Abstract
Background
Hypertrophic cardiomyopathy (HCM) is caused by sarcomere mutations and characterized by left ventricular hypertrophy (LVH) with increased risk of heart failure and sudden death. HCM typically cannot be diagnosed early in life, although subtle phenotypes are present. Animal studies indicate alterations in intracellular calcium handling before LVH develops. Furthermore, early treatment with diltiazem appeared to attenuate disease emergence.
Objectives
To assess the safety, feasibility, and effect of diltiazem as disease-modifying therapy for at-risk HCM mutation carriers.
Methods
In a pilot, double-blind trial, we randomly assigned 38 sarcomere mutation carriers without LVH (mean age 15.8 years) to therapy with diltiazem 360 mg/day (or 5 mg/kg/day) or placebo. Treatment duration ranged from 12 to 42 months (median 25 months). Study procedures included electrocardiography, echocardiography, cardiac magnetic resonance imaging, and serum biomarker measurement.
Results
Diltiazem was not associated with serious adverse events. Heart rate and blood pressure did not differ significantly between groups. However, mean left ventricular end diastolic diameter improved towards normal in the diltiazem group but decreased further in controls (change in z-scores, +0.6 vs. −0.5; P<0.001). Mean LV thickness-to-dimension ratio was stable in the diltiazem group, but increased in controls (−0.02 vs. +0.15; P=0.04). Among MYBPC3 mutation carriers, LV wall thickness and mass, diastolic filling, and cardiac troponin I levels improved in those taking diltiazem compared with controls. Four participants developed overt HCM, two in each treatment group.
Conclusions
Preclinical administration of diltiazem is safe and may improve early LV remodeling in HCM. This novel strategy merits further exploration.
Keywords: Cardiomyopathy, Genetics, Hypertrophy, Translational research, Treatment, Trials
Introduction
Hypertrophic cardiomyopathy (HCM) is caused by mutations in sarcomere genes, most commonly cardiac β-myosin heavy chain (MYH7), myosin binding protein C (MYBPC3), and troponin T (TNNT2) (1–6). Most patients have normal longevity and manageable symptoms, but sudden cardiac death and heart failure are prominent features of disease (7).
The clinical diagnosis of HCM relies on identifying unexplained left ventricular hypertrophy (LVH), and management focuses on symptom palliation and risk stratification for sudden death. However, mutation carriers usually have normal left ventricular (LV) wall thickness until adolescence or later.(8,9) Little is known about the pathways leading from sarcomere mutation to overt disease or adverse outcomes. Consequently, disease-modifying therapies have not yet been developed.
Studies in animal models of HCM indicate that sarcomere mutations trigger early dysregulation of intracellular calcium handling (10–12). These changes have been linked to the development of LVH and myocardial fibrosis. In these models, early diltiazem treatment decreased disease emergence, but late treatment could not reverse established HCM.(11,13) We thus sought to determine the safety, feasibility, and efficacy of diltiazem in attenuating phenotypic emergence of HCM, targeting at-risk sarcomere mutation carriers without LVH (ClinicalTrials.gov identifier: NCT00319982).
Methods
The institutional review and ethics boards of three collaborating medical centers approved the study protocol. All participants provided written informed consent or assent if they were younger than 18 years old at enrollment.
Study Design and Subjects
A double-blind, randomized, placebo-controlled pilot clinical trial was performed at Brigham and Women’s Hospital (Boston, MA), Boston Children’s Hospital (Boston, MA), and Royal Prince Alfred Hospital (Sydney, Australia). Eligible participants were at least 5 years old, carried the pathogenic or likely pathogenic (14) sarcomere mutation presumed to cause HCM in their family, and had normal LV wall thickness (echocardiographic maximal LV wall thickness ≤12 mm in adults or z-score ≤3 in children <18 years old). Major exclusion criteria included contraindications to diltiazem; concomitant treatment with cardioactive medications; impaired renal function (estimated glomerular filtration rate <60 mL/min/1.73 m2); and pregnancy or lactation. Up to four relatives from a single family could participate.
Study Procedures
Enrolled participants were randomly assigned in a 1:1 ratio to receive diltiazem or placebo. The Investigational Drug Service at Brigham and Women’s Hospital performed block randomization using the website randomization.com and a block size of four. Because of the influence of age on phenotypic expression, assignment was stratified by age (5 to <15 years and ≥ 15 years). Participants, parents, and all individuals involved in study procedures or data analysis were blinded to treatment assignment until after the database was locked.
After assignment, participants received sustained-release diltiazem (90 mg for adults and 1.5 mg/kg for children) or matched placebo capsules once daily. Dose was titrated over 2 to 4 weeks to a target daily dosage of 360 mg for adults and 5 mg/kg for children. Heart rate and blood pressure were monitored weekly during titration and at each visit during treatment. To maximize time on-treatment, the duration of follow-up varied depending on enrollment date. Participants were treated for a minimum of 1 year and a maximum of 3 years; earliest enrolling participants received longest follow-up. Follow-up visits occurred at 6, 12, 24, and 36 months after enrollment. Participants between the ages of 8.5 and 16.5 years at enrollment underwent additional assessment 18 months after enrollment to capture potentially faster phenotypic progression during puberty. Participants enrolled in the first year had an additional follow-up visit 6 to 12 months after completing treatment.
