Abstract
The majority of prostate cancer (PCa) cases are diagnosed as a localized disease. Definitive treatment, active surveillance or watchful waiting are employed as therapeutic paradigms. The current standard of care for the treatment of metastatic PCa is either medical or surgical castration. Once PCa progresses in spite of castrate androgen levels it is termed ‘castration-resistant prostate cancer’ (CRPC). Patients may even exhibit rising PSA levels with possible bone, lymph node or solid organ metastases. In 2010, the only agent approved for the treatment of CRPC was docetaxel, a chemotherapeutic agent. It is now known that cells from patients with CRPC express androgen receptors (AR) and remain continuously influenced by androgens. As such, treatments with novel hormonal agents that specifically target the biochemical conversion of cholesterol to testosterone have come to the forefront. The use of cytochrome P450c17 (CYP17A1) inhibitor underlies one of the most recent advances in the treatment of CRPC. Abiraterone Acetate (AA) was the first CYP17A1 inhibitor approved in the United States. This review will discuss CRPC in general with a specific focus on AA and novel CYP17A1 inhibitors. AA clinical trials will be reviewed along with other novel adjunct treatments that may enhance the effectiveness of abiraterone therapy. Furthermore, the most recently identified CYP17A1 inhibitors Orteronel, Galeterone, VT-464, and CFG920 will also be explored.
Keywords: Abiraterone, Castration resistance, prostate cancer
INTRODUCTION
Since the discovery that Prostate Cancer (PCa) is influenced by androgen activity and could be treated by castration in 1941, the role of androgens in PCa has been the focus of studies and the basis of new therapies [1]. While enormous accomplishments have been made in the field, PCa remains the second most common cause of male cancer-related deaths in the United States [2]. Currently, 91% of men with PCa are diagnosed with localized, and mostly curable disease. However, a small percentage of men present with locally advanced disease, metastatic disease or have localized PCa that has become unresponsive to first line therapies [2, 3]. The primary treatment strategy for men with this type of advanced disease is Androgen Deprivation Therapy (ADT). Removal of androgens from the circulation is accomplished via surgical (bilateral orchiectomy) or medical (Gonadotropin Releasing Hormone agonist/antagonists or anti-androgens) modalities [4]. Unfortunately, ADT is rarely curative because only ~80% of patients respond, and the majority of ADT responders will inevitably progress to castration-resistant disease [5–7]. If left untreated, castration-resistant prostate cancer (CRPC) is invariably fatal within 9–12 months [7, 8].
Prior to 2010, the best treatment option for CRPC patients was chemotherapy with docetaxel in combination with prednisone – a regiment that yielded only a modest survival advantage of two to three months [9, 10]. Recent studies have found that CRPC actually remains dependent on androgen receptor (AR) signaling [11–14]. This discovery has led to the development of new strategies that inhibit AR signaling, thus diminishing CRPC growth. Indeed, by 2011, the United States Food and Drug Administration (FDA) approved Abiraterone Acetate (AA), the first specific inhibitor of the steroidogenic enzyme cytochrome P450, family 17, subfamily A, popypeptide 1 (CYP17A1 or P450c17). The basis for approval was several phase III clinical trials in men with CRPC that demonstrated an improved overall survival compared to placebo [11].
This review will discuss the role of androgens and the AR in PCa and the mechanisms by which men develop CRPC. Furthermore, specifically in regards to CYP17A1 inhibitors, the trials that led to the approval of CYP17A1 inhibitors, the pathways by which abiraterone exerts its effects, as well as novel CYP17A1 inhibitors currently under investigation will be reviewed.
ROLE OF ANDROGRENS AND AR IN PROSTATE CANCER
Androgens, through their effects of the AR, play a crucial role in cell proliferation and differentiation during prostate development. In normal prostatic tissue, cell growth and death are in equilibrium through the regulation of proliferation, differentiation and apoptosis [15]. The progression of prostatic epithelial cells into a malignant state involves the de-regulated expression of growth factors and their receptors, up-regulation of proto-oncogenes, and down-regulation of tumor suppression genes [16]. Furthermore, ARs are present in the majority of PCa cells and the relationship between carcinogenesis with androgens and ARs has been studied for decades [16]. In cells with AR, androgen stimulation enhances cellular proliferation through the increased expression of cyclin-dependent kinases 2 and 4 and decreased expression of the cyclin-dependent kinase inhibitor p16– resulting in cells entering the S phase of the cell cycle [15]. Additionally, androgen reduces apoptosis through the increased expression of p21, an anti-apoptotic factor, and by inhibiting caspase activation in the apoptosis pathways [17, 18]. Because the AR and its downstream signaling can tip the equilibrium towards proliferation, an increase in AR activity is one potential mechanism leading to androgen-dependent tumor-genesis or disease progression. In addition, chromosomal rearrangements are found in 70% of PCa, and the fusion of the androgen-regulated TMPRSS2 promoter to the oncogenic transcription factors ERG or ETV1 are indicated as early events in prostate carcinogenesis [13, 14]. ERG or ETV1 were overexpressed in 57% of PCa cases with over 90% of these cases demonstrating the gene fusion of ERG or ETV1 with TMPRSS2, implicating the fusions as the cause of overexpression [19]. Of note, AR can facilitate these fusions by inducing chromosomal proximity between TMPRSS2 and ERG or ETV1 and causing double stranded DNA breaks for subsequent recombination [13, 16]. Furthermore, chromosomal rearrangements have been detected in non-malignant prostate cancer epithelial cells after prolonged exposure to dihydrotestosterone (DHT) [20–22]. Thus, the formation of fusion proteins provides yet another potential mechanism for androgen-dependent tumor-genesis or disease progression. Due to the importance of androgen and AR in the development and progression of PCa, androgen deprivation continues to be the mainstay of therapy for advanced cancers and the target of future therapeutics.
CURRENT PCa TREATMENT PARADIGMS
Roughly 91% of the 234,460 men annually diagnosed with PCa in the United States will have localized disease [3]. These are men with T1 or T2 disease with no evidence of lymph node involvement or metastatic disease. For these men, a variety of surgical or radiotherapy options are available with treatment often being curative. Conservative management is considered for men with low-grade and small-volume tumors and/or a short (<10 years) life expectancy. Watchful waiting is best suited for patients not likely to benefit from treatment and involves forgoing treatment and providing palliative care if progression occurs [3]. Active surveillance involves following the patient for evidence of progression through combinations of periodic physical examinations, serum prostate specific antigen (PSA) testing or repeat biopsies with the initiation of treatment if progression occurs. Radical (curative) treatment options for these men include radical prostatectomy, interstitial brachytherapy, and external beam radiation [3].
