Abstract
Cyclosporine, a calcineurin inhibitor, is successfully used as an immunosuppressant in transplant medicine. However, the use of this pharmaceutical during pregnancy is concerning, since calcineurin is thought to play a role in neural development. The risk for human brain development is difficult to evaluate, because of a lack of basic information on the sensitive developmental times and the potentially pleiotropic effects on brain development and behavior. In the present study, we use zebrafish as a model system to examine the effects of embryonic cyclosporine exposures. Early embryonic exposures reduced the size of the eyes and brain. Late embryonic exposures did not affect the size of the eyes or brain, but did lead to substantial behavioral defects at the larval stages. The cyclosporine-exposed larvae displayed a reduced avoidance response to visual stimuli, low swim speeds, increased resting, an increase in thigmotaxis, and changes in the average distance between larvae. Similar results were obtained with the calcineurin inhibitor FK506, suggesting that most, but not all, effects on brain development and behavior are mediated by calcineurin inhibition. Overall, the results show that cyclosporine can induce either structural or functional brain defects, depending on the exposure window. The observed functional brain defects highlight the importance of quantitative behavioral assays when evaluating the risk of developmental exposures.
Keywords: Zebrafish, brain, behavior, calcineurin, cyclosporine, cyclosporin
1. Introduction
The discovery of cyclosporine A as an immunosuppressive drug has revolutionized the field of transplant medicine, allowing allograft organ transplants to become commonplace [1]. Cyclosporine acts as an immunosuppressant by inhibiting calcineurin, a calcium-dependent protein phosphatase that plays a critical role in T-cell activation [2-3]. By inhibiting calcineurin and suppressing T-cell activation, cyclosporine effectively reduces the rate of transplant rejection. Following its success in transplant medicine, cyclosporine has been used for the treatment of a wide variety of autoimmune diseases, including psoriasis and rheumatoid arthritis [3].
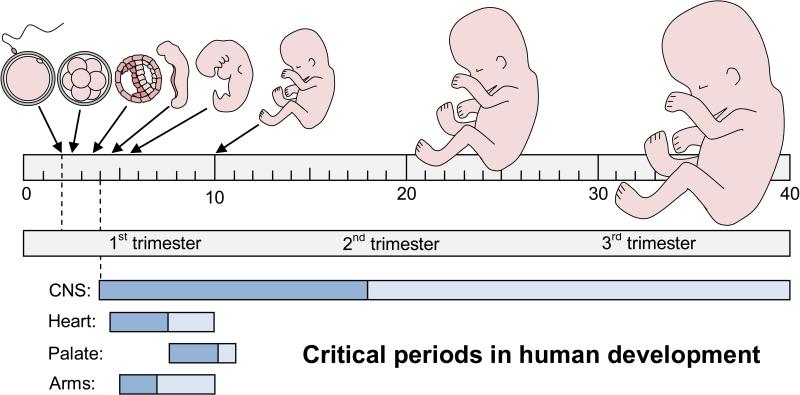
Cyclosporine is classified as a pregnancy category C drug by the United States Food and Drug Administration [4]. In Pregnancy Category C, ‘animal reproduction studies have shown an adverse effect on the fetus, there are no adequate and well-controlled studies in humans, and the benefits from the use of the drug in pregnant women may be acceptable despite its potential risks; or animal studies have not been conducted and there are no adequate and well-controlled studies in humans’ [5-6]. The labeling of Category C pharmaceuticals must state that the drug ‘should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus’ or ‘should be given to a pregnant women only if clearly needed’ [5]. Immunosuppressants are clearly needed in transplant medicine to prevent organ rejection and as a result cyclosporine treatment is continued during pregnancy despite the potential risk to the developing fetus [7]. The use of cyclosporine during pregnancy is concerning, since calcineurin is thought to play a role in neural development and axonal growth [8]. However, the risk for human brain development is difficult to evaluate, because of a lack of basic information on the sensitive developmental times and the potentially pleiotropic effects on brain development and behavior. Models of ‘critical periods’ in human development predict that different types of defects may be induced depending on the exposure window [9], i.e. early developmental exposures can lead to major structural brain defects, while late developmental exposures are likely to induce more subtle or functional brain defects (Fig 1).
Fig 1. Critical or sensitive periods in human development.
Most developing organs are sensitive to teratogens during the embryonic period from 4-10 weeks of gestation. A notable exception is the central nervous system (CNS), which remains sensitive throughout the fetal period from 10-40 weeks of gestation. To illustrate the extended period of sensitivity of the CNS, we redrew the textbook model from Moore et al. 2013 [9] on a linear 40-week scale (with permission). Dark blue = major structural defects, light blue = minor structural or functional defects. 40 weeks of gestation = 38 weeks of development.
In the current study, we examine the effects of cyclosporine on brain development and behavior using zebrafish as a model system. The signaling pathways that regulate brain development and neural function are conserved in vertebrate species [10-11] and the zebrafish has emerged as a powerful model system in behavioral neuroscience [12-14]. Hundreds of synchronously developing embryos can be collected from the bottom of a tank on a daily basis and exposures can be carried out in a culture dish. The embryos are transparent, which makes it possible to image the developing brain in living embryos using various state-of-the-art molecular tools [15-17]. Zebrafish embryos develop rapidly. At 24 hours post-fertilization (hpf), the embryos have a beating heart, a moving tail, two large eyes, and a brain with distinct brain regions [18]. The embryos hatch from their chorion between 2 and 3 days post-fertilization (dpf). At 5 dpf, the free-swimming larvae are approximately 4 mm long, have inflated swim bladders, and display a broad range of behaviors, which can be examined in multiwell or multilane plates [19-25].
In the present study, zebrafish embryos were exposed to cyclosporine during different stages in embryonic development. We found that early embryonic exposures led to a reduction in eye and brain size. Late embryonic exposures did not affect the size of the eyes and brain, but did lead to significant behavioral defects.