At each study visit, participants underwent physical examination, 12-lead electrocardiography (ECG), transthoracic echocardiography, and serum biomarker analysis. Cardiac magnetic resonance (CMR) imaging was performed at enrollment and at the end of treatment. Participants or their caregivers were called every 4 months and asked about adverse events and changes in symptoms. Adherence to study medication was monitored by pill count (15).
Echocardiographic Analysis
Standard two-dimensional, Doppler, and tissue Doppler images were obtained at each study visit. Cardiac dimensions and Doppler characteristics were recorded as the mean value of three cardiac cycles in accordance with the guidelines of the American Society of Echocardiography (16). If wall thickness was non-uniform, the location and greatest dimension were recorded after visual assessment from multiple views. Echocardiographic measures in all participants were also converted to z-scores to adjust for differences in age and body size.(17)
Left ventricular ejection fraction (LVEF) was calculated using Simpson’s method (16,18). Standard metrics of diastolic function included peak early (E) and late (A) transmitral velocities, E/A ratio, and E-wave deceleration time (19). Early myocardial tissue Doppler relaxation velocities (E′) were measured at the lateral, septal, anterior, and inferior aspects of the mitral annulus. Global E′ velocity was determined by averaging these four values.
All echocardiographic images were analyzed offline by two investigators (CYH and NKL), blinded to treatment assignment. Anticipating that some pediatric subjects may not be able to tolerate cardiac magnetic resonance imaging, echocardiography was designated as the primary modality to assess cardiac morphology and function.
Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance images were acquired at enrollment and at the final visit on study medication with a 3.0 T system (Tim Trio, Siemens, Erlangen, Germany), or 1.5 T scanners (Achieva, Philips Healthcare, Best, the Netherlands, or HDX Excite II, General Electric, Milwaukee, WI). Images were acquired with both cardiac-gating and breath-holding. The standard protocol consisted of cine steady-state free precession imaging for LV function and LV mass.(20) Left ventricular mass was derived by the summation-of-discs method after manual tracing of myocardial borders on short-axis cine images.(21) Left ventricular wall thickness was measured in at least four sections: anterior and posterior septal, lateral, and inferior.
A late gadolinium-enhancement (LGE) imaging protocol was used to detect focal myocardial fibrosis. A segmented inversion-recovery pulse sequence was started 10 to 15 minutes after the participant had received a 0.15-mmol/kg cumulative dose of gadolinium-DTPA (Bayer HealthCare Pharmaceuticals Inc, Wayne, New Jersey). Images were measured using a semi-automated gray-scale threshold technique using a cutoff of 6 standard deviations above the mean signal intensity (22). The quantity of LGE was expressed in grams and as a percentage of the total LV myocardial mass. All CMR analyses were performed using commercial software (QMassMR, version 7.4; Medis, Leiden, the Netherlands) by the same CMR physician (SAA), blinded to treatment assignment.
Serum Biomarkers Analysis
Blood samples (serum and K3-EDTA plasma) collected at enrollment and at each study visit were processed within 1 hour of phlebotomy and stored at −80 °C before analysis. All assays were performed using commercial reagents by personnel blinded to clinical and genetic status of participants. The following markers were analyzed: carboxy-terminal propeptide of procollagen type I (PICP; Quidel Corporation, San Diego, CA), amino terminal propeptide of B-type natriuretic peptide (NT-pro BNP; Roche, Indianapolis, IN), and cardiac troponin I (supersensitive assay, Singulex, Atlanta, GA).
Statistical Methods
Patient characteristics are summarized as means and standard errors or counts, as appropriate. Standard errors rather than standard deviations are presented because a generalized estimating equation approach, using the Genmod procedure in the SAS statistical package (version 9.2, SAS Institute, Cary, NC), was used to account for an exchangeable correlation structure within families. This assumption of equal correlation between any pair of family members is reasonable for the siblings that constitute almost all of our families, but may over-estimate the correlation in the sole aunt-niece pair. This same model, additionally adjusted for age, sex, genotype, and baseline value, was used to analyze echocardiographic, CMR, and biomarker outcomes. The covariates in our models were chosen a priori based on their potential roles as confounders or effect modifiers. Therefore they were included in all models despite the risk of overfitting and the associated potential for unstable estimates and false positive or negative results. Interaction terms between treatment and age, sex, and genotype were analyzed to identify any responsive subgroups. For safety analyses, counts of patients with adverse events were compared between groups using Fisher’s exact test.
As a pilot trial, all analyses were considered exploratory. Alpha was set at 0.05, and no adjustments were made for multiple testing. This trial was originally designed with global E′ as the primary outcome, and with the goal of detecting a 2- to 3-cm/s difference between the change in global E′ in controls and the change in global E′ in the diltiazem group. The final sample of 18 diltiazem and 20 placebo patients had a projected power of 83% to detect this difference, assuming a standard deviation of 2.5 cm/s. However, because of the small sample size, because early phenotypes of HCM are subtle and affect multiple pathways, and because the impact of treatment is unknown, this trial was changed prior to data analysis. Rather than having a primary focus on E′, we consider the trial to be a pilot effort to explore a broad range of imaging and biomarker features, to try to maximize the potential to detect phenotypic progression and treatment effect on complex disease biology.