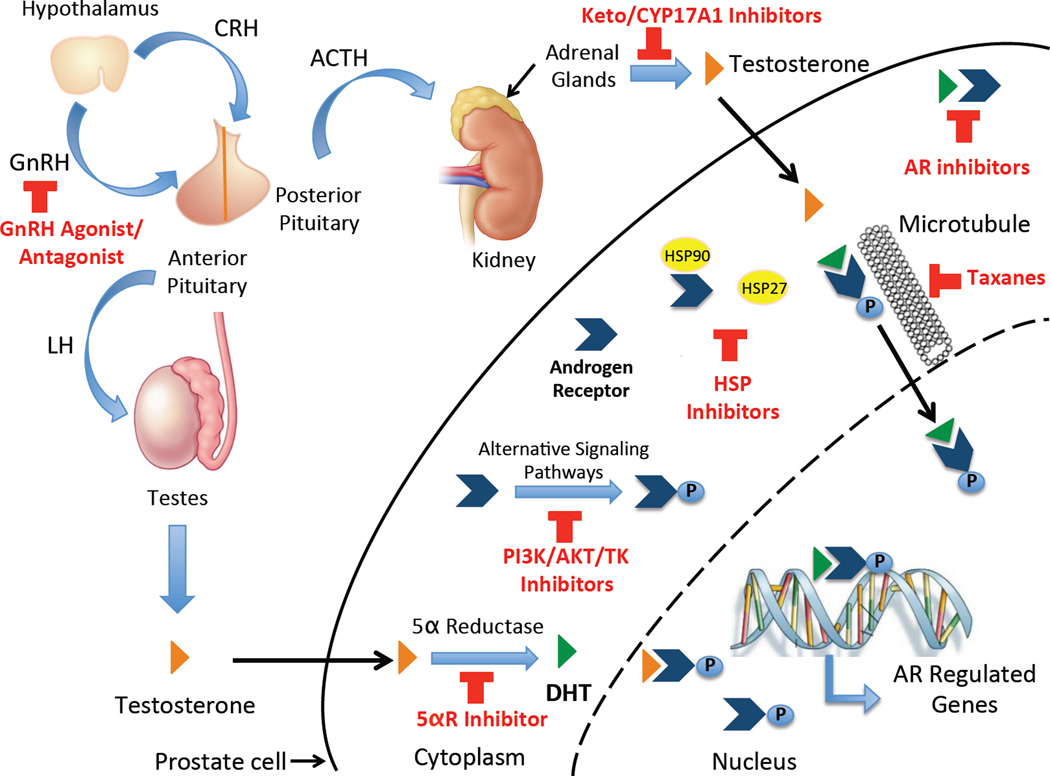
For patients with locally advanced disease (T3, evidence of cancer extension outside the prostate capsule) with no lymph node involvement and no metastatic disease, treatment includes medical ADT +/− radiotherapy. Finally, the standard of therapy for advanced disease is ADT either medically or surgically (used less frequently in the United States) [12]. Medical ADT options include GnRH agonists (i.e. leuprolide, goserelin, busererlin, triptorelin) or GnRH antagonists (i.e. degarelix) that both act at the level of the pituitary gland to reduce the release of gonadotropins leading to inhibition of androgen production (Figure 1) [23, 24]. These can be combined with anti-androgens (i.e. flutamide, bicalutamide, nilutamide) that competitively inhibit the AR from interaction with testosterone (T) and DHT (Figure 1) [12]. Unfortunately, this treatment option is not curative and following an initial response (decrease in PSA) there is an 80% risk of relapse, usually after a median of 24 months from ADT initiation. An increase in PSA after an initial response with ADT indicates disease progression, at which point the patient is diagnosed with CRPC [5–7].
Figure 1. Advanced prostate cancer treatment targets.
Gonadotropin releasing hormone (GnRH) agonist and antagonist inhibit the synthesis of androgens by blocking the release of gonadotropins from the pituitary. Ketoconazole (keto) and CYP17A1 Inhibitors block the synthesis of adrenal androgens to further decrease the amount of available testosterone (T) and other weak androgens that can potentially activate AR. In addition, 5α-reductase (5αR) inhibitors can be used to block the conversion of T to the more potent androgen, dihydrotestosterone (DHT), and AR inhibitors can prevent the binding of any remaining ligand. Phoshoinositide-3-Kinase (PI3K), Protein Kinase B (AKT), and Tyrosine Kinase (TK) inhibitors block alternative signaling pathways leading to activation of AR in the absence of ligand. Finally, heat shock protein (HSP) inhibitors are being studies as these can induce AR activation and nuclear localization independently of ligand (HSP90) and can protect cells against apoptosis (HSP27).
MECHANISMS OF PROGRESSION TO CRPC
Progression of PCa to CRPC involves a complex interaction between signaling molecules that collectively lead to a higher propensity for cell proliferation than cell death. It is important to recognize that while many mechanisms have been identified to cause tumor progression after ADT, they all lead to the activation of the AR signaling pathway. The six main categories for increased AR activation seen in patients with CRPC are as follows: 1) intracrine androgen production [25–27]; 2) AR amplification/overexpression [28, 29]; 3) AR gain of function mutations [30–32] 4) AR mRNA splice variants [33, 34], 5) changes in co-regulatory molecules [35, 36]; and 6) ligand independent AR activation by cytokines or through cross-talk with signaling pathways [37, 38].
Initially, it is important to recognize that while ADT reduces circulating levels of T and DHT, these androgens are present in PCa tissue at levels high enough to activate the AR [39]. While some of the remaining T may come from adrenal sources, the prostate and PCa cells can produce their own androgens from adrenal steroid precursors [40, 41]. When compared to primary tumors, tumors from patients with CRPC exhibit up-regulation of steroidogenic enzymes (i.e. CYP17A1 and CYP19A1) and genes that convert adrenal androgens to T, such the aldo-ketoreductase family 1, member C3 (AKR1C3), suggesting an increase in intracrine androgen production [25]. This is also supported by the fact that CRPC tumors are capable of intratumoral conversion of the precursor [14C] acetic acid to DHT through various steroid intermediates in both the classic and backdoor steroidogenic pathways [37].
