Abstract
To narrow the gap in our understanding of potential oxidative properties associated with Electronic Nicotine Delivery systems (ENDS) i.e. e-cigarettes, we employed semi-quantitative methods to detect oxidant reactivity in disposable components of ENDS/e-cigarettes (batteries and cartomizers) using a fluorescein indicator. These components exhibit oxidants/reactive oxygen species reactivity similar to used conventional cigarette filters. Oxidants/reactive oxygen species reactivity in e-cigarette aerosols was also similar to oxidant reactivity in cigarette smoke. A cascade particle impactor allowed sieving of a range of particle size distributions between 0.450 and 2.02 μm in aerosols from an e-cigarette. Copper, being among these particles, is 6.1 times higher per puff than reported previously for conventional cigarette smoke. The detection of a potentially cytotoxic metal as well as oxidants from e-cigarette and its components raises concern regarding the safety of e-cigarettes use and the disposal of e-cigarette waste products into the environment.
Keywords: electronic cigarettes, ENDS, ROS, oxidants, copper, EPR
1. Introduction
Investigation of the safety of electronic cigarettes (e-cigarettes) and related products, or Electronic Nicotine Delivery Systems (ENDS), is currently increasing (Printz, 2014). Classes of ENDS include disposable/ non-refillable e-cigarettes, refillable e-cigarettes, refillable e-pens, e-hookah pens, e-cigars and other fast-emerging products. While there is a growing body of research on the direct health hazards of e-cigarette use, there is a dearth of research on the potential environmental health hazards posed by improper disposal of these devices (Chang, 2014). Major components comprising a typical ENDS e-cigarette include a lithium-ion battery (LIB), light-emitting diode (LED) lights, microprocessor, metal casings, wires, plastics, and other absorbent polymers that stabilize components and secure vaporizable liquids to retain them inside the device. Most of these parts may come into contact with toxic contaminants detected in e-cigarettes, and should be disposed of properly (Goniewicz et al., 2014a; Goniewicz et al., 2014b). Disposal procedures and guidelines for ENDS are currently not subjected to government oversight which may be necessary to avoid potential public health risks that have yet to be fully assessed and made available to consumers. This potential problem is of particular concern given the continuing increase in the number of ENDS consumers (CDC, 2013).
Many ENDS replicate the size and appearance of conventional tobacco cigarettes while others involve “chambers” that are able to be easily refilled with larger volumes of vaporizable solvents. Each proprietor of ENDS may include variable ingredients, such as flavors, nicotine, and other additives to enhance the ENDS experience, as well as glycerin, propylene glycol, and polyethylene glycol that are predominantly used in the e-cigarette industry to produce aerosols (Drummond and Upson, 2014). The potential toxicities of these substances and other unregulated additives that could absorb into the skin or be vaporized for inhalation are incompletely understood (Orr, 2014).
With conventional cigarettes, the majority of known health related hazards are due directly to smoking and second hand exposure. Many of the dangers of tobacco use are well established, and proper tobacco cigarette disposal is an ongoing environmental concern (Novotny et al., 2009). The emergence of ENDS presents new challenges for public health officials both for individual users’ health and those near exposure ranges, and for how ENDS components are disposed of. Therefore, there is both a health risk concern as well as an environmental hazard concern. ENDS/e-cigarettes typically employ a battery power source, and both rechargeable and non-rechargeable varieties are readily available. It is difficult to forecast future consumption rates of both reusable (rechargeable) and disposable (non-rechargeable) ENDS or the technological evolution of vaporization/nicotine delivery methods and power sources.
Lithium-ion batteries (LIB) are the primary power source for ENDS as well as many other popular electronic devices. It is not clear which LIB technology is typically used in ENDS or if different manufacturers employ preferred LIB types. Some of the components found in LIBs include heavy metals that are known to have toxic effects on living organisms (Kang et al., 2013). The projected amount of disposal for LIBs may increase substantially with growing ENDS consumerism.
The second component that is liable to high rates of disposal comprises the replaceable cartridges (cartomizers) that contain the vaporizable liquid. The cartomizer combined with the battery in many instances is designed to resemble a conventional cigarette. Cartomizers would be expected to be discarded more frequently in regular waste or as litter relative to batteries. This would only add to ongoing cigarette filters (butt) disposal and potentially introduce new chemicals that accumulate alongside those in cigarette butts which are harmful to animals and flora (Moriwaki et al., 2009; Slaughter et al., 2011). Unfortunately, there is no information for ENDS litter to make comparisons to conventional cigarettes and their toxic impact on environments and habitats.
The present study investigated using a semi-quantitative measurement of oxidants/reactive oxygen species (ROS) from e-cigarette components (cartomizers and batteries) as compared to conventional cigarette butts to assess if there is a potential for environmental exposure to these materials which are expected to contribute to tobacco waste and environmental pollution. We extended our studies to detect the presence of oxidants/ROS associated with e-cigarette aerosols and measured aerosol particle size distribution and copper levels to assess if there is a potential concern for oxidants/ROS induced toxicity when inhaling ENDS/e-cigarette aerosols.
2. Materials and Methods
2.1. Cigarette products assessed
The following types of electronic vaporizing devices and conventional cigarettes, such as Blu® electronic cigarettes (Lorillard Technologies, Inc.), eGO Vision® Spinner (Vision High-Tech Electronics Limited, Shenzhen, Guangdong, China), Marlboro® 100s (Philip Morris USA Inc.), and Kentucky 3R4F reference cigarettes (Tobacco Research Institute, University of Kentucky, Lexington, KY) were used in this study.