Results
Participants
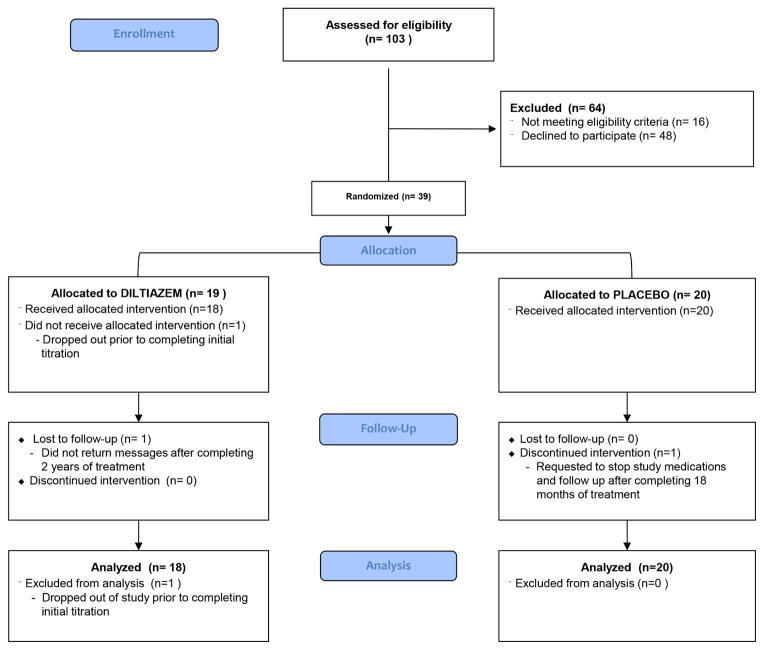
Of 103 sarcomere mutation carriers screened between July 2006 and June 2010, 16 were ineligible and 48 declined participation, primarily from concerns about taking daily medication or keeping study visits. Thirty-nine people (38% of those screened) were enrolled (Figure 1). All participants tolerated titration to target dose, although one withdrew from the study for personal reasons before completing titration. Thus, 38 participants, 18 in the diltiazem group and 20 controls, are included in analysis. Of the 38, 7 participants (ages 5 to 18 years at baseline) declined CMR imaging; 3 others declined intravenous cannula insertion to administer gadolinium contrast.
Figure 1. CONSORT Diagram (25).
Participant flow and dispositions in a randomized trial of hypertrophic cardiomyopathy sarcomere mutation carriers treated with diltiazem.
Baseline characteristics of the treatment and placebo groups were generally similar (Table 1). A listing of the specific sarcomere mutations present in the study cohort is provided in the supplemental table. For the overall study cohort, the mean (SD) age was 15.8 (8.6) years (range, 5 to 39 years), 58% were female, and all were healthy and had no cardiovascular symptoms or concomitant illnesses. Of the 28 participants who underwent gadolinium-contrast CMR imaging, none had late gadolinium enhancement at baseline. Twenty-nine families were represented of which 7 families had more than 1 relative enrolled, including 1 set of 4 siblings, 1 set of 3 siblings, 4 sets of 2 siblings, and 1 aunt-niece pair. Of the 17 related subjects, 10 were assigned to placebo and 7 to diltiazem.
Table 1.
Baseline Characteristics of the Study Participants
| Diltiazem n=18 |
Placebo n=20 |
|
|---|---|---|
| Age, years | 14.1 ± 1.7 | 17.3 ± 2.1 |
| Female/Male, n | 11/7 | 11/9 |
| Causal Gene, n | ||
| MYH7 | 10 | 11 |
| MYBPC3 | 6 | 6 |
| TNNT2 | 2 | 3 |
| Heart rate, bpm | 71 ± 4 | 73 ± 4 |
| SBP, mm Hg | 104 ± 3* | 113 ± 2 |
| DBP, mm Hg | 63 ± 2 | 66 ± 2 |
| Max LVWT, mm | 8.1 ± 0.4 | 8.1 ± 0.35 |
| Max LVWT z-score | 0.7 ± 0.4 | 0.2 ± 0.2 |
| LVEDD, cm | 4.2 ± 0.2 | 4.3 ± 0.15 |
| LVEDD z-score | −1.5 ± 0.2 | −1.5 ± 0.3 |
| LV thickness/Dimension Ratio | 1.76 ± 0.05 | 1.63 ± 0.10 |
| LVESD, cm | 2.4 ± 0.1 | 2.4 ± 0.1 |
| LVESD z-score | −2.7 ± 0.31 | −2.7 ± 0.4 |
| Echo LVEF, % | 70 ± 1 | 70 ± 2 |
| Global E′, cm/sec | 14.7 ± 0.6 | 14.2 ± 0.5 |
| E/E′ | 6.0 ±0.3 | 5.6 ± 0.2 |
| LA diameter, cm | 3.1 ± 0.2 | 3.2 ± 0.1 |
| LA z-score | −1.2 ± 0.2 | −1.1 ± 0.1 |
| CMR LA volume, ml | 57.1 ± 5.1 | 59.9 ± 7.0 |
| LA volume index, ml/m2 | 38.0 ± 3.1 | 34.4 ± 2.5 |
| TR maximal velocity, m/sec | 2.0 ± 0.1 | 1.9 ± 0.1 |
| CMR LV mass, g | 83.8 ± 10.0 | 80.3 ± 8.8 |
| CMR LV mass index, g/m2 | 49.9 ± 3.8 | 46.6 ± 3.4 |
| Troponin I, pg/mL | 2.2 ± 0.3 | 3.2 ± 0.8 |
| NT-proBNP, pg/mL | 64.6 ± 15.4 | 97.1 ± 34.7 |
| PICP, μg/liter | 130 ± 8 | 118 ± 9 |
Values represent means ± standard error, adjusted for family relations
p<0.01 comparing participants treated with diltiazem and placebo; otherwise no significant differences identified
bpm, beats per minute; SBP, systolic blood pressure; DBP, diastolic blood pressure; Max LVWT, maximal LV wall thickness, LVEDD, LV end-diastolic diameter; LVESD, LV end-systolic diameter; LVEF, LV ejection fraction; E′, tissue Doppler early myocardial relaxation velocity; CMR, cardiac magnetic resonance; LA, left atrium; TR, tricuspid regurgitation; NT-proBNP, N-terminal propeptide of B-type natriuretic peptide; PICP, C-terminal propeptide of procollagen type I
Safety and Adverse Events
Median treatment duration was 756 days in the diltiazem group and 755 days in controls (overall treatment duration ranged from 369 to 1280 days; Table 2). Mean systolic blood pressure did not change significantly in either treatment group; participants in the diltiazem group had a minor decrease in heart rate, consistent with drug effect (Table 3). No participants requested or required discontinuation of study medication for safety concerns, adverse events, side effects, or intolerance.