Furthermore, CRPC tumors have a 6 fold increased expression of AR when compared to primary untreated tumors and BPH [29]. Amplification of the AR gene leading to an increased gene copy number is one potential mechanism causing an increase in AR. While no amplifications were identified in primary tumors (N=33) or BPH (N=8), amplifications were identified in 31% (4 of 13) of CRPC tumors through the use of flourescence in situ hybridization [28, 37]. In addition, variations in the AR, increasing its activity, are commonly found in CPRC. While AR mutations are only found in 8% of hormone-naïve PCa, they are present in 15–45% of CRPC [30, 32, 42]. Some mutations, present in the ligand-binding domain, can lead to a “promiscuous” AR that, in addition to having a higher affinity to DHT, can also be activated through the binding of many other ligands such as estrogen, progesterone, adrenal androgens, and even AR antagonists [30, 32]. Another gain of function mutation, AR-E255K, found in CPRC leads to increased AR protein stability and nuclear localization in the absence of ligand [31]. Moreover, CRPC has a high content of splice variants (ie. AR-V7/A3) that lacks a ligand-binding domain and remains constitutively active in the absence of ligand [34].
Co-activators that enhance and co-repressors that suppress AR activity further modulate transcription [19]. Two co-activators, transcriptional intermediary factor 2 (TIF2) and steroid receptor co-activator 1 (SCR1), can be overexpressed in CRPC leading to increased trans-activation upon binding of adrenal androgens without altered steroid affinities. Furthermore, trans-activation can be further increased by the phosphorylation of p160 co-activators [36]. Lastly, overexpression of the growth factor HER-2 has been shown in CRPC when compared to hormone-naïve PCa [43, 44]. In addition to enhancing the magnitude of AR response to low levels of androgens, HER-2 overexpression can activate the AR independently of ligand by stabilizing the AR and promoting DNA binding leading to androgen-independent prostate tumor growth [38, 45].
TREATMENT OF CRPC
All of the aforementioned adaptive mechanisms that occur in PCa cells following ADT have left clinicians with the difficult challenge of treating CRPC. A condition that, if left untreated, will ultimately be fatal within 9–12 months [7, 8]. Few treatment options are available, and until 2010, chemotherapy with docetaxel and prednisone was the only therapy proven to prolong life in patients with CRPC. Unfortunately, this treatment regiment was only successful in prolonging survival to ~19.2 months while resulting in major adverse events including nausea/vomiting, stomatitis, alopecia, neuropathy, anemia and neutropenia [46, 47]. In addition, prior to 2010 there were no FDA approved treatment options for patients who progressed following docetaxel treatment.
In 2010, the FDA approved a novel taxane, cabazitaxel, for use in patients with CRPC progressing after docetaxel treatment [48]. Around the same time, sipuleucel-T was approved for the treatment of asymptomatic, or minimally symptomatic metastatic CRPC. This novel medication was a therapeutic cancer vaccine created by activating autologous peripheral antigen-presenting cells ex-vivo with a prostate antigen, prostatic acid phosphatase. When compared to placebo, Sipuleucel-T had a 22% relative reduction in the risk of death (HR=0.78; 95%CI, 0.61 to 0.98) and an improved median survival of 4.1 months (25.8 months vs. 21.7 months in controls) [49].
For decades, ketoconazole, an antifungal that inhibits adrenal androgen synthesis, was used for the treatment of CRPC (Figure 1 and 2). While treatment with ketoconazole caused a 50% decrease in PSA in 20–60 % of patients, its use was “off label” since this response was transient (~4–6 months) and failed to demonstrate a survival benefit [50, 51]. In addition, despite a similar efficacy with the use of lower doses (200mg 3x/day vs. 400mg 3x/day), the clinical use of ketoconazole was limited due to its side effects (ie. hepatotoxicity and adrenal insufficiency) and multiple drug interactions [50, 51]. Despite this, the growing knowledge of the mechanisms underlying the antitumor effects of ketoconazole and the importance of androgens and AR signaling in the progression of CRPC lead to the development of several drugs rationally designed to target different components of this pathway [52–54].
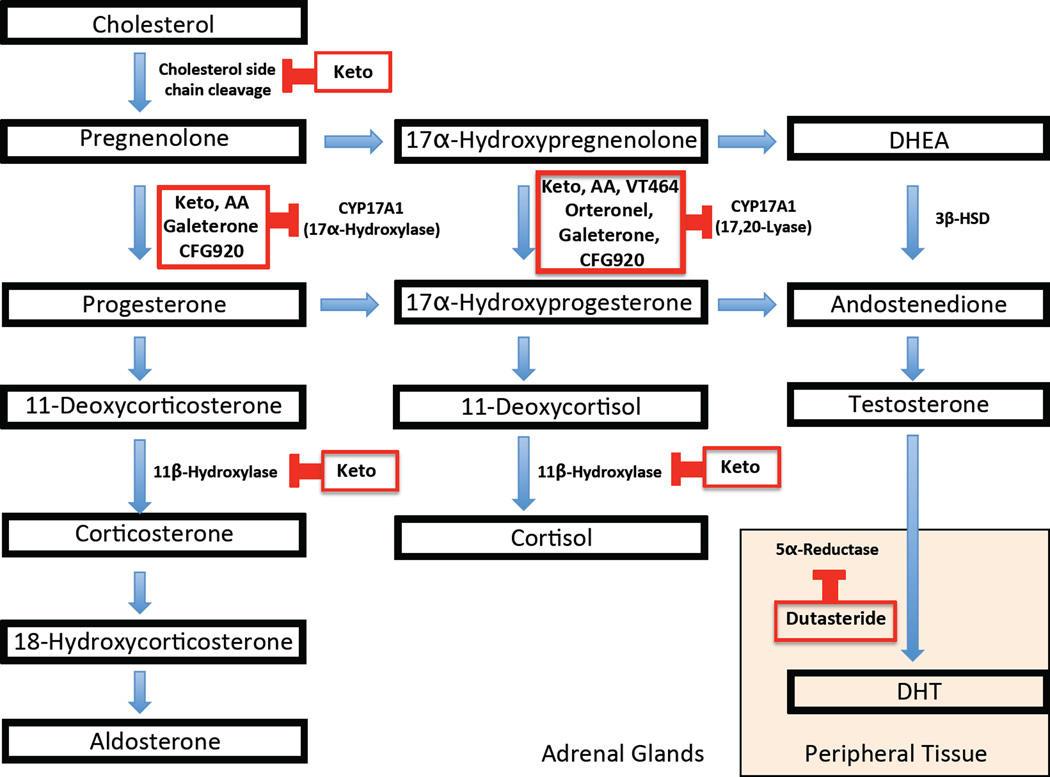
Figure 2. Steroid and Adrenal Androgen Synthesis Pathway.