2.2. E-cigarette components and detection of their oxidant reactivity
We obtained used cartomizers from e-cigarette users involved in a larger study examining e-cigarette emissions as a function of consumer behavior (Dr. Risa Robinson, Rochester Institute of Technology, Rochester, NY). The products were rechargeable Blu e-cigarettes (batteries, n=7 and cartomizer, n=17) used over a 24 hour period and then returned to the lab. E-cigarette cartomizers were disassembled and metal casings separated. All internal materials removed from the cartomizer metal housing, including polyfill absorbent material, wicking material, heating elements and electrical wires, silicone caps, and residual e-cigarette fluid absorbed were submerged in 2'-7'-dichlorodihydrofluorescein (DCFH) solution for 5 hours. For filter oxidant reactivity, conventional cigarette filters were removed from cigarettes either unused or following tobacco smoke filtering. A laboratory vacuum line was used to replicate puffs every 30 seconds for 4–5 seconds until the cigarette was consumed. Both unused and smoke exposed filters were placed in DCFH solution for 5 hours. Non-functional LIBs were placed in DCFH solution for 5 hours. All incubations carried out at room temperature in darkness. For cartomizers and LIBs, equal volumes of DCFH solution placed in test tubes alone for 5 hours were considered controls. The DCFH solution was measured for fluorescence as for e-cigarette vapor and conventional cigarette smoke (see section 2.3).
2.3. Cell-free oxidants/reactive oxygen species (ROS) assay
The relative levels of ROS produced from electronic cigarette vapor or smoke from filtered tobacco cigarettes was determined using a semi-quantitative measurements of oxidative/reactive oxygen species (ROS) by 2’,7’dichlorofluorescein diacetate (H2 DCF-DA) fluorogenic probe (EMD Bioscience, CA). Oxidation of DCFH (derived from H2 DCF-DA) converts it into fluorescent molecule (DCF) indicating the presence of free radicals such as ROS (i.e. H2O2) or potentially other reactive oxidants (Black and Brandt, 1974; Myhre et al., 2003). For each exposure, 5 ml of dichlorofluorescein-horse radish peroxidase (DCFH-HRP) solution developed to assess oxidant reactivity in cell free systems (Hung and Wang, 2001; Jiang et al., 2008), was loaded into a clean glass bubbler (prism research). A lab pump (FMI, Syosset, NY) with a flow range of 0–1296 ml/min was switch activated using an FMI stroke rate controller set at 60% flow to draw a steady stream of e-cigarette vapor/tobacco cigarette smoke directly through the DCFH solution (Fig. 1). E-cigarette vapor was puffed through DCFH solution in the bubbler at room temperature for 4–5 seconds at 30 second intervals for a total of 10 minutes (Hua et al., 2013). For filtered tobacco cigarettes, 4–5 seconds of smoke was drawn through DCFH at 30 second intervals for 5 minutes (approximately 2 cigarettes to minimize tar and particulate build up). All tobacco cigarettes (3R4F, Marlboro 100s) were combusted within an approved chemical flow hood and the samples protected from direct and ultraviolet light to prevent photo-auto-oxidation of DCFH. Following exposures, sample tubes were placed on ice and protected from light sources until analysis. A spectrofluorometer was used to measure oxidized dichlorofluorescein (DCF) fluorescence at an absorbance/emission maximum of 485 nm/535 nm. Hydrogen peroxide standards between 0 and 50 μM were created from 1 M stock and reacted at room temperature for 10 minutes with prepared DCFH solution in a total of 5 ml. These standards were then used to calibrate spectrofluorometer fluorescence intensity units to numerically match respective hydrogen peroxide concentrations that produce increasing amounts of DCF fluorescence in the presence of horseradish peroxidase (HRP).
Fig. 1. Activation of the pump initiates pressure changes within the e-cigarette which automatically activates the battery.
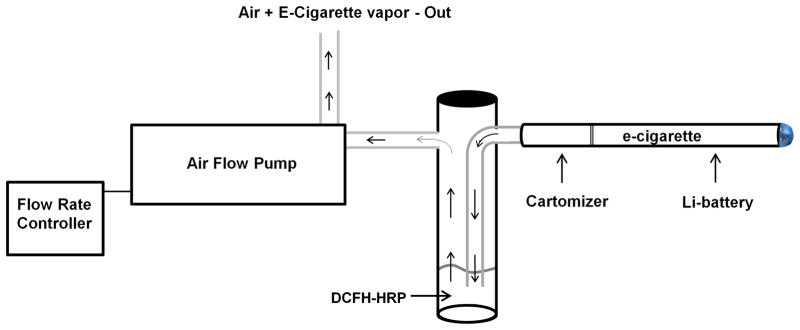
A current is subsequently delivered to the heating element within the cartomizer and the liquid housed within is vaporized. Air and vapor flow into the glass bubbler (impinger) containing the DCFH solution through tubing that is coupled to the cartomizer. The air and vapor finally passes through the DCFH solution, through the pump, and exits the apparatus via additional tubing.
All ENDS/e-cigarette DCFH reactions were carried out in a dark room at room temperature for 10 minutes which was adequate to produce stable fluorometric readings. The DCF data are expressed as μM H2O2 equivalents which refers to the exact concentration of the H2O2 added to the DCFH solution. Fluorescence values indicate the amount of non-fluorescent DCFH converted to fluorescent DCF involving H2O2 or possibly other ROS such as those associated with cigarettes (Huang et al., 2005; Myhre et al., 2003; Pryor and Stone, 1993).
