Abstract
Bacterial pathogens employ a myriad of strategies to alter host tissue cell functions for bacterial advantage during infection. Recent advances revealed a fusion of infection biology with stem cell biology by demonstrating developmental reprogramming of lineage committed host glial cells to progenitor/stem cell-like cells by an intracellular bacterial pathogen Mycobacterium leprae. Acquisition of migratory and immunomodulatory properties of such reprogrammed cells provides an added advantage for promoting bacterial spread. This presents a previously unseen sophistication of cell manipulation by hijacking the genomic plasticity of host cells by a human bacterial pathogen. The rationale for such extreme fate conversion of host cells may be directly linked to the exceedingly passive obligate life style of M. leprae with a degraded genome and host cell dependence for both bacterial survival and dissemination, particularly the use of host-derived stem cell-like cells as a vehicle for spreading infection without being detected by immune cells. Thus, this unexpected link between cell reprogramming and infection opens up a new premise in host-pathogen interactions. Furthermore, such bacterial ingenuity could also be harnessed for developing natural ways of reprogramming host cells for repairing damaged tissues from infection, injury and diseases.
Background
The body’s lineage-committed differentiated tissue cells are the residence of many bacterial pathogens that cause numerous human diseases. These pathogens often establish infection in their preferred niches by manipulating or subverting differentiated cell functions [1,2]. However, to accomplish these daunting tasks bacterial pathogens must fulfill several criteria [1,3]. For intracellular bacteria, many additional challenges and careful orchestrations are necessary to evade host immune attack, sustain bacterial survival and promote dissemination. Therefore, intracellular bacteria usually take precautions and reside within their favorable host niches for colonization and to gain full advantage of properties their preferred host cells offer. Although tissue niches with limited immune cell traffic are safe haven for propagation of intracellular bacteria, their dissemination, the next critical step of bacterial life cycle after colonization, particularly via systemic routes is challenging due to bacterial confinement to their specialized tissue niches. Better understanding of how intracellular bacteria overcome such challenges and pass infection to other tissues provide new tools for targeting the progression of bacterial infections.
New research continues to identify specific host cell functions and pathways that are required for many different bacterial pathogens during their infectious processes [4,5,6,7,8]. Developing strategies that target the critical host cell functions required for infection would have broad-spectrum efficacy and much less likelihood to permit pathogens to acquire resistant mutation and become drug resistant. Thus, usage of host-encoded functions essential for infection could be particularly timely, since the emergence of drug-resistant bacterial strains is a major concern for public health [9,10]. However, tackling such host-encoded functions as strategies for combating infection is challenging, since diverse pathogens use different tactics for their survival and propagation. Although tailor-made strategies for targeting individual pathogens with specific host requirements are possible, it is more beneficial and cost effective if we are able to identify common molecular host targets or pathways that can be applied to many bacterial pathogens simultaneously. Because pathogens are co-evolved alongside hosts with many common or evolutionary conserved strategies for cell manipulation, discovery of novel host cell modifying mechanisms from model organisms provide new insights into host-encoded functions that could be shared with many bacterial pathogens. It is likely that potentially effective common host-encoded functions can be identified from those bacterial pathogens, which are known to depend substantially or totally on host cell functions for every phase of their bacterial life cycle. Mycobacterium leprae, the causative organism for human leprosy, is one such intracellular pathogen that totally depends on host cells for maintaining bacterial survival and propagation [11], and thus could be a model organism for identifying both novel and common host-encoded functions.
One common property of host cells is the genomic plasticity, the extent to which host cells can alter their transcriptome in such a manner that allows these cells to adapt to changes in microenvironment [12]. Plasticity exists in adult tissue cells to varying degrees and this property is responsible for natural repair processes following tissue damage, often due to endogenous stem/progenitor cell populations [13,14]. It is now known that indeed adult tissue cell plasticity can be manipulated experimentally by changing expression of genes to reprogram somatic cells back to embryonic stage or change lineage commitment both in vitro and in vivo [15,16,17]. Plasticity of host cells can also be subjected to manipulation by intracellular bacterial pathogens. In this review, we describe how bacterial pathogens hijack plasticity of tissue cells to manipulate host cells during infection using ML and its preferred host niche, Schwann cells, as a model system. We also briefly discuss the implications of these findings for bacterial infectious diseases in general, and how such bacterial ingenuity can be employed as a potential strategy for converting somatic cells to stem cell-like cells for tissue regeneration.
Experimental manipulation of host cell plasticity
During mammalian development embryonic cells undergo a highly complex developmental program by acquisition, deletion or maintaining multiple transcriptional, epigenetic, and signaling programs to acquire various lineage-committed cell types of distinct functions. Although such terminally differentiated cells are stable in terms of operating their lineage committed programs in order to maintain the identity and specific cell functions in different tissues, recent advances have revealed that these committed programs of adult tissue cells are remarkably plastic and can easily be manipulated experimentally [12,16,18]. New areas of investigation for cell fate manipulation have rapidly evolved by the success of ectopic expression or deletion of critical genes required for either maintaining embryonic state or specific tissue lineage development, which are sufficient for reprogramming developmentally committed tissue cells all the way back to embryonic stem cells or converting into other cell phenotypes [19,20]. These revelations have attracted a wide range of interest as a strategy for cell manipulations which can eventually be harnessed for use in regenerative medicine, and also as research tools to gain new insights into basic developmental processes [21].
