Abstract
Problem
Effects of HIV infection on ovarian function and aging are unclear.
Method of Study
Anti-Müllerian Hormone (AMH) levels were analyzed in 2621 HIV-infected and 941 uninfected participants using left-censored longitudinal models.
Results
Age-adjusted AMH levels were 16% lower in women with undetectable viremia and 26% lower in detectable viremia, relative to uninfected women. Current CD4 count associated with higher AMH in both HIV-infected and HIV-uninfected women. After controlling for current and nadir CD4, AMH was ~15% higher in HIV-infected relative to uninfected women, regardless of HIV viremia. Gravidity, amenorrhea, and nadir total lymphocyte counts associated with higher AMH; hormonal contraceptive use and past weight loss associated with lower AMH.
Conclusions
CD4+ lymphocyte counts were associated with AMH in both HIV-infected and uninfected women. After adjustment for CD4 counts and age, HIV infection was associated with higher AMH. CD4 T cells and cellular activation may influence ovarian granulosa cell function.
Keywords: AMH, CD4 lymphocytes, HIV, menopause, ovarian follicle
Introduction
Anti-Müllerian Hormone (AMH, also known as Müllerian Inhibiting Substance, MIS) provides a measure of ovarian follicle mass that is stable through the ovulatory cycle, and proposed as a biomarker of ovarian reserve 1-6 and gonadal aging 7-10. The potential utility of AMH as an indicator of gonadal aging in women was recently reflected in the Stages of Reproductive Aging Workshop +10 that provided updated bleeding and endocrine criteria for staging reproductive aging, including low AMH as a supportive criterion for the late reproductive stage 11.
Determination of ovarian reserve and menopausal status in women with chronic illness is important for reproductive decision making and for researchers who seek to examine immunological and metabolic factors that may be influenced by gonadal function. Because clinical indicators of ovarian function, such as menstrual pattern, menopausal symptoms (for example, hot flashes, sleep disturbance) may be altered in chronic illness they are likely to be relatively non- specific in this setting. The ovulatory cycle phase must be considered for interpretation of the commonly used biomarkers of ovarian reserve, including FSH, estradiol and inhibin B, limiting the utility of these measures among women with amenorrhea or irregular cycles. Because AMH does not vary substantially during the ovulatory cycle, it can be used to assess ovarian reserve in women in whom commonly used measures are not well suited. AMH has been identified as a useful tool for the assessment of ovarian reserve after cancer chemotherapy in both women and girls 12-14.
We previously investigated the relationship between AMH and early follicular phase levels of inhibin B, FSH and estradiol in a cross-sectional study involving 263 participants (187 HIV-infected) of the Women's Interagency HIV Study (WIHS) who reported having regular menstrual cycles 15. Early follicular and random AMH levels were closely correlated with the other ovarian function markers assessed in early follicular phase. We also reported that African American women had 25% lower AMH levels ( 95%CI: −43.0 to −1.9, p=0.037) and faster rates of decline compared with Caucasian women16 in this subset of WIHS participants. We now present the results of a more extensive evaluation of AMH levels that were measured over time in a sample of 2621 HIV-infected and 941 uninfected WIHS participants who were not selected based on menstrual cycle characteristics. Using this larger representative sample, we examined the effects on AMH of factors related to both reproductive health and HIV morbidity.
Materials and Methods
Study Population
The WIHS is a longitudinal observational cohort study of HIV infection and related conditions in women. In brief, 3,766 women (2,791 HIV-infected and 975 HIV-uninfected) were enrolled in either 1994-1995 (n=2,623) or 2001-2002 (n=1,143) from 6 U.S. sites (Bronx/Manhattan, Brooklyn, Chicago, Los Angeles, San Francisco, and Washington, DC). 17 HIV-infected participants are representative of HIV-infected women in each community. 17 HIV-uninfected women were recruited based on exposures that put them at risk for HIV infection. Participants are interviewed and examined every six months. Women who contributed data to this report were enrolled in the first or second wave of WIHS.
Written informed consent was provided by all participants after approval of the human subjects protocols by internal review committees at each of the WIHS institutions. Study interviews included queries regarding menstrual periods, obstetrical history, gynecological surgery, tobacco and illicit drug use, use of exogenous steroids, use of other medications and medical conditions. HIV serology was performed at baseline and prospectively on women with negative results previously. Quantification of HIV RNA copy numbers (“viral load”) was performed on plasma and lymphocyte subsets (including determination of CD3+CD4+ and CD3+CD8+ cell counts) were measured in whole blood semiannually using laboratories that participate in the NIAID Division of AIDS Virology and Immunology Laboratory Quality Assurance Programs.
The present study was designed to investigate differences between HIV-infected and uninfected women and associated factors in levels of AMH. Our a priori hypothesis was that HIV infection would not substantially influence AMH level independent of other known factors. A total of 3,562 ethnically diverse women (2621 HIV-infected and 941 uninfected) provided serum samples for AMH measurement. Participants with history of cancer chemotherapy were excluded. The median number of AMH measurements for each participant was 2.0 (IQR 1, 4), and median follow-up between first and last AMH measure was 5.5 years (IQR 4.0, 7.6) for those with at least two measures.
Biomarker Analysis
Levels of AMH were determined using an ELISA kit purchased from Beckman Coulter Inc. (Chaska, MN). Serum samples were frozen at −80°C and not thawed prior to testing, which was conducted blind to HIV status. Inter-assay coefficients of variations were 8.2% at a dose of 2.8 ng/mL and 9.4% at a dose of 8.5 ng/mL. The lower limit of detection was 0.08 ng/mL.
Covariates
Candidate covariates of AMH level included age, ethnicity, lifestyle factors (smoking, crack/cocaine use), body mass index (BMI), waist circumference, factors related to fertility and menopause, and lymphocyte variables. Factors related to fertility and menopause included parity, gravidity, hormonal contraceptive use, history of irregular menses reported at the visit contributing AMH data, and age at menarche. We controlled for self reported amenorrhea (at the time of the WIHS visit contributing the AMH measurement), which can be an indicator both of the menopausal transition and of polycystic ovary syndrome (PCOS). HIV-related risk factors included HIV RNA level, history of weight loss (reported at the visit contributing AMH measurements), hepatitis C infection (defined by second-generation or third-generation enzyme linked immunoassay result on blood at WIHS entry), and use of potent combinations of antiretroviral drugs (highly active antiretroviral therapies or HAART) that were defined based on the current HIV treatment guidelines18, and CD4 count (current and nadir). Nadir cell counts were the lowest values measured at a WIHS visit and were identified for both HIV-infected and – uninfected women. Other lymphocyte variables included CD8, total lymphocyte, and WBC counts; all were modeled as both current and nadir.