2. Materials and Methods
2.1 Zebrafish embryos and exposures
Adult wild type zebrafish (Danio rerio) were originally obtained from Carolina Biological and have been maintained at Brown University as a genetically diverse outbred strain. For the analysis of structural brain defects, we used the Tg(elavl3:EGFP) line, which expresses the enhanced green fluorescent protein under control of a ubiquitous neuronal promotor [26]. Zebrafish embryos were collected within one hour after spawning and raised at 28.5°C in egg water, containing 60 mg/L sea salt (Instant Ocean) in deionized water and 0.25 mg/L methylene blue as a fungal inhibitor. Cyclosporine (cyclosporin A, Enzo Life Sciences) and FK506 (tacrolimus, Enzo Life Sciences) were diluted in egg water from 1000x stocks dissolved in dimethylsulfoxide (DMSO). The corresponding DMSO concentration (0.1% DMSO) was used as a control. Embryos were exposed from 2-26 hours post-fertilization (hpf), 26-50 hpf, or 50-74 hpf, washed four times in egg water, and grown in egg water for up to 5 days post-fertilization (dpf). The developing zebrafish are referred to as ‘embryos’ from 0-3 dpf and as ‘larvae’ afterwards [27].
2.2 Analysis of eye and brain defects
To examine eye size, wild type embryos were imaged at 3 dpf in a ventral view by standard bright-field microscopy on a Zeiss Axiovert 200M microscope, using a 10x objective. The eye length was measured in ImageJ, which can be downloaded at http://imagej.nih.gov/ij/download.html. Measurements of the left and right eyes were averaged in Microsoft Excel. These values were subsequently averaged over the number of embryos (n = number of embryos). To examine brain structure, Tg(elavl3:EGFP) embryos were imaged at 3 dpf by confocal or wide-field fluorescence microscopy. For confocal microscopy, the embryos were grown from 22-72 hpf in 0.003% 1-phenyl-2-thiourea (PTU) in egg water to suppress pigmentation. The 3 dpf embryos were oriented in 0.8% low-gelling temperature agarose. Neural patterns were imaged on a Leica SP2 AOBS confocal microscope using a 20x objective for a frontal view (transverse sections) or a 10x objective for a dorsal view (coronal sections). Z-stacks of 125 slices were acquired through 150 μm of the brain using a 2 Airy unit pinhole, a 488 nm laser for excitation, and a 510-600 nm filter. The data sets were examined by collapsing the stacks as maximum projections and by FluidVis 3D visualization [28]. For wide-field fluorescence microscopy, Tg(elavl3:EGFP) embryos were imaged at 3 dpf in a dorsal view on a Zeiss Axiovert 200M microscope, using 10x objective and a Hamamatsu ORCA-ER monochrome camera. Larvae were oriented in 2% methyl cellulose in egg water. Forebrain, midbrain, and hindbrain length, width and area were measured in ImageJ.
2.3 Head-trunk angles
To examine if embryos display developmental delays, we measured the head-trunk angle as described previously [18]. Embryos were oriented in 2% methyl cellulose in egg water and imaged in a side view. The angles were measured in ImageJ by drawing a line from the center of the eye to the center of the ear and a second line parallel to the notochord in the mid-trunk region.
2.4 Larval morphology
At 5 dpf, wild type larvae were oriented in 2% methyl cellulose in egg water and were imaged by standard bright-field microscopy on a Zeiss Axiovert 200M microscope, using a 5x objective and an AxioCam MRc5 color camera. A white reference image was acquired to avoid gradients in the background. Images from the anterior and posterior halves of the larvae were stitched with Fiji software [29], which can be downloaded at http://fiji.sc/. We used a linear blending method developed by Stephan Preibisch [30], which is available in the Fiji software under plugins, stitching, depreciated, 2D stitching by linear blending.
2.5 Analysis of behavior
The behavior of wild type zebrafish larvae was analyzed at 5 dpf. The 5 dpf larvae receive nutrients from their yolk sac and effects of feeding can be avoided at this time [21]. The larvae were imaged with a custom-built imaging system, as described previously [24-25]. In summary, the system includes a 15 megapixel Canon EOS Rebel T1i digital camera and an Acer Aspire 5517 laptop with a 15.6 inch screen to provide visual stimuli to the larvae. Larval behavior was examined in a ‘five-lane’ plate, with 5 larvae per lane (25 larvae per plate). The five-lane plate is made using a Nunc 1-well rectangular plate (Fisher 12-565-493), 50 ml of 0.8% agarose in egg water, and a CNC-milled plastic mold [25]. Each lane is 18 mm wide, 70 mm long and 3.5 mm deep and has 60° sloping edges to avoid shadows and blind spots along the perimeter of the swimming area. The lanes have ample space to examine larval interactions and measure avoidance of aversive visual stimuli. Larvae were first imaged for 15 minutes without visual stimuli and then for 15 minutes in the presence of a moving red bar, which is 1.3 cm wide and moves up and down at a speed of 17 mm/sec in the upper half of the lanes. Images were acquired every 6 seconds for a 30 minute period and were analyzed in ImageJ. We developed an ImageJ macro (version 25k) that automatically separates the color channels, subtracts the background, applies a threshold, identifies larvae based on particle size, and repeats these steps for subsequent images in a series. This macro can be downloaded from Clift et al. 2014 [21]. The macro generates a long list of X,Y coordinates indicating the location and orientation of the larvae over time. The list of X,Y coordinates is copied in a Microsoft Excel template, which calculates: a) the percentage of time that the larvae are located in the lower half of the lane, away from the visual stimuli, b) the swim speed, c) the percentage of time that the larvae rest, which is defined as the percentage of time the larvae move less than 1 mm in a 6 second interval, d) the average distance between larvae, e) the percentage of time that larvae are together, which is defined as less than 5 mm apart from the nearest neighbor, and f) the percentage of time that larvae are located along the edge of the lane, which is defined as the outer 3 mm perimeter of the swimming area.