Hijacking notable plasticity of adult Schwann cells by M. leprae
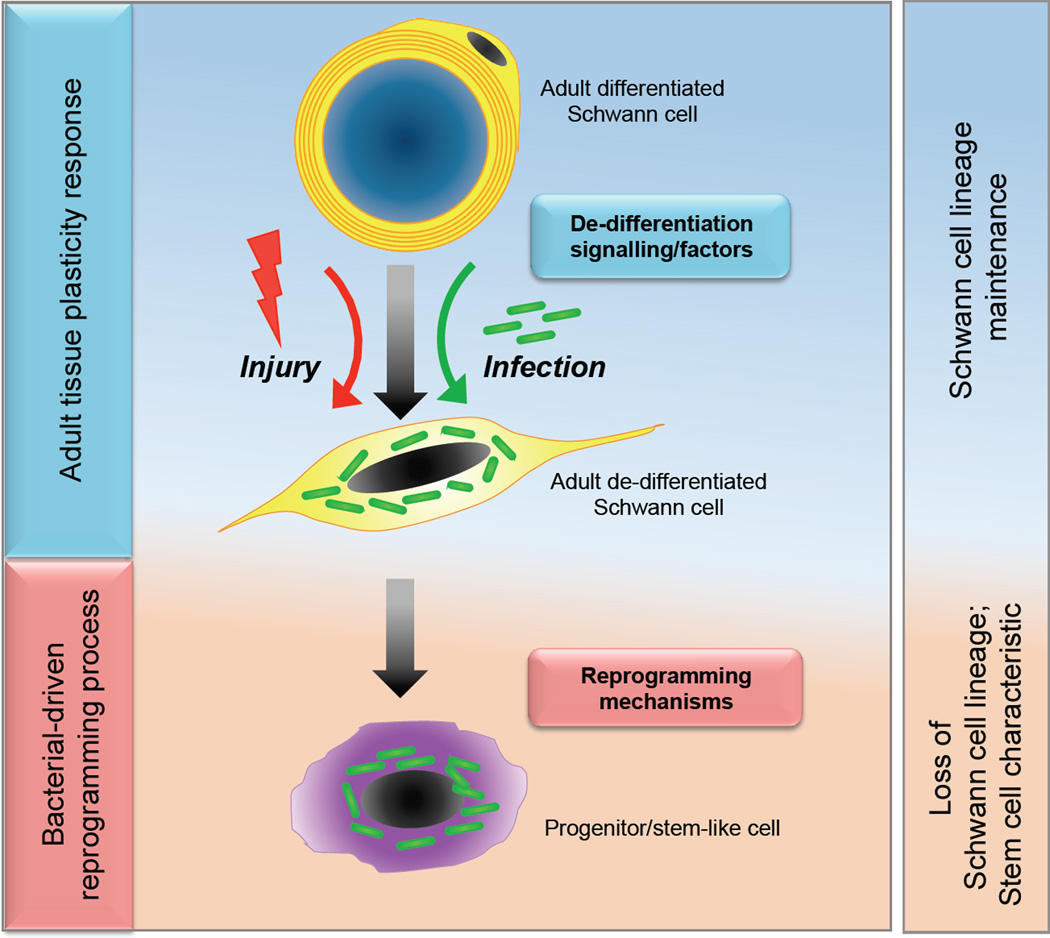
Among differentiated tissue cells, Schwann cells, the glial cells of the adult peripheral nervous system (PNS), which derive from neural crest precursors and comprise myelin-forming and non-myelin-forming phenotypes [22,23], can be considered as an example of sophistication in cell differentiation. Yet they show remarkable plasticity illustrated by the ability of adult Schwann cells to switch between differentiated and de-differentiated states following nerve injury [24,25]. In response to injury-induced signalling, myelinated Schwann cells switch off the myelination program following loss of axonal contact and acquire a phenotype resembling immature Schwann cells, re-enter cell cycle and de-differentiate (Fig. 1). These de-differentiated Schwann cells in turn promote regeneration of axons and the myelin sheath, and in this manner adult Schwann cell plasticity contributes to the regeneration capacity of adult PNS even after severe injury [26].
Figure 1. Setting the stage for host cell reprogramming: from terminal differentiation to de-differentiation and to stem-like cells.
M. leprae hijack the innate ability of adult Schwann cell plasticity - switching off the myelin program and adopt a de-differentiated program- for de-differentiation by activating receptor-mediated signalling pathways (top). Unlike in the injury process that re-differentiates de-differentiated Schwann cells back to the terminally differentiated state for completion of the nerve repair process, M. leprae infection (green rods) drives de-differentiated Schwann cells further out of lineage and to an immature state generating stem cell-like cells for bacterial advantage (bottom).
Intriguingly, leprosy bacteria use adult Schwann cells with this notable plasticity as the natural primary target for the establishment of infection within the PNS. ML is a strictly obligate intracellular pathogen with a severely degraded bacterial genome, unable to generate its own energy and metabolic needs fully and thus depends totally on host cell functions for bacterial survival [11]. By selecting Schwann cells, ML have acquired several survival advantages [11,27]. Recent studies have suggested that ML use the regeneration properties of the PNS for the expansion of the bacterial niche within Schwann cells [27,28,29].
Setting the stage: from terminal differentiation to de-differentiation
In adult peripheral nerves, Schwann cells are developmentally matured and have acquired the stage of terminal differentiation necessary for fully functional nerves. ML ‘simplifies’ this sophisticated terminal differentiation by initiating myelin damage pathways and inducing cells to re-enter the cell cycle by activation of canonical and non-canonical Erk1/2 signalling pathways [29,30]. In fact, this was the first example to show that demyelination can be caused by activating Schwann cell Erk1/2 signalling without nerve lesions or inflammatory responses [28,29]. Subsequent studies using transgenic mouse models in which Erk1/2 signalling is sustained in adult PNS have further confirmed that, indeed, like in infection with ML, transgenic activation of Erk1/2 signalling in adult nerves could also elicit demyelination without lesions [31].
As in experimentally induced or natural nerve injury responses, ML-induced demyelination also generates de-differentiated cells [28; Fig. 1]. These Schwann cells maintain their lineage commitment in an immature state lacking the myelin sheath, and are usually equipped with properties for promoting remyelination of damaged nerves [32]. Interestingly, such de-differentiated Schwann cells are highly susceptible for ML invasion and are also likely to be a more favourable phenotype for intracellular bacterial growth [27]. Additionally, these Schwann cells also serve as safe haven for ML, since the PNS blood-nerve barrier protects the organism from host immune assault [33,34], and thus initial ML propagation in Schwann cells is likely to occur progressively due to lack of resistance from immune cells. Such favorable conditions, which are assisted with the nontoxic, non-cytopathic, non-apoptotic nature of ML, permit bacterial residence within Schwann cells for a long period with moderate cell proliferation, but without causing any cell transformation or apoptosis [27,30,35]. Thus, it is likely that once invaded, ML maintain infected de-differentiated Schwann cells in a viable and active state so that essential host factors necessary for bacterial survival can be properly secured. Recent studies have shown that the fate of Schwann cells following such uniquely compatible host-pathogen adaptation is the reversal of the developmental program of these lineage-committed Schwann cells [36].
