Abstract
Expression of anger is associated with biological health risk (BHR) in Western cultures. However, recent evidence documenting culturally divergent functions of anger expression suggests that the link between anger expression and BHR may be moderated by culture. To test this prediction, we examined large probability samples of both Japanese and Americans with multiple measures of BHR including pro-inflammatory markers (Interleukin-6 and C-reactive protein) and indices of cardiovascular malfunction (systolic blood pressure and Total/HDL cholesterol ratio). We found that the positive link between anger expression and increased BHR was robust for Americans. As predicted, however, this association was diametrically reversed for Japanese, with anger expression predicting reduced BHR. The pattern was unique to the expressive facet of anger and remained after controlling for age, gender, health status, health behaviors, social status, and reported experience of negative emotions. Implications for socio-cultural modulation of bio-physiological responses are discussed.
Keywords: anger expression, inflammation, cardiovascular malfunctioning, culture
Is expressing anger always detrimental to health? Extant evidence suggests a robust positive association between anger expression/hostility and compromised health, particularly, cardiovascular morbidity and mortality (Chida & Steptoe, 2009a; Everson-Rose & Lewis, 2005; Schum, Jorgensen, Verhaeghen, Sauro, & Thibodeau, 2003; Smith, Glazer, Ruiz, & Gallo, 2004; Vandervoort, Ragland, & Syme, 1996). Consistent with the hypothesis that such effects may be mediated by a pathway of chronic inflammation (Miller, Chen, & Cole, 2009), recent studies reported similar positive associations between anger expression and inflammatory markers (Boylan & Ryff, 2013; Elovainio, Merjonen, & Pulkki-Råback, 2011; Graham et al., 2006; Marsland, Prather, Petersen, Cohen, & Manuck, 2008), especially for disadvantaged individuals such as those with low educational attainment (Boylan & Ryff, 2013) and low childhood socioeconomic status (Beatty & Matthews, 2009). Such health-compromising effect of anger has also been documented longitudinally (Kawachi, Sparrow, Spiro, Vokonas, & Weiss, 1996).
However, much of the prior literature is based on Western populations. Thus, the health-compromising effects of anger expression may not be evident across other cultural contexts, wherein expression of anger may serve different functions (Consedine, Magai, & Horton, 2005; Consedine et al., 2006). In the current work, using large probability samples of Americans and Japanese, we tested the hypothesis that the association between anger expression and biological health risk (BHR) is moderated by culture.
Our analysis draws on a formulation that anger expression has two separable facets (Park et al., 2013). First, anger expression sometimes reflects frustrating experiences. As argued by numerous scholars (Berkowitz, 1989), when faced with events that block one's goals and desires, individuals are likely frustrated, which in turn results in experience and expression of anger. Second, researchers both in ethology (Hurd & Enquist, 2001) and social psychology (Tiedens, 2001) also point out that expressing anger is a way to display one's dominance and to intimidate others. Anger expression in such contexts can therefore reflect one's dominance over others. Although related, the two aspects of anger are separable and can be differentially salient depending on a variety of contextual variables.
Culture is one way of framing such contextual influences. Culture is a set of symbolic beliefs, practices, and institutions that are recruited to define the meanings of social situations and to regulate social interactions (Adams & Markus, 2001; Kitayama, Markus, Matsumoto, & Norasakkunkit, 1997). These components of culture are shared across individuals within regions or groups that have common historical heritages such as North American cultures and East Asian cultures.
In Western cultural contexts, independence of the self is culturally sanctioned and, as a consequence, personal goals and agendas are highly salient (Kitayama & Uskul, 2011; Markus & Kitayama, 1991). Thus, if individuals lack sufficient resources to meet personal goals and agendas, they will likely become frustrated, which in turn can lead to expression of anger. Consistent with this analysis, Park et al. (2013) found that Americans with lower social status expressed more anger than their higher status counterparts and that this relationship was mediated by experiences of frustration. We suggest that if people experience and express anger primarily when frustrated, the frequency of anger expression in this cultural context may serve as a reliable index of frustrating personal experiences. We therefore hypothesized that anger expression within the U.S. would predict increased BHR indexed by pro-inflammatory responses (Irwin & Cole, 2011; Medzhitov, 2008; Miller et al., 2009), which are known to increase cardiovascular risks and, eventually, elevate risk for morbidity and mortality (Everson-Rose & Lewis, 2005; Medzhitov, 2008).
In contrast, in Asian cultural contexts, interdependence of the self is more strongly valued. The self is conceptualized as part of a hierarchically organized social group. In such settings, expression of anger is seen as socially disruptive and, as a consequence, there is a strong normative prohibition against it. An exception to the normative prohibition against anger expression comes from having power and dominance, such as being high in social status. Consistent with this view, Park et al. (2013) found that Japanese adults with high (vs. low) social status expressed more anger and, further, that this relationship was mediated by the amount of decision authority one had at work. We suggest that if people express anger primarily when feeling dominant and privileged, the frequency of anger expression in this cultural context may serve as a reliable index of social privileges. We therefore hypothesized that anger expression would predict reduced BHR in the Japanese cultural context. The sense of entitlement and power may likely relieve threats to the sense of the self as competent and in-control (Irwin & Cole, 2011; Medzhitov, 2008; Miller et al., 2009) and, thus, the Japanese adults who display more anger may be likely to enjoy reduced BHR compared to those who show little anger.