Statistical Analysis
We compared baseline demographic and clinical characteristics of HIV-infected and -uninfected women using Fisher's exact test for categorical variables and the Mann-Whitney U test for continuous variables, because several variables were found to be non-normally distributed.
We used multivariable linear mixed models with random intercepts and slopes to compare levels and rates of change in AMH between HIV-infected and -uninfected women. Because the assay has a lower limit of quantification, analysis of AMH is complicated by left-censoring of level below this limit (0.09 ng/mL). We therefore used a likelihood-based method as implemented in Thiébaut and Jacqmin-Gadda19using SAS Proc NLMIXED.
Separate models were constructed for HIV-infected and -uninfected women, adjusting for candidate covariates as listed above. We used stepwise backward selection with a significance level of α=0.05 to remove candidate covariates. Because of the essential role of age in AMH biology, we flexibly modeled and adjusted for age effects using linear splines, allowing the effect (per year increase in age) to vary within different age ranges. We evaluated interactions that addressed issues of interest and/or that had a priori biological plausibility.
All analyses were conducted using the SAS system, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of the HIV-infected and -uninfected groups
The baseline (enrollment) characteristics of WIHS participants providing data for this analysis are presented in Table 1. The HIV-infected women were slightly older than the uninfected women (median 38 vs. 37 years, p<.0001), but the racial distributions (58% African American) were similar in the two groups. Current smoking was less common in HIV-infected women than in uninfected women. HIV-infected women also had lower BMI, and were more likely to report a history of weight loss. The number of pregnancies and births were higher in HIV-infected women, while hormonal contraceptive use, history of irregular menses, and history of amenorrhea were less often reported by HIV-infected women. Both current and nadir CD4, WBC, and total lymphocyte counts were lower in HIV-infected women, while CD8 counts were higher than in uninfected comparison women.
Table 1.
Baseline characteristics of WIHS participants by HIV Status
| Parameter | HIV-infected (n=2621*) | HIV-uninfected (n=941) | P-value |
|---|---|---|---|
| Baseline Age (median, range) | 38 (19-80) | 37 (18-73) | <.0001 |
| Age<30 | 200 (8%) | 194 (21%) | |
| Age 30-40 | 1534 (59%) | 500 (53%) | |
| Age 40-50 | 751 (29%) | 217 (23%) | |
| Age>50 | 136 (5%) | 30 (3%) | |
| Race | |||
| African American | 1513 (58%) | 539 (57%) | 0.97 |
| Caucasian | 604 (23%) | 220 (23%) | |
| Other | 498 (19%) | 180 (19%) | |
| Cigarette smoking | |||
| Current | 1273 (49%) | 514 (55%) | 0.0049 |
| Past | 548 (21%) | 182 (19%) | |
| Never | 800 (31%) | 245 (26%) | |
| BMI (kg/m2) | 26 (23-31) | 28 (24-34) | <.0001 |
| Waist Circumference (cm) | 89 (80-101) | 91 (80-104) | 0.063 |
| Parity | |||
| 0-1 | 1010 (39%) | 426 (45%) | 0.0016 |
| 2 | 570 (22%) | 185 (20%) | |
| 3+ | 1037 (40%) | 329 (35%) | |
| Gravidity | |||
| 0-1 | 297 (11%) | 174 (19%) | <.0001 |
| 2-4 | 822 (31%) | 276 (29%) | |
| 5+ | 1497 (57%) | 489 (52%) | |
| Hormonal Contraceptive Use (current) | 176 (7%) | 85 (9%) | 0.024 |
| Hormonal Contraceptive Use (Ever) | 532 (20%) | 285 (30%) | <.0001 |
| Hx of Irregular Menses | 1555 (59%) | 643 (68%) | <.0001 |
| Hx of Amenorrhea | 1752 (67%) | 743 (79%) | <.0001 |
| Age at Menarche | 12 (11-14) | 12 (11-13) | 0.011 |
| Crack/cocaine use | 394 (15%) | 177 (19%) | 0.0071 |
| Hepatitis C | 748 (29%) | 156 (17%) | <.0001 |
| Hx of Weight Loss | 973 (37%) | 169 (18%) | <.0001 |
| Current CD4 (/ml) | 359 (191-564) | 1008 (803-1270) | <.0001 |
| Nadir CD4 (/ml) | 273 (128-423) | 850 (651-1069) | <.0001 |
| Current CD8 (/ml) | 755 (519-1066) | 516 (387-664) | <.0001 |
| Nadir CD8 (/ml) | 589 (394-859) | 435 (323-576) | <.0001 |
| Current Total WBC (×103/ml) | 4.4 (3.4-5.6) | 6.4 (5.1-7.9) | <.0001 |
| Nadir Total WBC (×103/ml) | 3.7 (2.8-4.6) | 5.5 (4.5-6.8) | <.0001 |
| Current Total Lymphocyte/ml | 152 (108-201) | 205 (167-255) | <.0001 |
| Nadir Total Lymphocyte/ml | 122 (85-165) | 178 (140-220) | <.0001 |
| Plasma HIV RNA (copies/ml) | |||
| ≤80 | 619 (24%) | ||
| 81-1999 | 432 (17%) | ||
| 2000-9999 | 421 (16%) | ||
| >10000 | 1126 (43%) | ||
| Current HAART use | 829 (32%) |
Data are presented as Median (IQR) or numbers (percent).
Abbreviations: IQR, interquartile range; Hx, history; WBC, white blood cell
HIV+ sample size is slightly larger in the longitudinal analyses below due to seroconversion
At baseline, the majority of HIV-infected women had detectable HIV RNA in plasma (using an assay sensitive to 80 copies of RNA per ml). Of note, 43% had RNA levels of 10,000 copies or greater. 32% of HIV-infected participants reported taking a HAART regimen at the time of enrollment; the majority of these women were enrolled in the second WIHS enrollment wave since HAART regimens were not in use during the initial wave of WIHS enrollment.