2.4. Electron paramagnetic resonance (EPR) analysis
ENDS/e-cigarette aerosols (Federal Trade Commission protocol) using a CSM-SSM machine (CH-Technologies Inc.) were collected in quartz EPR sample tubes (4 mm inner diameter, Wilmad LabGlass, Buena NY). Quartz wool (30 mg) was packed into the bottom 2.5 cm of each sample tube, and the tube was then immersed in liquid nitrogen. The aerosols were then pumped through long glass pasture pipettes into the bottoms of the EPR sample tubes. Pumping was performed for durations of 4–5 seconds at a time with 30 second intervals in between for a total of 10 minutes. After sample collection was complete, each EPR sample tube was quickly transferred into a liquid nitrogen finger Dewar situated in the EPR dual cavity (Varian). Thus radicals produced in the aerosols were kept between 80–90K for the duration of the experiment. EPR spectra were obtained using a Bruker 200ER X-band EPR spectrometer. A Cu (EDTA) standard was used to quantify the spin concentration of each sample. An ambient air blank was also obtained using the same collection scheme as for the ENDS/e-cigarette aerosols to verify that the collection method did not introduce any background signal.
2.5. Determination of particle size distribution and copper in e-cigarette aerosol
A cascade particle impactor ( consisting of 7 deposition stages with effective cut-off diameters (ECDs) of 0.45, 0.72, 1.27, 2.02, 3.38, 5.58, and 9.15 microns was setup with a consistent flow rate of 5.0 L/min of medical grade air. A lab pump was used to pulse two 4 second Blu e-cigarette puffs through the impactor at the same setting flow rate set for DCF measurements on the stroke rate controller. Particle deposition for each stage was weighed and the mass median aerodynamic diameter (MMAD) determined after generating a log probability plot using the particle weighed values (Christopher et al., 2010). To determine if copper particles are present in the Blu e-cigarette, 4 puffs (4 seconds/puff) of aerosols using the Federal Trade Commission protocol setting using a CSM-SSM machine (CH-Technologies Inc.) were collected on a methyl-cellulose filter for quantitative analysis using a Pinnacle 900 Atomic Absorption Spectrometer (PerkinElmer, MA).
2.6. Statistical Analysis
Probability of significance compared to control was based on unpaired 2-tail t-tests and indicated in figure legends. All data are shown as means ± SD.
3. Results
3.1. Detection of oxidants/ROS from cartomizer components
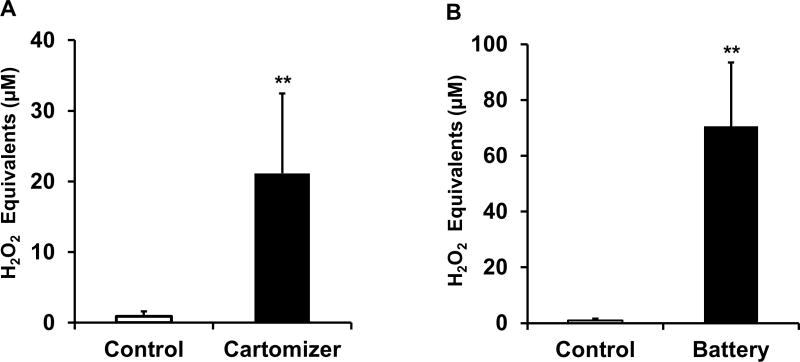
Cartomizer components were immersed into DCFH solution for 24 hours. Following immersion, we observed a significant increase in DCF (oxidized DCFH) fluorescence indicating the presence of ROS (Fig. 2A,B). Levels of ROS from used cartomizer components as compared to unused ones were not significantly different (data not shown), suggesting that both new and used cartomizers are a potential source of oxidants.
Fig. 2. Oxidants/ROS associated with e-cigarette components.
Immersion of e-cigarette components in DCFH solution (see Materials and Methods). Y axis - measurement of DCF fluorescence, (A) Components removed from cartomizer casing and placed in DCFH solution (n=12), Control; DCFH solution alone (n=3) (B) Non-functional lithium-ion battery of e-cigarette placed in DCFH solution (n=4) Control; DCFH solution alone (n=3). Data are shown as mean ± SD. **P< 0.01. Significant compared to control based on unpaired 2-tail t-test.
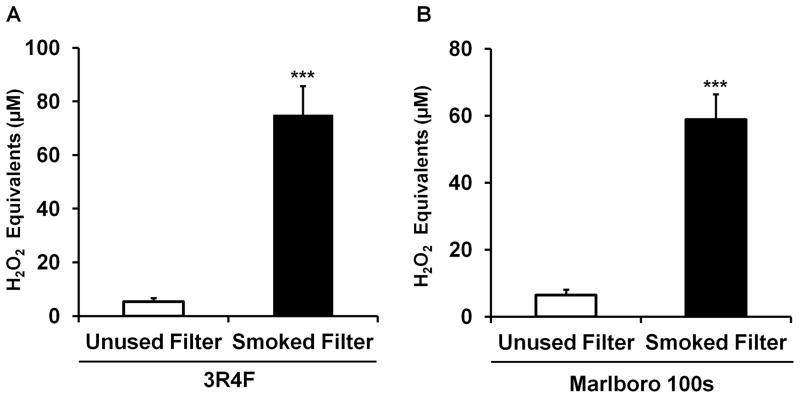
We next compared ROS reactivity in two different brands of used conventional cigarette filters (Moerman and Potts, 2011; Novotny et al., 2009; Novotny and Zhao, 1999; Patel et al., 2013; Pryor et al., 1998). As expected, ROS from smoked cigarette filters/butts produced a significant increase in DCF fluorescence for research grade reference cigarette (3R4F) and a significant increase in DCF fluorescence for Marlboro 100s (Fig. 3A,B). Together, these data suggest that both used conventional cigarette filters/butts and replaceable e-cigarette components harbor oxidative properties. Thus, oxidants remain inside the e-cigarette cartomizer and potentially on the outside after an individual takes a draw from the e-cigarettes.
Fig. 3. Oxidants/ROS levels in conventional cigarette filters.