Earlier evidence by Consedine and colleagues (2005, 2006) is consistent with the current emphasis on cultural differences in the link between anger and health. These researchers conducted large-scale surveys on multi-ethnic, community-dwelling women in Brooklyn, NY and found that trait anger was associated with poor self-reported health among US-born European Americans. In contrast, among women from all ethnic minority groups the relationship was reversed, with trait anger associated with better self-reported health. Conversing with the current analysis, these individuals from ethnic minority groups are likely to be more interdependent, as compared to European Americans (Oyserman, Coon, & Kemmelmeier, 2002). In the present work, we extended the Condesine et al. evidence in three important ways. First, we examined whether anger would be linked to a reduced health risk among Japanese in Japan, a majority group within the society. Second, whereas Condesine et al. tested only trait anger, we assessed both anger expression and other aspects of anger including trait anger, anger suppression, and anger control. Third, and most importantly, whereas Consedine et al. examined self-reported health, we tested objective measures of BHR.
Method
To test the prediction that anger expression would be associated with increased BHR for Americans, but with reduced BHR among Japanese, we used matched surveys from Japan and the U.S. To assess BHR, we employed two indices each for inflammation (Interleukin-6 [IL-6] and C-reactive protein [CRP]) and cardiovascular malfunctioning (systolic blood pressure [SBP] and the ratio of total to high-density lipoprotein cholesterol [Total/HDL cholesterol]). We controlled for several variables including age, gender, waist-to-hip ratio, chronic health conditions, smoking status, alcohol consumption, social status, and experience of negative emotions, all of which have been linked to these biomarkers in previous work (Coe et al., 2011; O'Connor et al., 2009). We also examined whether the predicted moderation of the association between anger expression and BHR would be moderated by social status.
Participants
American participants were a subset from the Midlife in the United States (MIDUS) survey. A national probability sample of 4,244 adults was initially recruited through random digit dialing in 1995-1996. The participants completed both a telephone interview and a self-administered questionnaire. Using the same assessments, a follow-up survey was conducted in 2004 (MIDUS II; response rate = 75%, adjusted for mortality). Biological data were collected from a subset of the MIDUS II participants, who traveled to one of three General Clinical Research Centers (GCRCs) for an overnight visit. Biomarker data were available from 1,054 participants (476 males, 578 females; Mage = 58.04 years, SDage = 11.62). The parallel survey, the Midlife in Japan (MIDJA), was conducted in 2008 with 1,027 participants randomly selected from the Tokyo metropolitan area. These participants completed a self-administered questionnaire. A subset of the MIDJA participants was recruited to participate in biological data collection (N = 382; 168 males, 214 females; Mage = 55.47 years, SDage = 14.04). These participants visited a medical clinic near the University of Tokyo. For detailed protocols used for biomarker collection and assaying, see Coe et al. (2011).
Measures
Anger expression
Anger expression was assessed with an 8-item anger-out subscale of the Anger Expression Inventory (AX; Spielberger, 1996). Participants rated how often (1 = almost never, 4 = almost always) they expressed angry feelings through verbally or physically aggressive behaviors when they felt furious and angry (e.g., I slam doors, I say nasty things; αs = .75 and .84 for Americans and Japanese, respectively). Park et al. (2013) performed a confirmatory factor analysis to establish measurement equivalence of anger expression between the two cultural groups. When a multi-group factor model was constrained so that factor loadings of the pertinent items on the latent variable were equal between the two cultural groups, the fit of the model was no worse compared to when these constraints were removed. This establishes factor equivalence across the two cultural groups (Cheung & Rensvold, 2002).
Other facets of anger
To determine whether our primary predictions would apply only to the expressive aspect of anger as shown by Park et al. (2013), or alternatively, they would extend to other facets of anger (e.g., Consedine et al., 2005; 2006), we considered three different facets of anger including trait anger, anger suppression, and anger control, all of which were assessed with the State-Trait Anger Expression Inventory (Spielberger, 1996). The 15-item trait anger subscale assessed one's chronic propensity toward anger. Participants indicated how well each of the items described themselves (e.g., I have a fiery temper, I am a hotheaded person; αs = .83 and .90 for Americans and Japanese, respectively) (1 = not at all, 4 = very well). The 8-item anger-in subscale measured the extent to which anger is held in or suppressed (1 = almost never, 4 = almost always) (e.g., I withdraw from people, I keep things in; αs = .81 and .74 for Americans and Japanese, respectively). Finally, the 4-item anger control subscale measured the extent to which people attempt to control the expression of anger (1 = almost never, 4 = almost always) (e.g., I control my tempter, I keep my cool; αs = .69 and .65 for Americans and Japanese, respectively). See Table 1 for inter-correlations among the four facets of anger (anger expression, trait anger, anger suppression, and anger control) for both cultural groups.