2.6 Statistical Analyses
The averages and standard errors of the mean (± SEM) were calculated and graphed in MS Excel. The eye and brain defects were averaged on a per-embryo basis (N= number of embryos). To assure independence of measurements in the behavioral analyses, larval behavior was averaged on a per-lane basis (N= number of lanes). Differences between the treated groups and the corresponding DMSO controls were tested for significance with a t-test (two-tailed, unequal variance). For the initial dose-response studies, the data were analyzed with a one-way ANOVA (p<0.01) using a post-hoc Bonferroni correction for multiple comparisons (four cyclosporine concentrations vs. a single DMSO control).
3. Results
3.1 Cyclosporine exposures affect eye size
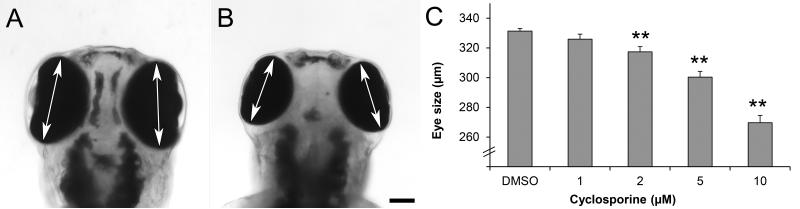
Since calcineurin subunits are expressed in the developing brain and eye [31], we first examined if cyclosporine induces morphological defects in these organ systems. To examine the effects of cyclosporine on eye development, wild type embryos were exposed to 1, 2, 5 and 10 μM cyclosporine from 2-26 hpf and imaged at 3 dpf by standard bright-field microscopy. The length of the eyes was 331 μm (±2) in the controls vs. 326 μm (±3), 317 μm (±3), 300 μm (±4) and 270 μm (±5) in embryos treated with 1, 2, 5, and 10 μM cyclosporine, respectively (Fig. 2). The ANOVA was <0.01 and the post-hoc analysis revealed that embryos exposed to 2, 5, or 10 μM cyclosporine have significantly smaller eyes than the DMSO-treated controls (8×10−4, 2×10−8, 8×10−13, N=25 embryos per group). In contrast, the 1 μM cyclosporine exposure did not affect eye size (p=0.15, N=25). The smaller eye size with 2, 5 and 10 μM cyclosporine (compared to the DMSO controls), corresponds to a 4%, 9% and 19% change, which can be reliably detected due to the low variability in the measurements of eye size. The 10 μM cyclosporine concentration was used in subsequent experiments.
Fig 2. Cyclosporine exposures affect eye size.
A) Control embryo, exposed to 0.1% DMSO from 2-26 hpf and imaged at 3 dpf. B) Embryo exposed to 10 μM cyclosporine from 2-26 hpf and imaged at 3 dpf. C) Measurements of eye size in embryos exposed to various concentrations of cyclosporine. Arrows indicate the maximum eye diameter, which was used as a measure of eye size. ** = p<0.01 / 4 (two-tailed t-test with Bonferroni correction for multiple comparisons). Scale bar = 100 μm.
3.2 Cyclosporine exposures affect brain size
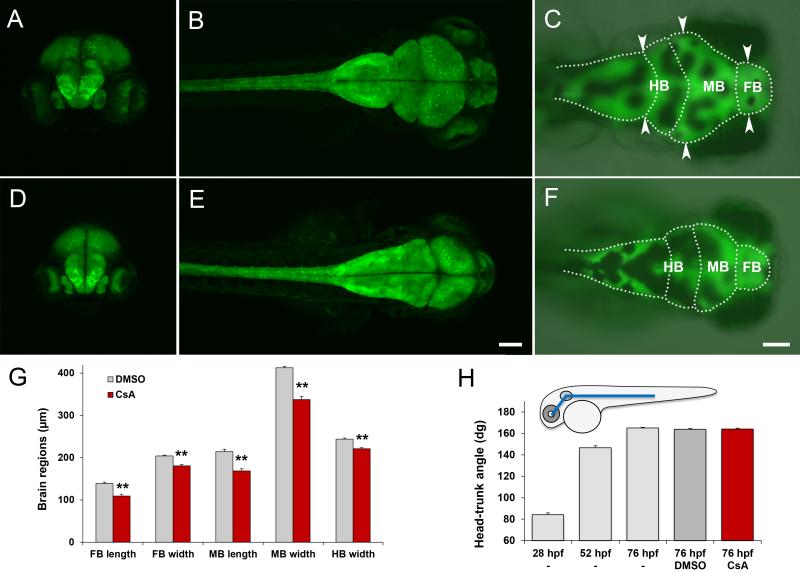
The effect of cyclosporine on the developing brain was examined in Tg(elavl3:EGFP) embryos, exposed to 10 μM cyclosporine from 2-26 hpf and imaged at 3 dpf by confocal and wide-field fluorescence microscopy. Confocal microscopy, combined with 3D visualization of the resulting data sets, is ideally suited for exploratory analyses of neural patterns [28] and revealed a cyclosporine-induced reduction in brain size (Fig. 3). Once identified, this effect was efficiently quantified by wide-field fluorescence microscopy (Fig 3C,F,G,H). The length of the forebrain was 139 μm (±3) in the DMSO-treated controls vs. 109 μm (±4) in the cyclosporine-treated embryos (p=1×10−5, NDMSO=16, NCsA=10). The width of the forebrain, measured at the broadest forebrain region, was 204 μm (±2) in the DMSO-treated controls vs. 180 μm (±3) in the cyclosporine-treated embryos (p=2×10−5, NDMSO= 16, NCsA=10). The length of the midbrain was 214 μm (±6) in the DMSO-treated controls vs. 169 μm (±5) in the cyclosporine-treated embryos (p=5×10−6, NDMSO=16, NCsA=10). The width of the midbrain, measured at the broadest midbrain region, was 413 μm (±3) in the DMSO-treated controls vs. 338 μm (±7) in the cyclosporine-treated embryos (p=1×10−7, NDMSO=16, NCsA=10). The width of the hindbrain, measured at the boundary between the cerebellum and medulla, was 244 μm (±2) in the DMSO-treated controls vs. 221 μm (±2) in the cyclosporine-treated embryos (p=1×10−6, NDMSO=16, NCsA=10). In summary, cyclosporine induced a significant decrease in eye size (Fig 2) and induced a significant decrease in all measured lengths and widths of the brain (Fig 3G). To examine if these effects could be attributed to a general delay in development, we measured the embryonic head-trunk angles, which is a standard method for staging zebrafish embryos [18]. We found that embryos exposed to 10 μM cyclosporine from 2-26 hpf do not display significant differences in the head-trunk angle compared to untreated or DMSO-treated controls (Fig 3H). Based on these results, we conclude that early embryonic cyclosporine exposures cause a reduction in eye size and a reduction in brain size that cannot be explained by a general developmental delay.