AMH levels in HIV-infected and -uninfected women
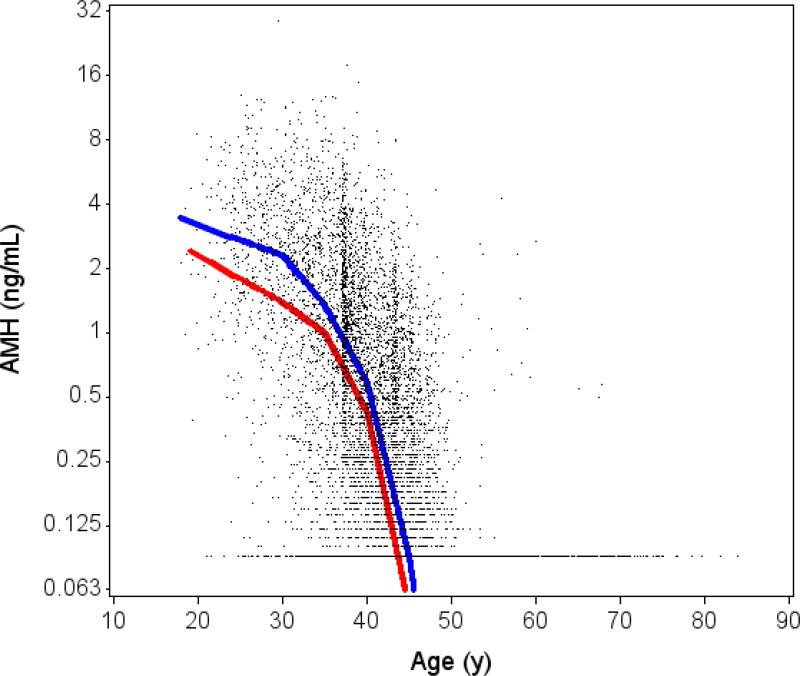
Figure 1 illustrates observed AMH levels by age according to HIV infection status. HIV-infected and -uninfected women showed a similar rate of change in AMH over time, with a consistent increasing rate of decline after 35 years of age. However, AMH levels were consistently lower at all ages among the HIV-infected women compared with -uninfected women. For example, the estimated geometric mean level of AMH at age 40 was 0.42 ng/mL in HIV-infected women (95% CI 0.39 to 0.47) and 0.63 ng/mL in -uninfected women (95% CI 0.53 to 0.74).
Figure 1. Unadjusted association of age with AMH levels by HIV status.
Solid lines denotes level of AMH from unadjusted linear piecewise model including left-censoring, with random intercept and slope to handle repeated AMH measures within a subject (red=HIV+, blue=Control). Plotted points include multiple values for individual subjects at different timepoints. The test for age by HIV interaction did not reach statistical significance (p=0.14).
HIV infection was associated with lower levels of AMH in women with both detectable and undetectable plasma HIV RNA (Table 2) in a model that adjusted only for age. Being HIV-infected and having undetectable plasma HIV RNA was associated with 15.7% lower levels of AMH (95%CI: −25.3 to −4.8, p=0.0058) than being HIV-uninfected. Being HIV-infected and having detectable plasma HIV RNA was associated with 25.6% lower levels of AMH (95%CI: −33.5 to −16.8, p<.0001). Multivariable analysis was conducted controlling for age, race, current smoking, current hormonal contraceptive use, history of amenorrhea, history of weight loss, current CD4 count, nadir CD4 count, nadir CD8 count, and nadir T lymphocyte count (Table 2). In fully adjusted analysis, the association of HIV infection with lower AMH levels was reversed: among HIV-infected women, both those with undetectable HIV RNA and those with detectable HIV RNA had ~30% higher levels of AMH compared with uninfected women. Being HIV seropositive with undetectable HIV RNA was associated with 27.7% higher levels of AMH (95%CI: 10.4 to 47.9, p=0.0010), and having detectable HIV RNA was associated with 31.2% higher levels of AMH (95%CI: 13.5 to 51.6, p=0.0002) compared with -uninfected women.
Table 2.
Association of HIV infection and HIVRNA levels with AMH levels
| Model | HIV-infected |
Uninfected Controls (N = 941) | |
|---|---|---|---|
| Undetectable HIVRNA (N = 698*) | Detectable HIVRNA (N =1946) | ||
| Unadjusted AMH level** | 0.56 (0.48, 0.65) | 0.37 (0.33, 0.42) | 0.63 (0.53, 0.74) |
| % Estimate (95%CI)‡ | % Estimate (95%CI)‡ | ||
| Adjusted for age | −15.7 (−25.3, −4.8), p=0.0058 | −25.6 (−33.5, −16.8), p<.0001 | Reference |
| Multivariable-adjusted§ | 27.7 (10.4, 47.9), p=0.0010 | 31.2 (13.5, 51.6), p=0.0002 | Reference |
sample size given at baseline. The numbers within each group may change during follow-up as a result of increases or decreases in HIVRNA levels, as well as seroconversion among participants who were uninfected at baseline.
estimated geometric mean AMH level (ng/mL) at age 40, with 95%CI.
AMH is log-transformed; results are back-transformed to produce estimated percentage differences in AMH attributable to each factor. Estimates are from mixed models for left-censored repeated measures.
Adjusted for age, race, current smoking, current hormonal contraceptive use, history of amenorrhea, history of weight loss, current CD4 count, nadir CD4 count, nadir CD8 count, and nadir T lymphocyte count.