Modeling the fate of infected Schwann cells
Considering the total host cell dependence for driving the bacterial life cycle to establish a productive infection, it is likely that ML has evolved to further sophisticatedly manipulate adult de-differentiated Schwann cells for bacterial advantage. However, in the case of human infection, it is unknown how long ML reside in de-differentiated Schwann cells, since these cells usually re-differentiate back to myelinated and non-myelinated Schwann cells in vivo, as in nerve injury [32; Fig. 1]. It is possible that ML occupy de-differentiated cells for a transient period until infection establishes within their preferred non-myelinated Schwann cells, as demonstrated in nerve biopsies from leprosy patients with high bacterial load [33].
As in nerve injury-like responses, it is inevitable that initial human infection of adult peripheral nerves undergoes a similar de-differentiation program because ML overtime produce highly unfavorable conditions that deregulate the well-regulated Schwann cell-axon communication system essential for maintaining functional PNS [27,28]. This signals terminally differentiated Schwann cells to generate de-differentiated cells as a part of natural property of plasticity, which is hijacked by ML. Although this early stage of ML infection cannot be studied in humans as there is no justifiable evidence for clinical manifestation of infection or nerve damage in affected individuals, these events can be recapitulated in model systems since plasticity of adult PNS and de-differentiation program following nerve injury or injury-like responses is highly conserved in human, rodents and other mammals including the natural animal host of ML, the nine-banded armadillo [37,38]. Indeed, new evidence suggests that nerve damage can be recapitulated in armadillo models by systemic infection with ML [38].
Infection reprograms committed Schwann cell fate to stem cell-like stage
Recent studies have recapitulated the early events of ML infection in a mouse model in order to understand the fate of these de-differentiated Schwann cells in response to long-term exposure to intracellular bacteria. Masaki et al. isolated de-differentiated Schwann cells from adult wild-type and Sox2-GFP transgenic mice after injury and separated Schwann cells from axons, which spontaneously generated de-differentiated Schwann cells. They then purified cells by FACS sorting using an antibody to Schwann cell surface marker p75NTR or GFP expression under the control of Sox2 promoter, respectively. The latter was important because it shows that isolated cells are GFP+/Sox2+, which is expressed in adult peripheral nerves only after Schwann cells undergo de-differentiation following injury [39]. Although Sox2, which is a stem cell marker (16), is expressed transiently in these adult de-differentiated cells in vivo they are lineage committed Schwann cells marked by master regulators of Schwann cell lineage such as Sox10 (22; Fig. 1). By using both purified pool and clonal de-differentiated Schwann cells infected with ML, Masaki et al showed that infected Schwann cells, as compared to uninfected/control cells, gradually ‘turn off’ Schwann cell differentiation/myelination- and lineage-associated genes and ‘turn on’ numerous developmental genes. The latter comprises mostly the mesoderm development including homeodomain/Hox, EMT (epithelial mesenchymal transition), as well as neural crest related genes, such as Hox-d, -a, -b gene clusters, Twist, Snai and Msx2 transcripts [36]. These findings suggested that ML gradually shut down the Schwann cell differentiation program and lead lineage-committed Schwann cells to a highly immature stage resembling progenitor/stem cell-like cells (pSLC). Indeed, properties of the stem cell-like phenotype and behavior of pSLC have been shown based on their reprogrammed stem cell-like transcriptome and the capacity to re-differentiate into multiple tissue types including bones, smooth muscles, skeletal muscle and adipocytes [36].
Conversion of infected Schwann cells to an early neural/mesoderm development program
A major tissue remodeling program that is central to early mesoderm development during embryogenesis is epithelial-mesenchymal transition (EMT), which is orchestrated by a number of developmentally regulated transcription factors (TFs) and generates cells that act as progenitors of different mesenchymal tissues [40]. Interestingly, master regulators of EMT, Twist-1, -2, Snail2 and Msx2, which are capable of inducing EMT in epithelial cells and converting them to mesenchymal stem-like phenotypes [41] , were among the highly upregulated TF genes in ML infected Schwann cells (36). Interestingly, these key EMT genes such as Twist and Snail1/2 are also expressed in neural crest stem cells [42]. Demethylation of the promoter region of Twist1 in these reprogrammed cells further suggests the change in cell fate accompanies a change in epigenetic status. It is possible that ML hijack an EMT-like process in de-differentiated Schwann cells and change the cell fate to a neural crest/mesenchymal phenotype. However, these ML-driven cell fate changes in Schwann cells are highly complex as infection involves upregulation of multiple developmental genes of both mesenchymal and neural crest associated genes. Also of particular interest is the modulation of TFs of the homeodomain/Hox family and Sox family in Schwann cells in response to ML. It is known that the fate of somatic cells can be altered by forced expressions of Hox genes [43]. On the other hand, Sox2, as described above, is a critical TF involved in maintaining embryonic stem (ES) stem cells, neural stem cells and neural crest stem cells, as well as one of the factors required for reprogramming fibroblasts to induced pluripotent stem (iPS) cells with ES cell-like properties [16, 44,45,46,47], suggesting good reasons why ML target these embryonic TFs during Schwann cell reprogramming. An important finding is that these ML-induced events are not associated with the tumor suppressor genes like p53 or Schwann cell tumor associated gene NF1, which is inactivated in neurofibromatosis type 1, suggesting reprogramming events in Schwann cells are not associated with tumor formation [36]. Indeed, previous studies using human primary adult Schwann cells infected with ML have shown that infected human cells do not undergo any transformation even after long-term incubation despite moderate cell proliferation and lack of apoptosis induction [30, 35].
Bacterial strategy: keeping the wanted and removing the unwanted
In addition to the activation of developmental genes, silencing regulators of Schwann cell lineage/differentiation may also be critical for reprogramming Schwann cells out of the lineage and to the pSLC state. One key early event in reprogramming is the bacterial-induced removal of nuclear Sox10 [36], the master regulator of Schwann cell lineage, identity and differentiation/myelination [48]. Genes encoding myelin proteins that provide a unique status for differentiated Schwann cells are direct targets of Sox10, and the down regulation of myelin genes during ML infection may be associated at least partly with nuclear Sox10 removal [22, 48]. This bacterial strategy may be of significant advantage for ML propagation, since the myelin sheath occupation within almost the entire Schwann cell cytoplasm is unfavorable for ML, which reside strictly within the cytoplasm for bacterial multiplication. Nuclear Sox10 removal accompanies the silencing of Sox10, which is directly correlated with highly significant DNA methylation of Sox10 promoter region in reprogrammed cells, suggesting epigenetic regulation of Sox10 mediated by intracellular ML.
