Abstract
A recent study by our lab found high accumulation of extracellular adenosine triphosphate (ATP) in the center of healthy porcine intervertebral discs (IVD). Since ATP is a powerful extracellular signaling molecule, extracellular ATP accumulation may regulate biological activities in the IVD. Therefore, the objective of this study was to investigate the effects of extracellular ATP on the extracellular matrix (ECM) biosynthesis of porcine IVD cells isolated from two distinct anatomical regions: annulus fibrosus (AF) and nucleus pulposus (NP). The ATP treatment significantly promoted the ECM deposition and corresponding gene expression (aggrecan and type II collagen) by both cell types in 3-dimensional agarose culture. A significant increase in ECM accumulation was found in AF cells at a lower ATP treatment level (20 µM) compared to NP cells (100 µM), indicating that AF cells may be more sensitive to extracellular ATP than NP cells. NP cells also exhibited higher ECM accumulation and intracellular ATP than AF cells under Control and treatment conditions, suggesting that NP cells are intrinsically more metabolically active. Moreover, the ATP treatment also augmented the intracellular ATP level in NP and AF cells. Our findings suggest that extracellular ATP not only promotes ECM biosynthesis via molecular pathway but also increases energy supply to fuel that process.
Keywords: intervertebral disc, extracellular matrix, ATP
INTRODUCTION
Low back pain is a condition which causes distress and suffering to patients. The impact of low back pain creates a major socio-economic burden in industrialized societies as well. As the leading cause of disability, low back pain affects more than 80% of the US population at some point in life (How-Ran, et al., 1999). Intervertebral disc (IVD) degeneration has been closely associated with low back pain, stimulating interest in finding the causes that lead to IVD degeneration. Therefore, understanding the mechanisms involved in the maintenance of IVD composition may shed light to development of novel therapies for IVD degeneration and low back pain.
The IVD provides the mechanical properties that allow flexion, bending, and torsion of the spine, and transmission of loads through the spinal column. These biomechanical properties are maintained by the composition and organization of the disc’s extracellular matrix (ECM). The interplay of the two ECM main macromolecules, the highly hydrated proteoglycan (PG) gel and the fibrillar collagen network, determines the mechanical response of the IVD (Roughley, 1976). The IVD cells, which populate the discs at low densities, are responsible for maintaining the proper homeostatic balance of biosynthesis, breakdown, and accumulation of ECM constituents (Ohshima, et al., 1995). These cellular processes determine the quality and integrity of the ECM and thus, the disc’s mechanical response (Buschmann, et al., 1995). In addition, decreasing PG concentration was found with increasing grade of IVD degeneration (Pearce, et al., 1987). In an in vitro study, disc aggrecan (part of the PG family) inhibited nerve growth, which is linked with the development of low back pain (Johnson, et al., 2002). Therefore, it is suggested that detrimental changes in the ECM are associated with IVD degeneration and low back pain.
Maintenance of the ECM is a high energy demanding process that requires glucose and oxygen consumption to produce energy in the form of adenosine triphosphate (ATP). Nutrients are supplied mainly by diffusion from blood vessels at the margins of the disc due to the avascular nature of the IVD and transported through the dense ECM to IVD cells (Urban, et al., 2004). This mechanism of transport may be restricted by factors such as calcification of the endplate or changes in the composition of the ECM (Grunhagen, et al., 2011) which results in detrimental effects on essential cellular activities (e.g., ATP production). A previous study reported that intracellular ATP level declined during the development of spontaneous knee osteoarthritis in guinea pigs, indicating that depletion of ATP is associated with cartilage degeneration (Johnson, et al., 2004). Hence, cellular energy production for the proper synthesis of ECM molecules may be crucial to sustain the integrity and function of the IVD.
