in the Rotarod data (Fig. 5D) than ML thickness, qPCR and MRS, leading to lower AUC values in ROC analysis. The outcomes of behavioral assessments can be affected by the individual administering the test and other environmental factors, both in rodents and humans. We took measures to minimize these effects (see Methods), however still observed a large variability in the Rotarod data (Fig. 5D) and differences between treated vs. untreated SCA1 mice remained at trend level. We previously had estimated a great reduction in sample size necessary for a 2-arm interventional trial when using MRS as an outcome measure vs. the standardized ataxia scale used for SCAs (Öz et al., 2011b). The demonstration of a higher sensitivity with MRS than motor testing to detect treatment effects in the current study supports this prediction. Note however that a relatively crude and variable motor function test like the Rotarod is not fully representative of a detailed clinical rating by an experienced clinician. Consistently, MRS-measured NAA, Ins and glutamate levels correlated significantly with scores on the Scale for the Assessment and Rating of Ataxia (Öz et al., 2010a), but they did not correlate with Rotarod outcomes in the current study. Therefore the sensitivities to disease- or treatment-related change over time of MRS vs. clinical ataxia scales need to be further evaluated in humans. Along these lines, we recently reported that MRS measures obtained at 3T are more sensitive to progression of neurodegeneration than clinical assessment in a preliminary analysis of a currently active, longitudinal study of patients with SCA1 (Deelchand et al., 2014b).
Challenges with clinical translation
We used an ultra-high field strength (9.4T) MRI scanner, which is not widely available for clinical trials in humans. However, 3T scanners are widely available in academic centers and all major university hospitals. In fact our patient studies that demonstrated the feasibility to detect neurochemical alterations in patients with SCAs were all performed at 4T (Öz et al., 2010a; Öz et al., 2011a) and recently reproduced at 3T (Emir et al., 2012). Thus, most of what is demonstrated here can be accomplished with 3T technology as long as optimized methods are used for MRS data acquisition and analysis. Another consideration for applicability of an imaging method for trials targeting a rare disease like SCA1 is that it can be utilized in a multi-site setting. We have recently demonstrated that identical neurochemical profiles are obtained from age- and gender-matched healthy populations by different operators at different 3T sites and that multi-site 3T MRS data collected from the cerebellum and the brainstem can be pooled (Deelchand et al., 2014a). Finally, an important limitation of MRS that needs to be addressed prior to utilizing the technique in multi-site clinical trials is the operator-dependence of voxel selection. While the voxel selection was very consistent in the abovementioned 2-site 3T study performed in the research setting (Deelchand et al., 2014a), it may be more variable in a multi-site clinical trial setting, where VOI are selected by rotating MR technologists. Therefore automated methods for VOI selection, as well as protocol execution, are critically needed in the field.
In clinical trials for neurodegenerative diseases like SCA1 some treatments may affect symptoms, but not prevent the progression of the underlying pathology. Conversely, subtle effects of treatments on the pathology may be obscured by the many confounds that may affect behavioral assessments. Therefore, symptomatic assessments that are typically used in clinical trials need to be supplemented by objective measures that reflect the brain pathology and gene expression when targeting the expression of the mutated gene. This study demonstrates the high potential of MRS in this respect.
HIGHLIGHTS.
We studied a conditional transgenic mouse model of SCA1
We compared outcome measures for sensitivity to treatment effects in the brain
In vivo MRS detects treatment effects with sensitivity similar to histology and qPCR
Motor behavioral testing has lower sensitivity
In vivo MRS reflects degree of transgene expression
Acknowledgments
This work was supported by the National Institute of Neurological Diseases and Stroke grants R01 NS070815 and R37 NS022920. Additional support was received from the NINDS Center Core at the University of Minnesota P30 NS062158, National Center for Research Resources (NCRR) biotechnology research resource grant P41 RR008079, National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant P41 EB015894, the Institutional Center Cores for Advanced Neuroimaging award P30 NS076408 and WM Keck Foundation. We thank the staff of the Center for MR Research for maintaining and supporting the MR system, LuAnn Anderson for expert technical help with histology and Drs. Teresa Macheda and Mark Thomas for guidance on the Rotarod assessments.