Key steps for the synthesis of steroids and adrenal androgens are shown along with the enzymes inhibited by drugs used for treatment of CRPC including ketoconazole (Keto) and CYP17A1 inhibitors: Abiraterone acetate (AA), Orteronel (Ort), Galetertone (Gal), VT464, and CFG920. CYP17A1 catalyzes the conversion of pregnenolone to 17α-pregnenolone and progesterone to 17α-hydroxyprogesterone through its 17α-hydroxylase activity pulling steroids down the pathway for androgen synthesis. In addition, CYP17A1 catalyzes the final steps in androgen synthesis through its C17,20-lyase activity, converting 17α-hydroxypregnenolone to dehydroepiandrosterone (DHEA) and 17α -hydroxyprogestrone to androstenedione. The enzyme 3β-Hydroxysteroid Dehydrogenase (3β-HSD) is also important in androgen production as it catalyzes the conversion of DHEA to androstenedione.
The FDA approved AA, the first in this new generation of drugs, in 2011 for the use in patients with metastatic CRPC who failed docetaxel therapy. This indication was expanded in 2012 to patients with metastatic CRPC without prior docetaxel therapy. Most recently, in 2012, the FDA approved the use of enzalutamide for patients with metastatic CRPC who failed docetaxel therapy. Enzalutamide is a second-generation anti-androgen specifically designed to bind and inhibit the AR. A recent phase III clinical trial showed an overall survival benefit of 5.2 months when compared to placebo along with minimal adverse events [55].
The remainder of this review will focus on the development, physiology, and supportive clinical trials (efficacy) of CYP17A1 inhibitors including AA as well as four other drugs that are not currently FDA-approved: orteronel, galeterone, VT-464, and CFG920.
ABIRATERONE ACETATE DEVELOPMENT AND PHYSIOLOGY
While generalized ADT is effective at inhibiting the testicular production of T, the levels of T and DHT in CRPC remain high enough to activate AR signaling [39]. Although adrenal androgens account for 5–10% of circulating testosterone, another important source is the intracrine action of hormones from the prostate itself [40]. Indeed, prostatic tissue has the capacity to efficiently transform the inactive steroids DHEA-S, DHEA, and 4-dione into T and DHT without releasing them into circulation [41]. Furthermore, CRPC tissue may become more efficient at this function as suggested by the up-regulation of the steroidogenic enzymes CYP17A1, HSD3B1, HSD17B3, CYP19A1, and UGT2B17 [25]. Thus, there is a need to further suppress androgen synthesis is patients with CRPC.
As previously mentioned, ketoconazole has been used for the treatment of CRPC because it inhibits adrenal androgen synthesis at several steps in the steroidogenic pathway, and its weak inhibition of CYP17A1 mediates the desired antitumor effects seen [56, 57]. This particular enzyme catalyzes the conversion of pregnenolone to 17α -pregnenolone and progesterone to 17α -hydroxyprogesterone through its 17α-hydroxylase activity, and also catalyzes the final steps in androgen synthesis through its C17,20-lyase activity (Figure 2) [58]. However, ketoconazole also inhibits the first step of cholesterol cleavage that converts cholesterol to pregnenolone and the 11β–hydroxylase enzyme that catalyzes a critical step for cortisol synthesis, causing adrenal insufficiency requiring replacement doses of steroids in all patients, among other significant side effects [40] (Figure 2). Given ketoconazole’s poor selectivity, drug developers have recognized the need for more potent and selective adrenal inhibitors.
Along this vein, the chemists at The Institute of Cancer and Research (Sutton, Surrey, United Kingdom) developed a method to screen for small molecule chemical inhibitors of CYP17A1 by using radiolabeled CYP17A1 steroid substrates and identifying steroid products by high-performance liquid chromatography. This technique led to the development of abiraterone, a potent, selective, and irreversible inhibitor of CYP17A1 [59, 60]. The pro-drug (Abiraterone Acetate) was then developed to increase bioavailability after oral administration, which must be de-acetylated in vivo to its active form. Preclinical studies showed that AA had a 10–30 times greater inhibition of CYP17A1 than ketoconazole [60]. Further work in mice found that AA decreased androgenic steroids downstream of CYP17A1 and caused a decrease in the weights of the ventral prostate, testicles, and seminal vesicles [59].
In addition, predictions of the side effects were made on the basis of studies examining the effects of congenital CYP17A1 deficiencies. Patients with 17α-hydroxylase deficiencies have no production of cortisol and sex steroids. However, the low level of cortisol causes a compensatory increase in ACTH that drives the synthesis of 11-deoxycorticosterone (has mineralocorticoid activity) and corticosterone (a weak glucocorticoid) leading to mineralocorticoid excess [61]. In addition, cortisol production remains intact with defects in C17,20-lyase activity [62]. Thus, patients treated with AA were predicted to have mineralocorticoid excess versus the adrenal insufficiency seen with ketoconazole treatment. Given the mild toxicities predicted (ie. hypertension and edema seen with mineralocorticoid excess), a solid safety profile was developed and the medication progressed to human clinical studies.
CLINICAL TRIALS WITH ABIRATERONE ACETATE
The first human studies, conducted by O’Donnell et al. [63], were a series of phase I clinical trials that showed that a dose of 800 mg was sufficient to suppress T levels. However, this suppression was not sustained in all patients due to a compensatory rise in Luteinizing Hormone (LH). As such, it was suggested that higher levels of AA or concomitant use with GnRH agonists should be considered [63]. This initial study led to an open-label, dose-escalation phase I trial, published by Attard et al., that examined the safety, tolerability, and recommended dose in ketoconazole naïve, castrate men with CRPC [64]. Amongst the trial patients, AA was well tolerated with no treatment-related grade 3 or 4 toxicities. As expected, side effects included hypertension, hypokalemia, and lower-limb edema- all symptoms of secondary mineralocorticoid excess. These side effects were easily controlled with either eplerenone (a mineralocorticoid antagonist), or daily low-dose dexamethasone used to suppress ACTH production. Anti-tumor effects were observed through declines in PSA (of >50%) in 12 of 21 patients (57%). Partial radiographic responses were seen in 5 of 8 patients (62%) leading to symptom improvement and a reduction of analgesic use in 8 of 11 patients [64]. However, the noted improvements were temporary with resistance eventually developing. The addition of 0.5 mg dexamethasone once daily reversed this resistance in 4 of 15 patients who had progressed by Prostate Specific-Antigen Working Group (PSAWG) criteria. Additionally, due to the plateau of endocrine effects at doses greater than 750 mg, a dose of 1000 mg was chosen as the recommended dose for the expansion of this study to a Phase II trial [64].
This first phase II trial was an open-label, two-stage, single-arm trial examining the effects of daily administration of 1000 mg AA in chemotherapy-naïve CRPC patients who experienced progression after multiple treatments [59]. At the time of disease progression, patients were given dexamethasone (5 mg daily) in addition to AA. The treatment was well tolerated and side effects were consistent with the previous phase I study. Only two patients (5%) developed asymptomatic transaminase elevation that resolved upon treatment interruption. A decline in PSA (of ≥50%) was found in 67% of patients and a decline in circulating tumor cell counts was noted in 59% of patients [59]. Dexamethasone did not uniformly or permanently reverse the resistance to AA.