Table 2.
Treatment Duration, Adherence, and Adverse Events Possibly Related to Study Medication
| Characteristic | Diltiazem n=18 |
Placebo n=20 |
P value |
|---|---|---|---|
| Length of treatment, median [IQR], days | 756 [736–818] | 755 [735–1099] | 0.85* |
| Adherence, % (SD) | 83 (10.8) | 90 (6.6) | 0.08* |
| Participants reporting adverse events, n | 10 | 12 | 1.0†,b |
| Number of adverse events, N | 29‡ | 23 | 0.37x |
| Most common adverse events | |||
| Shortness of breath/exertional dyspnea, n/N | 6/9§ | 1/1 | 0.03x |
| Lightheadedness or orthostasis, n/N | 3/5 | 5/6 | 0.92x |
| Nausea or GI symptoms, n/N | 1/1 | 4/4 | 0.19x |
| Fatigue, n/N | 1/1 | 2/2 | 0.62x |
| Headache, n/N | 0/0 | 1/2 | 0.34x |
| Chest Pain, n/N | 3/3 | 1/1 | 0.25x |
IQR, interquartile range; n/N, number of participants/number of events
Wilcoxon rank sum test
Fisher’s exact test
5 events occurred in a 9-year old girl with a baseline BP of 95/51 mm Hg and intermittent, asymptomatic recordings of SBP 86–89 mm Hg during follow up
Chi-square trend test, allowing for multiple events per participant
5 events with self-limited symptoms occurred in 2 participants
Table 3.
Effect of Diltiazem Treatment on Hypertrophic Cardiomyopathy Sarcomere Mutation Carriers.
| Change in Value (Final - Baseline) | Diltiazem N=18 |
Placebo N=20 |
P-value |
|---|---|---|---|
| Heart rate, bpm | −4.9 ± 2.2* | 2.0 ± 2.6 | 0.06 |
| Systolic blood pressure, mm Hg | −1.4 ± 1.7 | 2.1 ± 1.8 | 0.15 |
| Diastolic blood pressure, mm Hg | −0.1 ± 1.1 | 2.7 ± 1.3* | 0.08 |
| Max LVWT, mm | 0.9 ± 0.5 | 1.6 ± 0.6* | 0.32 |
| Max LVWT z-score | 0.52 ± 0.44 | 1.4 ± 0.53* | 0.22 |
| LVEDD, cm | 0.26 ± 0.06* | −0.02 ± 0.06 | 0.001 |
| LVEDD z-score | 0.60 ± 0.17* | −0.53 ± 0.16* | <0.001 |
| LV Thickness/Dimension Ratio | −0.02 ± 0.05 | 0.15 ± 0.06* | 0.04 |
| LVESD, cm | 0.19 ± 0.05* | 0.08 ± 0.06 | 0.14 |
| LVESD z-score | 0.61 ± 0.22* | −0.02 ± 0.27 | 0.05 |
| Echo LVEF, % | −0.22 ± 0.86 | −0.72 ± 0.95 | 0.72 |
| Global E′, cm/s | −0.06 ± 0.27 | −0.21 ± 0.42 | 0.75 |
| E/E′ | 0.02 ± 0.17 | −0.25 ± 0.15 | 0.29 |
| LA diameter, cm | 0.06 ± 0.09 | 0.10 ± 0.07 | 0.70 |
| LA z-score | −0.02 ± 0.14 | −0.01 ± 0.11 | 0.93 |
| LA Volume, ml | 4.5 ± 5.9 | 1.7 ± 6.1 | 0.73 |
| LA Volume Index, ml/m2 | −0.51 ± 2.6 | 0.88 ± 2.8 | 0.71 |
| TR maximal velocity, m/sec | 0.02 ± 0.05 | −0.07 ± 0.10 | 0.42 |
| CMR LV mass, g | −3.7 ± 3.6 | −7.5 ± 3.3* | 0.49 |
| CMR LV mass index, g/m2 | −0.96 ± 2.3 | −4.2 ± 1.3* | 0.25 |
| Troponin I, pg/mL | 0.22 ± 0.88 | 0.64 ± 0.56 | 0.72 |
| NT-proBNP, pg/mL | 22.3 ± 26.6 | −17.3 ± 13.4 | 0.24 |
| PICP, μg/ml | −2.9 ± 7.8 | −21.3 ± 5.1* | 0.08 |
Values represent mean changes ± standard error and are adjusted for age, sex, genotype, family relations, and baseline value
Significant change over time, P<0.05, within the designated treatment group
bpm, beats per minute; SBP, systolic blood pressure; DBP, diastolic blood pressure; Max LVWT, maximal LV wall thickness, LVEDD, LV end-diastolic diameter; LVESD, LV end-systolic diameter; LVEF, LV ejection fraction; E′, tissue Doppler early myocardial relaxation velocity; CMR, cardiac magnetic resonance; LA, left atrium; TR, tricuspid regurgitation; NT-proBNP, N-terminal propeptide of B-type natriuretic peptide; PICP, C-terminal propeptide of procollagen type I
One participant in the diltiazem group was lost to follow-up after 2 years. One control participant withdrew after 18 months of treatment. Adherence to the protocol, assessed by pill counts, averaged 83% in the diltiazem group and 90% in controls (P=0.08; Table 2).
