Abstract
Gonadectomy in adult male rats significantly impairs spatial working memory, behavioral flexibility and other functions associated with the prefrontal cortex (PFC). However, the mechanisms through which this occurs are largely unknown. In this study, intracortical drug challenge with the selective N-methyl-D-aspartate glutamate receptor (NMDAR) antagonist D(-)-2-amino-5-phosphonopentanoic acid (APV) was combined with Barnes maze testing, gonadectomy and hormone replacement (17β estradiol, testosterone propionate) to explore the contributions of NMDAR-mediated activity within the PFC to hormone effects on spatial cognition in adult male rats. Previous studies have shown that Barnes maze testing reveals significant estrogen-dependent, gonadectomy-induced deficits in spatial working memory and androgen-sensitive, gonadectomy-induced deficits in spatial search strategy. Here we found that bilateral infusion of APV into the medial prefrontal cortex prior to testing significantly improved both sets of behaviors in gonadectomized rats and significantly worsened performance measures in gonadally intact controls. In hormone-replaced cohorts, we further found that behaviors that are normally similar to controls were significantly disrupted by APV, and those that are normally similar to gonadectomized rats were rescued by intracortical APV infusion. There were, however, no residual effects of APV on retention testing conducted 24 hours later. Together these findings suggest that hormone regulation of NMDAR-mediated activity specifically within the PFC may be fundamental to the effects of gonadal steroids on spatial cognition in males. Our findings further identify NMDAR antagonists as potentially novel, non-steroidal means of attenuating the cognitive deficits that can accompany gonadal hormone decline in human males in aging, clinical cases of hypogonadalism and in certain neurologic and psychiatric illnesses. Accordingly, it may be important to obtain in males the kind of detailed knowledge concerning hormone effects on, for example, the channel and electrophysiological properties of NMDAR that currently exists for the female brain.
Keywords: working memory, Barnes maze, estrogen, androgen, schizophrenia, executive function
Among sex differences identified for cognitive functions in healthy humans are robust findings of male superiority in spatial cognitive tasks including mental object rotation and virtual radial arm maze and route learning tasks (Astur et al., 1998, Moffat et al., 1998, Astur et al., 2004, Coluccia and Louse, 2004, Woolley et al., 2010). Males also tend to be more often and more severely affected by the higher order cognitive deficits associated with certain neurological and neuropsychiatric disorders including Parkinson’s disease and schizophrenia (Leung and Chue, 2000, Miller and Cronin-Golomb, 2010, Vaskinn et al., 2011, Han et al., 2012, Moore et al., 2013).
Roles for gonadal hormones in influencing spatial cognitive ability in males are supported by findings of significant positive correlations between serum testosterone levels and spatial cognition in healthy men and between low testosterone levels and cognitive dysfunction in aging, clinical cases of hypogonadalism, schizophrenia, Parkinson’s disease and other disorders (Gordon and Lee, 1986, Christiansen and Knussmann, 1987, Janowsky et al., 1994, Silverman et al., 1999, Duff and Hampson, 2000, Cherrier et al., 2001, Cherrier et al., 2005, Janowsky, 2006, Cherrier et al., 2007, Moore et al., 2013) . However, while there are indications of promise for hormone replacement in treating these deficits (Cherrier et al., 2001, Okun et al., 2006, Ko et al., 2008{Okun, 2006 #261) there are also significant carodiovascular contraindications for hormone replacement in males(Vigen et al., 2013). These could be mitigated by identifying the non-steroidal, neural mediators of hormone influence over cognitive information processing in the male brain. Here, gonadally intact, gonadectomized and hormone-supplemented adult male rats were used to investigate roles for prefrontal cortex (PFC) glutamate (GLU) systems in this capacity.
Gonadectomy (GDX) in adult male rats significantly impairs spatial working memory, behavioral flexibility and other functions associated with the PFC (Ceccarelli et al., 2001, Kritzer et al., 2001, Daniel et al., 2003, Kritzer et al., 2007, Gibbs and Johnson, 2008, Spritzer et al., 2008, Hasegawa and Mochizuki, 2009, Hawley et al., 2013). These and other cognitive operations are also disrupted by induced PFC hyper- or hypodopaminergia(Kalsbeek et al., 1989, Stam et al., 1989, Murphy et al., 1996, Zahrt et al., 1997) as well as by local, intra-PFC infusion of N-methyl D-aspartate (NMDA) GLU receptor antagonists (Baldwin et al., 2000, Bohn et al., 2003, Stefani et al., 2003, Murphy et al., 2005, Stefani and Moghaddam, 2005, Murphy et al., 2012). Recent studies from this lab suggest that these findings may be linked. Specifically, in addition to adverse effects on spatial cognition, GDX in adult male rats also induces a tonic, near two-fold increase in extracellular PFC dopamine (DA) levels (Aubele and Kritzer, 2011) which was recently found to be a consequence of GDX-induced disruption of the tonic, intracortical NMDA receptor (NMDAR)-mediated influences that normally hold PFC DA levels in check. Thus, while reverse dialysis infusion of NMDAR antagonists into the medial PFC has been repeatedly shown to increase DA levels in intact males (Feenstra et al., 1995, Jedema and Moghddam, 1996, Takahata and Moghaddam, 1998, Del Arco and Mora, 1999, Wu et al., 2002, Aubele and Kritzer, 2012, Locklear et al., 2014), infusion of the selective NMDAR antagonist D(-)-2-amino-5-phosphonopentanoic acid (APV) decreases PFC DA levels in GDX rats (Aubele and Kritzer, 2012). Given the sensitivity of PFC operations to intracortical DA, to NMDAR mediated GLU activity and perhaps especially to interactions between these two transmitter systems (Sesack et al., 2003, Tseng and O'Donnell, 2004, Del Arco and Mora, 2005, Javitt, 2007), it is possible that GDX-induced dysregulation of NMDAR mediated signaling plays causal roles in the effects of GDX on spatial cognition. If so, intra-PFC NMDAR antagonism could also represent a novel means of attenuating GDX-induced cognitive deficits. This was tested by comparing the effects of bilateral, intra-PFC infusion of APV in gonadally intact control, GDX, and GDX rats supplemented with testosterone propionate or 17β estradiol prior to behavioral testing using a Barnes maze paradigm that is sensitive to GDX and hormone replacement (Locklear and Kritzer, 2014).
1.0 EXPERIMENTAL PROCEDURES
1.1 Animals
A total of 52 male Sprague-Dawley rats (Taconic Farms, Germantown, NY) were used. Of these, 13 were gonadectomized (GDX), 13 were GDX and supplemented with testosterone propionate (GDX-TP), 11 were GDX and supplemented with 17β-estradiol (GDX-E), and 15 received sham surgeries (CTRL) 28 days prior to behavioral testing. All were housed in same treatment pairs under a 12 h, non-reversed light-dark cycle, in standard-sized cages. Cages and water bottles (Lab Products, Inc., Seaford, DE) were made of a bisphenol–free plastic (Zyfone), ground corncob bedding (Bed O’ Cobs, The Anderson Inc., Maumee, OH) was used and animals had free access to food (Purina PMI LabDiet: ProLab RMH 3000) and water at all times. Rats were 2–3 months of age and weighed 325–350g at time of testing. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Stony Brook University and are in accord with the U.S. Public Health Service Guide for Care and Use of Laboratory Animals. All procedures were designed to minimize animal use and discomfort.
1.2 Surgeries
Aseptic surgical procedures were performed using intraperitoneal injections of ketamine (0.9 mg/kg) and xylazine (0.5 mg/kg) as anesthesia. A single, subcutaneous injection of buprenorphine (0.03 mg/kg) was given post-operatively as analgesia.
Twenty-eight days prior to behavioral testing, male rats underwent GDX or sham surgery. For both surgeries, an incision was made into the scrotum. For GDX, the vas deferens was bilaterally ligated (sterile, non-absorbable, 6-0 silk sutures) and both testes were removed. For hormone-supplemented animals, slow-release pellets containing either testosterone propionate (TP) or 17β -estradiol (E, Innovative Research of America, Sarasota, FL) were implanted within the tunica. Incisions were closed with wound clips, which were removed after 10 days.
Five days before behavioral testing, rats were surgically implanted with bilateral injection cannulae (14 mm, Plastics One, Roanoke, VA). For this procedure, rats were anesthetized and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Craniotomies were performed and burr holes were drilled to place the ends of the injection cannulae in deep layers of the left and right pregenual medial PFC near the border of areas PL and IL (Fig 1). Cannulae were lowered into place and secured to the skull with set screws and dental cement.
Figure 1.