While 39 of the 54 patients were started on dexamethasone upon disease progression, only 30 had received the combination therapy for≥12 weeks by the end of the study and were therefore included in the statistical analysis. Eleven of these patients had received and progressed on dexamethasone prior to the study, while the others were dexamethasone naïve [59]. Consequently, the addition of dexamethasone also increased the median ‘time to PSA progression’ (TTPP) from 229 days (95% CI, 157 to 301 days) with AA treatment alone to 420 days (95% CI, 259 to 580 days) with the addition of dexamethasone upon disease progression [59]. These findings supported the need for combination therapy with AA and low-dose corticosteroids to maximize the efficacy and minimize the toxicity.
Another single-arm, open-label, multicenter phase II study in chemotherapy and ketoconazole-naïve patients with metastatic CRPC demonstrated the benefits of combining AA and low dose corticosteroids [65]. In this study, patients received AA (1000 mg daily) with prednisone (5 mg twice daily). All measured parameters were improved with PSA declines of ≥50% seen in 79% of patients with a median TTPP of 16.3 months [65]. Radiographic disease stability or improvement was also seen in 11 of 12 patients with measurable disease. In addition, the toxicities related to mineralocorticoid excess seen in previous studies were rare [65]. In 2010, two multicenter phase II clinical trials also demonstrated the efficacy of AA in patients with progressive metastatic CRPC post-docetaxel. Importantly, the safety profile of these studies was consistent with the results seen in trials with CRPC patients prior to docetaxel treatment [66, 67]. These promising results led to randomized and controlled phase III clinical trials.
One of these, COU-AA-301, was a multinational, randomized double-blinded, placebo controlled study in patients with metastatic CRPC after chemotherapy [68]. Enrolled patients (n=1,195) were randomized to receive AA plus prednisone (n=797) or placebo plus prednisone (n=398) [68]. Treatment with AA caused a 35.4% reduction in the risk of death and an increase in overall survival to 14.8 months from the 10.9 months seen with placebo. The treatment group also had a significantly longer TTPP (10.2 months vs. 6.6 months), longer progression-free survival (5.6 months vs. 3.6 months), and a higher PSA response rate (29% vs. 6%) [68]. The number of adverse events was not significantly different between the two groups [68].
The second trial, COU-AA-302, was also a multinational, randomized double-blinded, placebo controlled phase III trial. This trial was conducted in patients with metastatic CRPC who had mild cancer-related symptoms [69]. In this study 1,088 patients were enrolled and randomized to receive AA and prednisone (n=546) or placebo and prednisone (n=542) [69]. Treatment with AA improved overall survival (25% decreased risk of death) and a radiographic progression free survival of 16.5 months vs. 8.3 months on prednisone alone [69]. Further benefits of delayed cognitive decline, decreased opiate use and a prolongation until the initiation of chemotherapy all contributed to the FDA expanding the indications for AA in combination with prednisone to include men with metastatic CRPC prior to chemotherapy. In 2011, based on the results of a multitude of studies, the US Food and Drug Administration approved the use of AA with prednisone for the treatment of metastatic CRPC in patients who had failed previous docetaxel therapy.
ALTERNATIVE STRATEGIES TO ENHANCE AA THERAPY
Although previous studies demonstrated a clear and significant improvement with AA, the effects are finite, and disease progression will inevitably occur. To overcome this, future studies are required to ascertain mechanisms of resistance and solutions to overcome these pathways. One potential mechanism for AA resistance is via increased T/DHT by enzymes involved in steroidogenesis or through the use of an alternative pathway of DHT synthesis. Indeed, preclinical studies have shown increased CYP17A1 expression in xenographs treated with AA [70, 71]. Furthermore, an increase in AKR1C3 and HSD17B3 expression (involved in conversion of adrenal androgens into T) has been identified in tissues treated with AA [71]. A recent study examining T and DHT levels in the blood of PCa patients with bone metastases receiving AA found that T and DHT levels remained suppressed even after disease progression. This suggests that while AA remains active, a ligand-independent mechanism may be driving progression [72].
The AR overexpression seen in patients with CPRC is further enhanced following AA treatment, and some CRPC tumors show increased expression of the full length AR (ARFL) as well as AR splice variants [29, 34]. This is important since a 3–5 fold increase in AR is enough to compensate for low androgen levels by acting to sensitize cells to minute amounts of androgens while promoting AR-driven growth [71]. In addition, the formation of AR mutants also results in a widened affinity of the AR to other steroids and/or ligands. For example, steroids that are ‘upstream’ of CYP17A1 (i.e. progesterone) can activate the T877A mutant AR. Furthermore, the T877A mutant AR can be activated by other medications administered alongside AA including eplerenone (a mineralocorticoid antagonist) and exogenous glucocorticoids (i.e. prednisone) [70].
Several clinical studies have examined the inhibition of AR activation. Since increased AR levels may enhance response to low ligand concentrations, a further decrease of the most potent AR agonist, DHT, could help inhibit AR activation. This theory is currently under investigation using combination therapy of AA and dutasteride, a 5 alpha-reductase inhibitor (Figure 1, NCT01393730). While the addition of dutasteride will likely decrease DHT levels further, it may have little or no effect on AR signaling due to ligand-independent AR activation. Therefore, blocking AR activation is more likely to enhance the effects of AA and reduce resistance. Interestingly, this can be done with AA alone, as AA binds to and inhibits wild type and mutant AR at doses that are clinically achievable (2000 mg/day) [73]. Furthermore, higher doses of AA and its 3-keto metabolite also inhibit 3β-Hydroxysteroid Dehydrogenase (3β-HSD) at high concentrations, blocking the conversion of DHEA to androstenedione, and subsequently decreasing the synthesis of DHT (Figure 2) [74]. Thus, higher levels of AA may benefit patients with initial or acquired AA resistance, and a current trial examining the effects of these higher AA doses (2000 mg/day) in patients not responding to standard doses (NCT01637402) will put this issue to rest. Lastly, several trials are currently examining the combination of AA with AR antagonists including enzalutamide (NCT01946165/ NCT01650194/ NCT01949337) and the competitive AR antagonist, ARN-509 (NCT01792687). Results from these clinical trials are still pending.