(A,B) Filters removed from cigarettes following consumption and immersed in DCFH solution (n=3). Unused cigarette filters immersed in DCFH solution (n=3) serve as reference control. Data are shown as the mean ± SD. ***P<0.001.
3.2. Oxidants/ROS levels of defunct rechargeable e-cigarette lithium-ion battery
In order to test if oxidants might be associated with e-cigarette battery components, we inundated four of the used e-cigarette batteries with DCFH solution and submerged them in the same solution for 24 hours. We observed that the used batteries exhibited evidence of ROS reactivity similar to both e-cigarette cartomizers (Fig. 2A,B), and used conventional cigarette filters (Fig. 3A,B). Although we do not have detailed information referring to the major components of the e-cigarette battery compartment, these results suggest that ENDS lithium-ion batteries undergo local oxidation-reduction activity when placed in an aqueous environment.
3.3. Detection of Oxidants/ROS in e-cigarette aerosols
Since we detected ROS associated with e-cigarette cartomizers, LIBs, and used conventional cigarette filters (butts), we next investigated whether or not e-cigarette aerosols produced from e-liquid vaporization also contains oxidants/ROS that might be inhaled by consumers. To probe for oxidizing agents in ENDS/e-cigarette aerosols produced from e-cigarettes, the aerosols were drawn directly into the DCFH solution. To both activate the e-cigarette battery and subsequently draw aerosols into DCFH solution, an apparatus including an electrically controlled air flow pump was assembled such that the mouthpiece of the e-cigarette (cartomizer containing vaporizable fluid and vaporizing components) is coupled to a glass bubbler containing the DCFH solution (Materials and Methods) by rubber tubing (Fig. 1). Travel distance of the aerosols drawn from the e-cigarette into the DCFH solution is approximately 20 cm which replicates the average distance from the mouth to the bifurcation of the trachea in adult humans (Sitzwohl et al., 2010). Exposure of DCFH solution to e-cigarette aerosols resulted in a significant increase in DCF fluorescence relative to an equivalent volume of ambient air (Fig. 4A). As expected, a significant increase in DCF fluorescence was also measured following DCFH exposure to conventional cigarette smoke from both research grade reference cigarettes and Marlboro 100s (Fig. 4B). Overall, these data suggests that ROS are produced by a popular e-cigarette and these oxidants travel concurrently within the ENDS aerosols over a distance similar to that of the distance from the mouth to the lung.
Fig. 4. Detection of ROS in vapor produced from e-cigarettes.
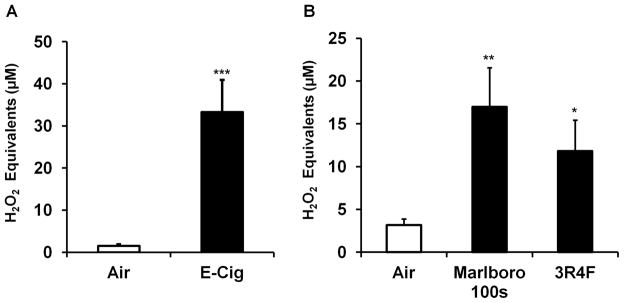
E-cigarette vapor or conventional cigarette smoke bubbled into DCFH solution and DCF fluorescence measured as described in Materials and Methods. (A) E-cigarette (E-Cig) aerosols (10 minute exposure period) produced from a single Classic Tobacco flavor cartomizer with nicotine (n=3), compared to ambient air control (n=4) (B) Conventional cigarette smoke (5 minute exposure period) from brand name or research grade cigarettes (n= 3), compared to air sham control (n=3). Data are shown as means ± SD.*P<0.05; **P<0.01; ***P<0.001 vs. Air group.
3.4. Detection of free radical species from ENDS/e-cigarette aerosols by electron paramagnetic resonance
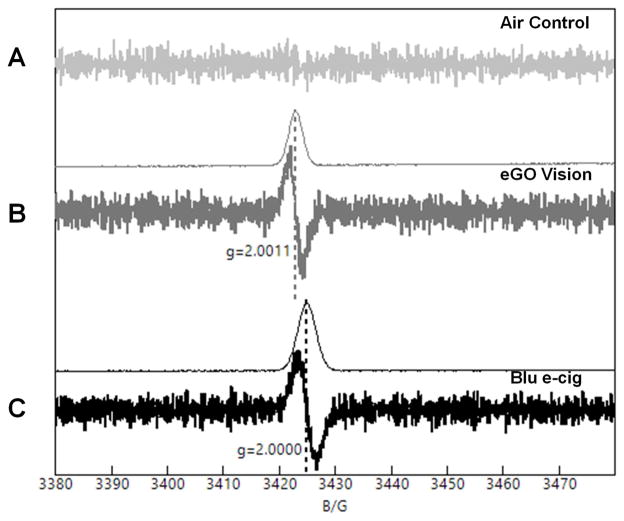
Electron paramagnetic resonance (EPR) is a sensitive technique for the detection and quantification of species that contain unpaired electrons, including organic radicals and paramagnetic metal ions. We used EPR to detect radicals produced in aerosols from a Blu e-cigarette (Classic tobacco, 16 mg nicotine) and refillable ENDS vaporizer (eGO Vision spinner) loaded with ECTO tobacco flavored e-liquid (18 mg nicotine). Aerosols produced by the devices were collected by adsorption onto quartz wool inside an EPR sample tube. EPR spectra of the resulting samples showed that both devices produced aerosols containing radical species (Fig. 5 Panel A–C). The EPR g-values of both samples are consistent with the production of organic, carbon-centered radicals. A comparison of the spectra for the two devices shows that the spectrum of the Blu sample (Fig. 5C) shows a slightly wider EPR signal than the eGo vaporizer sample (Fig. 5B). This suggests that the aerosols produced by the Blu e-cigarette contain a greater variety of free radical species than the eGo vaporizer. The spin concentration was estimated by double integration and comparison with a Cu(EDTA) standard to be 4.8 μmol and 7.7 μmol, respectively, for the eGo and Blu devices. This indicates that the device construction can have a significant impact on the types and amounts of radical species produced.