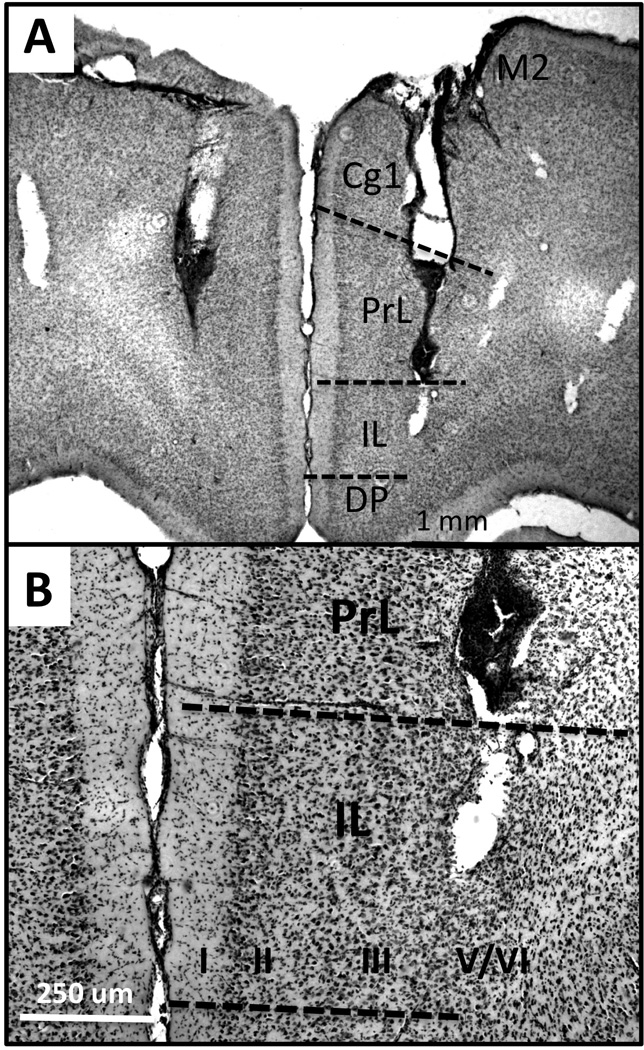
Representative low power (A) and higher power photomicrographs (B) showing the locations of bilateral cannulae and sites of drug delivery in relation to cytoarchitecture (hatched lines, B,C) of the prefrontal cortex. Cannulae were implanted to avoid damage to the saggital sinus and deliver drug to deep layers of the ventral, pregenual medial prefrontal cortex. Tissue sections (A, B) counterstained with cresyl violet show signs of visible damage from the cannulae. Cytoarchitecture reveals that the bottoms of the cannulae reside in deep cortical layers (roman numerals) near the border between prelimbic (PrL) and infralimbic (IL) pregenual medial prefrontal cortex. Additional abbreviations: Cg1, anterior cingulate cortex; DP, dorsal peduncular cortex; M2, premotor cortex; OLF, olfactory bulb, wm, white matter.
1.3 Hormone Replacement
Rats were implanted with slow release pellets at the time of GDX. The testosterone propionate (TP) pellets used released 3–4 ng of TP per milliliter of blood per day and the 17β-estradiol (E) pellets used released 25 pg of E per milliliter of blood per day; both have been used previously in this and other labs and have been shown to produce sustained plasma hormone levels falling within physiological ranges (Collins et al., 1992, Adler et al., 1999, Kritzer, 2000). The efficacies of GDX and hormone replacement were verified in quantitative analyses of the weights of animals’ androgen-sensitive bulbospongiosus muscles (BSM).
1.4 Behavioral Testing
1.4.1 Apparatus
The Barnes maze apparatus was a white, laminate, circular platform, 122 cm in diameter that was mounted on a rotatable pedestal. The surface of the maze was 78 cm above the ground and had 12 evenly spaced holes (10 cm in diameter, 17 cm apart, 1.3 cm from the platform edge) around its perimeter. A removable black acrylic goal box (23×17×10.2 cm) was located under one of these holes. The maze pedestal was draped in black fabric to make all holes including the goal location appear dark from the maze surface. A removable opaque open cylinder (20.3 cm in diameter, 17.8 cm high) was used as a start box to position animals at the center of the maze at the beginning of each trial.
The maze was located in the center of a 12 ft square, sound-attenuated, evenly and ambiently illuminated room. One wall in the room had fixed high contrast spatial cues (2 large, 10 inch, black triangles). During testing, the investigator stood in a fixed position located 1.5 m from the maze. A video camera (Logitech High Definition Webcam) was suspended 144.8 cm above the center of the maze and the laptop computer operating the camera was located on the floor next to the investigator. The testing room was located within a dedicated behavioral suite that included a separate, central room where rats were kept in home cages before and in between trials.
1.4.2 Testing procedures
Habituation to the maze was a step-wise process. On the day before testing began, rats were transported in home cages to the central room of the testing suite. After a one-hour acclimatization, rats were taken into the testing room and placed directly into the recessed goal box (located at a randomized, fixed position) where they remained undisturbed for 2 min. Rats were then returned to home cages in the outer room for a 15 min waiting period. After this period, rats were returned to the testing room and were placed on the maze surface directly adjacent to the goal hole. If rats did not spontaneously enter the goal box, they were gently guided into it. Once in the chamber, rats remained there undisturbed for 2 min. Finally, after an additional 15 min interval in the home cage, rats were placed at the maze center within a temporarily constructed, opaque walkway that led directly to the goal hole location. If rats did not spontaneously approach and enter the goal box, they were gently guided into it. Once inside, rats remained undisturbed in the recessed chamber for a final 2 min.
Behavioral testing began the day after habituation. The goal box was positioned 180 degrees from its location during habituation. For Day 1 acquisition testing, rats acclimated to the testing suite (central room, 1 h). 15 min before the start of the first trial, they were gently held with a cloth while infusion cannulae (Plastics One) were inserted into guide cannulae. Infusions of APV (10 µg/µL) or saline were infused at a rate of 0.5 µL/min for 1 min via a syringe pump and controller (Bioanalytical Systems Inc, West Lafayette, IN). Fifteen min after infusion, rats were placed in the start cylinder at the center of the maze for the first trial. Following a 10 sec delay, the cylinder was lifted away and rats were given 3 min to explore the maze and locate and enter the goal box. Upon entering the chamber, rats remained undisturbed for 2 min. If rats failed to find the goal within the 3 min trial, they were gently guided to and into it and allowed to stay for 2 min. Rats were then returned to home cages for a 15 min inter-trial interval in between each of a subsequent three Day 1 trials. All maze and goal box surfaces were cleaned with 70% ethanol in between these trials. The maze itself was also rotated between every trial—while keeping the goal box in the same relative position in space to further minimize the use of olfactory cues. Twenty-four hours later, rats received an additional four retention trials. These trials were as above, but with no drug or saline infusion prior to testing.
1.5 Euthanasia, Tissue Preparation and Histology
All rats were euthanized after testing by rapid decapitation. Brains were removed, post-fixed for 2–3 days in 10% buffered formaldehyde containing 30% sucrose, rapidly frozen in powdered dry ice and serially sectioned in a coronal plane on a freezing microtome (40 µm). A 1 of 4 series sections the extended from the mid-olfactory bulb to the genu of the corpus callosum were slide mounted and counter-stained with 0.5% cresyl violet. Light microscopic evaluation was used to map cannulae locations in relation to cortical cytoarchitecture. Only those cases where cannulae were confirmed to have spanned the deep layers at the border of prelimbic and infralimbic medial PFC were included in the analysis (Fig 1).
1.6 Data Analysis
Behavior was scored off-line from video-recordings by a single observer (MFK) who was blind to animal group. Videos were scored for each measure listed and described in Table 1. In addition, rats’ paths were also traced using the nose as a reference point (Tracker 4.62, Open Source Physics). These traces were converted to binary images (Image J, open source, NIH) and numbers of black pixels were used to quantify total path lengths in centimeters. Path tracings were also used to categorize rats’ search strategies on per trial bases as follows:
Table 1.
Definitions of quantitative measures of Barnes maze performance.
| OUTCOME MEASURE | UNITS | DESCRIPTION |
|---|---|---|
| Path Length | Centimeters | Total distance traveled, from start at maze center to the goal box. |
| Primary errors | Number | Total numbers of first-time investigations of incorrect holes per trial. |
| Secondary errors | Number | Total numbers of reinvestigations of incorrect hole locations per trial. |
| Latency to find goal | Seconds | Total time from trial start to front paws landing in the goal box. |
Direct-to-goal
Scored when all investigations involved holes were within two holes from the goal AND fewer than 3 total errors were made.
Serial search
Scored when at least 3 errors were made AND 75% of holes investigated were adjacent or within 1 hole of each other AND when animals searched them in order.
Random search
Scored when at least 3 errors were made AND when 50% of holes investigated were non-adjacent or non-localized to a given maze quadrant (4-hole span) OR when animals made more than 2 changes of direction or traverses across the maze center.