Fig 3. Cyclosporine exposures affect brain size.
A-C) Control embryos exposed to 0.1% DMSO from 2-26 hpf and imaged at 3 dpf. D-F) Embryos exposed to 10 μM cyclosporine from 2-26 hpf and imaged at 3 dpf. A,D) frontal view by confocal microscopy. B,E) dorsal view by confocal microscopy. C,F) dorsal view by fluorescence microscopy, with outlines of the forebrain (FB), midbrain (MB) and hindbrain (HB). G) Length and width of forebrain, midbrain and hindbrain. H) Measurements of the head-trunk angle. The cyclosporine-treated embryos do not display a significant developmental delay compared to the DMSO-treated or untreated controls. For imaging brain size, we used elav:GFP embryos, which express GFP in the brain and spinal cord. Arrow heads = measurements of brain width. CsA=cyclosporine exposure. Scale bars = 100 μm. ** p<0.01 (two-tailed t-test).
3.3 Sensitive periods
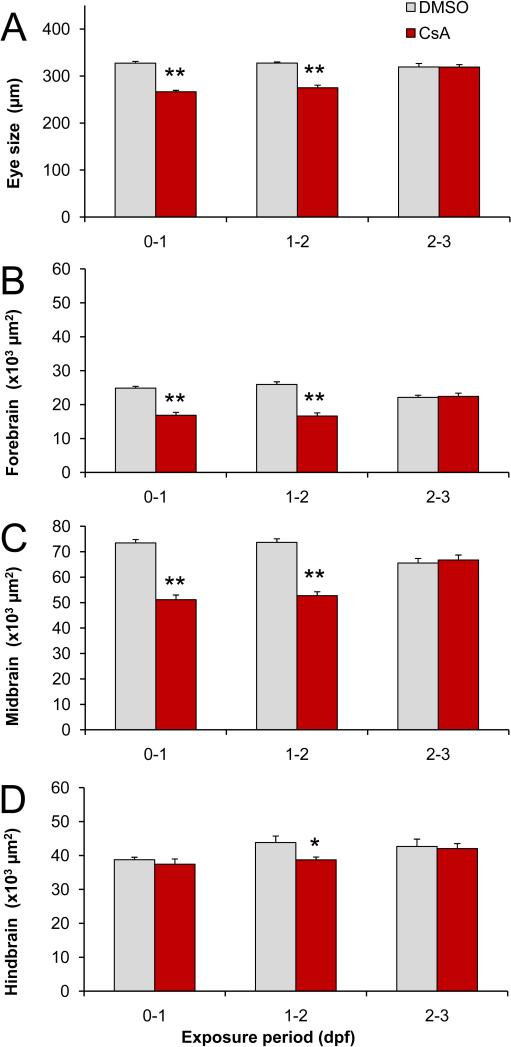
To determine if there is a sensitive period for the eye and brain defects, embryos were exposed to 10 μM cyclosporine from 0-1 dpf (day 1), 1-2 dpf (day 2), or 2-3 dpf (day 3). We measured the eye length at 3 dpf (Fig 4A). In addition, since prior analyses showed effects on brain length and width, we measured the area of the forebrain (Fig 4B), area of the midbrain (Fig 4C) and the area of the cerebellum in the anterior-dorsal region of the hindbrain (Fig 4D). Day 1 exposures induced a significant decrease in the size of the eyes, forebrain, and midbrain. The eye length was 327 μm (±4) in the DMSO-treated controls vs. 266 μm (±3) in the cyclosporine-treated embryos (p=2×10−14, NDMSO=20, NCsA=20). The forebrain area was 24,864 μm2 (±534) in the DMSO-treated controls vs. 16,870 μm2 (±865) in the cyclosporine-treated embryos (p=8×10−7, NDMSO=16, NCsA=10). The midbrain area was 73,465 μm2 (±1,331) in the DMSO-treated controls vs. 51,128 μm2 (±1852) in the cyclosporine-treated embryos (p=1×10−8, NDMSO=16, NCsA=10). Day 2 exposures induced a significant decrease in the size of the eyes, forebrain, midbrain and hindbrain (cerebellum). The eye length was 328 μm (±2) in the DMSO-treated controls vs. 275 μm (±6) in the cyclosporine-treated embryos (p=4×10−9, NDMSO=20, NCsA=20). The forebrain area was 25,936 μm2 (±801) in the DMSO-treated controls vs. 16,637 μm2 (±917) in the cyclosporine-treated embryos (p=1×10−7, NDMSO =13, NCsA=12). The midbrain area was 73,651 μm (±1,434) in the DMSO-treated controls and vs. 52,723 μm2 (±1,564) in the cyclosporine-treated embryos (p=1×10−9, NDMSO=13, NCsA=12). The area of the cerebellum was 43,824 μm2 (±1,917) in the DMSO-treated controls vs. 38,722 μm2 (±837) in the cyclosporine-treated embryos (p=0.03, NDMSO=13, NCsA=12). In summary, day 1 cyclosporine exposures led to a significant 19%, 32% and 30% decrease in the size of the eyes, forebrain and midbrain and day 2 cyclosporine exposures led to a significant 16%, 36%, 28%, and 12% decrease in the size of the eyes, forebrain, midbrain and cerebellum, respectively. In contrast, day 3 cyclosporine exposures did not induce significant changes in the size of the eyes, forebrain, midbrain, or cerebellum. Based on these results, we conclude that the size of eyes and the size of the brain are sensitive to cyclosporine exposures during early embryonic development (day 1 and 2), but not during late embryonic development (day 3).