Table 1.
Intercorrelations among the four facets of anger for Americans (top) and Japanese (down)
| Americans | N | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| 1. Anger expression | 1053 | - | .53*** | .20*** | −.30*** |
| 2. Trait anger | 1050 | - | .49*** | −.28*** | |
| 3. Anger suppression | 1052 | - | −.16*** | ||
| 4. Anger control | 1053 | - |
| Japanese | N | 1 | 2 | 3 | 3 |
|---|---|---|---|---|---|
| 1. Anger expression | 381 | - | .49*** | .42*** | .09† |
| 2. Trait anger | 362 | - | .43*** | .11* | |
| 3. Anger suppression | 380 | - | .27*** | ||
| 4. Anger control | 379 | - |
p < .10
p < .05
p < .001.
BHR
We assessed two theoretically linked facets of BHR: inflammation and cardiovascular malfunction. We analyzed two inflammatory measures: a) IL-6 and b) CRP. Frozen blood samples were shipped on dry ice from the 3 GCRC sites in the U.S. and from Tokyo to a single testing laboratory (MIDUS Biocore Laboratory; University of Wisconsin, Madison, WI). Serum IL-6 levels were determined by high-sensitivity enzyme-linked immunosorbent assay (ELISA) (Quantikine, R&D Systems, Minneapolis, MN), with a lower sensitivity of detection at 0.16 pg/mL. All values were quantified in duplicate; any value over 10 pg/mL was re-run in diluted sera to fall on the standard reference curve. Plasma CRP levels were determined using the BNII nephelometer (Dade Behring Inc., Deerfield, IL), utilizing a particle enhanced immunonepholometric assay. To reduce the effect of extreme outliers, a small number of high IL-6 and CRP (n = 7) and CRP (n = 4) were winsorized at three standard deviations from the mean within each culture separately (see Boylan & Ryff, 2013; Miyamoto et al., 2013 for similar approaches). Because the distributions of both markers were positively skewed, values were log-transformed.
Cardiovascular risk was assessed with a) SBP and b) Total/HDL cholesterol. Resting blood pressure was assessed three times in a seated position and the two most similar readings were averaged to yield an index of SBP. The total and the HDL cholesterol were assayed at Meriter Labs (Madison, WI), using a Cobas Integra analyzer (Roche Diagnostics, Indianapolis, IN) in the U.S. and at Showa Medical Science in Japan. A few outliers of SBP (n = 1) and the total cholesterol (n = 3) values were winsorized at three standard deviations from the mean in each culture. The distributions of SBP and Total/HDL cholesterol ratio were positively skewed and were log-transformed to reduce skewness.
Because inflammation is a major risk factor for cardiovascular disease, we anticipated that the four biomarkers would be inter-related. As predicted, these four indices were positively correlated within each culture (see Table 2). A principal component analysis showed that all four indices loaded on a single factor. The result did not differ when the principal component analysis was conducted within each culture. The factor score for biological health risk stemming from the more encompassing analysis thus constitutes our primary dependent variable. A higher number indicates increased BHR (i.e., greater inflammation and higher cardiovascular risk).
Table 2.
Intercorrelations among the four biomarkers for Americans (top) and Japanese (bottom).
| Americans | N | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| 1. Log IL-6 | 1044 | - | .49*** | .13*** | .11*** |
| 2. Log CRP | 1040 | - | .15*** | .19*** | |
| 3. Log SBP | 1053 | - | .13*** | ||
| 4. Log Total/HDL cholesterol | 1043 | - |
| Japanese | N | 1 | 2 | 3 | 3 |
|---|---|---|---|---|---|
| 1. Log IL-6 | 382 | - | .50*** | .38*** | .25*** |
| 2. Log CRP | 382 | - | .27*** | .28*** | |
| 3. Log SBP | 382 | - | .32*** | ||
| 4. Log Total/HDL cholesterol | 382 | - |
Note. IL-6 = Interleukin 6, CRP = C-reactive protein, SBP = systolic blood pressure, Total/HDL cholesterol = the ratio of total to HDL cholesterol. IL-6, CRP, SBP, and total cholesterol scores were winsorized at three standard deviations from the mean in each culture and all biomarkers were log-transformed.
p < .001.
We also conducted a confirmatory factor analysis to ensure that the data were consistent with the assumption that all four measures are indicators of a single latent variable, BHR. Consistent with this assumption, a single factor model had a good fit to the data in both the U.S. [X2(1, n = 1037) = 6.42, CFI = .985, NFI = .982, GFI = .997, RMSEA = .072] and Japan [X2(1, n = 382) = 3.84, CFI = .987, NFI = .983, GFI = .995, RMSEA = .086]. Moreover, in both countries, this model had a significantly better fit than an alternative model that assumed that the four variables are distinct, X2(4) = 334.60, p < .0001 in the U.S. and X2(4) = 201.10, p < .0001 in Japan.