1.7 Statistics
Statistical analyses were conducted using SPSS, Version 22 (SPSS Inc, Chicago, IL). Descriptive statistics were applied to all data to assess means, variance and equality of variance across groups. Bulbospongiosus weights were compared across groups using a one-way analysis of variance (ANOVA). Behavioral metrics were first compared in within-drug, across hormone treatment groups (i.e., CTRL-s vs. GDX-s, etc) using two-way ANOVAs with repeated measures designs. Two-way ANOVAs (repeated measures) were also used to compare behavioral measures across saline-, apv- and non-infused hormone treatment groups. Mauchly’s test of sphericity of the covariance matrix was applied to all of these data and degrees of freedom were corrected as indicated using the Huyhn-Feldt epsilon. In all cases, group served as the independent factor and Trial served as the repeated measure. When significant Group, Trial, or Group-by-Trial interactions were identified, post hoc Bonferroni analyses were used to identify groups that over or underperformed (collapsing across Trial), changes in trial-to-trial performance indicative of learning (collapsing across Groups) or trials in which groups’ performances diverged, respectively. Paired t-tests were also used in within-groups assessments to compare outcome measures derived from the last trial of Day 1 vs. the first trial of Day 2 testing, and Chi-square tests were used for across group comparisons of categorical variables of search strategy. In all cases p < 0.05 was accepted as significant. The comparative data from non-infused subjects (CTRL, n=7; GDX n=8; GDX-E, n=7; GDX-TP, n=8) were obtained from a separate study in which testing took place 4–6 months prior to testing of the infusion groups (Locklear and Kritzer, 2014).
2.0 RESULTS
2.1 Effectiveness of Hormone Treatments
The weights of the androgen sensitive bulbospongiosus muscles (BSM) showed group differences that paralleled expected differences in circulating androgen levels. Thus, muscle weights of the APV and saline infused CTRL rats (CTRL-apv, CTRL-s) were on average 1.78g and 1.77g, respectively, and those of the APV and saline infused GDX-TP groups (GDX-TP-apv, GDX-TP-s) were on average 1.64g and 1.66g, respectively (Fig 2). In contrast, in both the APV and saline infused GDX and GDX-E groups, average BSM weights were between 0.33g and 0.46g (Fig 2). Statistical comparisons of individual rats’ muscle weights (one-way ANOVA) confirmed that there were significant main effects of Group [F(7,39) = 49.55, p < 0.001] on muscle mass. The allowed post hoc comparisons further showed that BSM weights of saline and APV-infused CTRL and GDX-TP rats were all similar to each other; that the BSM weights of saline- and APV-infused GDX and GDX-E rats were all similar to each other; and that mean muscle weights of both the saline- and APV-infused CTRL and GDX-TP groups were significantly larger than those of both the saline- and APV-infused GDX and GDX-E groups (p < 0.001, Fig 2).
Figure 2.
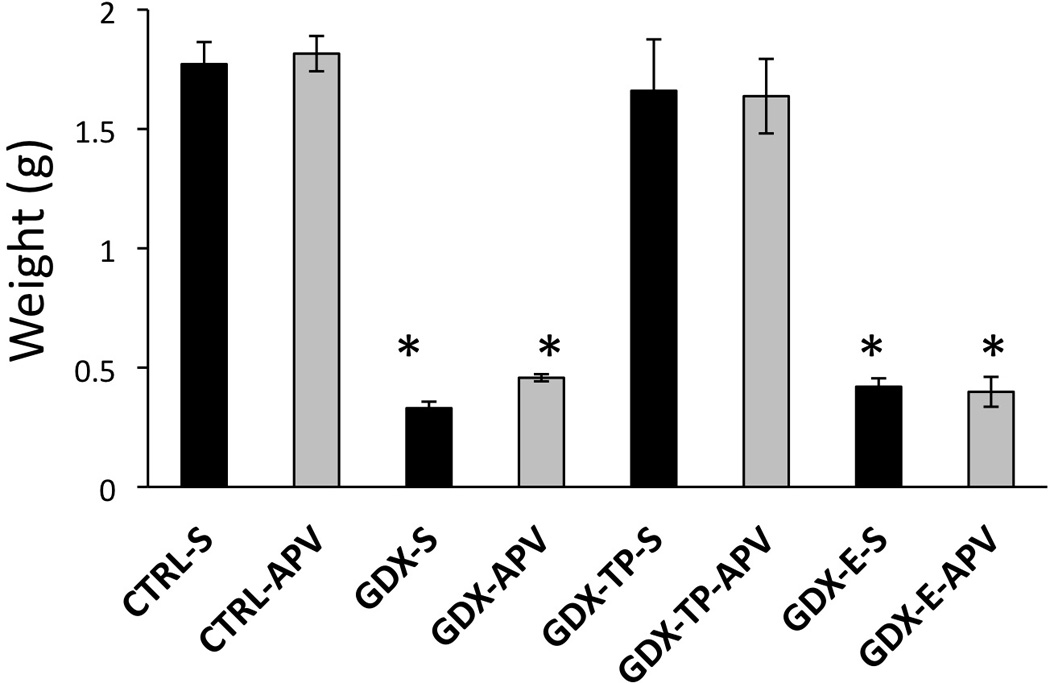
Bar graphs showing group average bulbospongiosus muscle weights in grams (g) plus standard errors of the mean for rat groups that were infused with saline (black bars) or with APV (gray bars) prior to Barnes maze testing. The mean weights from gonadally intact control (CTRL-S, CTRL-APV), gonadectomized (GDX-S, GDX-APV), and gonadectomized male rats supplemented with testosterone propionate (GDX-TP-S, GDX-TP-APV) or estradiol (GDX-E-S, GDX-E-APV) are shown. Muscle weights of the two CTRL and GDX-TP groups were similar to each other and were significantly greater than those of the two GDX and GDX-E groups. Muscle weights of the two GDX and GDX-E groups were also similar to each other. Asterisks denote significant differences from CTRL-S for post-hoc testing at the p < 0.05 level.
2.2 Barnes Maze Testing: Path Lengths, Errors and Latencies to Goal
Previous studies have shown that during Day 1 testing, GDX rats follow significantly longer routes, make significantly more errors (primary and secondary) and take significantly longer to locate the goal than CTRL, GDX –E or GDX-TP rats (Locklear and Kritzer, 2014). Saline vehicle injections prior to testing had no effect on these group differences (Figs 3–5, left panels). Thus, at the conclusion of Day 1 testing, in comparison to saline-infused CTRL, GDX-E and GDX-TP groups the GDX-s cohort followed longer average path lengths (GDX-s ⋍ 300cm; CTRL-s, GDX-E-s ⋍ 120 cm; GDX-TP-s ⋍ 240 cm, Fig 3A), committed higher mean numbers of errors (primary errors: GSX-s ⋍ 8 errors, CTRL-s, GDX-E-s, GDX-TP-s ⋍ 3–4 errors, Fig 4C: secondary errors: GSX-s ⋍ 3 errors, CTRL-s, GDX-E-s, GDX-TP-s ⋍ 0–1 error, Fig. 4C) and had longer mean latencies in locating the goal (GSX-s ⋍ 70 seconds, CTRL-s, GDX-E-s, GDX-TP-s ⋍ 30–40 seconds, Fig 5A). Analyses of variance (two-way, repeated measures) identified significant main effects of Group for path length (F3,13 = 3.77, p=0.038), primary errors (F3,13 = 10.35, p<0.001) and latency to goal (F3,13 = 4.14, p=0.029) and significant main effects of Trial for path length (F3,39 = 6.74, p=0.001), secondary errors (F3,39 = 9.96, p<0.001) and latency to goal (F2.03,26.44 = 5.69, p=0.009). Interactions between Group and Trial were not significant for any outcome measure. Allowed post hoc comparisons further defined the significant main effects of Trial as driven by differences in performance during Trials 3 and/or 4 compared to Trials 1 and 2 (p <0.001–0.05). The significant main effects of Group were also found to be driven by significant differences in performances of GDX-s rats compared to all groups for primary errors, secondary errors and latency to goal, by significant differences of GDX-s rats compared to CTRL-s and GDX-E-s rats (p = 0.001–0.05) for path length. Finally, comparisons with data previously collected from non-infused subjects (Locklear and Kritzer, 2014), identified significant main effects of Trial for all measures (Table 2) that were driven by significant differences in performance during Trials 1 and 2 compared to Trials 3 and/or 4, and significant main effects of Group for path length, primary errors and latency to goal (Table 2) that were driven by significant differences that parsed along lines of hormone treatment, i.e., there were no significant differences among groups of like-hormone treatment that were or were not vehicle-infused (Table 2).
Figure 3.