On the other hand, sustaining nuclear Sox2 expression is critical for maintaining infected Schwann cells in the de-differentiated state or perhaps promoting to the pSLC stage [36]. Because Sox2 is also a negative regulator of myelination [39,44,49], maintaining Sox2 expression, which is otherwise expressed only transiently in adult peripheral nerves after injury (39), in infected cells is critical not only for down-regulating differentiation but also retaining Schwann cells at the de-differentiated stage. However, it is the removal of Sox10 and concomitant expression of Sox2 together with other developmental TFs that are likely to be the initial driving force for reprogramming of Schwann cells to pSLCs. Indeed, Sox factors and their partner proteins can play sequential roles in development [50], and similarly their interplay at target genes could also tip the balance of cellular maturity. Perhaps whilst the normal injury environment favors re-differentiation after injury, mediated with the help of Sox10 when conditions are appropriate, infection conditions that lose Sox10 may then tip the balance towards Sox2 transcriptional networks that favor the pSLC state. Underlying mechanisms by which ML perform these tasks should provide new insights into not only targeting ML infection before infection spreads within the PNS but also the regulation of Schwann cell de-differentiation and remyelination.
Bacterially reprogrammed host cells as a vehicle for dissemination of infection
A critical step of the bacterial life cycle after colonization in the primary host niche is dissemination via systemic routes. This is particular challenging for an extremely passive bacterium like ML with a strictly obligate intracellular life style, residing in a specialized and complex host niche such as the adult PNS. ML appear to overcome these challenges by conversion of parent Schwann cells to pSLC with acquired characteristics of differentiation to mesenchymal tissues as well as migratory and immunomodulatory properties [36,51]. Host cell dependence of ML for bacterial survival is well documented but host cell dependence for bacterial dissemination was unknown until recently. Converting parent host cells to stem-like cells with a migratory property provide ML with a vehicle to disseminate infection. Since pSLC are host derived, ML take the unique advantage of these migratory stem cell-like cells as a hideout during the hostile journey of transmission to other preferred niches without being detected by immune cells that normally traffic throughout the body. By converting Schwann cells to pSLC with neural crest/mesenchymal stem cell-like properties, ML take advantage not only to migrate but also transfer infection to skeletal and smooth muscles by re-differentiating stem cells into these tissues without systemic involvement (Fig. 2A). In leprosy patients, disseminated ML have been demonstrated in several mesenchymal tissues including skeletal muscles and smooth muscles [52,53,54]. These findings together provide a possible mechanism by which ML disseminate to smooth and skeletal muscle during human infection. This also provides an intriguing strategy for an extremely passive intracellular pathogen like ML to overcome challenges faced during dissemination.
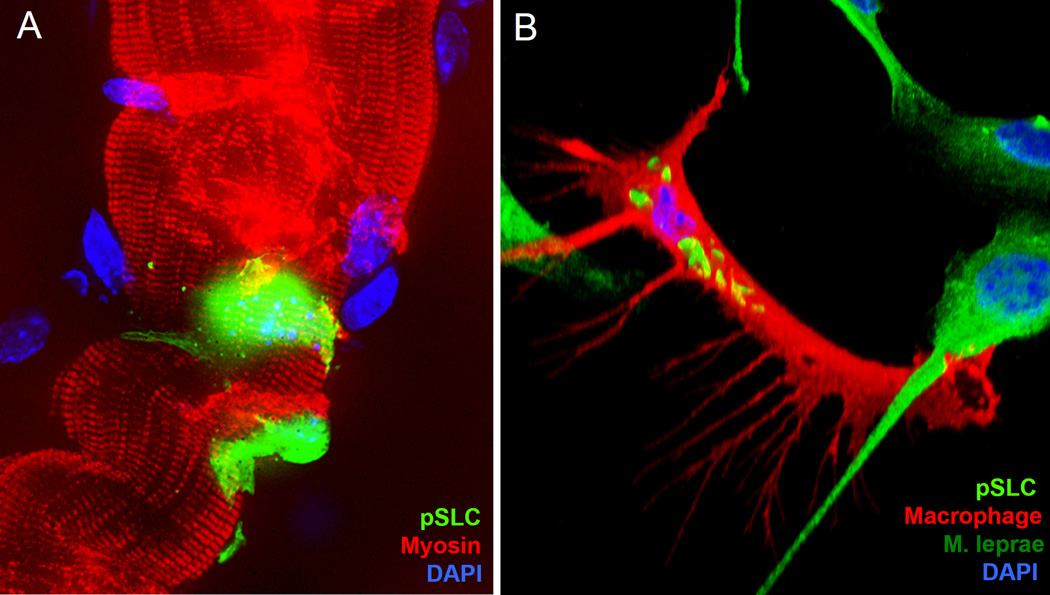
Figure 2. Stem cells generated by host cell reprogramming contribute to bacterial dissemination.
(A) Leprosy bacteria reprogram primary adult Schwann cells to progenitor/stem-like cells (pSLC) (GFP/green) and use stem cell-like properties to fuse and differentiate directly to adult skeletal muscles in vivo (red; myosin labeling; DAPI in blue shows muscle nuclei), and thus spread the infection passively to skeletal muscles. (B) pSLC acquire immunomodulatory and efficient bacterial transfer capabilities during reprogramming and use these characteristics, particularly the release of chemokines and cytokines, to attract macrophages and transfer infection to them. Shown is bacterial transfer to a macrophage (F4/80 marker in red; ML is in green rods) by pSLC (green) in an in vitro model (Adapted from Masaki et al; 36)
Another striking property that ML take advantage of by reprogramming Schwann cells is the use of the efficient capacity of pSLC to transfer bacteria to other cell types. Before reprogramming, primary Schwann cells of both rodent and human origin usually retain intracellular ML after infection [28–30; 55]. Such initial bacterial retention capacity in adult Schwann cells may be of functional significance during human infection, since Schwann cells in leprosy patients are known to harbor ML for an extensive period, which may be critical for extremely slow bacterial expansion (ML doubling time is about 14 days), within this privileged niche [56,57]. However, once colonized, Schwann cells undergo a reprogramming process, which acquires a property of effective ML transfer to skin fibroblasts and neural fibroblasts [55]. The latter is of particular significance, since they are abundant in the peripheral nerve microenvironment and thus could serve as an immediate target for ML during dissemination. Also, ubiquitous distribution of fibroblasts in almost all body tissues types suggests that pathogens are most likely to take advantage of these cells in order to reach or exit from their specific tissue niches.