Social status indicators
We examined two indices of social status (objective and subjective) used in the Park et al. study (2013) as covariates as well as moderators in our analysis. In our prior work, objective social status was assessed as a composite of educational attainment and occupational status (Park et al., 2013). We adopted this composite index as our primary measure of objective social status, although we also performed the same analysis with educational attainment and occupational status as alternative indices to ensure that the results did not differ.
Since the educational system is different across cultures, educational attainment was originally assessed on a culture-specific scale ranging from 1 (8th grade, junior high school) to 12 (Ph.D., or other professional degree) in the U.S., and from 1 (8th grade, junior high school graduate) to 8 (graduate school) in Japan. To make the scales comparable for both cultural groups, we rescaled the scores to a 7-point scale for both cultures (1 = 8th grade, junior high school, 7 = attended or graduate from graduate school) as in the Park et al. study (2013; see also Curhan et al., in press). Current occupational status was assessed on a 3-point scale (1 = manual, blue'collar, or service, 2 = non-manual, white-collar, or clerical, 3 = managerial or professional). To yield a single indicator of objective social status, the two indices were standardized and averaged within culture.
Subjective social status was assessed with a ladder instrument. Following prior research (e.g., Adler et al., 2000), participants were presented with a picture of a ladder that had 10 rungs (1 = lowest, 10 = highest; Goodman et al., 2001), and asked to choose one corresponding to their standing in their “own community.” What community meant was left open so that participants could base their choice on what made sense to them. The participants were thus allowed to employ culturally relevant criteria in judging their relative status (see also Leu et al., 2008) because social status is likely to have greater impact when it is defined with respect to a community that is most meaningful to each individual (Conley, 2008).
Control variables
We controlled for several confounding variables that have been linked to inflammation and/or cardiovascular risk (O'Connor et al., 2009)). These included age, gender, health status, adiposity, and health behaviors of participants. For example, health status and inflammatory biomarkers are associated with obesity and central adiposity (indexed by waist-to-hip ratio [WHR]) and other chronic health problems can also be specifically linked to inflammation and cardiovascular functioning (e.g., diabetes) (Monhamed, Winn, Rampal, Rashid, & Mustaffa, 2005). Chronic health conditions were assessed based on the number of health problems respondents self-reportedly experienced for the past 12 months (up to 30; e.g., diabetes, asthma, tuberculosis). To reduce the effects of outliers (n = 2) and correct for positively skewed distribution, WHR was log-transformed after winsorizing at three standard deviations from the mean in each culture. Health behavior was assessed with smoking and alcohol consumption. Smoking status was categorized as never smoker, former smoker, and current smoker, while alcohol consumption was assessed in terms of the number of drinks consumed per week. To reduce the effect of outliers for alcohol consumption (n = 29), the values were winsorized at three standard deviations from the mean within each culture.
In a prior report (Miyamoto et al., 2013), we documented that one of the biomarkers used in the current work, IL-6, is related to experience of negative emotions. Whereas experience of negative emotions was linked to increased levels of IL-6 for Americans, there was no such relationship for Japanese. The negative emotional experience index used in the Miyamoto et al. (2013) report did not include anger. Nevertheless, to ensure that the effects predicted for anger expression are unique and distinct from the effects documented by Miyamoto et al. (2013) for negative emotional experience, we controlled for negative emotional experience in the current analysis. Participants rated how often (1 = none of the time, 5 = all the time) they felt each of the six negative emotions (i.e., so sad nothing could cheer you up, nervous, restless or fidgety, hopeless, that everything was an effort, and worthless) during the past 30 days (for details about the source, see Mroczek & Kolarz, 1998; αs = .85 and .86 for Americans and Japanese, respectively).
Demographic variables and descriptive statistics for the key variables are presented in Table 3 for each cultural group.
Table 3.