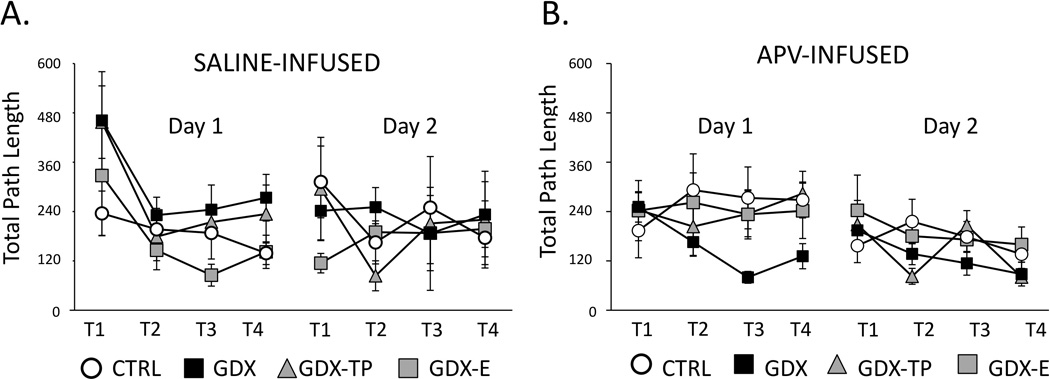
Line graphs showing average total path lengths, measured in centimeters (cm), during the four trials (T1-T4) of Day 1 (acquisition) and the four trials of Day 2 (retention) testing for gonadally intact control (CTRL, open circles), gonadectomized (GDX, black squares), and gonadectomized male rats supplemented with testosterone propionate (GDX-TP, gray triangles) or estradiol (GDX-E, gray squares) that were infused with saline (A) or with APV (B) prior to Day 1 testing. Error bars represent standard errors of the mean. During Day 1 testing, the saline-infused CTRL (n=6), GDX-E (n=3) and GDX-TP (n=3) groups showed trial-by-trial shortening of the distances traveled while path lengths of GDX rats (black squares, n =5) were consistently longer. However, among APV-infused rats, the GDX group (n=8) showed trial-by-trial shortening of the distances travel to the goal; the CTRL (n=9), GDX-E (n= 8) and GDX-TP (n=10) groups consistently followed longer routes. Analyses of variance performed on path length data identifying significant main effects of Group and of Trial and revealing significant differences in GDX compared to other hormone-treatment groups for both the saline and APV treated cohorts are described in the text. During Day 2 testing, path lengths were similarly short and consistent across trials for all saline (A) and APV (B) infused cohorts. There were no significant main effects of Group or Trial among these data.
Figure 5.
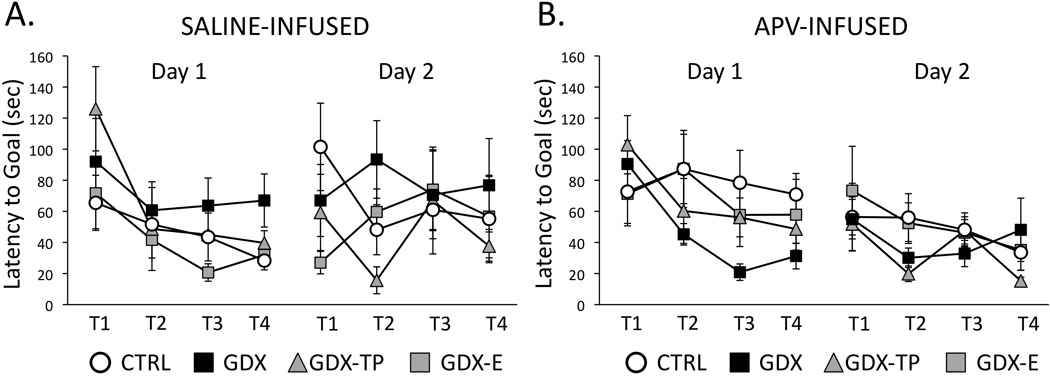
Line graphs showing average latencies in seconds to locate the goal across the four trials (T1-T4) of Day 1 (acquisition) and the four trials of Day 2 (retention) testing for gonadally intact control (CTRL, open circles), gonadectomized (GDX, black squares), and gonadectomized male rats supplemented with testosterone propionate (GDX-TP, gray triangles) or estradiol (GDX-E, gray squares) that were infused with saline (A, C) or with APV (B, D) prior to Day 1 testing. Error bars represent standard errors of the mean. During Day 1 testing, the saline-infused CTRL (n=6), GDX-E (n=3) and GDX-TP (n=3) groups took less and less time to locate the goal across trials, whereas GDX rats (black squares, n=5) continued to take longer times to reach the goal. However, among APV-infused rats, the GDX group (n=8) showed greater reduction in the amount of time taken to locate the goal compared to the times taken by all other groups [CTRL (n=9), GDX-E (n=8) and GDX-TP (n=10)]. Analyses of variance performed on latency data identifying significant main effects of Group and of Trial and revealing significant differences in GDX compared to other hormone-treatment groups for both the saline and APV treated cohorts are described in the text. During Day 2 testing, latencies to locating the goal for all groups were similar, relatively short and consistent across trials for both the saline (A) and APV (B) infused cohorts. There were no significant main effects of Group or Trial among these data.
Figure 4.
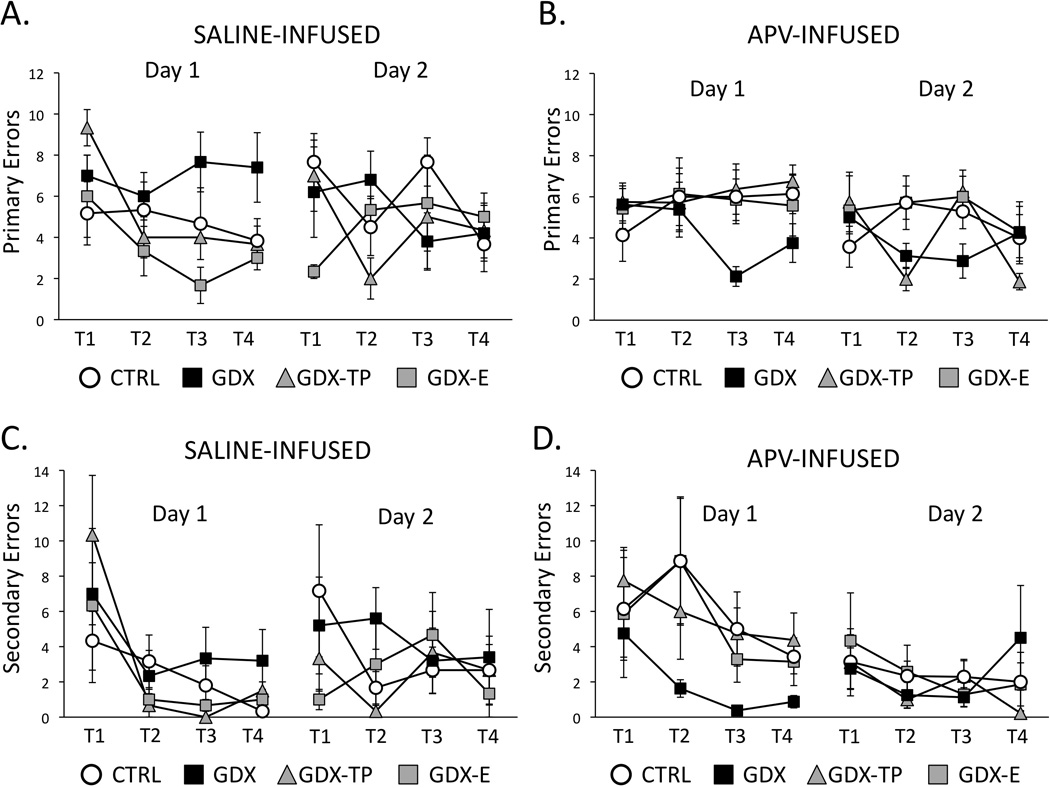
Line graphs showing average numbers of primary errors (A, B) and average numbers of secondary errors (C, D) committed during the four trials (T1-T4) of Day 1 (acquisition) and Day 2 (retention) testing for gonadally intact control (CTRL, open circles), gonadectomized (GDX, black squares), and gonadectomised male rats supplemented with testosterone propionate (GDX-TP, gray triangles) or estradiol (GDX-E, gray squares) that were infused with saline (A, C) or with APV (B, D) prior to Day 1 testing. Error bars represent standard errors of the mean. During Day 1 testing, the saline-infused CTRL (n =6), GDX-E (n=3) and GDX-TP (n=3) groups showed similar, consistent decreases in primary (A) and secondary (C) errors across trials, while GDX rats (n=5) continued to make more errors of both types. However, among APV-infused rats, the GDX group (black squares, n =8) consistent decreases in primary (B) and secondary errors (D) across Day 1 trials while the CTRL (n=9), GDX-E (n=8) and GDX-TP (n=10) groups continued to commit larger numbers of errors. Analyses of variance performed on error data identifying significant main effects of Group and of Trial and revealing significant differences in GDX compared to other hormone-treatment groups for both the saline and APV treated cohorts are described in the text. During Day 2 testing, the commission of primary and secondary errors was similar, low and consistent across trials among all saline (A, C) and APV-infused (B, D) groups. There were no significant main effects of Group or Trial among these data.
TABLE 2.