Reprogrammed cells contribute to bacterial dissemination by granuloma formation
The changes in Schwann cells induced by ML appear to have unexpected bacterial advantages. The primary tissue niche of ML, adult Schwann cells, are non-immune neural tissue cells whose major functions are to produce the unique myelin sheath and support neurons for maintaining a proper functional nervous system [22]. Conversion of Schwann cells to pSLCs with immunomodulatory properties, releasing numerous chemokines and tissue remodeling factors, may set the stage for ML to disseminate infection via systemic routes.
By using the capacity to secrete multiple chemoattractants and survival factors, ML may use reprogrammed cells to create a secondary niche, recruit macrophages, transfer infection and establish a new habitat for further bacterial expansion and dissemination [36]. An important finding is the contribution of innate immune factors during Schwann cell reprogramming and their expression to the highest levels at pSLC stage [36,51]. Although early innate immune factors upregulated in the early stage of infection are likely to promote a reprogramming process, release of these immune factors particularly chemokines/cytokines are more prominent at the pSLC stage [51]. This was demonstrated by the capacity of pSLCs to recruit macrophages and form granuloma-like structures in both in vitro and in vivo models, resembling typical granulomas seen in tissue lesions from leprosy and tuberculosis patients [58,59,60]. Interestingly, some of the immune factors/chemokines released from pSLC are already known to foster granuloma formation [61,62].
Although mycobacterial granulomas are considered to be essential for containment of infection, recent studies on Zebrafish models have suggested that macrophage granulomas may also promote mycobacterial spread during early infection [58–60; 63]. However, unlike other pathogenic mycobacteria, ML use adult Schwann cells as primary non-immune tissue cells for initial colonization [34]. These findings suggest that once colonized, ML maintain Schwann cells in an active and viable state and take full advantage of Schwann cell plasticity to convert parent cells to pSLCs with the capacity to produce chemoattractants and trophic factors, which in turn promote the recruitment and survival of macrophages [36]. In vivo and in vitro analyses also provide further evidence that ML-laden macrophages in the granulomas contribute to the spread of the infection. In this manner it is possible that ML take a long route via remodeling events of host cells using multiple complex mechanisms to get access to final systemic dissemination of infection once established in a privileged niche like Schwann cells. This shows a striking example of how a human bacterial pathogen potentially makes use of its own remodeled host niche for dissemination of infection to other tissues. Ironically, the above-described bacterial strategies are a lesson from a neglected pathogen causing a neglected human disease, leprosy, which still remains a major public health issue worldwide [64]. However, the sophistication the leprosy bacterium displays to manipulate its host cell niche is unprecedented and cannot be ignored.
Conclusions
Recent advances on bacterial-host cell interactions described above open up a new theme of fusion of infection biology and stem cell biology fields. These advances, by connecting tissue cell reprogramming to bacterial infections, may have implications on how we approach fundamental research into bacterial infectious diseases, which are becoming an ever more challenging global task than before due to emerging drug resistant bacteria. Undoubtedly, future studies on this direction with many bacterial pathogens will enhance our understanding of biology of both host-pathogen interaction and their symbiosis and co-evolution with much more sophistication. The striking example of adult Schwann cell reprogramming by leprosy bacilli provides a new level of sophistication in bacterial-host interaction that can be harnessed for studying many aspects of both pathogen and host biology. Remodeling normal host cells to stem-like cells that provide advantage for safe bacterial dissemination reveals an unexpected adaptation of host cell dependence from a bacterial pathogen. Also, mechanisms by which ML reprogram host cells will allow us to identify common mechanisms that we can adapt to dissect other bacterial-host interactions. Such directions may provide clues for identifying common host-encoded functions required for many bacterial infections and target them for developing strategies as alternative to or use in combination with antibiotics for halting the progression of bacterial infections. In the case of leprosy bacteria, reprogramming adult Schwann cells is likely to be an early critical event during infection of adult peripheral nerves way before complex inflammation-mediated neurological damage begins. Molecular basis of how ML perform these tasks should provide new insights into targeting ML infection before infection spread within the PNS, which would enormously benefit leprosy sufferers and prevent devastating nerve damage that disables these patients. The extent of host cell reprogramming capabilities of ML may also add new insights into current methods of artificially changing cell fate reversal used by overexpressing TFs using transgenic methods [18,19]. iPS cells generated by this technology are now widely used for understanding many aspects of biology, drug discovery and regenerative medicine [21,65]. However, the efficiency of reprogramming adult tissue cells to ES-like iPS cells remains extremely low, and the full potential of this technology for disease modeling and cell therapy has yet to be realized [18,66]. Although how ML reprograms host cells is a vastly complex process and unknown, certainly the long incubation time within cells gives greater opportunity to alter cellular dynamics without causing oncogenic transformation. Many studies have documented that various bacterial pathogens use a range of strategies to modulate host cell behavior, including secretion of nucleomodulins to alter host chromatin state [67], epigenetic modifications [68,69], hijacking host gene transcription [70], influencing metabolism [71,72], immune responses [5] and signaling pathways [4,73,74]. In addition, existing tissue progenitor/stem cells can also be modified by commensal bacteria in the gut [75] and other invading pathogens like E.coli, which mobilize hematopoietic stem cells [76]. Together, some commonality can be extracted from these examples and reprogramming induced by ML. Therefore, elucidating mechanisms by which bacterial pathogens modulate the host cell machinery is not only valuable for developing strategies targeting host-encoded functions for prevention of infectious process, but also manipulate host cells for generating new approaches of medical importance for health benefit. As with the discovery of how bacterial pathogens protect themselves against infectious phages and plasmids using CRISPR/cas9 system as a mechanism for bacterial immunity [77,78], which later developed into a powerful method for gene editing and bioengineering [79], it is possible that unraveling the molecular detail of the mechanisms of bacterial-induced host cell reprogramming may provide us with new tools for tissue cell manipulations in medicine and tissue repair in regenerative medicine.