Fig 4.
Sensitive periods for eye and brain defects. A) Eye size. B) Forebrain area. C) Midbrain area. D) Anterior hindbrain area (cerebellum). Embryos exposed to 10 μM cyclosporine (CsA) from 0-1 and 1-2 dpf display a reduction in eye and brain size. In contrast, embryos exposed from 2-3 dpf do not display a reduction in eye and brain size. The wild type (A) and elav:GFP embryos (B-D) were imaged at 3 dpf. * p<0.05, ** p<0.01 (two tailed t-test).
3.4 Late embryonic exposures lead to changes in behavior
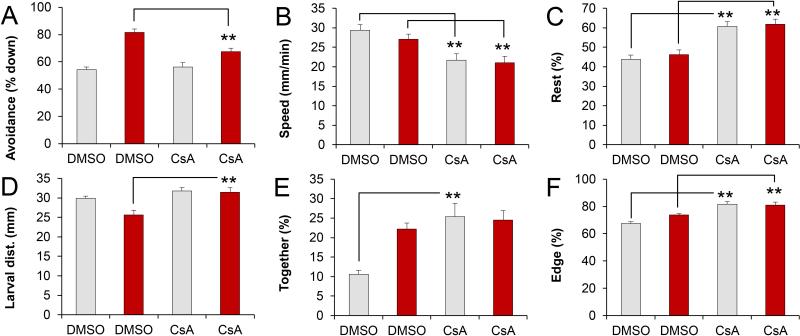
To examine if day 3 cyclosporine exposures induce functional brain defects, we grew DMSO and cyclosporine-exposed larvae to 5 dpf for behavioral analyses. At 5 dpf, the DMSO-treated and cyclosporine-treated larvae are indistinguishable by morphological criteria (Fig 5). However, the cyclosporine-exposed larvae displayed various behavioral defects in a 5-lane moving bar assay (Fig 6). This assay has two components. During the first 15 minutes, behaviors are analyzed without visual stimuli. During the subsequent 15 minutes, behaviors are analyzed in the presence of an aversive visual stimulus, a red moving bar, which the larvae avoid [21, 25]. We found that day 3 cyclosporine exposures affected the avoidance response (Fig 7A), swim speed (Fig 7B), resting (Fig 7C), the average distance between larvae (Fig 7D), the percentage of time that larvae are close together (Fig 7E), and the percentage of time the larvae spend on the edge of the swimming area (Fig 7F). Specifically, the time that larvae spent down in the lane, away from the visual stimulus, was 82% (±3) in the DMSO-treated controls vs. 67% (±3) in the cyclosporine-treated embryos (p=2×10−4, NDMSO=40, NCsA=37). The swim speed without visual stimuli was 29 mm/min (±2) in the DMSO-treated controls vs. 22 mm/min (±2) in the cyclosporine-treated embryos (p=0.001, NDMSO=40, NCsA=37). Similarly, the swim speed with visual stimuli was 27 mm/min (±1) in the DMSO-treated controls vs. 21 mm/min (±2) in the cyclosporine-treated embryos (p=0.005, NDMSO=40, NCsA=37). The percentage of time that larvae rest without visual stimuli was 44% (±2) in the DMSO-treated controls vs. 61% (±3) in the cyclosporine-treated embryos (p=3×10−6, NDMSO=40, NCsA= 37). Similarly, the percentage of time that larvae rest with visual stimuli was 46% (±2) in the DMSO-treated controls vs. 62% (±3) in the cyclosporine-treated embryos (p=2×10−5, NDMSO=40, NCsA=37). The larval distance in the presence of visual stimuli was 26 mm (±1) in the DMSO-treated controls vs. 32 mm (±1) in the cyclosporine-treated embryos (p=8×10−4, NDMSO=40, NCsA=37). The percentage of time that larvae were together (<5 mm apart), without visual stimuli, was 11% (±1) in the DMSO-treated controls vs. 25% (±3) in the cyclosporine-treated embryos (p=2×10−4, NDMSO=40, NCsA=37). The percentage of time that the larvae were located on the outer edge of the swimming area, without visual stimuli was 67% (±1) in the DMSO-treated controls vs. 82% (±2) in the cyclosporine-treated embryos (p=1×10−7, NDMSO=40, NCsA=37). Similarly, the percentage of time that the larvae were located on the outer edge of the swimming area, with visual stimuli was 74% (±1) in the DMSO-treated controls vs. 81% (±2) in the cyclosporine-treated embryos (p=0.001, NDMSO=40, NCsA=37). We conclude that cyclosporine exposures during late embryonic development lead to significant behavioral changes in free-swimming zebrafish larvae.
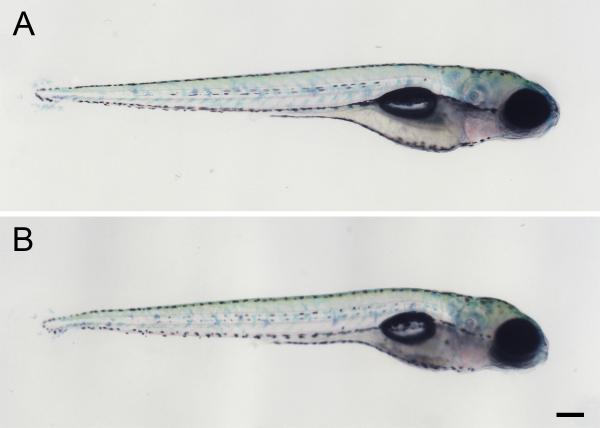
Fig 5.
Larval morphology at 5 dpf. A) Control larva, exposed to 0.1% DMSO from 2-3 dpf. B) Larva exposed to 10 μM cyclosporine from 2-3 dpf. These late embryonic cyclosporine exposures do not induce gross morphological defects. Scale bar = 200 μm.
Fig 6.