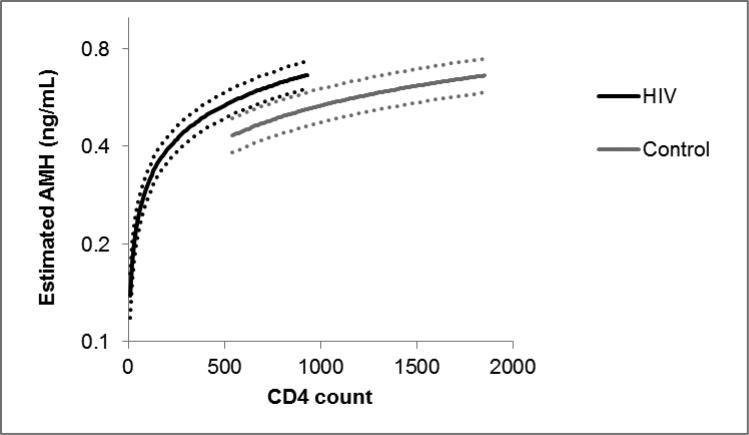
Additional analysis revealed that the HIV-related decrease in AMH levels appeared to be due primarily to the depletion of CD4 lymphocytes (Supplemental Table 1). When we controlled only for age, current CD4 cell count, and nadir CD4 cell count, we found that HIV infection was associated with 15-16% higher AMH levels, regardless of whether HIV viral load was detectable or not. This finding is estimating how AMH levels would differ between HIV-infected and uninfected women who have identical CD4 counts, as illustrated in Figure 2. When we removed the lymphocyte variables from the model, HIV infection was again associated with lower AMH levels in those with both undetectable (−19.7, 95%CI: −28.8, −9.4) and detectable viremia (−26.5, 95%CI: −34.3, −17.9).
Figure 2. Estimated AMH levels at age 40 by HIV status and CD4 count.
Solid lines denote estimated level of AMH from linear piecewise model including left-censoring, with random intercept and slope to handle repeated AMH measures within a subject. CD4 ranges shown extend from 5th to 95th percentile for HIV-infected and uninfected participants separately. Dotted lines indicate 95% confidence bands. Model controls for age, HIV status, and CD4 count.
We also investigated whether participants who experienced large increases in CD4 cell count during the course of the study (usually associated with use of antiretroviral treatment regimens) had a smaller age-related decline in AMH levels over the period of increase than women who did not have a large increase in CD4 cell count. Among the 478 HIV-infected participants with increases in CD4 cell count of 200 cells/μl or more, we observed an age-adjusted increase of 21% (95%CI 9.1, 34.8, p=0.0004) in AMH levels that coincided with the period of the CD4 cell count increase. This increase appeared to be larger in Caucasians (29%, 95%CI 4.2, 60.0, p=0.020) than in African-Americans (15%, 95%CI −0.03, 32.4, p=0.050), but this difference was not statistically significant (p=0.38). We observed an age-adjusted AMH decline of 13% (95% CI −24.3, −0.91, p=0.036) among 515 HIV-infected participants with an increase in CD4 cell count of 100-199 cells/μl but who never had an increase of 200 or more cells. Thus the extent of treatment related increases in CD4 cell counts appears have influenced AMH levels adding further support to our finding that CD4 cell counts are a important influence on AMH production. We also investigated whether there was a period effect due to recruitment cohort. 68% of the participants in our analysis were in the original cohort (1994-95), and 32% were in the 2001-02 recruitment cohort. When tested in our model, the effect of being in the original cohort was associated with 3.1% lower AMH levels, but the association was not statistically significant (95%CI: −13.4, 8.4, p=0.58).
Interactions between race and HIV status were evident in both age-adjusted and fully adjusted analyses. In age-adjusted analysis, HIV infection was associated with 8.6% lower levels of AMH in Caucasians and 28.8% lower in African-Americans (test for HIV by race interaction: p=0.022). In fully adjusted analysis, HIV infection was associated with 50.8% higher levels of AMH (95% CI: 14.6, 98.5, p=0.0035) in Caucasians and 7.6% higher in African-Americans (95% CI: −12.0, 31.5, p=0.48), although the test interaction for the effect HIV status by race on AMH level did not reach statistical significance (p=0.15).
Factors associated with AMH in HIV-infected and -uninfected women Among HIV-infected women, factors independently associated with lower levels of AMH (Table 3) included African American race, older age, increasing waist circumference, current hormonal contraceptive use, and a history of weight loss (which may be related to HIV infection). Greater BMI, gravidity, history of amenorrhea, and higher current CD4 and higher nadir CD4 counts were independently associated with higher levels of AMH in HIV-infected women.
Table 3.
Multivariable-adjusted associations with level of AMH in HIV-infected and uninfected WIHS participants
| Predictor | HIV-infected* | HIV-uninfected** | ||
|---|---|---|---|---|
| % Estimate (95%CI) | P-value | % Estimate (95%CI) | P-value | |
| Age effect per year | ||||
| within ages <30 | −10.5 (−17.0, −3.5) | 0.0041 | −5.4 (−10.3, −0.18) | 0.043 |
| within ages 30-35 | −7.9 (−11.8, −3.7) | 0.0003 | −8.5 (−13.4, −3.2) | 0.0018 |
| within ages 35-40 | −15.9 (−18.4, −13.3) | <.0001 | −14.6 (−18.9, −10.1) | <.0001 |
| within ages 40-45 | −32.3 (−34.2, −30.3) | <.0001 | −30.2 (−33.4, −26.8) | <.0001 |
| within ages 45-50 | −51.2 (−53.5, −48.6) | <.0001 | −47.7 (−51.6, −43.5) | <.0001 |
| within ages >50 | −25.4 (−32.0, −18.0) | <.0001 | −32.3 (−39.8, −23.9) | <.0001 |
| African American vs. Caucasian | −16.5 (−28.8, −1.97) | 0.028 | 29.9 (5.2, 60.4) | 0.015 |
| Other vs. Caucasian | −13.9 (−29.9, 5.7) | 0.15 | 37.0 (3.1, 81.9) | 0.030 |
| BMI (kg/m2) | 1.75 (0.60, 2.9) | 0.0027 | −1.63 (−3.2, −0.016) | 0.048 |
| Waist Circ (per 10 cm) | −5.1 (−9.9, −0.023) | 0.049 | 3.6 (−3.8, 11.7) | 0.35 |
| Gravidity | 2.6 (1.20, 4.0) | 0.0003 | −1.71 (−3.5, 0.16) | 0.073 |
| Current hormonal contraceptive use | −19.8 (−30.5, −7.5) | 0.0025 | −5.0 (−22.9, 17.1) | 0.63 |
| Hx of amenorrhea | 20.2 (6.7, 35.5) | 0.0025 | 25.0 (−0.32, 56.6) | 0.053 |
| History of weight loss | −16.1 (−24.4, −6.8) | 0.0011 | −15.0 (−30.2, 3.5) | 0.11 |
| Current CD4 (per doubling) | 16.7 (12.4, 21.2) | <.0001 | 19.4 (4.2, 36.8) | 0.011 |
| Nadir T Lymph (per doubling) | 12.5 (3.2, 22.5) | 0.0074 | 9.1 (−8.8, 30.6) | 0.34 |
AMH is log-transformed; results are back-transformed to produce estimated percentage differences in AMH attributable to each factor.