Growth factors can activate AR in a ligand independent manner through the PI3K (phosphoinositide-3-kinase)/ AKT/ MAPK (mitogen-activated protein kinase) pathways via direct phosphorylation, and a high level of phosphorylated AKT in PCa tissue is a strong predictor of biological recurrence [75]. In addition, anti-androgen therapy leads to decreased AKT phosphatase activity, due to the loss of negative feedback, leaving higher levels of phosphorylated AKT to directly activate the AR [76]. As such, inhibition of any of the aforementioned pathways can improve the effects of AA. Several studies are currently being conducted to examine the efficacy of combining AA with: tyrosine kinase inhibitors (cabozantinib-NCT01574937, dasantinib- NCT01685125, sunitinib-NCT01254864), PI3K inhibitors (BEZ235-NCT01717898, BKM120-NCT01741753, both-NCT01634061), and AKT inhibitors (GDC-0980/GDC-0068- NCT01485861). These studies appear promising given that preclinical studies in prostate cancer cells and mouse models have shown improved responses with the combination of AR plus PI3K/AKT inhibitors versus monotherapy [76].
Heat Shock Proteins (HSPs) are important for chaperone-mediated folding of AR and stabilization of AR in the cytoplasm. In addition, HSP90 assists with ligand-independent AR nuclear translocation [77]. HSP27 is also of interest, given that its primary function is to protect cells from apoptosis, and it is uniformly overexpressed in patients with CRPC [78]. In vitro HSP90 inhibition leads a decrease in androgen-induced AR activation by disrupting the AR hormone-binding activity and stability, providing another potential mechanism for AR signaling disruption (Figure 1) [79]. Indeed, both in vitro and in vivo studies have shown inhibition of tumor growth following treatment with HSP inhibitors [80–82]. Currently, there are two ongoing clinical trials examining treatment of CRPC with AA in combination with either the HSP27 inhibitor OGX-427 (NCT01681433) or the HSP90 inhibitor, AT13387 (NCT01685268) (Figure 1).
Lastly, several clinical trials are examining combination therapy of AA with other chemotherapy drugs. Studies have shown that taxol chemotherapy has an AR-inhibitory effect that could potentially improve AA therapy [83]. To this effect, three clinical trials are examining the effects of combining AA with the taxanes docetaxel (NCT01400555) and cabazitaxel (NCT01511536, NCT01845792). Other trials combining AA with inhibitors of cellular proliferation like veliprarib (PARP inhibitor, NCT 01576172) and alisertib (Aurora A Kinase inhibitor, NCT01848067) or angiogenesis inhibitor AMG386 (NCT01553188) are being conducted. Finally, AA is also being combined with immunomodulation therapies like ipilimumab (human anti-CTLA-4, NCT01688492) and sipuleucel-T (an autologous cellular immunity therapy, NCT01487863) in hopes of developing further gains in the treatment of CRPC.
NOVEL CYP171A INHIBITORS
A more recently designed CYP17A1 inhibitor, Orteronel (TAK-700; Millennium Pharmaceuticals), was derived from naphthylmethylimidazole and formulated to have highly selective c17,20-lyase inhibition [84]. Preclinical in vitro (human and monkey adrenal cells) and in vivo (monkeys) studies have documented a 5.4 times more potent inhibition of c17,20-lyase than 17α-hydroxylase as well as a greater reduction in T and DHEA compared to cortisol [85]. The potential benefit for this greater c17,20-lyase inhibition is a reduction in mineralocorticoid excess and thus, a lowered requirement for corticosteroids. Preliminary results from phase I/II studies found a 63% PSA response rate at 12 weeks with patients given 300 mg twice daily. However, despite demonstrating lower 17α-hydroxylase inhibition in preclinical studies, the side effect profile for orteronel is similar to AA requiring concomitant use of corticosteroids [86]. Therefore, whether the c17,20-lyase selectivity seen in preclinical trials occurs in humans in vivo remains unknown. Furthermore, the benefits of orteronel over AA must be investigated further given their similarity in efficacy and side effects seen in the preliminary data. Currently, there are two ongoing phase III trials comparing orteronel with prednisone to placebo with prednisone in CRPC patients that have either progressed after docetaxel (NCT01193257) or are chemotherapy naïve (NCT01193244). In addition, a trial combining orteronel plus docetaxel had recently been completed (NCT01084655) with results pending and a trial combining orteronel with radiation therapy is currently recruiting (NCT01546987).
Galeterone (VN/124-1, TOK-001; Tokai Pharmaceuticals) is another CYP17A1 inhibitor derived from 3β-acetoxyandrost-5-en-17-one that also functions as an AR antagonist. In addition, a sustained increase in calcium from the endoplasmic reticulum (ER) has been observed with galeterone leading to phosphorylation of eIF2a, which inhibits the translation of several pro-growth genes (i.e. cyclin D1) [87, 88]. Pre-clinical studies have demonstrated that galeterone inhibits PCa cell growth, and causes marked suppression of PCa growth in mice to a greater degree than observed with castration alone [89]. A phase I trial showed a decrease in PSA of ≥30% in 49% of patients with only mild side effects like nausea, fatigue, diarrhea, and asymptomatic transaminase elevation [11]. At the time of this review, recruitment has commenced for a phase II trial in patients with CRPC (NCT01709734).
Finally, two additional drugs; VT-464 (Viamet Pharmaceuticals) and CFG920 (Novartis Pharmaceuticals), are in early stages of development. VT-464 is thought to be a selective c17,20-lyase inhibitor that is currently in phase I/II testing in men with CRPC (EudraCT number: 2011-004103-20). CFG920 is another CYP17A1 inhibitor and recruitment has begun for a phase I/II in men with CRPC (NCT01647789) [90].
CONCLUSION
This review has focused on CRPC and the novel therapies designed to treat this advanced stage of PCa. In 2010, the only agent approved for therapy was docetaxel; however, treatments with novel hormonal agents that specifically target the biochemical conversion of cholesterol to testosterone have recently come to the forefront. Specifically, the CYP17A1 inhibitor abiraterone has emerged as an important therapeutic modality in CRPC. Current clinical trials examining other novel CYP17A1 inhibitors, such as Orteronel and Galeterone, hint at the potential that these medications have in the treatment of men with CRPC.
HIGHLIGHTS.
Androgen receptors and the current treatment of prostate cancer will be reviewed.
Progression to castrate resistant prostate cancer (CRPC) and treatment are discussed.
Basics of abiraterone acetate (AA) physiology are highlighted with clinical trials summarized.
Alternative strategies to enhance AA therapy and novel CYP171A inhibitors are summarized.
Acknowledgments
Financial Support: JRK is a Male Reproductive Health Research Career (MHRH) Development Physician-Scientist Scholar (K12, HD073917-01) awarded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program to DJL. DJL also holds NIH grants P01HD36289 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development at the National Institutes of Health and 1R01DK078121 from the National Institute of Kidney and Digestive Diseases.