Fig. 5. Electron paramagnetic resonance detection of radicals in ENDS aerosols.
EPR Spectra of aerosols produced from (A) Air (control), and the experimental groups: (B) eGO Vision refillable ENDS (filled with ECTO tobacco e-liquid 18 mg nicotine), and (C) Blu e-cigarette (Classic tobacco, 16 mg nicotine). Spectra were obtained on an a Bruker 200ER X-Band EPR spectrometer using the following instrument parameters: Modulation: 100 kHz, Microwave Freq.: 9.587 GHz, Power: 2 mW, Modulation Amplitude: 4.0 G, Scan range: 100 G, Time constant: 40.96 msec, Sweep Time: 167.77 seconds, Receiver gain: 3.56 x 104. The thin lines above each spectrum represent the first integral of the data (the calculated absorbance spectrum). The Y-axis represents the first derivative of the EPR signal from the static magnetic field. The X-axis represents the range of increasing magnetic field strength in which the EPR signal is detected.
3.5. Particle size distribution and detection of copper in e-cigarette aerosols
Particle sizes measured in aerosols from the Blu e-cigarette resulted in stage impactor distribution patterns ranging from 0.45 to 0.72 μm. The impactor stage cutoff values resulted in a 1.03 Mass Median Aerodynamic Diameter (MMAD). Therefore, 50% of the particles by mass in the e-cigarette aerosol fall within the submicron size range since they are less than 1 μm in diameter. The polydispersity of the aerosols (spread of different size particles) is reflected by a Geometric Standard Deviation (GSD) of 1.71. Next, we sought to detect if copper is among the aerosol constituents since it has been detected in e-cigarette aerosols from a different brand of e-cigarette that has similar levels of copper compared to levels of copper in tobacco smoke (Williams et al., 2013). The amount of copper in Blu e-cigarette aerosols analyzed by atomic absorption averaged 116.79 ± 83.59 ng/puff (Fig. 6). This data includes a relatively large range of copper ng values/puff between experimental replicates. Aerosol copper levels varying from 24.3 to 224.7 ng/puff were observed.
Fig. 6. Detection of copper nanoparticles in vapors produced by e-cigarettes.
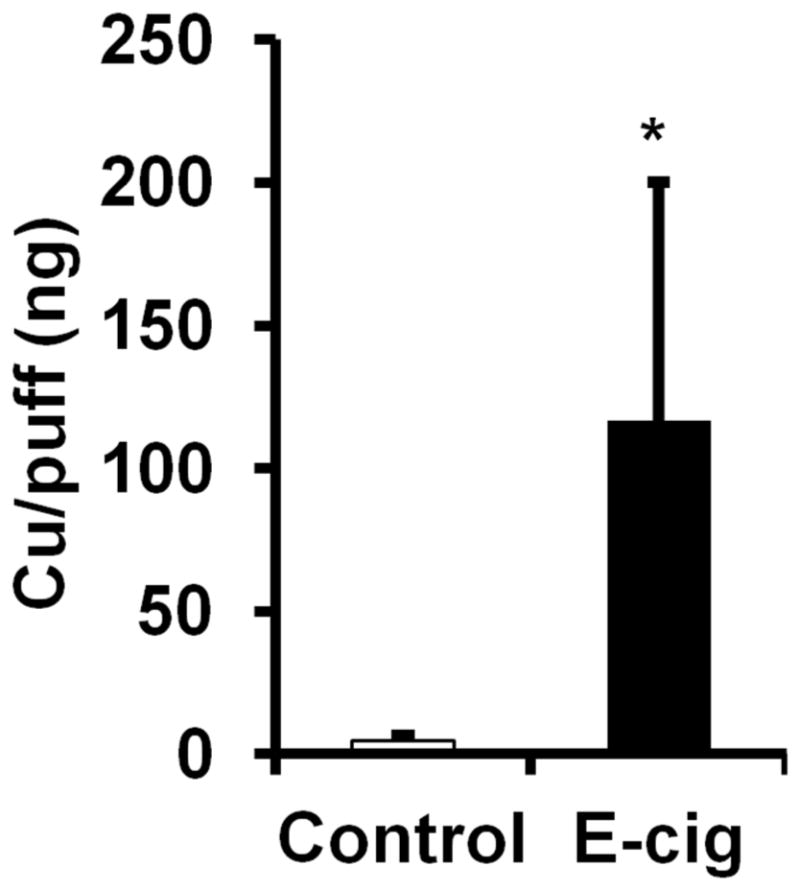
Amount of copper measured in Blu e-cigarette (E-cig) aerosols per 4 second puff (n=4), Control; amount of copper measured on nitrocellulose filter not exposed to aerosols (n=4). Data are shown as mean ± SD, *P<0.05 significant compared to control.
4. Discussion
In this study, we tested for the presence of oxidants/ROS in ENDS products (aerosols, components). The ROS detected are associated with popular ENDS/e-cigarette devices and also accompany the disposal of exhausted components (cartomizers and batteries). Although there are very little experimental data for health care professionals or environmental scientists to attain a strong position regarding recommendations for ENDS use and disposal, the oxidants accompanying the e-cigarette tested might pose unforeseen future public health and environmental risks that warrant further study initiatives.