Results of 2-way analyses of variance (ANOVA) with repeated measures design for each measure across the four acquisition trials. Significant main effects of Group and/or Trial were found for most measures. There were no significant interactions between these two factors or any behavior.
| SALINE-Infused and NO INFUSION Groups | |||
|---|---|---|---|
| BEHAVIOR | MAIN EFFECTS OF GROUP | MAIN EFFECTS OF TRIAL | INTERACTIONS |
| Total Path Length | (F7,39= 11.82,p<0.001) | (F2.38,92.32= 17.32,p<0.001) | (NS) |
| Primary Errors | (F7,38= 5.33,p<0.001) | (F3,117= 5.29,p=0.002) | (NS) |
| Secondary Errors | (F7,39= 1.45,p=0.216) | (F2.04,79.48= 12.51,p<0.001) | (NS) |
| Latency to Goal | (F7,39= 3.43,p=0.007) | (F2.39,93.20= 12.49,p<0.001) | (NS) |
| Results of post hoc tests (Bonferroni) comparing Saline and No Infusion Groups across the four acquisition trials. The p-values for each group comparison are reported. | |||
| SALINE-Infused and NO INFUSION Groups | |||||
|---|---|---|---|---|---|
| Total Path Length | Primary Error | Secondary Error | Latency to Goal | ||
| CTRL vs. CTRL-S | 0.721 | 0.349 | 0.780 | 0.282 | |
| GDX vs. GDX-s | 0.059 | 0.079 | 0.536 | 0.209 | |
| GDX-TP vs. GDX-TP-s | 0.091 | 0.309 | 0.272 | 0.903 | |
| GDX-E vs. GDX-E-s | 0.439 | 0.811 | 0.607 | 0.599 | |
Performance in APV infused rats also improved across Day 1 acquisition trials. However, improvements were greatest in the GDX-apv compared to all other groups. Thus, by session’s end in comparison to the CTRL-apv, GDX-E-apv and GDX-TP-apv groups, the GDX-apv cohort had shorter mean path lengths (GSX-apv ⋍ 120 cm, CTRL-apv, GDX-E-apv, GDX-TP-apv ⋍ 240–300 cm, Fig 3B), had lower average numbers of errors (primary errors: GSX-apv ⋍ 3 errors, CTRL-apv, GDX-E-apv, GDX-TP-apv ⋍ 5–7 errors, Fig 4b; secondary errors: GSX-apv ⋍ 0–1 error, CTRL-apv, GDX-E-apv, GDX-TP-apv ⋍ 3–5 errors, Fig. 4D) and had shorter average latencies in locating the goal (latency to goal: GSX-apv ⋍30 seconds, CTRL-apv, GDX-E-apv, GDX-TP-apv ⋍ 50–70 seconds, Fig 5B). Analyses of variance (two-way, repeated measures) identified significant main effects of Group for primary and secondary errors (F3,26 = 3.29, p=0.036 and F3,26 = 4.81, p=0.009, respectively), and main effects of path length and latency to goal that approached significance (F3,26 = 2.88, p=0.055 and F3,26 = 2.45, p=0.086, respectively). Main effects of Trial were also significant for latency to goal (F2.34,60.87 = 3.27, p=0.038) and approached significance for secondary errors (F2.06,53.44 = 2.45, p=0.069). Interactions between Group and Trial were not significant for any behavioral measure. Allowed post hoc comparisons showed that the main effects of Trial were driven by significant differences in latencies during Trials 3 and/or 4 compared to Trials 1 and 2 (p =0.001–0.05) and that the main effects of Group were driven by significant differences in performance of GDX-apv rats compared to all other APV-infused groups (p =0.001–0.05). Finally, comparisons with the saline infused groups also identified significant main effects of Group for all outcome measures (path length: F7,39 = 2.73, p=0.021; primary error: F7,39 = 3.39, p=0.006; secondary error: F7,39 = 3.84, p=0.003; latency to goal: F7,39 = 2.46, p=0.034) and significant main effects of Trial for path length (F3,110 = 7.30, p=0.007 ), secondary errors ( F3,110 = 6.24, p<0.001) and latency to goal (F2.34, 91.42 = 5.82, p<0.001). The interactions between Group and Trial were not significant. Post hoc testing revealed that the main effects of Trial were related to performance measures in Trials 1 and 2 compared to Trials 3 and/or 4, and that the main effects of Group were driven by significant group differences among CTRL and GDX cohorts that parsed across drug treatment within hormone treatment groups, i.e., CTRL-s vs. CTRL-apv, GDX-s vs. GDV-apv and across hormone treatments within drug-treatment groups, i.e., CTRL-s vs. GDX-s, CTRL-apv, vs. GDX-apv (p < 0.025–0.03).
During Day 2 testing, within-groups performances during the first few trials largely overlapped those recorded during Trial 4 on the previous testing day (Figs 3–5). Day 2 performances were also overlapping and more or less asymptotic across all drug treatment groups and regardless of hormone status. A series of two-way ANOVAs with repeated measures design found no significant main effects of Group or of Trial and no significant interactions between these two factors for any performance measure in either the saline-or APV-infused groups. Within groups, paired t-tests also confirmed that there were no significant differences in performance on the last trial from Day 1 compared to the first trial from Day 2 for any group.
2.3 Barnes Maze Testing: Search Strategies
Previous studies identified group-specific transitions in search strategies used by CTRL, GDX and hormone supplemented GDX rats across Barnes maze Day 1 acquisition trials (Locklear and Kritzer, 2014). Similar transitions were observed in the saline-infused groups. Thus, within a trial or two a majority of the CTRL-s and GDX-TP-s rats replaced random maze investigations with serial and/or direct-to-goal searches. In contrast, most of the GDX-s and GDX-E-s continued to employ random search strategies across all four Day 1 trials (Fig 6, left). In contrast, among the APV-infused rats, it was a majority of the GDX-apv and GDX-E-apv rats that quickly adopted serial and/or direct-to-goal search strategies and the CTRL-apv and GDX-TP-apv groups where most rats persisted in using random search strategies across all four trials (Fig 6, right). Chi-square comparisons of categorical values of Group (saline- or APV-infused hormone treatment groups) and strategy (random, serial and direct-to-goal) identified group effects on spatial learning as non-significant trends for the saline-infused rats and as significant among the APV-infused groups for Trial 3 [χ2 (22, N=17) =33.95, p = 0.05] and Trial 4 [χ2 (22, N=35) =34.20, p = 0.047]. By the end of Day 2 testing, however, the majority of rats from all groups utilized serial or direct-to-goal searches (Fig 6).
Figure 6.
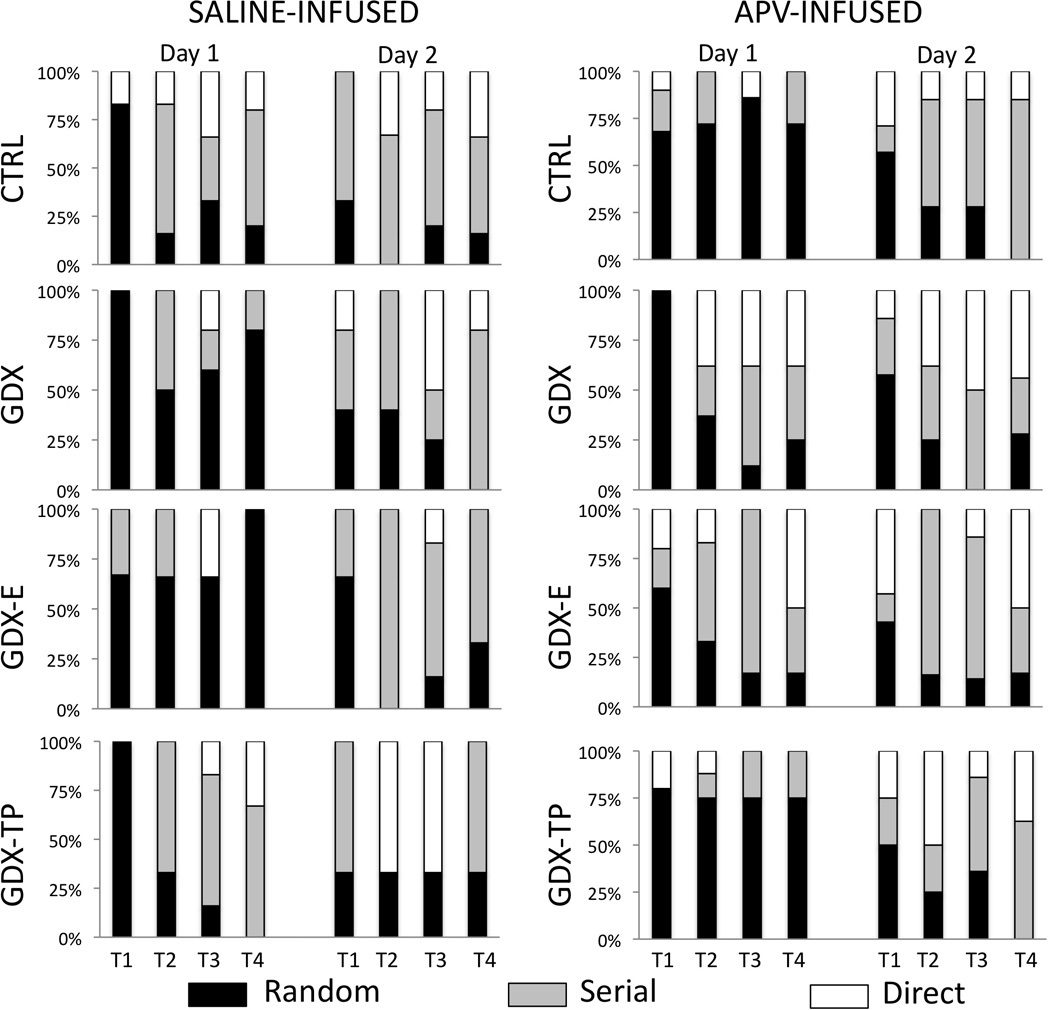
Stacked bar graphs depicting percentages of animals utilizing random (black bars), serial (gray bars), or direct-to-goal (white bars) search strategies in locating the goal across the four trials (T1-T4) of Day 1 (acquisition) and the four trials of Day 2 (retention) testing for gonadally intact control (CTRL), gonadectomized (GDX), and gonadectomized male rats supplemented with testosterone propionate (GDX-TP) or estradiol (GDX-E) that were infused with saline (left hand columns) or with APV (right hand columns) prior to Day 1 testing During Day 1 testing, among saline-infused subjects, the majority of CTRL (n=6) and GDX-TP rats (n=3) rapidly replaced random searches (black bars) with serial (gray bars) or direct (white bars) search strategies, whereas the majority of GDX (n=5) and GDX-E rats (n=3) continued to explore holes randomly. However, among APV-infused rats, it was the majority of GDX (n=8) and GDX-E rats (n=8) that rapidly adopted serial (gray bars) or direct hole searches (white bars) and the majority of CTRL (n=9) and GDX-TP (n=10) rats that continued to explore the maze randomly (black bars). Across Day 2 trials, the majority of subjects in all groups utilized either serial (gray bars) or direct searches (white bars), regardless of drug or vehicle treatment prior to Day 1 testing.