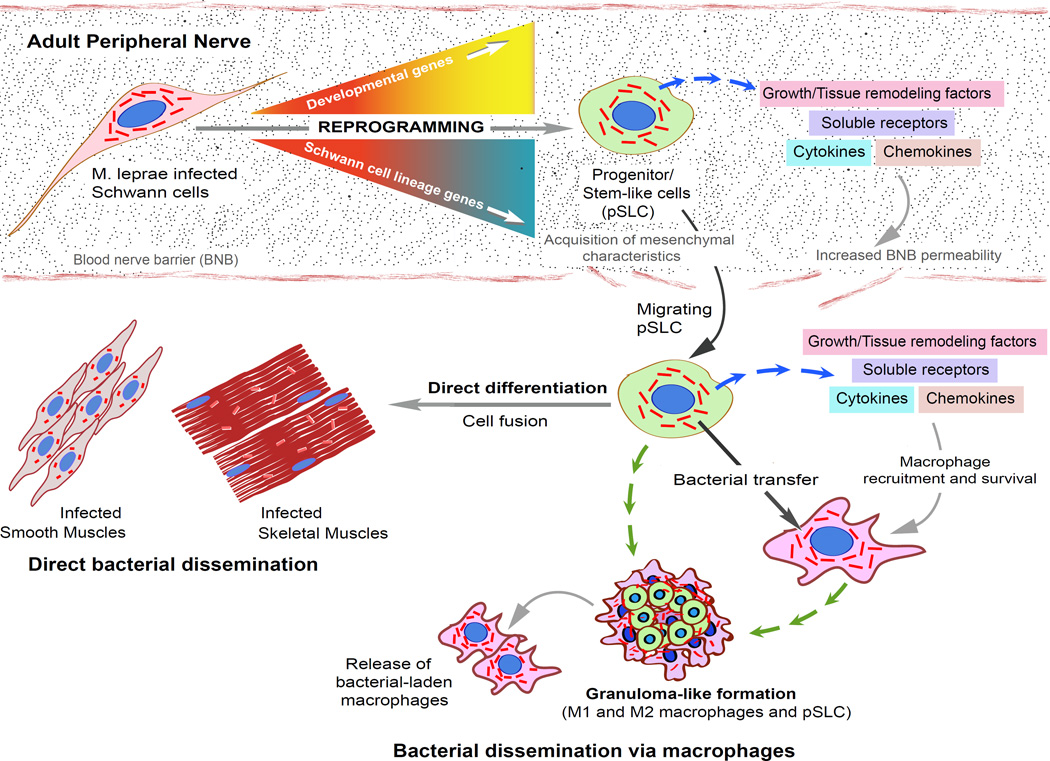
Figure 3. The proposed model for reprogramming adult Schwann cells to stem cell-like cells by intracellular M. leprae and subsequent events leading to dissemination of infection.
Schwann cells in the adult peripheral nerves infected with ML undergo a reprogramming process that convert Schwann cells to pSLC by turning off Schwann cell differentiation/myelination program-associated genes and upregulating embryonic genes of mesenchymal and neural crest development. Reprogramming renders pSLC to acquire migratory properties and immunomodulatory characteristics - releasing numerous chemokines, cytokines and growth/remodeling factors, which not only increased permeability of blood nerve barrier (BNB) but also attract macrophages. Acquired migratory properties promote ML-laden pSLC to exit breached BNB and disseminate to other preferred tissue niches such as smooth muscles and skeletal muscles where they can undergo direct differentiation, and thus transfer bacteria passively to these tissues. Chemoattractants released from pSLC recruit macrophages, transfer ML and form typical granuloma-like structures, which then release bacterial-laden macrophages, a mechanism by which reprogrammed cells may channel bacterial dissemination via systemic routes.
Highlights.
Induction of stem cells by M. leprae shows a fusion of infection biology with stem cell biology
Stem-like cells acquire migratory and immunomodulatory properties and promote dissemination
Reprogramming Schwann cells may be an early critical event during M. leprae infection
Bacterial-induced host cell reprogramming may have applications in regenerative medicine
Acknowledgements
We thank present and past lab members and collaborators who contributed for many years of work, which are described and cited here; we particularly acknowledge the contribution of Toshihiro Masaki. Research presented here was funded in part by grants from NINDS, NIAID, The Order of MALTALEP Foundation, the Rockefeller University, the University of Edinburgh, and Wellcome Trust Institutional Strategic Support Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991;65(7):1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- 2.Mostowy S, Cossart P. From pathogenesis to cell biology and back. Cell Host Microbe. 2009;5(6):510–513. doi: 10.1016/j.chom.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Finlay BB, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alto NM, Orth K. Subversion of cell signaling by pathogens. Cold Spring Harb Perspect Biol. 2012;4(9):a006114. doi: 10.1101/cshperspect.a006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxt LA, Garza-Mayers AC, Goldberg MB. Bacterial subversion of host innate immune pathways. Science. 2013;340(6133):697–701. doi: 10.1126/science.1235771. [DOI] [PubMed] [Google Scholar]
- 6.Cossart P, Roy CR. Manipulation of host membrane machinery by bacterial pathogens. Curr Opin Cell Biol. 2010;22(4):547–554. doi: 10.1016/j.ceb.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carabeo R. Bacterial subversion of host actin dynamics at the plasma membrane. Cell Microbiol. 2011;13(10):1460–1469. doi: 10.1111/j.1462-5822.2011.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alix E, Mukherjee S, Roy CR. Subversion of membrane transport pathways by vacuolar pathogens. J Cell Biol. 2011;195(6):943–952. doi: 10.1083/jcb.201105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Curr Opin Pharmacol. 2014;18C:56–60. doi: 10.1016/j.coph.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145. doi: 10.3389/fpubh.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honoré N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. Massive gene decay in the leprosy bacillus. Nature. 2001;409(6823):1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 12.Theise ND, Wilmut I. Cell plasticity: flexible arrangement. Nature. 2003;425(6953):21. doi: 10.1038/425021a. [DOI] [PubMed] [Google Scholar]
- 13.Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344(6189):1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas-Ríos P, González-Reyes A. Concise review: The plasticity of stem cell niches: a general property behind tissue homeostasis and repair. Stem Cells. 2014;32(4):852–859. doi: 10.1002/stem.1621. [DOI] [PubMed] [Google Scholar]
- 15.Gurdon JB. Adult frogs derived from the nuclei of single somatic cells. Dev Biol. 1962;4:256–273. doi: 10.1016/0012-1606(62)90043-x. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 18.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143(4):508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang JC, Gao X, Lu L, Liu P. Cellular reprogramming by transcription factor engineering. Curr Opin Genet Dev. 2014;28C:1–9. doi: 10.1016/j.gde.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Ladewig J, Koch P, Brüstle O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat Rev Mol Cell Biol. 2013;14(4):225–236. doi: 10.1038/nrm3543. [DOI] [PubMed] [Google Scholar]