Automated analysis of behavior in a five-lane plate. Five-day-old larvae are imaged for 15 minutes without visual stimuli (left panel) and then for 15 minutes in the presence of a red bar, which moves up and down in the upper half of the lanes (right panel). The larvae swim towards the lower half of the lanes, away from the visual stimulus.
Fig 7.
Behavioral defects at 5 dpf. A) Avoidance of visual stimuli. B) Swim speed. C) Resting (larvae move less than 1 mm / 6 sec interval). D) Average distance between larvae. E) Together (larvae are less than 5 mm apart). F) Edge preference or thigmotaxis (larvae are less than 3 mm from the perimeter). The cyclosporine-exposed embryos display significant behavioral defects, as compared to the DMSO-treated controls (** p< 0.01, t-test). DMSO = Control embryos were exposed to 0.1% DMSO from 2-3 dpf. CsA = Embryos were exposed to 10 μM cyclosporine from 2-3 dpf. Gray bars = without visual stimuli. Red bars = with visual stimuli.
3.5 The calcineurin inhibitor FK506 induces similar defects
We examined whether similar developmental defects may be induced by FK506, another calcineurin inhibitor that is used as an immunosuppressant in transplant medicine [2-3]. We found that embryos exposed to 1 μM FK506 from 2-26 hpf displayed a similar decrease in eye size as embryos exposed to 10 μM cyclosporine. The length of the eyes was 327 μm (±2) in the DMSO-treated controls vs. 279 μm (±7) in the FK506-treated embryos (p=2×10−6, NDMSO=18, NFK506= 18 embryos). Embryos exposed to 1 μM FK506 from 2-26 hpf displayed a reduction in brain size, similar to the reduction in brain size observed in cyclosporine-exposed embryos. The area of the forebrain was 21,908 μm2 (±480) in the DMSO-treated controls vs. 17,575 μm2 (±739) in the FK506-treated embryos (p=2×10−4, NDMSO=10, NFK506=10). The area of the midbrain was 67,347 μm2 (±1,683) in the DMSO-treated controls vs. 57,530 μm2 (±2,006) in the FK506-treated embryos (p=0.002, NDMSO=10, NFK506=10). The area of the hindbrain (cerebellum) was not significantly reduced in the FK506-treated embryos. In summary, FK506 induces a significant 15% reduction in eye length, 20% reduction in forebrain area, and 15% reduction in midbrain area.
Embryos exposed to 1 μM FK506 during day 3 displayed similar behavioral defects as embryos exposed to 10 μM cyclosporine during day 3. Specifically, the percentage of time that larvae avoid the visual stimulus was 79% (±3) in the DMSO-treated controls vs. 63% (±2) in the FK506-treated embryos (p=7×10−5, NDMSO=33, NFK506=30). The percentage of time that the larvae were located on the outer edge of the swimming area, without visual stimuli was 71% (±2) in the DMSO-treated controls vs. 83% (±1) in the FK506-treated embryos (p=1×10−6, NDMSO=33, NFK506=30). Similarly, the percentage of time that the larvae were located on the outer edge of the swimming area, with visual stimuli, was 73% (±2) in the DMSO-treated controls vs. 80% (±1) in the FK506-treated embryos (p=0.002, NDMSO=33, NFK506=30). The larval distance in the presence of visual stimuli was 26 mm (±1) in the DMSO-treated controls vs. 33 mm (±1) in the FK506-treated embryos (p=2×10−5, NDMSO=33, NFK506=30). While the FK506-induced changes in behavior are similar to the cyclosporine-induced changes in behavior, the behavioral profiles of FK506 and cyclosporine are not identical. The swim speed without visual stimuli was 22 mm/min (±2) in the DMSO-treated controls vs. 27 mm/min (±2) in the FK506-treated embryos (p=0.052, NDMSO=33, NCsA=30). The percentage of time that larvae rest without visual stimuli was 56% (±3) in the DMSO-treated controls vs. 53% (±3) in the FK506-treated embryos (p=0.5, NDMSO=33, NCsA=30). Thus, the effects of FK506 on swim speed and resting are not significant and show a trend in the opposite direction compared to the effects of cyclosporine on swim speed and resting. In summary, FK506 induced a significant decrease in avoidance of visual stimuli, increase in thigmotaxis, and increase in larval distance in the presence of visual stimuli, similar to the behavioral defects induced by cyclosporine exposures. However, the cyclosporine-induced decrease in swim speed and increase in resting were not observed after FK506 exposure.
4. Discussion
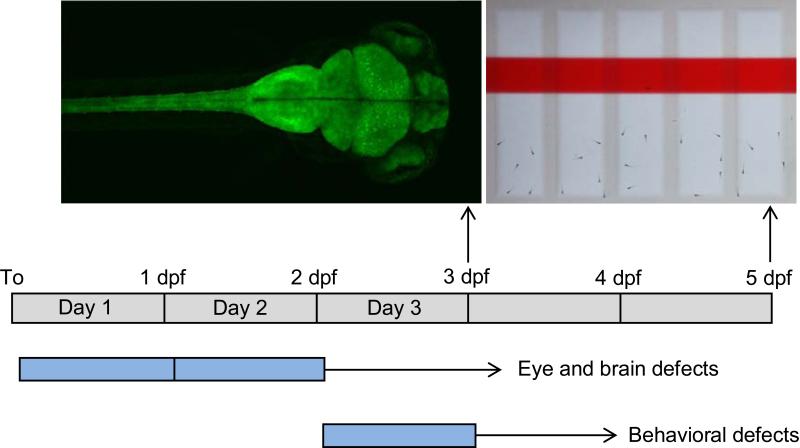
The results of this study show that cyclosporine exposures during embryonic development can induce structural or behavioral defects, depending on the exposure window (Fig 8). Early embryonic cyclosporine exposures (day 1 or day 2) led to a reduction in eye size and brain size. Late embryonic exposures (day 3) did not affect eye and brain size, but did lead to significant behavioral defects in free-swimming zebrafish larvae.
Fig 8.
Summary of sensitive periods in zebrafish development. Early embryonic exposures to cyclosporine lead to structural brain defects. Late embryonic exposures to cyclosporine lead to behavioral defects. Blue = window of cyclosporine exposure.