Descriptive statistics for the demographic, psychological, and biological variables for Americans (left) and Japanese (right)
| Americans |
Japanese |
|||||
|---|---|---|---|---|---|---|
| Variable | N | M | SD | N | M | SD |
| Demographic variables | ||||||
| Age | 1054 | 58.04 | 11.62 | 382 | 55.47 | 14.04 |
| Gender (% famale) | 54.8% | 382 | 56.0% | |||
| Educational attainment | 1050 | 4.97 | 1.61 | 378 | 4.38 | 1.63 |
| Health status | ||||||
| Chronic conditions | 1054 | 2.30 | 2.34 | 377 | 2.31 | 2.02 |
| Waist-to-Hip Raito (WHR) | 1052 | .89 | .10 | 382 | .83 | .08 |
| Log WHR | 1052 | −.05 | .05 | 382 | −.08 | .04 |
| Health behaviors | ||||||
| Smoking status | 1044 | 382 | ||||
| Never-smoker | 600 | 56.9% | 185 | 48.4% | ||
| Former-smoker | 342 | 32.4% | 89 | 23.3% | ||
| Current-smoker | 112 | 10.6% | 82 | 21.5% | ||
| Missing | 0 | 0% | 26 | 6.8% | ||
| Alcohol consumption | 1052 | 3.14 | 5.52 | 379 | 7.24 | 11.75 |
| Experience of negative emotions | 1050 | 1.49 | .55 | 381 | 1.70 | .65 |
| Social status | ||||||
| Objective social status | 1050 | 0 | 1.00 | 378 | 0 | 1.00 |
| Subjective social status | 1042 | 6.59 | 1.72 | 374 | 6.24 | 2.04 |
| Anger index | ||||||
| Anger expression (anger-out) | 1053 | 12.79 | 3.13 | 381 | 12.25 | 2.63 |
| Trait anger | 1050 | 23.75 | 5.21 | 362 | 26.25 | 6.96 |
| Anger suppression (anger-in) | 1052 | 14.60 | 4.07 | 380 | 14.44 | 3.68 |
| Anger control | 1053 | 10.09 | 2.22 | 379 | 8.04 | 2.50 |
| Biological health risk (BHR) factor score | 1037 | .29 | .85 | 382 | −.78 | .96 |
| Interleukin-6 (IL-6) | 1044 | 2.79 | 2.79 | 382 | 1.64 | 2.11 |
| Log IL-6 | 1044 | .32 | .32 | 382 | .04 | .36 |
| C-reactive protein (CRP) (ug/mL) | 1040 | 2.70 | 4.28 | 382 | .76 | 2.00 |
| Log CRP | 1040 | .14 | .50 | 382 | −.45 | .42 |
| Systolic blood pressure (SBP) (mmHg) | 1053 | 131.01 | 17.87 | 382 | 121.64 | 19.95 |
| Log SBP | 1053 | 2.11 | .06 | 382 | 2.08 | .07 |
| Total cholesterol (mg/dL) | 1045 | 187.12 | 40.00 | 382 | 205.81 | 38.21 |
| HDL cholesterol (mg/dL) | 1043 | 54.63 | 17.61 | 382 | 71.24 | 21.28 |
| Total/HDL cholesterol | 1043 | 3.75 | 1.43 | 382 | 3.15 | 1.15 |
| Log Total/HDL cholesterol | 1043 | .55 | .15 | 382 | .47 | .14 |
Note. BHR factor score was obtained from a principal component analysis based on the four biomarkers (IL-6, CRP, SBP, Total/HDL cholesterol), which yielded a single factor. WHR, alcohol consumption, IL-6, CRP, SBP, and total cholesterol are the raw data before winsorizing. Educational attainment was assessed on the culturally matched 7-point scale (1 = 8th grade, junior high school, 7 = attended or graduate from graduate school).
Results
To test our prediction that the relationship between anger expression and BHR would be moderated by culture, we carried out a series of multiple regression analyses. In Step 1, the social status variables, as well as all control variables, were entered along with the main effects of both culture and anger expression. In Step 2, the interaction between culture and anger expression was tested. In Step 3, we tested whether the Culture x Anger expression interaction would be moderated by social status (both subjective and objective). The results of these analyses are summarized in Table 4.
Table 4.
Unstandardized regression coefficients in predicting biological health risk (BHR) as a function of culture and anger expression (Model 1), the interaction between culture and anger expression (Model 2), and the interactions between culture, anger expression, and social status (Model 3).
| Predictors | Model 1 all main effects |
Model 2 + 2-way interaction term |
Model 3 + 3-way interaction term |
||||||
|---|---|---|---|---|---|---|---|---|---|
| b | β | t-test | b | β | t-test | b | β | t-test | |
| Culture | −.79 | −.35 | −14.44*** | −.81 | −.35 | −14.72*** | −.81 | −.35 | −14.43*** |
| Anger expression | .01 | .02 | 1.03 | .02 | .07 | 2.78** | .02 | .07 | 2.79** |
| Gender | .25 | .13 | 4.16*** | .26 | .13 | 4.30*** | .26 | .13 | 4.27*** |
| Age | .01 | .16 | 6.62*** | .01 | .15 | 6.50*** | .01 | .15 | 6.51*** |
| Log WHR | 8.79 | .42 | 13.58*** | 8.77 | .42 | 13.61*** | 8.76 | .42 | 13.59*** |
| Chronic conditions | .03 | .07 | 2.87** | .03 | .07 | 2.83** | .03 | .07 | 2.88** |
| Smoking status (never vs. former) | −.05 | −.02 | −.92 | −.05 | −.02 | −.95 | −.05 | −.02 | −.94 |
| Smoking status (never vs. current) | .16 | .05 | 2.31* | .16 | .06 | 2.36* | .16 | .06 | 2.41* |
| Alcohol consumption | .00 | .01 | .49 | .00 | .01 | .54 | .00 | .01 | .46 |
| Experience of negative emotions | −.06 | −.04 | −1.48 | −.06 | −.03 | −1.34 | −.06 | −.04 | −1.42 |
| Subjective social status | −.02 | −.03 | −1.35 | −.02 | −.03 | −1.21 | −.02 | −.03 | −1.23 |
| Obective social status | −.09 | −.09 | −3.92*** | −.08 | −.08 | −3.81*** | −.08 | −.08 | −3.79*** |
| Culture × Anger expression | −.05 | −.09 | −3.56*** | −.05 | −.09 | −3.43*** | |||
| Culture × Anger expression × Subjective social status | −.01 | −.02 | −1.03 | ||||||
| Culture × Anger expression × Objective social status | −.00 | −.01 | −.22 | ||||||
| R2 change | .413*** | .006*** | .001 | ||||||
Note. N = 1351.
p < .05
p < .01
p < .001.