3.0 DISCUSSION
Intracortical drug challenge and Barnes maze testing were combined to explore the contributions of intra-PFC NMDAR-mediated signaling to the effects of GDX and hormone replacement on spatial cognition in adult male rats. The Barnes maze paradigm used has recently been shown to reveal significant, GDX-induced deficits in spatial working memory and in spatial learning/search strategy (Locklear and Kritzer, 2014). Here, we found that bilateral intra-PFC infusion of the NMDAR antagonist APV prior to Barnes maze testing reversed these group differences in behavior. That is, performance in GDX-apv rats was signficantly different from GDX-s rats and statistically indistinguishable from that of saline- or non-infused CTRLS. There were no residual effects of APV on retention testing conducted 24 hours later in any of the groups. As discussed below, these findings suggest that behavioral constructs of spatial cognition tapped in Barnes maze acquisition depend on intact intra-PFC, NMDAR mediated activity and that circulating gonadal steroids exert behaviorally critical influence over this activity. Findings in the hormone replacement groups further suggest that behaviorally relevant intra-PFC NMDAR mediated activity is sensitive to both estrogen and androgen stimulation, and that androgen vs. estrogen impact on PFC NMDAR systems affects behavior in construct-specific ways and according to mechanisms that may also differ in the extent to which they engage DA/GLU interactions.
3.1 NMDAR activity and spatial cognition: Comparisons of APV-infusion in gonadally intact CTRL rats to previous studies
Numerous studies have combined systemic drug challenge with behavioral testing to tie NMDAR mediated activity to an array of higher order functions. For example, in healthy human subjects, systemic administration of NMDAR antagonist drugs such as phencyclidine or ketamine at low or sub-anesthetic doses has been shown to induce cognitive and mnemonic deficits that in part resemble those associated with schizophrenia and other forms of psychosis (Ghoneim et al., 1985, Oye et al., 1992, Krystal et al., 1994, Malhotra et al., 1997, Tamminga, 1998). In rodents, systemic administration of these and other NMDA receptor antagonists, including APV, dizolcilpine (MK801), 3-[(R)-2-carboxypiperazine-4-yl]-propyl-1-phosphoric acid [(R)-CPP] and others likewise induce an array of cognitive impairments. These include deficits in acquisition and extinction of conditioned passive avoidance (Mele et al., 1996, Adriani et al., 1998), in spatial working and spatial reference memory (Pontecorvo et al., 1991, Shapiro and O'Connor, 1992, Verma and Moghaddam, 1996, Moghaddam et al., 1997) and in discrimination and reversal learning, behavioral flexibility, attentional set shifting and response inhibition (Jentsch et al., 1997, Stefani et al., 2003, Stefani and Moghaddam, 2005, Egerton et al., 2008, Dalton et al., 2011).
In contrast to systemic drug challenges, a smaller number of studies using intracerebral routes of drug administration have identified more delimited sets of behavioral deficits that are directly ascribable to NMDA-R signaling in the medial PFC. For example, studies in which MK801 was infused into the medial PFC prior to plus maze testing showed no impact on acquisition or retention of a discrimination rule for maze arm selection, but did find a significant deficit in rats’ abilities to switch to new guiding criteria (Stefani et al., 2003, Stefani and Moghaddam, 2003, 2005). In Barnes maze testing of CTRL rats, we also found that APV infusion had no effects on task retention but that it did produce what may be corresponding deficits in animals’ behavioral flexibility. Specifically, following APV-infusion CTRL rats persisted in following relatively long and circuitous routes to search for the goal. This contrasts with the more spatially circumscribed serial or place strategies that male rats typically adopt within a trial or two of testing in Barnes, radial arm, Morris water and other spatial mazes (Sandstrom et al., 1998, Sava and Markus, 2005, Spritzer et al., 2013, Locklear and Kritzer, 2014).
In other studies, bilateral infusion of the NMDAR antagonist (R)-CPP into the medial PFC prior to 5-choice serial reaction time task testing was shown to decrease animals’ response accuracy, increase numbers of omitted trials and when infused specifically within infralimbic cortex, to increase animals’ premature responding (Murphy et al., 2005, Murphy et al., 2012). These findings indicate that in addition to behavioral flexibility (above), processes of visuospatial attention and behavioral inhibition also depend on intact NMDAR signaling within the PFC. The present findings showing that bilateral intra-PFC infusion of APV in CTRL rats significantly increased their re-examinations of incorrect hole locations (secondary errors) further suggests spatial working memory as a behavioral element that is reliant on intra-PFC, NMDAR-mediated activity. This conclusion is also consistent with data from recent fear conditioning studies in rats that demonstrated selective disruption of trace (but not delay or contextual) conditioning by bilateral infusion of the NR2B NMDAR antagonist Ro25-6981 {Gilmartin, 2013 #457}. These findings dovetail with those from iontophoretic studies carried out in non-human primates performing an oculomotor delayed response task. While these studies showed that local NMDAR blockade in the dorsolateral PFC decreased firing rates in neurons selectively tuned to cue, delay and/or response portions of trials (Wang et al., 2013) they also added to the evidence suggesting specific roles for the slow kinetics of NR2B-containing NMDAR in the persistent activity of delay cells thought to be critical for representational knowledge, working memory and other PFC operations (Compte et al., 2000, Wang, 2001, Wang et al., 2008, Cui et al., 2011). These receptor subtypes could also be important for the present findings of hormone sensitivity in the PFC NMDAR axis in the adult male rat. While developmentally down regulated in most brain regions, NR2B containing NMDAR are relatively retained in the PFC and other mnemonic brain areas (Dumas, 2005), are linked to executive operations of behavioral flexibility in rats (Dalton et al., 2011) and have been repeatedly implicated in schizophrenia and other disorders where cognitive processes are at risk in sex specific ways (Bi and Sze, 2002, Kristiansen et al., 2010a, Kristiansen et al., 2010b). In female rats, NR2B-containing NMDAR have also been identified as critical mediators of estradiol’s neuro-protective effects (Liu and Zhao, 2013) as well as estradiol’s effects on synaptic plasticity (Smith and McMahon, 2006). More recent evidence for pubertal shifts in NR2B-mediated modulation of plasticity in the developing male rats PFC (Flores-Barrera et al., 2014) suggests that these receptor subunits might also shape hormone sensitivity of NMDAR in the adult male PFC.
3.2 NMDAR activity and hormone sensitivity of spatial cognition: Divisions of labor and predicted mechanisms
Recent work from this lab has shown that cognitive operations engaged in Barnes maze testing are sensitive to GDX in adult male rats (Locklear and Kritzer, 2014). The present studies extend these observations by showing that the behavioral deficits induced by GDX are significantly attenuated by intra-PFC infusion of the NMDAR antagonist APV prior to testing. This suggests that the behavioral impairments caused by GDX stem from an induced over-activity of intra-PFC NMDAR activity. Although currently more extensively studies in females, there are small number of studies in males with findings that are consistent with this scenario. For example, GDX in adult male rats has been shown to increase neuronal sensitivity to MK801-induced excitoxicity in retrosplenial cortex (de Olmos et al., 2008) and to increase 123I MK801 binding and lower the threshold for induction of NMDA-dependent LTP (Kus et al., 1995, Handa et al., 1997, Harley et al., 2000) in hippocampus.