Estimates from mixed models for longitudinal left-censored repeated measures.
2624 participants and 7430 observations.
941 participants and 2415 observations.
In sensitivity analyses that replaced current hormonal contraceptive use with any history of use, we found little association of any history of hormonal contraception with AMH levels (−5% 95%CI: −15.8, 7.2, p=0.40 in age-adjusted analysis and −8.2% 95%CI: −19.9, 5.3, p=0.22 in fully-adjusted analysis). We also examined the interaction of age with current hormonal contraceptive use on AMH levels. This analysis found that in women over 40 years of age, current hormonal contraception was associated with even lower levels of AMH (−32.6%, 95%CI: −48.8, −11.2; p=0.0050).
Among HIV-uninfected women, age, Caucasian ethnicity, and greater BMI were independently associated with lower levels of AMH, while higher current CD4 counts were independently associated with higher levels of AMH (Table 3). History of amenorrhea was associated with 25% higher levels of AMH, but the association did not reach statistical significance (p=0.053). Among HIV-uninfected women aged greater than 40, current hormonal use was associated with 19% lower levels of AMH, although the association did not reach statistical significance (95%CI: −53.0, 40.7; p=0.46).
We found little evidence of an association of current CD8 counts with levels of AMH in fully adjusted analysis in either HIV-infected (−1.0, 95%CI: −7.4, 5.8; p=0.76) or -uninfected women (−5.4, 95%CI: −15.3, 5.5, p=0.32). The association of BMI with AMH weakened when waist circumference was removed from the model in both uninfected (−0.68, 95%CI: −1.64, 0.28, p=0.17) and infected (+0.89, 95%CI: 0.19, 1.60, p=0.013) women.
Discussion
In both HIV-infected and -uninfected WIHS participants, current CD4+ lymphocyte count was a strong and independent predictor of serum AMH level. This finding was unexpected, and appeared to explain the association of HIV infection with lower AMH levels seen in the comparisons that were adjusted only for age. In our analyses, the interaction between AMH and leukocyte counts was focused upon CD3+/CD4+ lymphocyte subset and total lymphocyte count nadir values in HIV-infected women. It should be noted that in both untreated and treated HIV infection, CD4 cell counts can range widely, and occur within the normal range. The normal range for CD4 cell counts is broad, roughly from 450 cells to 1500 cells/ml and our results indicate that variation within the normal range is associated with AMH level; our findings are not limited to profoundly depleted CD4 cells. There was no substantial effect of CD3+/CD8+ lymphocyte counts on AMH levels in fully adjusted analyses. Among HIV infected participants who had AMH measures during an antiretroviral treatment-related expansion of CD4+ cell counts (i.e., increases in CD4 cell count of 200 cells/μl or more), the age related decline in AMH level was slowed during the expansion. These findings indicate that CD4+ lymphocytes may have a role in the ovarian follicle, more specifically in the function of granulosa cells that are the exclusive source of AMH in females5.
The idea that leukocytes play an important role in the physiology of ovarian follicles is not new20, 21. A range of studies indicate that interactions between leukocytes and ovarian follicles and inflammatory responses are important for ovulation and the fertilization of oocytes22-26. A large number of studies have shown that HIV infection results in a significant depletion of CD4 lymphocytes in tissues, which is partially reversed by the long term administration of effective antiretroviral therapy. Early studies of leukocytes in the ovary found that CD4+ cells were major constituents of lymphocytes in the corpus luteum, and tend to be located surrounding the granulosa cell layer27. Luteinizing granulosa cells produce stromal cell-derived factor −1 (SDF-1 or CXCL12), which reduces early apoptosis of these cells28 and improves the quality of embryos. CD4+ lymphocytes are recruited to the follicle by ovarian granulosa cells. When evaluated via follicular aspirates, CD4+ and CD8+ lymphocytes migrate towards ovarian granulosa cells, whereas other lymphocytes do not. The presence of SDF-1 protects granulosa cells from early apoptosis, and this effect is eliminated when lymphocytes are not present28. An influx of splenic leukocytes occurs after LH surge, indicating hormonal regulation of ovarian leukocyte populations29. In the mouse model, ovarian chemokine secretion results in the recruitment of a specialized CD8+ cell population to the ovary, an occurrence required for optimal fertility30. The inflammatory cells likely participate in both ovulatory follicular rupture and resolution of the corpus luteum21.
While this study is unique in assessing the influence of peripheral blood CD4 lymphocyte counts on AMH levels, there is some indication that women with iatrogenic leukopenia may have reduced AMH levels. AMH levels rapidly decline during cancer chemotherapy14, though the individual contributions of ovarian injury and lymphocyte depletion have not been assessed. Lawrenz and colleagues compared AMH levels in 33 premenopausal systemic lupus erythematosis (SLE) patients with 33 age-matched controls, and found that AMH levels were lower in the SLE patients31. Because several of the commonly used therapies for SLE cause leukopenia, it is possible that the difference in AMH levels found in this study may be explained by the effects of lymphopenia rather than other effects of SLE. Indeed, Lawrenz observes that the drugs used to treat SLE are not known to reduce the number of ovarian follicles. Several studies have reported the rapid decline in AMH levels after initiation of cancer treatment in girls, with recovery among girls who received ovary-sparing treatments; the specific effect of leukocyte depletion was not examined in these studies32, 33}.