- 21.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12(2):79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 22.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 23.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13(5):529–536. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Kim HA, Mindos T, Parkinson DB. Plastic fantastic: Schwann cells and repair of the peripheral nervous system. Stem Cells Transl Med. 2013;2(8):553–557. doi: 10.5966/sctm.2013-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salzer JL. Switching myelination on and off. J Cell Biol. 2008;181(4):575–577. doi: 10.1083/jcb.200804136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radtke C, Vogt PM. Peripheral nerve regeneration: a current perspective. Eplasty. 2009;9:e47. [PMC free article] [PubMed] [Google Scholar]
- 27.Rambukkana A. Usage of signaling in neurodegeneration and regeneration of peripheral nerves by leprosy bacteria. Prog Neurobiol. 2010;91(2):102–107. doi: 10.1016/j.pneurobio.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science. 2002;296(5569):927–931. doi: 10.1126/science.1067631. [DOI] [PubMed] [Google Scholar]
- 29.Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12(8):961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- 30.Tapinos N, Rambukkana A. Insights into regulation of human Schwann cell proliferation by Erk1/2 via a MEK-independent and p56Lck-dependent pathway from leprosy bacilli. Proc Natl Acad Sci U S A. 2005;102(26):9188–9193. doi: 10.1073/pnas.0501196102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73(4):729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Miko TL, Le Maitre C, Kinfu Y. Damage and regeneration of peripheral nerves in advanced treated leprosy. Lancet. 1993;342(8870):521–525. doi: 10.1016/0140-6736(93)91647-5. [DOI] [PubMed] [Google Scholar]
- 33.Job CK. Nerve damage in leprosy. Int J Lepr Other Mycobact Dis. 1989;57(2):532–539. [PubMed] [Google Scholar]
- 34.Stoner GL. Importance of the neural predilection of Mycobacterium leprae in leprosy. Lancet. 1979;2(8150):994–996. doi: 10.1016/s0140-6736(79)92564-9. [DOI] [PubMed] [Google Scholar]
- 35.Lahiri R, Randhawa B, Krahenbuhl JL. Infection of mouse macrophages with viable Mycobacterium leprae does not induce apoptosis. J Infect Dis. 2010;201(11):1736–1742. doi: 10.1086/652499. [DOI] [PubMed] [Google Scholar]
- 36. Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell. 2013;152(1–2):51–67. doi: 10.1016/j.cell.2012.12.014. ** This study showed for the first time that a bacterial pathogen reprograms the fate of a lineage committed host cell to a stem-cell like state. Evidence suggests this facilitates the bacterial dissemination, showing a remarkably sophisticated and dynamic interaction between bacteria and host.
- 37.Truman R. Leprosy in wild armadillos. Lepr Rev. 2005;76(3):198–208. [PubMed] [Google Scholar]
- 38. Truman RW, Ebenezer GJ, Pena MT, Sharma R, Balamayooran G, Gillingwater TH, Scollard DM, McArthur JC, Rambukkana A. The armadillo as a model for peripheral neuropathy in leprosy. ILAR J. 2014;54(3):304–314. doi: 10.1093/ilar/ilt050. * The pathology of leprosy in nine-banded armadillos, the natural host of M.leprae, and use as a model organism for this disease is discussed. As in vivo studies of early-stage infection in humans is unfeasible, these animals could provide further insight into the early infectious process.
- 39.Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci USA. 2005;102(7):2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 41.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaBonne C, Bronner-Fraser M. Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol. 1999;15:81–112. doi: 10.1146/annurev.cellbio.15.1.81. [DOI] [PubMed] [Google Scholar]
- 43.Schneuwly S, Klemenz R, Gehring WJ. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. Nature. 1987;325(6107):816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- 44.Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136(14):2311–2322. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42(3):421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Thomas S, Thomas M, Wincker P, Babarit C, Xu P, Speer MC, Munnich A, Lyonnet S, Vekemans M, Etchevers HC. Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum Mol Genet. 2008;17(21):3411–3425. doi: 10.1093/hmg/ddn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnston AP, Naska S, Jones K, Jinno H, Kaplan DR, Miller FD. Sox2-mediated regulation of adult neural crest precursors and skin repair. Stem Cell Reports. 2013;1(1):38–45. doi: 10.1016/j.stemcr.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bösl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189(4):701–712. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56(14):1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 50. Bergsland M, Ramsköld D, Zaouter C, Klum S, Sandberg R, Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25(23):2453–2464. doi: 10.1101/gad.176008.111. * This study revealed an interesting temporal network of Sox family transcription factor activity to guide differentiation, highlighting the interplay and coordination of transcription factors has a key role in programming cell fate.