Studies in the PFC have also shown that GDX disrupts NMDAR mediated effects on DA overflow in a manner that is consistent with an exaggeration of NMDAR mediated excitation of PFC pyramidal cells (Aubele and Kritzer, 2012). Given the close ties of not only NMDAR and DA systems but also DA/GLU interactions to cognitive information processing in the PFC (Sesack et al., 2003, Tseng and O'Donnell, 2004, Del Arco and Mora, 2005, Javitt, 2007), hormone effects on NMDAR-mediated mechanisms of PFC DA homeostasis may be well poised to influence male abilities in spatial cognition and male vulnerabilities to cognitive dysfunction in disorders such as schizophrenia where anomalies in PFC DA and NMDAR function are suspected (Moghaddam and Jackson, 2003, Lewis and Moghaddam, 2006, Javitt, 2010, Moghaddam and Javitt, 2012). However, the effects of GDX on the NMDAR/DA overflow axis were found to be androgen-sensitive and estrogen-insensitive; that is, the effects of GDX were attenuated in GDX rats supplemented with TP but not E (Aubele and Kritzer, 2012). This is a somewhat atypical pattern of hormone sensitivity that is shared by only some of the GDX-induced deficits identified in Barnes testing, namely those related to adopting efficient search strategies; in contrast, the relatively high incidence or errors, the relatively long distances traveled and the relatively large amounts of time taken by GDX rats to locate the goal were similarly attenuated in GDX rats supplemented with either TP or E (Locklear and Kritzer, 2014), implicating estrogens or estrogen metabolites in the underlying hormone processes. Thus, the rescue of all GDX-induced behavioral deficits-- whether estrogen or androgen sensitive, by infusion of APV suggests a common root cause of induced dysregulation of NMDAR activity in the PFC. However, in addition to suggesting the involvement of multiple hormone signaling streams, based on prior microdialysis studies (Aubele and Kritzer, 2012) findings in GDX-E and GDX-TP rats may further predict that the androgen stimulation affecting animals’ search strategies does so by modulating the functional couplings between the PFC’s DA and NMDAR-mediated GLU systems (Sesack et al., 2003, Tseng and O'Donnell, 2004, Del Arco and Mora, 2005, Tseng and O'Donnell, 2005, Javitt, 2007, O'Donnell, 2010, Flores-Barrera et al., 2014). However, the estrogen-sensitive effects of GDX on spatial working memory and other aspects of spatial cognition may be more likely to interfere with intra-PFC, NMDAR-mediated actions that modulate non-DA systems such as the amino acid transmitters (Del Arco and Mora, 2000, 2002, Murphy et al., 2012).
4.0 Conclusions
Whether by DA/GLU interactions or by other means, intra-PFC NMDAR activity plays vital roles in defined aspects of cognitive information processing. The present studies show that this activity is powerfully modulated by circulating gonadal steroids in the male brain, that this modulation is behaviorally relevant and that the actions of NMDAR-selective drugs effectively counter the adverse effects of hormone manipulations on spatial cognition in this sex. These interactions suggest that hormone impact on NMDAR is a fundamental part to the neurobiology of spatial cognitive information processing in the PFC, and could have bearing on the debilitating cognitive dysfunction in disorders such as schizophrenia that disproportionately afflict males. The dramatic changes in NMDAR-mediated activity that coincide with puberty in the male rat, including the functional coupling of NMDAR and DA D1 receptor signaling (Tseng and O'Donnell, 2004, 2005, O'Donnell, 2010, Flores-Barrera et al., 2014) and the emergence of NR2B function in late adolescence (Flores- Barrerra GOA) suggest a strong likelihood for this scenario. Nonetheless, it is important to acquire in males the same level of detailed knowledge concerning hormone effects on, for example, the channel properties, subunit compositions and electrophysiological properties of NMDAR that currently exist for the female brain. With the additional promise of identifying safe, non-steroidal means of attenuating the cognitive dysfunction that accompanies hormone depletion in human males in aging, clinical cases of hypogonadalism and in certain neurologic and psychiatric illnesses, converging evidence from developing and mature and from gonadally intact and hormone manipulated subjects alike point to the NR2B subunit containing NMDARs as a particularly fruitful place to start.
NMDA receptor block in prefrontal cortex impairs Barnes maze performance in male rats
NMDA receptor block in prefrontal cortex improved cognition in gonadectomised rats
Estrogen and androgen modulate cognition through NMDA receptors in prefrontal cortex
ACKNOWLEDGEMENTS
The authors thank Mr. Ramon Cabrera for his excellent technical assistance in areas of animal handling/care and histology.
FUNDING
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (R01-NS41966 to M.F.K.).
ABBREVIATIONS
- ANOVA
analysis of variance
- APV, apv
D(-)-2-amino-5-phosphonopentanoic acid
- BSM
bulbospongiosus muscle
- CTRL
control
- DA
dopamine
- E
estradiol
- GDX
gonadectomy
- GLU
glutamate
- MK801
dizolcilpine
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- PFC
prefrontal cortex
- (R)-CPP
3-[(R)-2-carboxypiperazine-4-yl]-propyl-1-phosphoric acid
- s
saline
- TP
testosterone propionate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Adriani W, Felici A, Sargolini F, Roullet P, Usiello A, Oliverio A, Mele A. N-methyl-D-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp Brain Res. 1998;123:52–59. doi: 10.1007/s002210050544. [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Gonadectomy and hormone replacement affects in vivo Basal extracellular dopamine levels in the prefrontal cortex but not motor cortex of adult male rats. Cereb Cortex. 2011;21:222–232. doi: 10.1093/cercor/bhq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level. Cereb Cortex. 2012;22:1799–1812. doi: 10.1093/cercor/bhr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav Neurosci. 2000;114:84–98. doi: 10.1037//0735-7044.114.1.84. [DOI] [PubMed] [Google Scholar]
- Bi H, Sze CI. N-methyl-D-aspartate receptor subunit NR2A and NR2B messenger RNA levels are altered in the hippocampus and entorhinal cortex in Alzheimer's disease. J Neurol Sci. 2002;200:11–18. doi: 10.1016/s0022-510x(02)00087-4. [DOI] [PubMed] [Google Scholar]
- Bohn I, Giertler C, Hauber W. NMDA receptors in the rat orbital prefrontal cortex are involved in guidance of instrumental behaviour under reversal conditions. Cereb Cortex. 2003;13:968–976. doi: 10.1093/cercor/13.9.968. [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, Scaramuzzino A, Aloisi AM. Effects of gonadal hormones and persistent pain on non-spatial working memory in male and female rats. Behav Brain Res. 2001;123:65–76. doi: 10.1016/s0166-4328(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, LaTendresse S, Craft S. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, Peskind ER, Raskind MA. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32:72–79. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18:27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- Collins WF, 3rd, Seymour AW, Klugewicz SW. Differential effect of castration on the somal size of pudendal motoneurons in the adult male rat. Brain Res. 1992;577:326–330. doi: 10.1016/0006-8993(92)90292-h. [DOI] [PubMed] [Google Scholar]
- Coluccia E, Louse G. Gender differences in spatial orientation: A review. Journal of environmental psychology. 2004;24:329–340. [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Cui Y, Jin J, Zhang X, Xu H, Yang L, Du D, Zeng Q, Tsien JZ, Yu H, Cao X. Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PLoS One. 2011;6:e20312. doi: 10.1371/journal.pone.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Ma LM, Phillips AG, Floresco SB. Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology (Berl) 2011;216:525–535. doi: 10.1007/s00213-011-2246-z. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- de Olmos S, Bueno A, Bender C, Lorenzo A, de Olmos J. Sex differences and influence of gonadal hormones on MK801-induced neuronal degeneration in the granular retrosplenial cortex of the rat. Brain Struct Funct. 2008;213:229–238. doi: 10.1007/s00429-008-0186-0. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Effects of endogenous glutamate on extracellular concentrations of GABA, dopamine, and dopamine metabolites in the prefrontal cortex of the freely moving rat: involvement of NMDA and AMPA/KA receptors. Neurochem Res. 1999;24:1027–1035. doi: 10.1023/a:1021056826829. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Endogenous dopamine potentiates the effects of glutamate on extracellular GABA in the prefrontal cortex of the freely moving rat. Brain Res Bull. 2000;53:339–345. doi: 10.1016/s0361-9230(00)00353-1. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. NMDA and AMPA/kainate glutamatergic agonists increase the extracellular concentrations of GABA in the prefrontal cortex of the freely moving rat: modulation by endogenous dopamine. Brain Res Bull. 2002;57:623–630. doi: 10.1016/s0361-9230(01)00758-4. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Glutamate-dopamine in vivo interaction in the prefrontal cortex modulates the release of dopamine and acetylcholine in the nucleus accumbens of the awake rat. J Neural Transm. 2005;112:97–109. doi: 10.1007/s00702-004-0172-5. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Dumas TC. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol. 2005;76:189–211. doi: 10.1016/j.pneurobio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McGregor S, Cochran SM, Morris BJ, Pratt JA. Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology (Berl) 2008;198:37–49. doi: 10.1007/s00213-008-1071-5. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, van der Weij W, Botterblom MH. Concentration-dependent dual action of locally applied N-methyl-D-aspartate on extracellular dopamine in the rat prefrontal cortex in vivo. Neurosci Lett. 1995;201:175–178. doi: 10.1016/0304-3940(95)12164-1. [DOI] [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Heng LJ, Cass DK, Caballero A, Tseng KY. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biol Psychiatry. 2014;75:508–516. doi: 10.1016/j.biopsych.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoneim MM, Hinrichs JV, Mewaldt SP, Petersen RC. Ketamine: behavioral effects of subanesthetic doses. J Clin Psychopharmacol. 1985;5:70–77. [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HW, Lee PA. A relationship between gonadotropins and visuospatial function. Neuropsychologia. 1986;24:563–576. doi: 10.1016/0028-3932(86)90100-4. [DOI] [PubMed] [Google Scholar]