U.S is coded as the referent group for the culture variable.
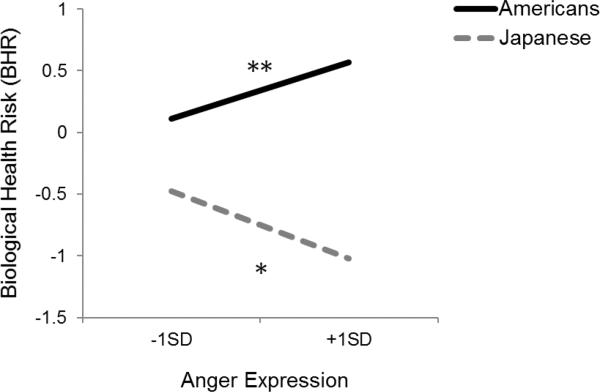
As predicted, the interaction between culture and anger expression was statistically significant in Step 2, b = −.05, t(1337) = −3.56, p < .001. As illustrated in Figure 1, replicating the previous pattern obtained in previous Western studies, anger expression was related to increased BHR among Americans, b = .02, t(1337) = 2.78, p < .01. However, as also predicted, anger expression in Japan was linked to reduced BHR, b = −.03, t(1337) = −2.29, p < .05. As shown in Table 4, this interaction was not moderated by social status indicators, ts(1335) < − 1,03, ps > .30.
Figure 1.
Biological health risk (BHR) factor score as a function of anger expression for Americans (solid line) and Japanese (dotted line). Higher number on the y-axis indicates greater biological health risk. Demographic variables, health status, health behaviors, experience of negative emotions, and social status indicators are controlled. Statistical significance is indicated by asterisk (*p < .05, **p < .01).
Next, we tested whether the Culture x Anger interaction would be observed for other facets of anger. To begin, we found a significant interaction between culture and anger suppression, b = −.03, t(1337) = −2.42, p < .05. The pattern obtained was similar to, but somewhat attenuated as compared to the one observed for anger expression. Anger suppression was associated with reduced BHR for Japanese, b = −.02, t(1337) = −2.03, p < .05. Unlike in anger expression, however, the association between anger suppression and BHR was negligible for Americans, b = .01, t(1337) = 1.08, p = .28. Further, when we examined the two relevant interaction terms (Culture x Anger expression, Culture x Anger suppression) simultaneously as predictors of BHR, the Culture x Anger expression interaction remained significant, b = −.04, t(1335) = −2.81, p < .01, but the Culture x Anger suppression interaction did not, b = −.02, t(1335) = −1.35, p > .18. No significant interaction effects were observed for trait anger and anger control, ts < −1.67, ps > .10. These findings indicate that the hypothesized cultural moderation in the relationship between anger and BHR is evident primarily for the expressive facet of anger.
Discussion
The most important contribution of the present study is to provide evidence for a cultural moderation of the link between anger expression and BHR. As found in previous studies conducted in Western cultures, anger expression was associated with increased BHR for Americans. Importantly, however, it was associated with reduced BHR for Japanese. This pattern was quite robust for the expressive facet of anger, but weak for anger suppression and negligible for trait anger and anger control.
Our work extended the pioneering work by Consedine and colleagues (2005, 2006). First, whereas Consedine et al. suggested that anger might empower people with disadvantaged social standings (i.e., ethnic minorities with low socioeconomic standings in the U.S. society), our data show that anger is also related to improved health among Japanese in Japan (a majority group in Japan). Second, we tested both anger expression and trait anger and showed that while trait anger can contribute to anger expression, this facet of anger is unlikely to constitute the primary health correlate. Instead, it is the expressive facet of anger that is linked more directly to health effects. Third, whereas Consedine et al. assessed self-reported health, we used objectively measured biological risk factors as the health outcome.
The current evidence for the cultural moderation of the association between anger expression and BHR was obtained after controlling for a number of variables known to be associated with BHR, including age, gender, health status, adiposity, health behaviors, and social status. Moreover, we also controlled for experience of negative emotions. In our earlier report, Miyamoto et al. (2013) showed that experience of negative emotions (which did not include anger) was positively linked to BHR, assessed with a single biomarker, IL-6, for Americans (Pressman, Gallagher, & Lopez, 2013), but there was no such association among Japanese (Curhan et al., in press). The positive effect of anger expression on BHR observed in the present work for Americans is analogous to the one observed in the Miyamoto et al. study. However, the current finding was observed above and beyond the effect of negative emotions and thus is distinct. Moreover, unlike the Miyamoto et al. study, the current work showed a contrastingly negative relationship between anger expression and BHR among Japanese. This novel finding casts doubt on the currently dominant assumption that anger expression and hostility have adverse effects on health.