- 51.Masaki T, McGlinchey A, Cholewa-Waclaw J, Qu J, Tomlinson SR, Rambukkana A. Innate immune response precedes Mycobacterium leprae-induced reprogramming of adult Schwann cells. Cell Reprogram. 2014;16(1):9–17. doi: 10.1089/cell.2013.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearson JM, Rees RJ, Weddell AG. Mycobacterium leprae in the striated muscle of patients with leprosy. Lepr Rev. 1970;41(3):155–166. doi: 10.5935/0305-7518.19700023. [DOI] [PubMed] [Google Scholar]
- 53.Job CK, Chehl SK, Hastings RC. Transmission of leprosy in nude mice through thorn pricks. Int J Lepr Other Mycobact Dis. 1994;62(3):395–398. [PubMed] [Google Scholar]
- 54.Werneck LC, Teive HA, Scola RH. Muscle involvement in leprosy. Study of the anterior tibial muscle in 40 patients. Arq Neuropsiquiatr. 1999;57(3B):723–734. doi: 10.1590/s0004-282x1999000500001. [DOI] [PubMed] [Google Scholar]
- 55. Masaki T, McGlinchey A, Tomlinson SR, Qu J, Rambukkana A. Reprogramming diminishes retention of Mycobacterium leprae in Schwann cells and elevates bacterial transfer property to fibroblasts. Version 3. F1000Res. 2013b;2:198. doi: 10.12688/f1000research.2-198.v1. * This study demonstrated a cell-state dependency for bacterial infection, where M.leprae have to remodel their host before transferring to other tissues. This not only shows a complex host-pathogen partnership, but also hints at a target pathway for preventing infection spread.
- 56.Pereira JA, Lebrun-Julien F, Suter U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012;35(2):123–134. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Shetty VP, Antia NH, Jacobs JM. The pathology of early leprous neuropathy. J Neurol Sci. 1988;88(1–3):115–131. doi: 10.1016/0022-510x(88)90210-9. [DOI] [PubMed] [Google Scholar]
- 58.Modlin RL, Rea TH. Immunopathology of leprosy granulomas. Springer Semin Immunopathol. 1988;10(4):359–374. doi: 10.1007/BF02053846. [DOI] [PubMed] [Google Scholar]
- 59.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 60.Bold TD, Ernst JD. Who benefits from granulomas, mycobacteria or host? Cell. 2009;136(1):17–19. doi: 10.1016/j.cell.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu B, Frait KA, Reich F, Komuniecki E, Chensue SW. Chemokine expression dynamics in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation. Am J Pathol. 2001;158(4):1503–1515. doi: 10.1016/S0002-9440(10)64101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiu BC, Freeman CM, Stolberg VR, Hu JS, Komuniecki E, Chensue SW. The innate pulmonary granuloma: characterization and demonstration of dendritic cell recruitment and function. Am J Pathol. 2004;164(3):1021–1030. doi: 10.1016/S0002-9440(10)63189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136(1):37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodrigues LC, Lockwood DNj. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis. 2011;11(6):464–470. doi: 10.1016/S1473-3099(11)70006-8. [DOI] [PubMed] [Google Scholar]
- 65.Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol. 2012;13(11):713–726. doi: 10.1038/nrm3448. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Yao L, Yu X, Ou J, Hui N, Liu S. A poor imitation of a natural process: a call to reconsider the iPSC engineering technique. Cell Cycle. 2012;11(24):4536–4544. doi: 10.4161/cc.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bierne H, Cossart P. When bacteria target the nucleus: the emerging family of nucleomodulins. Cell Microbiol. 2012;14(5):622–633. doi: 10.1111/j.1462-5822.2012.01758.x. [DOI] [PubMed] [Google Scholar]
- 68. Silmon de Monerri NC, Kim K. Pathogens hijack the epigenome: a new twist on host-pathogen interactions. Am J Pathol. 2014;184(4):897–911. doi: 10.1016/j.ajpath.2013.12.022. * An insightful review into the diversity of bacterial ability to manipulate host cell epigenome to alter cell function.
- 69.Sinclair SH, Rennoll-Bankert KE, Dumler JS. Effector bottleneck: microbial reprogramming of parasitized host cell transcription by epigenetic remodeling of chromatin structure. Front Genet. 2014;5:274. doi: 10.3389/fgene.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hannemann S, Gao B, Galán JE. Salmonella modulation of host cell gene expression promotes its intracellular growth. PLoS Pathog. 2013;9(10):e1003668. doi: 10.1371/journal.ppat.1003668. * Another striking example of how Salmonella alter host transcriptome for promoting bacterial growth inside host cells.
- 71.Vromman F, Subtil A. Exploitation of host lipids by bacteria. Curr Opin Microbiol. 2014;17:38–45. doi: 10.1016/j.mib.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 72.van der Meer-Janssen YP, van Galen J, Batenburg JJ, Helms JB. Lipids in hostpathogen interactions: pathogens exploit the complexity of the host cell lipidome. Prog Lipid Res. 2010;49(1):1–26. doi: 10.1016/j.plipres.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tahoun A, Mahajan S, Paxton E, Malterer G, Donaldson DS, Wang D, Tan A, Gillespie TL, O'Shea M, Roe AJ, Shaw DJ, Gally DL, Lengeling A, Mabbott NA, Haas J, Mahajan A. Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe. 2012;12(5):645–656. doi: 10.1016/j.chom.2012.10.009. ** Another example of bacterial-induced change in cell phenotype. In this case, a single factor, SopB, could induce an EMT process, demonstrating the power of tools bacteria can have at their disposal.
- 74.Pizarro-Cerdá J, Kühbacher A, Cossart P. Phosphoinositides and host-pathogen interactions. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbalip.2014.09.011. pii: S1388-1981(14)00189-9. [DOI] [PubMed] [Google Scholar]
- 75. Nigro G, Rossi R, Commere PH, Jay P, Sansonetti PJ. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15(6):792–798. doi: 10.1016/j.chom.2014.05.003. ** This highlights a host-pathogen symbiosis between bacteria and stem cells of the intestine. Microbiota products can stimulate stem cell survival, provide protection against stress and trigger regeneration.
- 76. Burberry A, Zeng MY, Ding L, Wicks I, Inohara N, Morrison SJ, Núñez G. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe. 2014;15(6):779–791. doi: 10.1016/j.chom.2014.05.004. * This study shows how Escherichia coli mobilizes functional hematopoietic stem cells using NOD- and Toll-like receptors and how stem cells contributed to limit secondary infection via innate immune cells.
- 77.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327(5962):167–170. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 79.Charpentier E, Marraffini LA. Harnessing CRISPR-Cas9 immunity for genetic engineering. Curr Opin Microbiol. 2014;19:114–119. doi: 10.1016/j.mib.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]