Our results indicate that the mechanism underlying the association of HIV infection with lower AMH levels is CD4 lymphocyte depletion: HIV causes CD4 cell depletion, which in turn causes reduced AMH levels. Unexpectedly, we found that once AMH levels were adjusted for CD4 cell count, HIV infection was associated with higher AMH levels, with suggestive evidence that this reversed relationship may be stronger in Caucasian women. The estimated effect of HIV when controlled for CD4 count is estimating how AMH levels would differ between HIV-infected and uninfected women who have identical CD4 counts. This association with HIV infection held regardless of the presence of viremia. HIV infection is closely associated with lymphocyte activation and increased production of inflammatory mediators34-36, which may be involved in ovarian signaling and granulosa cell function. Race differences in patterns of immune activation are well recognized, and could explain the possible race-based differences we observed in the relationship between HIV infection and increased AMH level. Race differences in immune response likely involve multiple mechanisms, but result in substantial differences in responses to anti-inflammatory treatment37, allograft outcomes in solid organ transplant38, the occurrence of autoimmune diseases39 and lymphoma.40
We considered the possibility that CD4 cell counts could have been influenced by both HIV (certainly true) and AMH, a so-called “collider stratification bias”. 41 We are not aware of any evidence or biological reasoning that supports a causal effect of AMH level on CD4 cell counts; this possibility appears to be less likely than the mechanisms noted above. Another scenario is that of unknown confounders that influence both CD4 count and AMH. Our inability to control for these could influence our estimated effect of CD4 on AMH and thereby distort the estimated effect of HIV on AMH when controlled for CD4. We are not aware of any likely candidates for such confounders, but this cannot be ruled out. Based on the above considerations, we believe that there is reasonable evidence that most or all of HIV's total association with lower AMH is due to its influence on CD4 cell counts and CD4 cells’ influence on AMH production, while a possible positive influence of HIV on AMH via other mechanisms is more speculative. AMH has been considered as a means to determine ovarian reserve in the setting of cancer chemotherapy and other treatments that may influence fertility.32, 33, 42, 43 Our findings indicate that leukocyte counts may need to be considered in the interpretation of AMH levels. For women who experience transient leukopenia, such as recipients of cyto-reductive chemotherapies, AMH levels should either be adjusted for CD4 cell counts or measured after leukocyte recovery.
Age was independently associated with lower AMH levels among both the HIV-infected and HIV -uninfected women in our study, a finding that is consistent with its relationship to age related ovarian follicle depletion demonstrated in many previous studies. The issue of whether exogenous hormones influence serum AMH levels has been debated in the literature, with some studies finding an effect and others not44-46. In our study, current use of hormonal contraceptives was independently associated with lower serum AMH levels among the larger group of HIV-infected women, while ever-use showed a much weaker association. This difference may provide a clue to the nature of the interaction between exogenous sex steroid and AMH, because hormonal contraceptives are used to treat some perimenopausal symptoms47, 48 such as menorrhagia49. The current users in our study were older, and perhaps more likely to be taking hormonal contraceptives to control perimenopausal symptoms than they might have been during use earlier in life.
We found that, when adjusted for age and other relevant factors, race altered the relationship between HIV infection and AMH. When other key variables were adjusted for, AMH levels were 50% higher among HIV-infected Caucasian women compared to HIV-uninfected Caucasian women, while HIV-infected African American women had a much more modest increase in AMH levels, relative to -uninfected women. Studies of AMH and reproductive aging tend to focus on Caucasian women, or do not report the race of participants. Our study benefitted from ethnically diverse enrollment, and thus could address how race influenced AMH levels. We previously reported the findings of a smaller WIHS study that found AMH levels were 25.2% lower in African American (p=0.037) and 24.6% lower in Hispanic women (p=0.063) than Caucasian women16 in analyses that controlled for age, body mass, HIV status, and smoking but not CD4 lymphocyte counts or hormonal contraception. This present study likely provides a more comprehensive picture of the factors that determine AMH levels, and supports the finding that women of European ancestry with HIV infection have higher AMH levels than other groups of HIV-infected women.
In this study self- reported amenorrhea was associated with 20-25% higher AMH levels, a finding which may seem paradoxical since amenorrhea is an indicator of the menopausal transition. However, amenorrhea is also commonly reported by women with polycystic ovary syndrome (PCOS). High AMH values are characteristic, and perhaps, diagnostic of PCOS; AMH levels were found to range from approximately 6-15ng per ml among women with diagnosed with PCOS according to standard criteria versus 1-3 ng per ml among women without PCOS 50, 51 a cut off AMH value of 5ng/ml has also been proposed52. In our study women who reported amenorrhea had a wide range in AMH values (0.09 to 28.8), indicating that some study participants who reported amenorrhea may have had PCOS. Thus women who report amenorrhea may be a mixed group including those entering the menopausal transition and women with PCOS.
Among the HIV-infected women, a history of weight loss, which is common in women with progressive HIV disease, was associated with 16% lower AMH levels. While AMH level should be independent of the energy imbalance factors that can influence gonadotropins such as FSH, weight loss in HIV is often concurrent with immunologic injury. Thus, the association of AMH decline and weight loss in this study may reflect altered but unmeasured CD4 cell functions or other factors. The association of BMI with AMH weakened when waist circumference was removed from the model in both uninfected and infected women. This suggests that BMI may represent overall adiposity beyond what is reflected by increased waist circumference, and when controlled for waist circumference it represents peripheral (noncentral) adiposity. BMI may be a marker of health in HIV-infected women, because depleted fat stores (lipoatrophy) would be associated with lower AMH due in part to lower CD4 cell counts. Additionally, increased waist circumference may be and indicator of polycystic ovary traits, a condition that is associated with increased AMH levels.
Our findings have important limitations. Our sample was primarily midlife, only 8% were under age 30 years. Thus our findings do not address younger women, whose ovarian function is likely to be more consistently robust. As in all observational cohort studies, there may be important unknown confounders for which we did not adjust or incompletely captured in our multivariable analysis. Some of the data, such as menstrual and medication histories, are self-reported, and thus subject to recall error. Additionally, we were unable to fully explain our unexpected finding that HIV-infected women had higher AMH levels than uninfected women after controlling for CD4 cell counts. However, a major strength of this study is that it utilizes an ethnically diverse sample that was not selected on the basis of infertility, menstrual pattern or known fertility. Another strength of the study is the availability of extensive data on risk factors and lymphocyte counts that enabled adjustment for potential confounders, which was our major aim. Furthermore, we measured AMH at multiple visits over time in our participants, which enabled us to compare age-related changes in HIV-infected and -uninfected women. Finally, AMH is more stable over the ovulatory cycle than other biomarkers of ovarian reserve and can be measured accurately even in women with irregular cycles or amenorrhea induced by chronic illness, which improves its utility as a biomarker of ovarian reserve and gonadal aging. The WIHS group recently reported a positive correlation between levels of Vitamin D and AMH among women in a subset of 141 women aged≥40 53, a finding that could shed light on the race differences we report in the interactions between AMH, HIV and CD4 cell count. Given the high prevalence of vitamin D deficiency in HIV-infected persons, future studies should investigate whether this deficiency may mediate the association of CD4 cell count with AMH levels.