Abbreviations
- AA
abiraterone acetate
- ADT
androgen deprivation therapy
- CRPC
castration-resistant prostate cancer
- PCa
prostate cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. The Journal of urology. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. The Journal of urology. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. The New England journal of medicine. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 6.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. The New England journal of medicine. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 7.Eltayb A, Wadenberg ML, Schilstrom B, Svensson TH. Topiramate augments the antipsychotic-like effect and cortical dopamine output of raclopride. Naunyn-Schmiedeberg’s archives of pharmacology. 2005;372:195–202. doi: 10.1007/s00210-005-0014-5. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberger MA, Simon R, O’Dwyer PJ, Wittes RE, Friedman MA. A reevaluation of nonhormonal cytotoxic chemotherapy in the treatment of prostatic carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1985;3:827–841. doi: 10.1200/JCO.1985.3.6.827. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka K, Tazaki A, Kitayama A, Ueno N, Watanabe K, Mochii M. Identification of asymmetrically localized transcripts along the animal-vegetal axis of the Xenopus egg. Development, growth & differentiation. 2005;47:511–521. doi: 10.1111/j.1440-169X.2005.00826.x. [DOI] [PubMed] [Google Scholar]
- 10.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. The New England journal of medicine. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 11.Ferraldeschi R, Pezaro C, Karavasilis V, de Bono J. Abiraterone and novel antiandrogens: overcoming castration resistance in prostate cancer. Annual review of medicine. 2013;64:1–13. doi: 10.1146/annurev-med-121211-091605. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay AK, Leung HY. Signalling pathways in prostate carcinogenesis: potentials for molecular-targeted therapy. Clinical science. 2009;117:209–228. doi: 10.1042/CS20080391. [DOI] [PubMed] [Google Scholar]
- 13.Jagla M, Feve M, Kessler P, Lapouge G, Erdmann E, Serra S, et al. A splicing variant of the androgen receptor detected in a metastatic prostate cancer exhibits exclusively cytoplasmic actions. Endocrinology. 2007;148:4334–4343. doi: 10.1210/en.2007-0446. [DOI] [PubMed] [Google Scholar]
- 14.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer cell. 2009;16:458–462. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Lu S, Tsai SY, Tsai MJ. Regulation of androgen-dependent prostatic cancer cell growth: androgen regulation of CDK2, CDK4, and CKI p16 genes. Cancer research. 1997;57:4511–4516. [PubMed] [Google Scholar]
- 16.Djakiew D. Dysregulated expression of growth factors and their receptors in the development of prostate cancer. The Prostate. 2000;42:150–160. doi: 10.1002/(sici)1097-0045(20000201)42:2<150::aid-pros10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Lu S, Liu M, Epner DE, Tsai SY, Tsai MJ. Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Molecular endocrinology. 1999;13:376–384. doi: 10.1210/mend.13.3.0254. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, Markowski M, Bowen C, Gelmann EP. Androgen blocks apoptosis of hormone-dependent prostate cancer cells. Cancer research. 2001;61:5611–5618. [PubMed] [Google Scholar]
- 19.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 20.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin C, Yang L, Tanasa B, Hutt K, Ju BG, Ohgi K, et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009;139:1069–1083. doi: 10.1016/j.cell.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mani RS, Tomlins SA, Callahan K, Ghosh A, Nyati MK, Varambally S, et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science. 2009;326:1230. doi: 10.1126/science.1178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandow J, Von Rechenberg W, Jerzabek G, Stoll W. Pituitary gonadotropin inhibition by a highly active analog of luteinizing hormone-releasing hormone. Fertility and sterility. 1978;30:205–209. doi: 10.1016/s0015-0282(16)43461-8. [DOI] [PubMed] [Google Scholar]
- 24.Johnson ES, Gendrich RL, White WF. Delay of puberty and inhibition of reproductive processes in the rat by a gonadotropin-releasing hormone agonist analog. Fertility and sterility. 1976;27:853–860. doi: 10.1016/s0015-0282(16)41963-1. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer research. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer research. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 27.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer research. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 28.Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer research. 1999;59:803–806. [PubMed] [Google Scholar]
- 29.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer research. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 30.Buchanan G, Yang M, Harris JM, Nahm HS, Han G, Moore N, et al. Mutations at the boundary of the hinge and ligand binding domain of the androgen receptor confer increased transactivation function. Molecular endocrinology. 2001;15:46–56. doi: 10.1210/mend.15.1.0581. [DOI] [PubMed] [Google Scholar]
- 31.Steinkamp MP, O'Mahony OA, Brogley M, Rehman H, Lapensee EW, Dhanasekaran S, et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer research. 2009;69:4434–4442. doi: 10.1158/0008-5472.CAN-08-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. The New England journal of medicine. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 33.Bergerat JP, Ceraline J. Pleiotropic functional properties of androgen receptor mutants in prostate cancer. Human mutation. 2009;30:145–157. doi: 10.1002/humu.20848. [DOI] [PubMed] [Google Scholar]
- 34.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Hsu CL, Chang C. Androgen receptor corepressors: an overview. The Prostate. 2005;63:117–130. doi: 10.1002/pros.20170. [DOI] [PubMed] [Google Scholar]
- 36.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer research. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 37.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocrine-related cancer. 2008;15:841–849. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nature medicine. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 39.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 40.Salem M, Garcia JA. Abiraterone acetate, a novel adrenal inhibitor in metastatic castration-resistant prostate cancer. Current oncology reports. 2011;13:92–96. doi: 10.1007/s11912-011-0153-4. [DOI] [PubMed] [Google Scholar]
- 41.Labrie F. Adrenal androgens and intracrinology. Seminars in reproductive medicine. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- 42.Marcelli M, Ittmann M, Mariani S, Sutherland R, Nigam R, Murthy L, et al. Androgen receptor mutations in prostate cancer. Cancer research. 2000;60:944–949. [PubMed] [Google Scholar]
- 43.Osman I, Scher HI, Drobnjak M, Verbel D, Morris M, Agus D, et al. HER-2/neu (p185neu) protein expression in the natural or treated history of prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:2643–2647. [PubMed] [Google Scholar]
- 44.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. Journal of the National Cancer Institute. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 45.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 46.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 47.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. The New England journal of medicine. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 48.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 49.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 50.Harris KA, Weinberg V, Bok RA, Kakefuda M, Small EJ. Low dose ketoconazole with replacement doses of hydrocortisone in patients with progressive androgen independent prostate cancer. The Journal of urology. 2002;168:542–545. [PubMed] [Google Scholar]