No serious adverse events were reported. Twenty-two subjects reported fifty-two mild adverse events possibly related to diltiazem (Table 2). Non-limiting dyspnea, lightheadedness, and gastrointestinal upset were most frequently described. In the diltiazem group, 5 adverse events were related to one young female subject whose systolic blood pressure was less than 90 mm Hg. At enrollment, she was 9 years old and had a blood pressure of 95/51 mm Hg. After titration, her blood pressure was 90/60 mm Hg. During treatment, her systolic blood pressure varied between 86 and 89 mm Hg. These values are normal for her age and size. She was asymptomatic, pursued vigorous activities without difficulty and so continued in the study.
Shortness of breath and exertional dyspnea were more frequent in the diltiazem group (9 events vs. 1, P=0.03). However, 5 of these 9 events occurred in two participants, a 12-year-old female and a 17-year-old male. Both had mild and inconsistent symptoms that resolved spontaneously.
Response to Treatment
We assessed changes in values (difference between final and baseline values) to gauge treatment response or disease progression over the course of the trial (Table 3). There was no significant change in systolic blood pressure or heart rate over time between treatment groups. Among imaging and biomarker characteristics, only changes in mean LV end diastolic diameter (LVEDD) differed significantly between groups. At baseline, participants in both groups had below-average LV cavity size (mean LVEDD z-score = −1.5). During treatment, LVEDD z-scores improved slightly towards normal in 14 of 18 participants treated with diltiazem (78%) but in only 5 of 20 placebo-treated participants (25%). Collectively, controls showed a further small decrease in mean (SE) cavity size during treatment (0.53 decrease in LVEDD z-score; P<0.001) (Table 3). Neither baseline heart rate nor change in heart rate affected this difference between the changes in LVEDD (data not shown). Although the treatment groups had comparable LV wall thickness after treatment, the posterior wall thickness-to-LVEDD ratios indicate differences in LV remodeling. At baseline, these ratios were similar (Table 1). With treatment, the ratio remained stable in the diltiazem group, but increased significantly in controls (+0.15 ± 0.06; P<0.001; Table 3). Our original primary outcome, Global E′, showed small declines in both groups, with no significant difference between them (p= 0.75; Table 3).
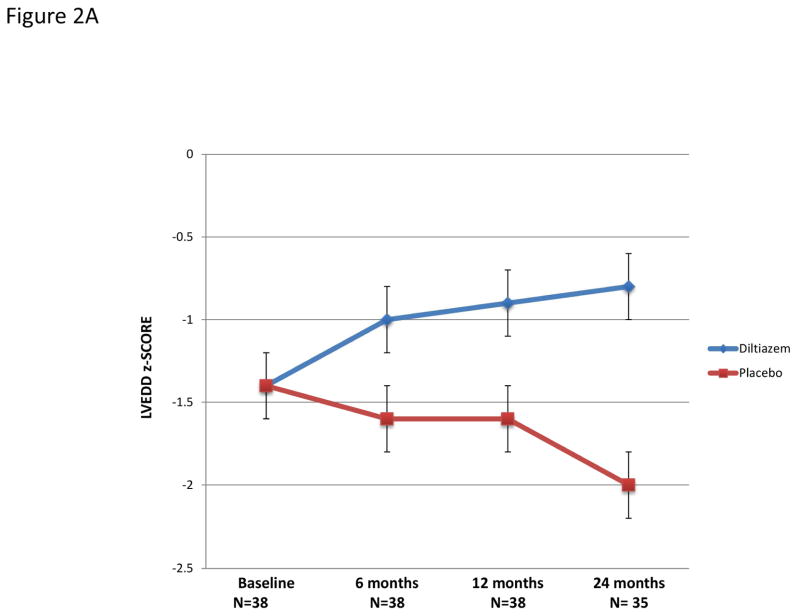
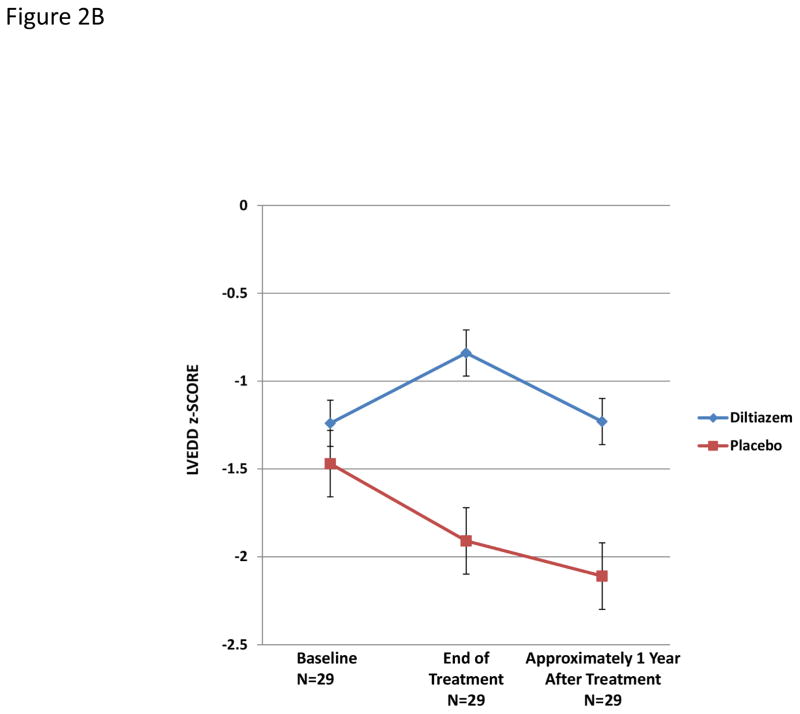
Hypertrophic cardiomyopathy is typically characterized by small LV cavity size; therefore, the increase in LVEDD in diltiazem-treated participants may reflect a beneficial treatment response (Figure 2a). Among 29 participants who received additional follow up approximately year after treatment ended, LV cavity size decreased after diltiazem was discontinued (Figure 2b).