Eye and brain defects were induced by early exposures to either cyclosporine or FK506. Both calcineurin inhibitors are immunosuppressants, but act through different mechanisms: cyclosporine inhibits calcineurin via cyclophilin and FK506 inhibits calcineurin via FKBP [2]. The similar defects induced by different calcineurin inhibitors, suggests that the observed effects are mediated by an inhibition of calcineurin signaling. It remains to be established if these effects are cell autonomous or if calcineurin signaling in other embryonic tissues influence development of the eye and brain. Calcineurin subunits are expressed in the developing brain and in the retina of the eye [31], suggesting that cell-autonomous processes might be important.
The late embryonic cyclosporine exposures led to various behavioral defects in free-swimming zebrafish larvae. The cyclosporine-exposed larvae displayed a reduced avoidance response, lower swim speeds, increased resting, and an increased preference for the edge of the swimming area. In addition, cyclosporine exposure affected larval interactions. The cyclosporine-exposed larvae remain further apart in the presence of visual stimuli, but spent more time close together without visual stimuli. It is possible that this broad range of behavioral defects is caused by an overarching syndrome. Previous studies have shown that thigmotaxis, a preference for the edge of a swimming area, is an anxiety-related behavior in zebrafish larvae [32-33]. In addition, the observed increase in immobility or ‘resting’ and low swim speed may reflect an increase in ‘freezing’ behavior, similar to larval freezing induced by a novel visual stimulus [34] and anxiety-related freezing behaviors in adult zebrafish [35]. Thus, the cyclosporine-induced increase in thigmotaxis and immobility could be indicative of an anxiety-related syndrome. However, it is also possible that the various behavioral defects are not linked by an overarching mechanism. For example, the organophosphate pesticide chlorpyrifos induces low swim speeds and decreased thigmotaxis [36], showing that the low swim speed and elevated thigmotaxis observed in the present study are not necessarily linked. In addition, the observed changes in larval interactions suggest that cyclosporine may induce a multifaceted syndrome.
Since day 3 cyclosporine and FK506 exposures induce similar behavioral defects, these defects are likely caused by calcineurin inhibition. The underlying mechanisms may include calcineurin-dependent axonal growth and guidance or calcineurin-dependent neuronal apoptosis in the developing brain, similar to the roles of calcineurin in other systems [8, 37]. A better understanding of these basic developmental mechanisms could provide novel insights in various neurodevelopmental disorders. For example, in Down syndrome, calcineurin/NFAT signaling is thought to be suppressed by RCAN1 and DYRK1A located on chromosome 21 [38-39], but little is known about the neural mechanisms that are affected by this suppression of calcineurin/NFAT signaling. In addition to the behavioral defects induced by both cyclosporine and FK506, we found that cyclosporine induced a few behavioral defects that were not observed with FK506. The reduced swim speeds and increased resting were observed after cyclosporine exposure, but not after FK506 exposure. Possibly, this subset of behavioral defects is caused by off-target effects, i.e. effects that are not mediated by calcineurin inhibition. Since the behavioral assays may be used to identify both calcineurin-mediated effects and off-target effects, the assays could be valuable for the development novel pharmaceuticals with minimal off-target effects.
Cyclosporine is used to prevent organ rejection in transplant medicine and has been successfully used during pregnancy [7]. These pregnancies are considered high risk and close maternal and fetal surveillance is vital. The results of our study raise the question if cyclosporine induces developmental brain disorders when used during pregnancy. However, to what extent can our results in zebrafish be translated to developmental disorders in humans? On one hand, there are substantial differences between zebrafish and human embryos, for example in the speed of development and the environment surrounding the embryo. In addition, it is unclear how the 1-10 μM (1.2-12 mg/L) cyclosporine concentrations used in this study relate to the 0.8-2 mg/L in the serum of patients who take cyclosporine for immunosuppression [40] and the unknown cyclosporine concentrations in developing human embryos. On the other hand, the conserved signaling pathways that regulate brain development [10-11] and the substantial structural and functional brain defects that were observed in the current study suggest that there is reason for concern. Data from clinical studies and data from studies using various model systems can be taken into account when advising patients who have transplants or autoimmune diseases. In addition, if calcineurin inhibitors are used during pregnancy, it is prudent to carry out health assessment not only during pregnancy, but also during childhood and adolescence. Finally, our results highlight the importance of studying different exposure windows in zebrafish and other animal model systems. The different exposure windows, combined with quantitative analyses of behavior, provide a better basic understanding of the factors that can induce functional brain defects during specific sensitive periods in development.
Highlights.
We examined the effects of cyclosporine exposures during zebrafish development.
Early embryonic exposures led to a reduction in eye size and brain size.
Late embryonic exposures led to behavioral defects.
The use of cyclosporine during pregnancy is concerning.
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD060647). Holly Richendrfer, Ruth Colwill and Robbert Creton received funding from the National Institute of Environmental Health Sciences (F32 ES021342, R03ES017755, P42 ES013660) and Robert Thorn received funding from a NIH training grant in molecular biology, cell biology and biochemistry (T32 GM007601). We thank Dr. Bonkowsky for the transgenic zebrafish line and thank Sean Pelkowski for contributing to the eye size experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahan BD. Forty years of publication of transplantation proceedings--the second decade: the cyclosporine revolution. Transplant Proc. 2009;41:1423–37. doi: 10.1016/j.transproceed.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Liu JO. Calmodulin-dependent phosphatase, kinases, and transcriptional corepressors involved in T-cell activation. Immunol Rev. 2009;228:184–98. doi: 10.1111/j.1600-065X.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can't live without. J Immunol. 2013;191:5785–91. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 4.Babalola O, Strober BE. Management of psoriasis in pregnancy. Dermatol Ther. 2013;26:285–92. doi: 10.1111/dth.12073. [DOI] [PubMed] [Google Scholar]
- 5.FDA. U.S. Food and Drug Administration [Nov 2014];CFR - Code of Federal Regulations Title 21. http://wwwaccessdatafdagov/scripts/cdrh/cfdocs/cfcfr/CFRSearchcfm?fr=20157. 2014.