There is an extensive literature on harmful compounds found in conventional cigarette ingredients and smoke (Lofroth, 1989; Rodgman and Perfetti, 2013). Some of the major constituents of both cigarette smoke and tar are characterized as oxidants which are also associated with markers of oxidative damage in the lung tissue of smokers (Rahman and MacNee, 1999). Our data show that conventional cigarette smoke and Blu e-cigarette aerosols exhibited oxidant reactivity that fell within a mean total range of 11.8 μM to 33.3 μM of H2O2, the lowest mean value referring to research grade (3R4F) cigarettes (5 minute smoke exposure period to DCFH) and the highest mean value referring to the e-cigarette aerosols (10 minute aerosol exposure period to DCFH). Despite the reduced exposure time of cigarette smoke to DCFH solution, we initially yet expected much higher values of H2O2 μM equivalents (DCF fluorescence) as measured from the conventional cigarette smoke compared to e-cigarette aerosols due to the high concentration of oxidants that comprise cigarette smoke (Pryor and Stone, 1993).
Since smoked cigarette filters are one of the most littered and frequently disposed forms of polluted consumer waste (Moerman and Potts, 2011; Novotny et al., 2009; Novotny and Zhao, 1999; Patel et al., 2013), we compared the reactivity of the e-cigarette cartomizer to used cigarette filters/butts. Cigarette tar, which collects in the filters, is known to contain stable oxidants/ROS that can liberate highly reactive forms of ROS after the tar is immersed into an aqueous solution (Pryor et al., 1998). The oxidants/ROS we detected in e-cigarette components, and conventional cigarette smoke/filters, are all measurable below 100 μM H2O2 equivalents using this particular DCFH assay. Though collection methods slightly differed, our ROS values for conventional cigarette smoke and filters, are relatively close in measurement to cigarette ROS gas phase and ROS particle phase values reported previously by using the acellular DCFH approach (Zhao and Hopke, 2012). It should be noted that though Zhao and Hopke reported their ROS levels in total nmol, the values are exchangeable with μM concentration when converting their data based on the volume of DCFH solution they used (10 mL). It is also important to note that the comparative levels of fluorescence values obtained for both e-cigarette aerosols and conventional cigarette smoke for example, likely reflect the semi-quantitative aspect of the DCFH assay to measure oxidants (Bonini et al., 2006; Jakubowski and Bartosz, 2000). We ruled out the possibility of artifact in the oxidation of DCFH by ENDS/e-cigarette aerosols by utilizing electron paramagnetic resonance (EPR) as an orthogonal technique for quantification of radical species. Precise measurements of free radical levels in cigarette smoke have previously been determined using EPR (Nakayama et al., 1989; Pryor et al., 1983; Pryor and Stone, 1993). The detection of free radicals in ENDS/e-cigarettes aerosols by EPR supports the data of acellular DCFH approach to detect radical oxidants. Thus, the DCF fluorescence data should be interpreted as indicative of oxidant presence, but not an accurately direct measurement of specific ROS levels until further EPR studies are compared to increases in DCF fluorescence relative to the amount of ENDS aerosols exposed to the DCFH solution.
According to the manufacturer of the e-cigarette used in this study, each rechargeable LIB is designed to be charged and discharged approximately one thousand times or is expected to be functional for one year before replacement. Although we did not closely monitor charge/discharge cycles of the e-cigarette batteries, we have collected over five batteries that have ceased to retain the capacity to be recharged and have purchased less than ten rechargeable e-cigarette units total within the past year. We estimate that each of the five defunct batteries that have been collected from previous experiments in our hands have been recharged less than one hundred times before failing to hold a charge or produce vapor from their compatible cartomizers. Therefore, the potential for discarding or disposal of e-cigarette batteries could occur at higher than expected frequencies by consumers, and further aggravate the hazard on environmental pollution.
Copper is among other elemental constituents (such as iron, aluminum, and sodium) identified in aerosol volumes from a disposable e-cigarette (Williams et al., 2013). The level of copper we measured in Blu e-cigarette aerosols exceeds levels of copper reported by Williams et al by approximately an 83% increase (0.117 μg/puff vs. 0.020 μg/puff). Additionally, the average amount of copper we detect in Blu e-cigarette aerosol is 6.1 times higher than reported for conventional cigarette smoke (Stohs et al., 1997). We did not determine whether or not the copper particles specifically fell within nanoparticle size range (<100 nm) using the methods employed in this study. The particle within Blu e-cigarette aerosol ranged between 0.45 μm and 2.02 μm, with an MMAD of 1.03 μm. However, it is likely that some fraction of the aerosol particles that passed through the smallest impactor stage (0.45 μm) were nanosized particles, as these have been measured in e-cigarette vapors previously using a different sampling method (Schripp et al., 2013; Williams et al., 2013). Others report a different distribution pattern of particle sizes in aerosols from a refillable style ENDS device as peak modes generated from a fast mobility particle sizer (FMPS) indicating particle species as low as 10 nm (Fuoco et al., 2014; Schripp et al., 2013). Therefore, it is possible a smaller particle size distribution could be resolved from aerosols produced from the e-cigarette brand used for this study by applying a different technique, such as FMPS. However, since FMPS preparation methods may disrupt aggregates, this suggests many of the particles from e-cigarettes aerosols are deposited in agglomerated forms.