Figure 2. Changes in left ventricular end-diastolic z-scores over time in study participants.
A) Changes during treatment with diltiazem or placebo in the full cohort. The two treatment groups diverged over time (interaction between time and treatment, P=0.003), with significant pairwise differences at 12 (P=0.01) and at 24 (P<0.001) months.
B) Changes during the year after the end of treatment with diltiazem or placebo in the 29 subjects with available follow up. The two treatment groups again diverged over time (interaction between time and treatment, P=0.047), with a significant pairwise difference at the end of treatment (P=0.005). The pairwise difference 1 year after discontinuation of diltiazem was attenuated but remained significant (P=0.02). Error bars are standard errors.
Treatment Interactions
We examined baseline features that may affect the expression of sarcomere mutations, including age, sex, and underlying genotype. Anecdotal reports have suggested that disease expression can be earlier or more pronounced in males and during puberty (3,8,23,24). In females receiving diltiazem, E′ velocity improved more than in controls (+0.63 vs. −0.32 cm/s; P=0.049; interaction with sex P= 0.02). Additionally, diltiazem-treated participants less than 15 years old showed a slight decrease in LV thickness-to-dimension ratio (−0.10), whereas controls had a slight increase (+0.22; P<0.001; interaction with age, P= 0.02).
HCM is most commonly caused by mutations in MYH7 and MYBPC3. The clinical manifestations of MYH7 mutations have been postulated to be more severe (3,8). We analyzed MYBPC3 and MYH7 mutation carriers to assess for potential interaction between treatment and underlying genotype. Mean maximal LV wall thickness z-score decreased by 0.02 in diltiazem-treated MYBPC3 mutation carriers but increased by 2.6 in controls (P=0.01; Table 4). Similarly, LV mass as assessed by CMR decreased in diltiazem-treated MYBPC3 mutation carriers but increased in controls (P<0.001). The E/E′ ratio, which reflects LV filling pressure, decreased significantly more in diltiazem-treated MYBPC3 mutation carriers than in controls (p=0.001). Finally, serum cardiac troponin I levels decreased in diltiazem-treated MYBPC3 mutation carriers while it increased in controls (p=0.01). There was no suggestion of beneficial change in these parameters in diltiazem-treated MYH7 mutation carriers.
Table 4.
Change in Cardiac Measurements after 3 Years of Treatment with Diltiazem, by Genotype.
| Outcome | MYH7 carriers (n=21) | P-value | MYBPC3 (carriers n=12) | P-value | P for interaction | ||
|---|---|---|---|---|---|---|---|
| Diltiazem | Placebo | Diltiazem | Placebo | ||||
| Sample size (Echocardiography), n | 10 | 11 | 6 | 6 | |||
| Sample Size (CMR), n | 8 | 8 | 5 | 5 | |||
|
| |||||||
| Change in outcome measure | |||||||
| Echo MaxLVWT, mm* | + 1.4 ± 0.60 | + 0.84 ± 0.70 | 0.49 | − 0.02 ± 0.60 | + 3.2 ± 0.84 | 0.001 | 0.003 |
| Echo MaxLVWT, z-score* | + 0.92 ± 0.63 | +0.78 ± 0.62 | 0.87 | − 0.02 ± 0.45 | +2.6 ± 0.92 | 0.01 | 0.04 |
| E/E′, mean (SD), | + 0.53 ± 0.20 | − 0.03 ± 0.17 | 0.04 | − 0.97 ± 0.15 | −0.25 ± 0.17 | 0.001 | <0.001 |
| CMR LV mass, mean (SD), g† | − 1.8 ± 4.4 | − 8.8 ± 3.7 | 0.24 | − 13.4 ± 3.4 | + 4.0 ± 4.2 | <0.001 | <0.001 |
| CMR LV mass index, mean (SD)† | − 1.1 ± 3.1 | − 5.6 ± 1.6 | 0.21 | − 3.7 ± 2.3 | + 1.9 ± 1.4 | 0.008 | 0.01 |
| Troponin I, mean (SD), pg/mL | + 1.2 ± 1.2 | − 0.51 ± 0.59 | 0.24 | − 0.61 ± 0.70 | + 2.1 ± 1.1 | 0.01 | 0.02 |
Values represent mean changes ± standard error and are adjusted for age, sex, genotype, family relations, and baseline value
Measured by echocardiography
Measured by cardiac magnetic resonance imaging
Max LVWT, maximal LV wall thickness; LVEDD, LV end-diastolic diameter; E/E′, Mitral inflow E wave/E′
Development of HCM
Four unrelated participants, two in each treatment group, had substantial increases in LV wall thickness during follow up, leading to a diagnosis of HCM. All carried pathogenic mutations: MYH7 Arg719Gln (2 unrelated participants; female, age 9 years at baseline and male, age 12 years at baseline), MYH7 Arg663Cys (male, age 17 years at baseline), and TNNT2 Arg92Trp (male, age 17 years at baseline). None of these subjects reported symptoms or change in exercise capacity throughout the study.