- 6.Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34:389–95. doi: 10.1002/phar.1385. [DOI] [PubMed] [Google Scholar]
- 7.Armenti VT, Constantinescu S, Moritz MJ, Davison JM. Pregnancy after transplantation. Transplant Rev (Orlando) 2008;22:223–40. doi: 10.1016/j.trre.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T, Di Giovanni S. NFAT signaling in neural development and axon growth. Int J Dev Neurosci. 2008;26:141–5. doi: 10.1016/j.ijdevneu.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore KL, Persaud TVN, Torchia MG. The Developing Human: Clinically Oriented Embryology. 9th edition. Elsevier; Philadelphia, PA.: 2013. [Google Scholar]
- 10.Cavodeassi F, Houart C. Brain regionalization: of signaling centers and boundaries. Dev Neurobiol. 2012;72:218–33. doi: 10.1002/dneu.20938. [DOI] [PubMed] [Google Scholar]
- 11.Rinkwitz S, Mourrain P, Becker TS. Zebrafish: an integrative system for neurogenomics and neurosciences. Prog Neurobiol. 2011;93:231–43. doi: 10.1016/j.pneurobio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Gerlai R. Fish in behavior research: unique tools with a great promise! J Neurosci Methods. 2014;234:54–8. doi: 10.1016/j.jneumeth.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci. 2014;35:63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 2014;37:264–78. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Bene F, Wyart C. Optogenetics: a new enlightenment age for zebrafish neurobiology. Dev Neurobiol. 2012;72:404–14. doi: 10.1002/dneu.20914. [DOI] [PubMed] [Google Scholar]
- 16.Simmich J, Staykov E, Scott E. Zebrafish as an appealing model for optogenetic studies. Prog Brain Res. 2012;196:145–62. doi: 10.1016/B978-0-444-59426-6.00008-2. [DOI] [PubMed] [Google Scholar]
- 17.Weber T, Koster R. Genetic tools for multicolor imaging in zebrafish larvae. Methods. 2013;62:279–91. doi: 10.1016/j.ymeth.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 19.Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, Healey D, Kim S, Werdich AA, Haggarty SJ, Macrae CA, Shoichet B, Peterson RT. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6:231–7. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT, Schier AF. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–51. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clift D, Richendrfer H, Thorn RJ, Colwill RM, Creton R. High-throughput analysis of behavior in zebrafish larvae: effects of feeding. Zebrafish. 2014;11:455–61. doi: 10.1089/zeb.2014.0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colwill RM, Creton R. Imaging escape and avoidance behavior in zebrafish larvae. Rev Neurosci. 2011;22:63–73. doi: 10.1515/RNS.2011.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creton R. Automated analysis of behavior in zebrafish larvae. Behav Brain Res. 2009;203:127–36. doi: 10.1016/j.bbr.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 24.Pelkowski SD, Kapoor M, Richendrfer HA, Wang X, Colwill RM, Creton R. A novel high-throughput imaging system for automated analyses of avoidance behavior in zebrafish larvae. Behav Brain Res. 2011;223:135–44. doi: 10.1016/j.bbr.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richendrfer H, Creton R. Automated high-throughput behavioral analyses in zebrafish larvae. J Vis Exp. 2013:e50622. doi: 10.3791/50622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, Hong SK, Shin J, Yoo KW, Hibi M, Hirano T, Miki N, Chitnis AB, Huh TL. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol. 2000;227:279–93. doi: 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- 27. [Nov 2014];Zebrafish Developmental Staging Series. http://zfin.org/zf_info/zfbook/stages/
- 28.Lewis D. The CAVE artists. Nat Med. 2014;20:228–30. doi: 10.1038/nm0314-228. [DOI] [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25:1463–5. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammond DR, Udvadia AJ. Cabin1 expression suggests roles in neuronal development. Dev Dyn. 2010;239:2443–51. doi: 10.1002/dvdy.22367. [DOI] [PubMed] [Google Scholar]
- 32.Richendrfer H, Pelkowski SD, Colwill RM, Creton R. On the edge: pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav Brain Res. 2012;228:99–106. doi: 10.1016/j.bbr.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnorr SJ, Steenbergen PJ, Richardson MK, Champagne DL. Measuring thigmotaxis in larval zebrafish. Behav Brain Res. 2012;228:367–74. doi: 10.1016/j.bbr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 34.O'Neale A, Ellis J, Creton R, Colwill RM. Single stimulus learning in zebrafish larvae. Neurobiol Learn Mem. 2014;108:145–54. doi: 10.1016/j.nlm.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, Wu N, Wong K, Roy S, Suciu C, Goodspeed J, Elegante M, Bartels B, Elkhayat S, Tien D, Tan J, Denmark A, Gilder T, Kyzar E, Dileo J, Frank K, Chang K, Utterback E, Hart P, Kalueff AV. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc. 2010;5:1786–99. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- 36.Richendrfer H, Pelkowski SD, Colwill RM, Creton R. Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae. Neurotoxicol Teratol. 2012 doi: 10.1016/j.ntt.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara MR, Snyder SH. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol. 2007;47:117–41. doi: 10.1146/annurev.pharmtox.47.120505.105311. [DOI] [PubMed] [Google Scholar]
- 38.Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, Neilson JR, Chen L, Heit JJ, Kim SK, Yamasaki N, Miyakawa T, Francke U, Graef IA, Crabtree GR. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 39.Park J, Oh Y, Chung KC. Two key genes closely implicated with the neuropathological characteristics in Down syndrome: DYRK1A and RCAN1. BMB Rep. 2009;42:6–15. doi: 10.5483/bmbrep.2009.42.1.006. [DOI] [PubMed] [Google Scholar]
- 40.Keown PA. New concepts in cyclosporine monitoring. Curr Opin Nephrol Hypertens. 2002;11:619–26. doi: 10.1097/00041552-200211000-00008. [DOI] [PubMed] [Google Scholar]