Our results indicate that oxidants are associated with both ENDS/e-cigarette components (battery and cartomizer) and most strikingly, the aerosols that are inhaled by the user. Only a few studies have begun to assess the environmental impact of conventional cigarette smoke pollution (Micevska et al., 2006; Slaughter et al., 2011). A side by side comparison between electronic cigarette aerosols and conventional cigarette smoke suggests pollutants released from e-cigarette aerosols is much lower than cigarettes, and therefore poses a low health risk (McAuley et al., 2012). However, our results suggest there might be other constituents with oxidizing properties associated with e-cigarettes that are health hazards which warrant further examination. Cigarette tar, which collects in cigarette filters, contains oxidants, semiquinones, and aldehydes that are more stably maintained in the tar than many of the oxidants found in cigarette smoke (Pryor and Stone, 1993). Semiquinones promote free radical production, apart from tar and iron/copper deriving Fenton reaction to generate ROS, when introduced to aqueous solution (Stone et al., 1994). The materials and liquids used in various ENDS products, may contain heavy metals, aldehydes, plastics, or other chemicals that act as oxidants as well. When heated together, they produce ROS or accumulate semiquinones and carbonyls/aldehydes that could have deleterious health effects similar to those caused by conventional cigarettes (Goniewicz et al., 2014b), although this has not been extensively assessed. The ROS and particles we have detected in ENDS products have not been characterized. However, results from the present study complement those of Williams et al. who detected a number of heavy metal compounds in e-cigarette components and their aerosols which may have a role in generating reactive oxygen intermediates/ROS when exposed to healthy cells (Williams et al., 2013). Hence, a lack of regulatory oversight into the manufacturing of ENDS may only contribute to environmental hazards as the ENDS market grows.
In 2009, the FDA reported that lab analysis of e-cigarette samples had found carcinogens and toxic chemicals [www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm173222.htm]. Manufacturers do not currently release the details on ingredients or concentrations of chemicals, due in part to maintaining proprietary control of their formulas. The effect of these chemicals and potential reactions from mixing with nicotine is unknown. The effect of oxidants associated with ENDS/e-cigarette components and vapor on health and the environment requires further study. Nevertheless, there is a pressing need for strong regulation of e-cigarettes and their components, including regulation of their storage, leachates, recycling and disposal (Chang, 2014). The regulatory agency needs to regulate how these devices are disposed of in order to reduce potential environmental pollution or public health risks. Over time, the use of these devices may expand to next generation devices with modified forms of e-liquids/e-juices and the need for regulated and understandable disposal guidelines will become paramount. Thus, risks associated with unregulated or improper disposal, storage, and recycling of reusable and non-reusable products of ENDS need to be a focus of research.
Based on our findings, there are numerous important questions raised regarding the safety and disposal of e-cigarettes and their components that require further investigation. For example, nicotine and e-liquid/e-juice expire, but how quickly does this happen, and what are the potential harms of vaping expired liquid/juice with flavorings? Do the different chemicals/composition and flavorings that may be used in making e-juices affect expiration/stability, and how are they affected by the vaporization or dripping process especially with varying heating of filaments/e-juices/e-liquids? Are people who are susceptible to allergies and allergen induced airway responsiveness affected more due to the contaminants/pollutants released? Does the nicotine and flavorings in the e-cigarettes mix with other chemicals to create additional environmental and health hazards? How do these issues affect disposal and environmental hazard of e-cigarette litter/trash?
In conclusion, the oxidants/ROS detected in the present study are produced by a popular ENDS/e-cigarette device and also accompany the disposal of exhausted components (cartomizers and batteries). Although there are very little experimental data available to guide regulatory recommendations for ENDS use and disposal, the oxidants accompanying the e-cigarette components tested in the present study may pose a public health risks due to environmental pollution that warrant further research. Additionally, the detection of oxidants/ROS, nanoparticles, and copper metals associated with the e-cigarette aerosols intended for inhalation reinforces the urgency to improve the understanding of the effect of ENDs vapors deposition and comparative inhalation toxicity on tissues and cells of the oral mucosa and respiratory tract.
Highlights.
E-cigarettes disposal is associated with environmental health hazard/pollution
Oxidants associated with electronic cigarette components and aerosols
Metal copper and nanoparticles detected in electronic cigarette aerosols
Environmental disposal of e-cigarettes components must be regulated with guidelines
Acknowledgments
The authors thank Mr. Robert Gelein and Dr. Günter Oberdörster (University of Rochester) for conducting the copper measurements and for assistance with aerosol size distribution measurements. We also thank Kermit R. Mercer (University of Rochester EPR core facility) for EPR instrument technical support. This work was supported by the National Institute of Drug Abuse at the National Institutes of Health (R21DA036057 to R.R. and I.R.), (2R01HL085613 to I.R.), and the National Cancer Institute at the National Institutes of Health (R01CA132950 to D.J.O., and R01CA152093 to S.M.), pulmonary training grant T32 HL066988, University of Rochester CTSI 5UL1RR024160 incubator project, and NIEHS Environmental Health Science Center grant P30-ES01247. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Drug Abuse, the National Cancer Institute or the National Institutes of Health.