Discussion
We tested a novel, genotype-guided, disease-modifying intervention in individuals at risk for developing inherited cardiomyopathy, before diagnosis with disease. This unique strategy involved treating at-risk sarcomere mutation carriers with the intention of changing the natural history of HCM by starting therapy before potentially irreversible changes in cardiac structure and function developed.
Diltiazem was safely administered to this young, healthy population without detrimental effects. Mild adverse events were evenly distributed between treatment groups. Adherence to the study protocol was high, even in a potentially challenging cohort of adolescents.
Diltiazem may attenuate a longitudinal decrease in LV cavity size in HCM sarcomere mutation carriers. We suggest this result is notable because LVH with small LV cavity size is a distinctive feature of hypertrophic cardiomyopathy. Indeed, even in the absence of LVH, mutation carriers typically have relatively small LV cavities, reflected by the baseline mean LVEDD z-score of −1.5 in study participants. Left ventricular cavity size progressively improved towards normal in diltiazem-treated participants but progressively decreased in controls, without associated changes in heart rate or ejection fraction. The improvements were lost within a year off treatment. Additionally, the LV thickness-to-dimension ratio decreased slightly in the diltiazem group, but increased significantly in controls. Otherwise, the treatment groups did not differ markedly.
We postulate these geometric changes may have a greater functional impact than reflected by simple linear measurements. A fundamental detrimental effect of hypertrophy in HCM is decreased LV compliance. The general assumption is that poor ventricular compliance in HCM is due primarily to myocardial abnormalities that result in increased stiffness. However, geometric effects are also important because wall stress is proportional to the product of pressure and the LV dimension-to-thickness ratio. In HCM, the increase in LV thickness and concomitant decrease in LV cavity size reduce LV wall stress, and therefore higher diastolic pressures are required to sufficiently distend the ventricle. This purely geometric effect impacts filling, even if the muscle itself is normal. Reducing the thickness-to-dimension ratio improves ventricular compliance, independently of changing relaxation or myocardial compliance.
Secondary analyses suggested that MYBPC3 mutation carriers may be more responsive to disease-modifying treatment with diltiazem than MYH7 mutation carriers. Diltiazem-treated MYBPC3 mutation carriers had significantly less increase in LV wall thickness and mass over time than controls. Diltiazem-treated MYBPC3 mutation carriers also had lower serum levels of cardiac troponin I and a suggestion of improved LV filling pressures, as represented by a decrease in E/E′ values, compared to placebo-treated MYBPC3 mutation carriers. These beneficial effects were not seen in diltiazem-treated MYH7 mutation. Further investigation is needed to better elucidate mechanisms that may govern genotypic differences in treatment response. Such advances may ultimately allow more targeted and personalized therapy.
Limitations of the Study
This was a pilot, exploratory study to test a new approach to treating HCM. Firm conclusions about treatment efficacy cannot be drawn. The small number of participants and short follow-up duration are limitations of this study. We are unable to determine whether diltiazem may have more prominent effects if administered for longer periods or at higher doses. Additionally, the tools currently available to monitor treatment response and phenotypic progression are crude and may lack the resolution necessary to identify subtle changes in healthy young people. Identifying new robust, quantitative and dynamic phenotypes will be key to detect early disease emergence, progression and treatment effects. Furthermore, the penetrance of sarcomere mutations is variable, unpredictable, and not absolute. Some mutation carriers may not develop HCM, or do so only at an advanced age. More precise understanding of the natural history of early HCM is critically needed to better understand the factors that drive disease evolution and adverse outcomes. Such knowledge will help guide the development of disease-modifying therapy, and help direct such therapy to those at highest risk.
Conclusions
Genetic testing can identify individuals with sarcomere mutations that cause hypertrophic cardiomyopathy in advance of clinical disease. These at-risk mutation carriers may be targeted for therapies designed to modify the progression and emergence of disease. Long-term use of diltiazem is safe. This type of approach may be able to attenuate the phenotypic expression of HCM if given early in disease pathogenesis. Such strategies are worth exploring further in larger trials because of the potential to transform the care of delayed-onset genetic disease, like HCM. Although there are many challenges to overcome, success with these tactics would allow management to evolve from a largely passive strategy of symptom palliation and sudden death risk stratification, to a proactive strategy of disease modification and, perhaps, disease prevention.