- Han M, Huang XF, Chen da C, Xiu MH, Hui L, Liu H, Kosten TR, Zhang XY. Gender differences in cognitive function of patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:358–363. doi: 10.1016/j.pnpbp.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Hejna GM, Lorens SA. Androgen inhibits neurotransmitter turnover in the medial prefrontal cortex of the rat following exposure to a novel environment. Brain Res. 1997;751:131–138. doi: 10.1016/s0006-8993(96)01394-7. [DOI] [PubMed] [Google Scholar]
- Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–697. doi: 10.1002/1098-1063(2000)10:6<693::AID-HIPO1007>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Hasegawa N, Mochizuki M. Improved effect of Pycnogenol on impaired spatial memory function in partial androgen deficiency rat model. Phytother Res. 2009;23:840–843. doi: 10.1002/ptr.2702. [DOI] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63:559–565. doi: 10.1016/j.yhbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006;10:77–82. doi: 10.1016/j.tics.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 2010;47:4–16. [PubMed] [Google Scholar]
- Jedema HP, Moghddam B. Characterization of excitatory amino acid modulation of dopamine release in the prefrontal cortex of conscious rats. J Neurochem. 1996;66:1448–1453. doi: 10.1046/j.1471-4159.1996.66041448.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, de Bruin JP, Matthijssen MA, Uylings HB. Ontogeny of open field activity in rats after neonatal lesioning of the mesocortical dopaminergic projection. Behav Brain Res. 1989;32:115–127. doi: 10.1016/s0166-4328(89)80079-8. [DOI] [PubMed] [Google Scholar]
- Ko YH, Lew YM, Jung SW, Joe SH, Lee CH, Jung HG, Lee MS. Short-term testosterone augmentation in male schizophrenics: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2008;28:375–383. doi: 10.1097/JCP.0b013e31817d5912. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Bakir B, Haroutunian V, Meador-Woodruff JH. Expression the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophr Res. 2010a;119:198–209. doi: 10.1016/j.schres.2010.02.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Expression the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010b;64:495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase ovarian and testicular hormones. J Comp Neurol. 2000;427:617–633. [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kus L, Handa RJ, Hautman JM, Beitz AJ. Castration increases [125I]MK801 binding in the hippocampus of male rats. Brain Res. 1995;683:270–274. doi: 10.1016/0006-8993(95)00384-3. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Liu SB, Zhao MG. Neuroprotective effect of estrogen: role of nonsynaptic NR2B-containing NMDA receptors. Brain Res Bull. 2013;93:27–31. doi: 10.1016/j.brainresbull.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Locklear MN, Cohen AB, Jone A, Kritzer MF. Sex Differences Distinguish Intracortical Glutamate Receptor-Mediated Regulation of Extracellular Dopamine Levels in the Prefrontal Cortex of Adult Rats. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Kritzer MF. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav. 2014;66:298–308. doi: 10.1016/j.yhbeh.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Mele A, Castellano C, Felici A, Cabib S, Caccia S, Oliverio A. Dopamine-N-methyl-D-aspartate interactions in the modulation of locomotor activity and memory consolidation in mice. Eur J Pharmacol. 1996;308:1–12. doi: 10.1016/0014-2999(96)00266-x. [DOI] [PubMed] [Google Scholar]
- Miller IN, Cronin-Golomb A. Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord. 2010;25:2695–2703. doi: 10.1002/mds.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “virtual” maze: Sex differences and correlation with psychometric measures of spatial ability in humans. Evolution and Human Behavior. 1998;19:73–87. [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Jackson ME. Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci. 2003;1003:131–137. doi: 10.1196/annals.1300.065. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L, Kyaw M, Vercammen A, Lenroot R, Kulkarni J, Curtis J, O'Donnell M, Carr VJ, Shannon Weickert C, Weickert TW. Serum testosterone levels are related to cognitive function in men with schizophrenia. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl) 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18:306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- Okun MS, Fernandez HH, Rodriguez RL, Romrell J, Suelter M, Munson S, Louis ED, Mulligan T, Foster PS, Shenal BV, Armaghani SJ, Jacobson C, Wu S, Crucian G. Testosterone therapy in men with Parkinson disease: results of the TEST-PD Study. Arch Neurol. 2006;63:729–735. doi: 10.1001/archneur.63.5.729. [DOI] [PubMed] [Google Scholar]
- Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- Pontecorvo MJ, Clissold DB, White MF, Ferkany JW. N-methyl-D-aspartate antagonists and working memory performance: comparison with the effects of scopolamine, propranolol, diazepam, and phenylisopropyladenosine. Behav Neurosci. 1991;105:521–535. doi: 10.1037//0735-7044.105.4.521. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kaufman J, Huettel SA. Males and females use different distal cues in a virtual environment navigation task. Brain Res Cogn Brain Res. 1998;6:351–360. doi: 10.1016/s0926-6410(98)00002-0. [DOI] [PubMed] [Google Scholar]
- Sava S, Markus EJ. Intramaze cue utilization in the water maze: effects of sex and estrous cycle in rats. Horm Behav. 2005;48:23–33. doi: 10.1016/j.yhbeh.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, O'Connor C. N-methyl-D-aspartate receptor antagonist MK-801 and spatial memory representation: working memory is impaired in an unfamiliar environment but not in a familiar environment. Behav Neurosci. 1992;106:604–612. doi: 10.1037//0735-7044.106.4.604. [DOI] [PubMed] [Google Scholar]
- Silverman I, Kastuk D, Choi J, Phillips K. Testosterone levels and spatial ability in men. Psychoneuroendocrinology. 1999;24:813–822. doi: 10.1016/s0306-4530(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Fox EC, Larsen GD, Batson CG, Wagner BA, Maher J. Testosterone influences spatial strategy preferences among adult male rats. Horm Behav. 2013;63:800–812. doi: 10.1016/j.yhbeh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LA. Castration differentially affects spatial working and reference memory in male rats. Arch Sex Behav. 2008;37:19–29. doi: 10.1007/s10508-007-9264-2. [DOI] [PubMed] [Google Scholar]
- Stam CJ, de Bruin JP, van Haelst AM, van der Gugten J, Kalsbeek A. Influence of the mesocortical dopaminergic system on activity, food hoarding, social-agonistic behavior, and spatial delayed alternation in male rats. Behav Neurosci. 1989;103:24–35. doi: 10.1037//0735-7044.103.1.24. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Distinct contributions of glutamate receptor subtypes to cognitive set-shifting abilities in the rat. Ann N Y Acad Sci. 2003;1003:464–467. doi: 10.1196/annals.1300.064. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Glutamatergic regulation of basal and stimulus-activated dopamine release in the prefrontal cortex. J Neurochem. 1998;71:1443–1449. doi: 10.1046/j.1471-4159.1998.71041443.x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Vaskinn A, Sundet K, Simonsen C, Hellvin T, Melle I, Andreassen OA. Sex differences in neuropsychological performance and social functioning in schizophrenia and bipolar disorder. Neuropsychology. 2011;25:499–510. doi: 10.1037/a0022677. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigen R, O'Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- Woolley DG, Vermaercke B, Op de Beeck H, Wagemans J, Gantois I, D'Hooge R, Swinnen SP, Wenderoth N. Sex differences in human virtual water maze performance: novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res. 2010;208:408–414. doi: 10.1016/j.bbr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Wu WR, Li N, Sorg BA. Regulation of medial prefrontal cortex dopamine by alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptors. Neuroscience. 2002;114:507–516. doi: 10.1016/s0306-4522(02)00276-2. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