Abbreviations
- DCFH-DA
2'-7'-dichlorodihydrofluorescein diacetate
- DCFH
2'-7'-dichlorodihydrofluorescein
- e-cigarette
electronic cigarette
- ENDS
electronic nicotine delivery systems
- EPR
electron paramagnetic resonance
- LIB
lithium-ion battery
- ROS
reactive oxygen species
- MMAD
mass median aerodynamic diameter
Footnotes
Conflicting financial interests: The authors have declared that no conflicts of interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Black MJ, Brandt RB. Spectrofluorometric analysis of hydrogen peroxide. Anal Biochem. 1974;58:246–254. doi: 10.1016/0003-2697(74)90464-3. [DOI] [PubMed] [Google Scholar]
- Bonini MG, Rota C, Tomasi A, Mason RP. The oxidation of 2',7'-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med. 2006;40:968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- CDC. Electronic cigarette use among middle and high school students - United States 2011–2012. 2013. pp. 729–730. [PMC free article] [PubMed] [Google Scholar]
- Chang H. Research gaps related to the environmental impacts of electronic cigarettes. Tob Control. 2014;23(Suppl 2):ii54–58. doi: 10.1136/tobaccocontrol-2013-051480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher J, Dey M, Lyapustina S, Mitchell J, Tougas T, Oort M, Strickland H, Wyka B. Generalized Simplified Approaches for Mass Median Aerodynamic Determination. Pharmacopeial Forum. 2010;36(3) [Google Scholar]
- Drummond MB, Upson D. Electronic cigarettes. Potential harms and benefits. Ann Am Thorac Soc. 2014;11:236–242. doi: 10.1513/AnnalsATS.201311-391FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523–529. doi: 10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014a;109:500–507. doi: 10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014b;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22:103–106. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- Huang MF, Lin WL, Ma YC. A study of reactive oxygen species in mainstream of cigarette. Indoor Air. 2005;15:135–140. doi: 10.1111/j.1600-0668.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- Hung HF, Wang CS. Experimental determination of reactive oxygen species in Taipei aerosols. Journal of Aerosol Science. 2001;32:1201–1211. [Google Scholar]
- Jakubowski W, Bartosz G. 2,7-dichlorofluorescin oxidation and reactive oxygen species: what does it measure? Cell Biol Int. 2000;24:757–760. doi: 10.1006/cbir.2000.0556. [DOI] [PubMed] [Google Scholar]
- Jiang J, Oberdorster G, Elder A, Gelein R, Mercer P, Biswas P. Does Nanoparticle Activity Depend upon Size and Crystal Phase? Nanotoxicology. 2008;2:33–42. doi: 10.1080/17435390701882478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DHP, Chen MJ, Ogunseitan OA. Potential Environmental and Human Health Impacts of Rechargeable Lithium Batteries in Electronic Waste. Environmental Science & Technology. 2013;47:5495–5503. doi: 10.1021/es400614y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofroth G. Environmental tobacco smoke: overview of chemical composition and genotoxic components. Mutat Res. 1989;222:73–80. doi: 10.1016/0165-1218(89)90021-9. [DOI] [PubMed] [Google Scholar]
- McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhalation Toxicology. 2012;24:850–857. doi: 10.3109/08958378.2012.724728. [DOI] [PubMed] [Google Scholar]
- Micevska T, Warne MS, Pablo F, Patra R. Variation in, and causes of, toxicity of cigarette butts to a cladoceran and microtox. Arch Environ Contam Toxicol. 2006;50:205–212. doi: 10.1007/s00244-004-0132-y. [DOI] [PubMed] [Google Scholar]
- Moerman JW, Potts GE. Analysis of metals leached from smoked cigarette litter. Tob Control. 2011;20(Suppl 1):i30–35. doi: 10.1136/tc.2010.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki H, Kitajima S, Katahira K. Waste on the roadside, 'poi-sute' waste: its distribution and elution potential of pollutants into environment. Waste Manag. 2009;29:1192–1197. doi: 10.1016/j.wasman.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2',7'-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Church DF, Pryor WA. Quantitative analysis of the hydrogen peroxide formed in aqueous cigarette tar extracts. Free Radic Biol Med. 1989;7:9–15. doi: 10.1016/0891-5849(89)90094-4. [DOI] [PubMed] [Google Scholar]
- Novotny TE, Lum K, Smith E, Wang V, Barnes R. Cigarettes butts and the case for an environmental policy on hazardous cigarette waste. Int J Environ Res Public Health. 2009;6:1691–1705. doi: 10.3390/ijerph6051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny TE, Zhao F. Consumption and production waste: another externality of tobacco use. Tob Control. 1999;8:75–80. doi: 10.1136/tc.8.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MS. Electronic cigarettes in the USA: a summary of available toxicology data and suggestions for the future. Tob Control. 2014;23(Suppl 2):ii18–22. doi: 10.1136/tobaccocontrol-2013-051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Thomson GW, Wilson N. Cigarette butt littering in city streets: a new methodology for studying and results. Tob Control. 2013;22:59–62. doi: 10.1136/tobaccocontrol-2012-050529. [DOI] [PubMed] [Google Scholar]
- Printz C. Regulating E-cigarettes: A rule proposed by the FDA aims to extend authority to E-cigarettes, other tobacco products. Cancer. 2014;120:2069–2071. doi: 10.1002/cncr.28872. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. discussion 27–18. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Stone K, Zang LY, Bermudez E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 1998;11:441–448. doi: 10.1021/tx970159y. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol. 1999;277:L1067–1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. 2. CRC Press (Publisher); Boca Raton: 2013. [Google Scholar]
- Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25–31. doi: 10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- Sitzwohl C, Langheinrich A, Schober A, Krafft P, Sessler DI, Herkner H, Gonano C, Weinstabl C, Kettner SC. Endobronchial intubation detected by insertion depth of endotracheal tube, bilateral auscultation, or observation of chest movements: randomised trial. BMJ. 2010;341:c5943. doi: 10.1136/bmj.c5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter E, Gersberg RM, Watanabe K, Rudolph J, Stransky C, Novotny TE. Toxicity of cigarette butts, and their chemical components, to marine and freshwater fish. Tob Control. 2011;20(Suppl 1):i25–29. doi: 10.1136/tc.2010.040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D, Bagchi M. Toxicity of trace elements in tobacco smoke. Inhalation Toxicology. 1997;9:867–890. [Google Scholar]
- Stone KK, Bermudez E, Pryor WA. Aqueous extracts of cigarette tar containing the tar free radical cause DNA nicks in mammalian cells. Environ Health Perspect. 1994;102(Suppl 10):173–178. doi: 10.1289/ehp.94102s10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8:e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JY, Hopke PK. Concentration of Reactive Oxygen Species (ROS) in Mainstream and Sidestream Cigarette Smoke. Aerosol Science and Technology. 2012;46:191–197. [Google Scholar]