To account for these differing cultural patterns, we have hypothesized that the expression of anger in the two cultural contexts serves as a reliable index of a variety of different experiences. Whereas in the US, it may index the degree to which individuals are exposed to negative events (e.g., life difficulties, annoyances, and frustrations), in Japan it may index the degree to which individuals are empowered and entitled. Extending that line of inquiry, other factors as well might contribute to either negative experiences (in the case of the U.S.) or power and entitlement (in the case of Japan). For example, among those who are equally low in their social standing, one may experience additional life difficulties such as a more demanding boss at work. Within the U.S., this person may experience more frustration, which could lead to greater anger expression, relative to the other person. Likewise, among those who are identical in high social standing, one may have other sources of social dominance such as subordinates at work who are docile and meek. Within the Japanese context, this person may be more at liberty to display anger than the other person due to his/her enhanced dominance and power over the subordinates. The point is that anger expression is a complex phenomenon likely motivated by a variety of factors, many of which could be culture-specific. These cultural factors must be taken into account if we are to achieve a full understanding of the link between anger and health.
One limitation of the current work is the cross-sectional nature of the design. It will be important to examine the longitudinal influence of anger expression on morbidity and mortality over time across different cultures. Such work will provide explicit tests of the mechanistic pathways linked with the biological risk factors assessed herein. Nevertheless, the current work is the first that draws on large, population-based, cross-cultural samples to test theoretically driven predictions regarding the association between anger expression and health. Moreover, our focus on multiple indices of BHR (comprised of inflammatory measures, cholesterol, and blood pressure) will likely stimulate further work on the interface between socio-cultural processes and neurobiological processes. In particular, our finding that the association between anger expression and these health risks varies cross-culturally qualifies some of the simpler unidirectional conclusions about the relationship between anger and health (Chida & Steptoe, 2009b; Elovainio et al., 2011; Everson-Rose & Lewis, 2005). It thus conveys the importance of incorporating cultural perspectives into the analysis of anger expression, especially its effects on physical health.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (5R37AG027343) to conduct a study of Midlife in Japan (MIDJA) for comparative analysis with Midlife in the U.S. (MIDUS, P01-AG020166).
Footnotes
Author Contribution
Shinobu Kitayama (SK) and Jiyoung Park (JP) conceived the study. JP carried out all data analysis. SK and JP drafted the paper. Jennifer Morozink Boylan, Yuri Miyamoto, Cynthia S. Levine, Hazel Rose Markus, Christopher L. Coe, and Carol D. Ryff provided critical revisions. Mayumi Karasawa, Norito Kawakami, and Gayle D. Love played significant roles in Japanese data collection. All authors participated in discussions of the paper. They have approved the final version of the paper for submission.
References
- Adams G, Markus HR. Culture As Patterns: An Alternative Approach to the Problem of Reification. Culture & Psychology. 2001;7(3):283–296. doi:10.1177/1354067X0173002. [Google Scholar]
- Beatty DL, Matthews KA. Unfair treatment and trait anger-in relation to nighttime ambulatory blood pressure in African American and white adolescents. Psychosomatic Medicine. 2009;71:813–820. doi: 10.1097/PSY.0b013e3181b3b6f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz L. Frustration-aggression hypothesis: Examination and reformulation. Psychological Bulletin. 1989 doi: 10.1037/0033-2909.106.1.59. [DOI] [PubMed] [Google Scholar]
- Boylan JM, Ryff CD. Varieties of Anger and the Inverse Link Between Education and Inflammation: Toward an Integrative Framework. Psychosomatic Medicine. 2013;75(6):519–519. doi: 10.1097/PSY.0b013e31829683bd. doi:10.1097/PSY.0b013e3182a0f38e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GW, Rensvold RB. Evaluating Goodness-of-Fit Indexes for Testing Measurement Invariance. Structural Equation Modeling: a Multidisciplinary Journal. 2002;9(2):233–255. [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009a;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. doi:10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. The Association of Anger and Hostility With Future Coronary Heart DiseaseA Meta-Analytic Review of Prospective Evidence. Journal of the American College of Cardiology. 2009b;53(11):936–946. doi: 10.1016/j.jacc.2008.11.044. doi:10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Coe CL, Love GD, Karasawa M, Kawakami N, Kitayama S, Markus HR, et al. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain. 2011;25(3):494–502. doi: 10.1016/j.bbi.2010.11.013. doi:10.1016/j.bbi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley D. Reading class between the lines: A reflection on why we should stick to folk concepts of social class. In: Lareau A, Conley D, editors. Social class. Russell Sage Foundation; New York, NY: 2008. pp. 366–373. [Google Scholar]