List of Abbreviations
- SCA1
spinocerebellar ataxia 1
- MRS
magnetic resonance spectroscopy
- qPCR
quantitative polymerase chain reaction
- NAA/Ins
N-acetylaspartate-to-myo-inositol ratio
- AUC
area under the curve
- ROC
receiver operating characteristic
- RNAi
RNA interference
- tTA
tetracycline-responsive activator
- RARE
rapid acquisition with relaxation enhancement
- TR
repetition time
- TE
echo time
- VOI
volume-of-interest
- FASTMAP
fast automatic shimming technique by mapping along projections
- LASER
localization by adiabatic selective refocusing
- RF
radiofrequency
- VAPOR
variable power RF pulses with optimized relaxation delays
- CRLB
Cramér-Rao lower bounds
- tCr
total creatine
- tCho
total choline
- ML
molecular layer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, Duvick LA, Zoghbi HY, Orr HT. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–48. doi: 10.1016/0092-8674(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Clark HB, Burright EN, Yunis WS, Larson S, Wilcox C, Hartman B, Matilla A, Zoghbi HY, Orr HT. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17:7385–95. doi: 10.1523/JNEUROSCI.17-19-07385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelchand DK, Adanyeguh IM, Emir UE, Nguyen TM, Valabregue R, Henry PG, Mochel F, Öz G. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single voxel MRS at 3 T. Magn Reson Med. 2014a doi: 10.1002/mrm.25295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelchand DK, Emir UE, Hutter D, Gomez CM, Eberly LE, Bushara KO, Öz G. High field MRS is more sensitive to progression of neurodegeneration than clinical decline in spinocerebellar ataxia type 1 (SCA1). Proc Intl Soc Mag Reson Med; Milan, Italy. 2014b. p. 64. [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- Emir UE, Brent Clark H, Vollmers ML, Eberly LE, Öz G. Non-invasive detection of neurochemical changes prior to overt pathology in a mouse model of spinocerebellar ataxia type 1. J Neurochem. 2013;127:660–668. doi: 10.1111/jnc.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emir UE, Hutter D, Bushara KO, Gomez CM, Eberly LE, Öz G. MRS biomarkers of neurodegeneration in spinocerebellar ataxia type 1 (SCA1): Current and future potential. 20th Scientific Meeting of the ISMRM; Melbourne, Australia. 2012; p. 1802. [Google Scholar]
- Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153:155–77. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med. 1986;3:823–33. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Bonferroni Test Procedure. Scand J Statistics. 1979;6:65–70. [Google Scholar]
- Ifediba MA, Moore A. In vivo imaging of the systemic delivery of small interfering RNA. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:428–37. doi: 10.1002/wnan.1158. [DOI] [PubMed] [Google Scholar]
- Keiser MS, Boudreau RL, Davidson BL. Broad therapeutic benefit after RNAi expression vector delivery to deep cerebellar nuclei: implications for spinocerebellar ataxia type 1 therapy. Mol Ther. 2014;22:588–95. doi: 10.1038/mt.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser MS, Geoghegan JC, Boudreau RL, Lennox KA, Davidson BL. RNAi or overexpression: alternative therapies for Spinocerebellar Ataxia Type 1. Neurobiol Dis. 2013;56:6–13. doi: 10.1016/j.nbd.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, Artates JW, Weiss A, Cheng SH, Shihabuddin LS, Hung G, Bennett CF, Cleveland DW. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–44. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretsky AP. Is there a path beyond BOLD? Molecular imaging of brain function. Neuroimage. 2012;62:1208–15. doi: 10.1016/j.neuroimage.2012.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae-Poetscher LM, Bonekamp D, Barker PB, Brant LJ, Kaufmann WE, Horska A. Asymmetry and gender effect in functionally lateralized cortical regions: a proton MRS imaging study. J Magn Reson Imaging. 2004;19:27–33. doi: 10.1002/jmri.10429. [DOI] [PubMed] [Google Scholar]
- Öz G, Hutter D, Tkáč I, Clark HB, Gross MD, Jiang H, Eberly LE, Bushara KO, Gomez CM. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 2010a;25:1253–1261. doi: 10.1002/mds.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Iltis I, Hutter D, Thomas W, Bushara KO, Gomez CM. Distinct Neurochemical Profiles of Spinocerebellar Ataxias 1, 2, 6, and Cerebellar Multiple System Atrophy. Cerebellum. 2011a;10:208–17. doi: 10.1007/s12311-010-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Nelson CD, Koski DM, Henry PG, Marjanska M, Deelchand DK, Shanley R, Eberly LE, Orr HT, Clark HB. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2010b;30:3831–8. doi: 10.1523/JNEUROSCI.5612-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Vollmers ML, Nelson CD, Shanley R, Eberly LE, Orr HT, Clark HB. In vivo monitoring of recovery from neurodegeneration in conditional transgenic SCA1 mice. Exp Neurol. 2011b;232:290–8. doi: 10.1016/j.expneurol.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–5. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- Tkáč I, Starcuk Z, Choi I-Y, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Vandsburger MH, Radoul M, Cohen B, Neeman M. MRI reporter genes: applications for imaging of cell survival, proliferation, migration and differentiation. NMR Biomed. 2013;26:872–84. doi: 10.1002/nbm.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–20. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Orr HT. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1. J Biol Chem. 2009;284:7425–9. doi: 10.1074/jbc.R800041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, Orr HT. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J Neurosci. 2004;24:8853–61. doi: 10.1523/JNEUROSCI.2978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]