Supplementary Material
Supplemental Table. Sarcomere Mutations Present in the Study Cohort
PERSPECTIVES.
Competency in Medical Knowledge
Hypertrophic cardiomyopathy (HCM) is an important inherited cardiomyopathy associated with an increased risk of sudden death and heart failure. Current management focuses on symptom palliation and selection of appropriate patients for ICD therapy.
Translational Outlook
Genetic testing allows identification of patients at-risk for developing disease in the preclinical stage. This provides opportunities for studying early steps in pathogenesis that will foster the development of novel treatment strategies that aim to modify the natural history of disease.
Acknowledgments
Funding: The National Institutes of Health (K23 HL078901 to CYH) funded this study but was not involved with study design or analysis.
Additional support was provided by the agreement between FIMA and Unión Temporal de Empresas project CIMA (AG, BL, JD); the Ministry of Economy and Competitiveness, Spain (RIC and Ramon y Cajal Program) (AG, BL, JD) and the European Union (MEDIA and EU-MASCARA projects) (AG, BL, JD).
Abbreviations
- CMR
Cardiac magnetic resonance
- HCM
Hypertrophic cardiomyopathy
- E′
Tissue Doppler mitral annular early relaxation velocity
- LVH
Left ventricular hypertrophy
- MYH7
Cardiac β-myosin heavy chain gene
- MYBPC3
Cardiac myosin binding protein C gene
Footnotes
Disclosures: None
ClinicalTrials.gov Identifier: NCT00319982
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geisterfer-Lowrance AA, Kass S, Tanigawa G, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 2.Thierfelder L, MacRae C, Watkins H, et al. A familial hypertrophic cardiomyopathy locus maps to chromosome 15q2. Proc Natl Acad Sci U S A. 1993;90:6270–4. doi: 10.1073/pnas.90.13.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins H, Rosenzweig A, Hwang DS, et al. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy [see comments] N Engl J Med. 1992;326:1108–14. doi: 10.1056/NEJM199204233261703. [DOI] [PubMed] [Google Scholar]
- 4.Watkins H, MacRae C, Thierfelder L, et al. A disease locus for familial hypertrophic cardiomyopathy maps to chromosome 1q3. Nat Genet. 1993;3:333–7. doi: 10.1038/ng0493-333. [DOI] [PubMed] [Google Scholar]
- 5.Watkins H, McKenna WJ, Thierfelder L, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058–64. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 6.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–67. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 7.Gersh BJ, Maron BJ, Bonow RO, et al. Circulation. 2011. ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. [DOI] [PubMed] [Google Scholar]
- 8.Niimura H, Bachinski LL, Sangwatanaroj S, et al. Mutations in the gene for cardiac myosin-binding protein C and late- onset familial hypertrophic cardiomyopathy [see comments] N Engl J Med. 1998;338:1248–57. doi: 10.1056/NEJM199804303381802. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Seidman JG, Seidman CE. Proposal for contemporary screening strategies in families with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44:2125–32. doi: 10.1016/j.jacc.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Fatkin D, McConnell BK, Mudd JO, et al. An abnormal Ca(2+) response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J Clin Invest. 2000;106:1351–9. doi: 10.1172/JCI11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semsarian C, Ahmad I, Giewat M, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013–20. doi: 10.1172/JCI14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinto PJ, Haim TE, Dowell-Martino CC, Sibinga N, Tardiff JC. Temporal and mutation-specific alterations in Ca2+ homeostasis differentially determine the progression of cTnT-related cardiomyopathies in murine models. Am J Physiol Heart Circ Physiol. 2009;297:H614–26. doi: 10.1152/ajpheart.01143.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westermann D, Knollmann BC, Steendijk P, et al. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail. 2006;8:115–21. doi: 10.1016/j.ejheart.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Lakdawala NK, Funke BH, Baxter S, et al. Genetic testing for dilated cardiomyopathy in clinical practice. Journal of cardiac failure. 2012;18:296–303. doi: 10.1016/j.cardfail.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–57. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 17.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 18.Park SH, Shub C, Nobrega TP, Bailey KR, Seward JB. Two-dimensional echocardiographic calculation of left ventricular mass as recommended by the American Society of Echocardiography: correlation with autopsy and M-mode echocardiography. J Am Soc Echocardiogr. 1996;9:119–28. doi: 10.1016/s0894-7317(96)90019-x. [DOI] [PubMed] [Google Scholar]
- 19.Maron BJ, Spirito P, Green KJ, Wesley YE, Bonow RO, Arce J. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1987;10:733–42. doi: 10.1016/s0735-1097(87)80264-4. [DOI] [PubMed] [Google Scholar]
- 20.Steel K, Broderick R, Gandla V, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. The American journal of cardiology. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 22.Flett AS, Hasleton J, Cook C, et al. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. Jacc. 2011;4:150–6. doi: 10.1016/j.jcmg.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Niimura H, Patton KK, McKenna WJ, et al. Sarcomere protein gene mutations in hypertrophic cardiomyopathy of the elderly. Circulation. 2002;105:446–51. doi: 10.1161/hc0402.102990. [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig A, Watkins H, Hwang DS, et al. Preclinical diagnosis of familial hypertrophic cardiomyopathy by genetic analysis of blood lymphocytes [see comments] N Engl J Med. 1991;325:1753–60. doi: 10.1056/NEJM199112193252501. [DOI] [PubMed] [Google Scholar]
- 25.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Sarcomere Mutations Present in the Study Cohort