- Consedine NS, Magai C, Horton D. Ethnic Variation in the Impact of Emotion and Emotion Regulation on Health: A Replication and Extension. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(4):P165–P173. doi: 10.1093/geronb/60.4.p165. doi:10.1093/geronb/60.4.P165. [DOI] [PubMed] [Google Scholar]
- Consedine NS, Magai C, Kudadjie-Gyamfi EK, Longfellow JK, Ungar TM, King AR. Stress versus discrete negative emotions in the prediction of physical complaints: Does predictive utility vary across ethnic groups? Cultural Diversity & Ethnic Minority Psychology. 2006;12(3):541–557. doi: 10.1037/1099-9809.12.3.541. doi:10.1037/1099-9809.12.3.541. [DOI] [PubMed] [Google Scholar]
- Curhan KS, Sims T, Markus HR, Kitayama S, Karasawa M, Kawakami N, Love GD, Coe CL, Miyamoto Y, Ryff CR. Just how bad negative affect is for your health depends on culture. Psychological Science. doi: 10.1177/0956797614543802. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovainio M, Merjonen P, Pulkki-Råback L. Hostility, metabolic syndrome, inflammation and cardiac control in young adults: The Young Finns Study. > Psychology. 2011 doi: 10.1016/j.biopsycho.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005 doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain Behavior and Immunity. 2006;20(4):389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hurd PL, Enquist M. Threat display in birds. Canadian Journal of Zoology. 2001 [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology. 2011;11(9):625–632. doi: 10.1038/nri3042. doi:10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Sparrow D, Spiro A, Vokonas P, Weiss ST. A Prospective Study of Anger and Coronary Heart Disease. Circulation. 1996 doi: 10.1161/01.cir.94.9.2090. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Uskul AK. Culture, Mind, and the Brain: Current Evidence and Future Directions. Annual Review of Psychology. 2011;62(1):419–449. doi: 10.1146/annurev-psych-120709-145357. doi:10.1146/annurev-psych-120709-145357. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Markus HR, Matsumoto H, Norasakkunkit V. Individual and collective processes in the construction of the self: Self-enhancement in the United States and self-criticism in Japan. Journal of Personality and Social Psychology. 1997;72(6):1245–1267. doi: 10.1037//0022-3514.72.6.1245. doi:10.1037/0022-3514.72.6.1245. [DOI] [PubMed] [Google Scholar]
- Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion, and motivation. Psychological Review. 1991;98(2):224–253. doi:10.1037/0033-295X.98.2.224. [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behavior and Immunity. 2008;22(5):753–761. doi: 10.1016/j.bbi.2007.11.008. doi:10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. doi:10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009 doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Morozink Boylon J, Coe CL, Curhan KB, Levine CS, Markus HR, Park J, Kitayama S, Kawakami N, Karasawa M, Love GD, Ryff CD. Negative emotions predict elevated interleukin-6 in the United States but not in Japan. Brain, behavior, and immunity. 2013;34:79–85. doi: 10.1016/j.bbi.2013.07.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monhamed M, Winn T, Rampal G, Rashid AR, Mustaffa BE. A Prelimenary Result of the Cardiovascular Risk factors Intervention Study (Pikom Study): Diabetes Mellitus, Hypertension and their Associated Factors. The Malaysian Journal of Medical Sciences : MJMS. 2005;12(1):20. [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Oyserman D, Coon HM, Kemmelmeier M. Rethinking individualism and collectivism: evaluation of theoretical assumptions and meta-analyses. Psychological Bulletin. 2002 [PubMed] [Google Scholar]
- O'Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behavior and Immunity. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005. doi:10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kitayama S, Markus HR, Coe CL, Miyamoto Y, Karasawa M, et al. Social status and anger expression: The cultural moderation hypothesis. Emotion. 2013;13(6):1122–1131. doi: 10.1037/a0034273. doi:10.1037/a0034273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Gallagher MW, Lopez SJ. Is the Emotion-Health Connection a “First-World Problem”? Psychological Science. 2013;24(4):544–549. doi: 10.1177/0956797612457382. doi:10.1177/0956797612457382. [DOI] [PubMed] [Google Scholar]
- Schum JL, Jorgensen RS, Verhaeghen P, Sauro M, Thibodeau R. Trait Anger, Anger Expression, and Ambulatory Blood Pressure: A Meta-Analytic Review. Journal of Behavioral Medicine. 2003;26(5):395–415. doi: 10.1023/a:1025767900757. doi:10.1023/A:1025767900757. [DOI] [PubMed] [Google Scholar]
- Smith TW, Glazer K, Ruiz JM, Gallo LC. Hostility, Anger, Aggressiveness, and Coronary Heart Disease: An Interpersonal Perspective on Personality, Emotion, and Health. Journal of Personality >. 2004;72(6):1217–1270. doi: 10.1111/j.1467-6494.2004.00296.x. doi:10.1111/j.1467-6494.2004.00296.x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anger Expression Inventory: Professional Manual. Odessa; FL: 1996. [Google Scholar]
- Tiedens LZ. Anger and advancement versus sadness and subjugation: The effect of negative emotion expressions on social status conferral. Journal of Personality and Social Psychology. 2001;80(1):86–94. doi:10.1037/0022-3514.80.1.86. [PubMed] [Google Scholar]
- Vandervoort DJ, Ragland DR, Syme SL. Expressed and suppressed anger and health problems among transit workers. Current Psychology. 1996;15(2):179–193. doi:10.1007/BF02686950. [Google Scholar]