In conclusion, we found that circulating numbers of CD4 cells strongly influenced serum AMH levels in both HIV-infected and -uninfected women, indicating that T helper cells may have an important role in ovarian granulosa cell function and follicle physiology. CD4 cell depletion appeared to explain the lower levels of AMH in HIV-infected versus -uninfected women. When adjusted for age and CD4 cell count, AMH levels were actually higher in HIV-infected women, particularly Caucasian women. The findings of this study suggest that immune factors such as lymphocyte subgroups, immune activation and host immune genetics impact ovarian follicle functions, and could lead to new directions of research. Serum AMH measurement could be a useful biomarker in chronically ill women, whose menstrual pattern may be unreliable. Interpretation of AMH levels and ovarian reserve may require measurement of CD4 cell counts in women whose illness or treatment results in perturbation of lymphocyte populations.
Supplementary Material
Acknowledgments
The authors thank Cheryl Felber, Adam Cheng and Niloufar Ameli for their technical support. We also thank Linda Giudice, Peter Gilbert, Victor De Gruttola, and Sander Greenland for their critical reads of the manuscript. Finally we thank the participants of WIHS for their many contributions.
Funding Sources
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. These funding sources had no involvement in the design or execution of this study.
Footnotes
Disclosure
G.M. received unrestricted research support from Beckman Coulter, Inc.
References
- 1.van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-mullerian hormone as a marker of ovarian reserve. The Australian & New Zealand journal of obstetrics & gynaecology. 2005;45:20–24. doi: 10.1111/j.1479-828X.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 3.Fanchin R, Taieb J, Mendez Lozano D, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–927. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 4.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–4063. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 5.La Marca A, De Leo V, Giulini S, Orvieto R, Malmusi S, Giannella L, Volpe A. Anti-Mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Investig. 2005;12:545–548. doi: 10.1016/j.jsgi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Seifer DB, Maclaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88:539–546. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 7.van Rooij IA, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, Themmen AP, te Velde ER. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 8.van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, Broekmans FJ. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93:2129–2134. doi: 10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- 9.Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF., Jr. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–3483. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16:797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- 11.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. The Journal of clinical endocrinology and metabolism. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH. Anti-Mullerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. The Journal of clinical endocrinology and metabolism. 2012;97:2059–2067. doi: 10.1210/jc.2011-3180. [DOI] [PubMed] [Google Scholar]
- 13.Morse H, Elfving M, Lindgren A, Wolner-Hanssen P, Andersen CY, Ora I. Acute onset of ovarian dysfunction in young females after start of cancer treatment. Pediatr Blood Cancer. 2013;60:676–681. doi: 10.1002/pbc.24327. [DOI] [PubMed] [Google Scholar]
- 14.Dillon KE, Sammel MD, Prewitt M, Ginsberg JP, Walker D, Mersereau JE, Gosiengfiao Y, Gracia CR. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99:477–483. doi: 10.1016/j.fertnstert.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifer DB, Golub ET, Lambert-Messerlian G, Springer G, Holman S, Moxley M, Cejtin H, Nathwani N, Anastos K, Minkoff H, Greenblatt RM. Biologic markers of ovarian reserve and reproductive aging: application in a cohort study of HIV infection in women. Fertil Steril. 2007;88:1645–1652. doi: 10.1016/j.fertnstert.2007.01.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seifer DB, Golub ET, Lambert-Messerlian G, Benning L, Anastos K, Watts DH, Cohen MH, Karim R, Young MA, Minkoff H, Greenblatt RM. Variations in serum mullerian inhibiting substance between white, black, and Hispanic women. Fertil Steril. 2009;92:1674–1678. doi: 10.1016/j.fertnstert.2008.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacon M, Von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young M. The women's interagency HIV study (WIHS): an observational cohort brings clinical sciences to the bench. Clin Diag Lab Immunol. 2005 doi: 10.1128/CDLI.12.9.1013-1019.2005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AIDSinfo: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. US PHS. 2012 [Google Scholar]
- 19.Thiebaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Comput Methods Programs Biomed. 2004;74:255–260. doi: 10.1016/j.cmpb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Best CL, Pudney J, Welch WR, Burger N, Hill JA. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod. 1996;11:790–797. doi: 10.1093/oxfordjournals.humrep.a019256. [DOI] [PubMed] [Google Scholar]
- 21.Hedin L. Invaders from the spleen: an unexpected origin of the leukocytes participating in ovulation. Endocrinology. 2010;151:4096–4099. doi: 10.1210/en.2010-0669. [DOI] [PubMed] [Google Scholar]
- 22.Tamba S, Yodoi R, Segi-Nishida E, Ichikawa A, Narumiya S, Sugimoto Y. Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. Proc Natl Acad Sci U S A. 2008;105:14539–14544. doi: 10.1073/pnas.0805699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledee N, Lombroso R, Lombardelli L, Selva J, Dubanchet S, Chaouat G, Frankenne F, Foidart JM, Maggi E, Romagnani S, Ville Y, Piccinni MP. Cytokines and chemokines in follicular fluids and potential of the corresponding embryo: the role of granulocyte colony-stimulating factor. Hum Reprod. 2008;23:2001–2009. doi: 10.1093/humrep/den192. [DOI] [PubMed] [Google Scholar]
- 24.Skinner MK, Schmidt M, Savenkova MI, Sadler-Riggleman I, Nilsson EE. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev. 2008;75:1457–1472. doi: 10.1002/mrd.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole DH, Pate JL. Luteal microenvironment directs resident T lymphocyte function in cows. Biol Reprod. 2012;86:29. doi: 10.1095/biolreprod.111.092296. [DOI] [PubMed] [Google Scholar]