- 51.Small EJ, Baron AD, Fippin L, Apodaca D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. The Journal of urology. 1997;157:1204–1207. [PubMed] [Google Scholar]
- 52.Mohler JL. A role for the androgen-receptor in clinically localized and advanced prostate cancer. Best practice & research Clinical endocrinology & metabolism. 2008;22:357–372. doi: 10.1016/j.beem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. The American journal of pathology. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schweizer MT, Antonarakis ES. Abiraterone and other novel androgen-directed strategies for the treatment of prostate cancer: a new era of hormonal therapies is born. Therapeutic advances in urology. 2012;4:167–178. doi: 10.1177/1756287212452196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semenas J, Dizeyi N, Persson JL. Enzalutamide as a second generation antiandrogen for treatment of advanced prostate cancer. Drug design, development and therapy. 2013;7:875–881. doi: 10.2147/DDDT.S45703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayub M, Levell MJ. Inhibition of testicular 17 alpha-hydroxylase and 17,20-lyase but not 3 beta-hydroxysteroid dehydrogenase-isomerase or 17 beta-hydroxysteroid oxidoreductase by ketoconazole and other imidazole drugs. Journal Vof steroid biochemistry. 1987;28:521–531. doi: 10.1016/0022-4731(87)90511-5. [DOI] [PubMed] [Google Scholar]
- 57.Higashi Y, Omura M, Suzuki K, Inano H, Oshima H. Ketoconazole as a possible universal inhibitor of cytochrome P-450 dependent enzymes: its mode of inhibition. Endocrinologia japonica. 1987;34:105–115. doi: 10.1507/endocrj1954.34.105. [DOI] [PubMed] [Google Scholar]
- 58.Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Seminars in reproductive medicine. 2004;22:281–288. doi: 10.1055/s-2004-861545. [DOI] [PubMed] [Google Scholar]
- 59.Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer research. 2009;69:4937–4940. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- 60.Bryce A, Ryan CJ. Development and clinical utility of abiraterone acetate as an androgen synthesis inhibitor. Clinical pharmacology and therapeutics. 2012;91:101–108. doi: 10.1038/clpt.2011.275. [DOI] [PubMed] [Google Scholar]
- 61.Yanase T, Imai T, Simpson ER, Waterman MR. Molecular basis of 17alpha-hydroxylase/17,20-lyase deficiency. The Journal of steroid biochemistry and molecular biology. 1992;43:973–979. doi: 10.1016/0960-0760(92)90325-D. [DOI] [PubMed] [Google Scholar]
- 62.Collett-Solberg PF. Congenital adrenal hyperplasia: from genetics and biochemistry to clinical practice, Part 1. Clinical pediatrics. 2001;40:1–16. doi: 10.1177/000992280104000101. [DOI] [PubMed] [Google Scholar]
- 63.O'Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. British journal of cancer. 2004;90:2317–2325. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 65.Ryan CJ, Shah S, Efstathiou E, Smith MR, Taplin ME, Bubley GJ, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4854–4861. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. The New England journal of medicine. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer research. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Efstathiou E, Titus M, Tsavachidou D, Tzelepi V, Wen S, Hoang A, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer research. 2012;72:2176–2182. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li R, Evaul K, Sharma KK, Chang KH, Yoshimoto J, Liu J, et al. Abiraterone inhibits 3beta-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:3571–3579. doi: 10.1158/1078-0432.CCR-12-0908. [DOI] [PubMed] [Google Scholar]
- 75.Ayala G, Thompson T, Yang G, Frolov A, Li R, Scardino P, et al. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:6572–6578. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- 76.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saporita AJ, Ai J, Wang Z. The Hsp90 inhibitor, 17-AAG, prevents the ligand-independent nuclear localization of androgen receptor in refractory prostate cancer cells. The Prostate. 2007;67:509–520. doi: 10.1002/pros.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rocchi P, So A, Kojima S, Signaevsky M, Beraldi E, Fazli L, et al. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer research. 2004;64:6595–6602. doi: 10.1158/0008-5472.CAN-03-3998. [DOI] [PubMed] [Google Scholar]
- 79.Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell stress & chaperones. 2002;7:55–64. doi: 10.1379/1466-1268(2002)007<0055:eogoar>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burger AM, Fiebig HH, Stinson SF, Sausville EA. 17-(Allylamino)-17-demethoxygeldanamycin activity in human melanoma models. Anti-cancer drugs. 2004;15:377–387. doi: 10.1097/00001813-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Kelland LR, Sharp SY, Rogers PM, Myers TG, Workman P. DT-Diaphorase expression<and tumor cell sensitivity to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat shock protein 90. Journal of the National Cancer Institute. 1999;91:1940–1949. doi: 10.1093/jnci/91.22.1940. [DOI] [PubMed] [Google Scholar]
- 82.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:986–993. [PubMed] [Google Scholar]
- 83.Gan L, Chen S, Wang Y, Watahiki A, Bohrer L, Sun Z, et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer research. 2009;69:8386–8394. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaku T, Hitaka T, Ojida A, Matsunaga N, Adachi M, Tanaka T, et al. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorganic & medicinal chemistry. 2011;19:6383–6399. doi: 10.1016/j.bmc.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 85.Yamaoka M, Hara T, Hitaka T, Kaku T, Takeuchi T, Takahashi J, et al. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. The Journal of steroid biochemistry and molecular biology. 2012;129:115–128. doi: 10.1016/j.jsbmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Zhu H, Garcia JA. Targeting the adrenal gland in castration-resistant prostate cancer: a case for orteronel, a selective CYP-17 17,20-lyase inhibitor. Current oncology reports. 2013;15:105–112. doi: 10.1007/s11912-013-0300-1. [DOI] [PubMed] [Google Scholar]
- 87.Bruno RD, Gover TD, Burger AM, Brodie AM, Njar VC. 17alpha-Hydroxylase/17,20 lyase inhibitor VN/124-1 inhibits growth of androgen-independent prostate cancer cells via induction of the endoplasmic reticulum stress response. Molecular cancer therapeutics. 2008;7:2828–2836. doi: 10.1158/1535-7163.MCT-08-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vasaitis T, Belosay A, Schayowitz A, Khandelwal A, Chopra P, Gediya LK, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Molecular cancer therapeutics. 2008;7:2348–2357. doi: 10.1158/1535-7163.MCT-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Handratta VD, Vasaitis TS, Njar VC, Gediya LK, Kataria R, Chopra P, et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. Journal of medicinal chemistry. 2005;48:2972–2984. doi: 10.1021/jm040202w. [DOI] [PubMed] [Google Scholar]
- 90.Yin L, Hu Q, Hartmann RW. Recent progress in pharmaceutical therapies for castration-resistant prostate cancer. International journal of molecular sciences. 2013;14:13958–13978. doi: 10.3390/ijms140713958. [DOI] [PMC free article] [PubMed] [Google Scholar]