- 26.Molyneaux KA, Schaible K, Wylie C. GP130, the shared receptor for the LIF/IL6 cytokine family in the mouse, is not required for early germ cell differentiation, but is required cell-autonomously in oocytes for ovulation. Development. 2003;130:4287–4294. doi: 10.1242/dev.00650. [DOI] [PubMed] [Google Scholar]
- 27.Wang LJ, Pascoe V, Petrucco OM, Norman RJ. Distribution of leukocyte subpopulations in the human corpus luteum. Hum Reprod. 1992;7:197–202. doi: 10.1093/oxfordjournals.humrep.a137616. [DOI] [PubMed] [Google Scholar]
- 28.Kryczek I, Frydman N, Gaudin F, Krzysiek R, Fanchin R, Emilie D, Chouaib S, Zou W, Machelon V. The chemokine SDF-1/CXCL12 contributes to T lymphocyte recruitment in human pre-ovulatory follicles and coordinates with lymphocytes to increase granulosa cell survival and embryo quality. Am J Reprod Immunol. 2005;54:270–283. doi: 10.1111/j.1600-0897.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 29.Oakley OR, Kim H, El-Amouri I, Lin PC, Cho J, Bani-Ahmad M, Ko C. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology. 2010;151:4551–4559. doi: 10.1210/en.2009-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C, Wu J, Borillo J, Torres L, McMahon J, Lou YH. Potential roles of a special CD8 alpha alpha+ cell population and CC chemokine thymus-expressed chemokine in ovulation related inflammation. J Immunol. 2009;182:596–603. [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrenz B, Henes J, Henes M, Neunhoeffer E, Schmalzing M, Fehm T, Kitter I. Impact of systemic lupus erythematosus on ovarian reserve in premenopausal women: evaluation by using anti-Muellerian hormone. Lupus. 2011;20:1193–1197. doi: 10.1177/0961203311409272. [DOI] [PubMed] [Google Scholar]
- 32.Morse H, Elfving M, Lindgren A, Wolner-Hanssen P, Andersen CY, Ora I. Acute onset of ovarian dysfunction in young females after start of cancer treatment. Pediatric blood & cancer. 2013;60:676–681. doi: 10.1002/pbc.24327. [DOI] [PubMed] [Google Scholar]
- 33.Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH. Anti-Mullerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab. 2012;97:2059–2067. doi: 10.1210/jc.2011-3180. [DOI] [PubMed] [Google Scholar]
- 34.Fevrier M, Dorgham K, Rebollo A. CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses. 2011;3:586–612. doi: 10.3390/v3050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas A, Zimmermann K, Oxenius A. Antigen-dependent and -independent mechanisms of T and B cell hyperactivation during chronic HIV-1 infection. J Virol. 2011;85:12102–12113. doi: 10.1128/JVI.05607-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.d'Ettorre G, Paiardini M, Ceccarelli G, Silvestri G, Vullo V. HIV-associated immune activation: from bench to bedside. AIDS Res Hum Retrovir. 2011;27:355–364. doi: 10.1089/aid.2010.0342. [DOI] [PubMed] [Google Scholar]
- 37.Federico MJ, Covar RA, Brown EE, Leung DY, Spahn JD. Racial differences in T-lymphocyte response to glucocorticoids. Chest. 2005;127:571–578. doi: 10.1378/chest.127.2.571. [DOI] [PubMed] [Google Scholar]
- 38.Padiyar A, Hricik DE. Immune factors influencing ethnic disparities in kidney transplantation outcomes. Expert Rev Clin Immunol. 2011;7:769–778. doi: 10.1586/eci.11.32. [DOI] [PubMed] [Google Scholar]
- 39.Mori M, Yamada R, Kobayashi K, Kawaida R, Yamamoto K. Ethnic differences in allele frequency of autoimmune-disease-associated SNPs. J Hum Genet. 2005;50:264–266. doi: 10.1007/s10038-005-0246-8. [DOI] [PubMed] [Google Scholar]
- 40.Koshiol J, Lam TK, Gridley G, Check D, Brown LM, Landgren O. Racial differences in chronic immune stimulatory conditions and risk of non-Hodgkin's lymphoma in veterans from the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:378–385. doi: 10.1200/JCO.2010.30.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, Poole C. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–420. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su HI. Measuring ovarian function in young cancer survivors. Minerva Endocrinol. 2010;35:259–270. [PubMed] [Google Scholar]
- 43.Dillon KE, Sammel MD, Prewitt M, Ginsberg JP, Walker D, Mersereau JE, Gosiengfiao Y, Gracia CR. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99:477–483. doi: 10.1016/j.fertnstert.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li HW, Wong CY, Yeung WS, Ho PC, Ng EH. Serum anti-mullerian hormone level is not altered in women using hormonal contraceptives. Contraception. 2011;83:582–585. doi: 10.1016/j.contraception.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying the effect of the combined oral contraceptive pill on the functional ovarian reserve as measured by serum anti-Mullerian hormone and the small antral follicle count made using three-dimensional ultrasound. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011 doi: 10.1002/uog.10114. [DOI] [PubMed] [Google Scholar]
- 46.Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception. 2010;81:245–248. doi: 10.1016/j.contraception.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin KA, Manson JE. Approach to the patient with menopausal symptoms. J Clin Endocrinol Metab. 2008;93:4567–4575. doi: 10.1210/jc.2008-1272. [DOI] [PubMed] [Google Scholar]
- 48.Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204–212. doi: 10.1016/j.maturitas.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Marret H, Fauconnier A, Chabbert-Buffet N, Cravello L, Golfier F, Gondry J, Agostini A, Bazot M, Brailly-Tabard S, Brun JL, De Raucourt E, Gervaise A, Gompel A, Graesslin O, Huchon C, Lucot JP, Plu-Bureau G, Roman H, Fernandez H, Francais CCNdGeO. Clinical practice guidelines on menorrhagia: management of abnormal uterine bleeding before menopause. Eur J Obstet Gynecol Reprod Biol. 2010;152:133–137. doi: 10.1016/j.ejogrb.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 50.Skalba P, Cygal A, Madej P, Dabkowska-Huc A, Sikora J, Martirosian G, Romanik M, Olszanecka-Glinianowicz M. Is the plasma anti-Mullerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol. 2011;158:254–259. doi: 10.1016/j.ejogrb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941–945. doi: 10.1210/jc.2005-2076. [DOI] [PubMed] [Google Scholar]
- 52.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, Duhamel A, Catteau-Jonard S. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 53.Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, Golub ET, Young M, Karim R, Greenblatt R, Minkoff H. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: Women's Interagency HIV Study. Fertil Steril. 2012;98:228–234. doi: 10.1016/j.fertnstert.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.