During daily activities, the spine is subjected to mechanical forces that influence cell metabolism, gene expression and ECM synthesis in IVD cells (Kasra, et al., 2006, Korecki, et al., 2009, Maclean, et al., 2004, Ohshima, et al., 1995, Walsh and Lotz, 2004). Our recent studies have demonstrated that compressive loading promotes ATP production and release in IVD cells in a 3-dimensional agarose gel model (Czamanski, et al., 2011, Fernando, et al., 2011) and in-situ energy metabolism in the IVD (Wang, et al., 2013). Furthermore, high accumulation of extracellular ATP due to the disc’s avascular nature was found in the center of young healthy porcine IVD (Wang, et al., 2013). ATP is an extracellular signaling molecule that mediates a variety of cellular activities via purinergic pathways (Burnstock, 1997), including ECM production (Croucher, et al., 2000, Waldman, et al., 2010). Hence, ATP metabolism mediated by compressive loading and extracellular ATP accumulation could be a potential pathway that regulates crucial biological activities in the IVD. Therefore, the objective of this study was to investigate the effects of extracellular ATP on the ECM synthesis of porcine IVD cells isolated from two distinct anatomical regions: annulus fibrosus (AF) and nucleus pulposus (NP).
MATERIALS AND METHODS
Intervertebral Disc Cells Isolation and Samples Preparation
The IVDs were obtained from mature pigs (~250 lbs.) within 2 hours of sacrifice (Cabrera Farms, Hialeah, FL). NP and outer AF tissues were harvested and digested in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen Corp., Carlsbad, CA) containing 1 mg/ml type II collagenase (Worthington Biochemical Corp., Lakewood, NJ), and 0.6 mg/ml protease (Sigma-Aldrich, St. Louis, MO) for 24 hours at 37 °C, 5% CO2. The cells-enzyme solutions were filtered using a 70µm strainer (BD Biosciences, San Jose, CA) and cells were isolated by centrifugation. The IVD cells were then re-suspended in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen Corp.) and 1% antibiotic-antimycotic (Invitrogen Corp.). The cells were mixed at a 1:1 ratio with 4% agarose gel to obtain cell-agarose samples of 1 × 106 cells in 100 µl of 2% agarose. Freshly isolated cells were used since serial passaging was reported to cause phenotypic changes (Chou, et al., 2006). Three-dimensional culture was chosen because of its minimal binding interaction with cells (Knight, et al., 1998, Lee, et al., 2000) and capability to maintain cellular phenotype (Gruber, et al., 1997). All the samples were cultured at 37°C, 5% CO2 in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic for the duration of the experiments.
PG and Collagen Content Measurements
Our previous study found that NP cells reside in an environment which has an extracellular ATP level of ~165 µM (Wang, et al., 2013). In the literature, the dose range of 62.5 – 125 µM ATP favored ECM production in articular chondrocytes in 3-dimensional agarose culture (Usprech, et al., 2012). Therefore, experimental groups included: Control (no ATP), 20 µM and 100 µM ATP treatment groups (NP: n = 9; AF: n = 9 for each group). The samples were cultured for 21 days under ATP (Sigma-Aldrich). The cell culture medium was changed three times a week and ATP was administered in each medium change. The duration of the experiment was chosen based on a previous study of chondrocytes (Croucher, et al., 2000). After 21 days, each sample was lyophilized and then digested overnight in 1 ml of papain at 60°C. PG content was quantified using the dimethylmethylene blue (DMMB) dye binding assay as previously described in the literature (Farndale, et al., 1986). 150 µl of the samples were further hydrolyzed overnight with 6N hydrochloric acid at 105°C and assayed for hydroxyproline (HYP) content, as previously described in the literature (Neuman and Logan, 1950). As high HYP content is found in collagen, HYP levels were measured as an indicator of collagen content (Neuman and Logan, 1950). DNA content was quantified in samples digested in papain using a Quant-iT dsDNA HS Assay Kit (Invitrogen Corp.). The PG and collagen levels in each sample were normalized by its DNA content to account for variations in cell number. To evaluate the effects of long-term ATP treatment on PG and collagen contents, each ATP treatment group was normalized by its respective Control group. To evaluate the difference between NP and AF cells, values of the NP cells were normalized by the average of AF Control groups. One-way ANOVA followed by post hoc Student Newman Keuls test (SPSS Statistics 20, Chicago, IL) was performed to compare PG and collagen contents between different treatment groups of the same cell type. Student’s t-tests were performed to compare PG and collagen levels between NP and AF cells with the same treatment. Significance was taken at p < 0.05 in all statistical analysis. Additionally, cell viability was examined using LIVE/DEAD® Cell Viability Assay (Invitrogen Corp.) as instructed by manufacturer.
Gene Expression of Aggrecan and type II Collagen
Samples were cultured for 16 hours with 100 µM ATP (NP: n = 12; AF: n = 9 for Control and ATP treatment group). According to our pilot study, the highest increase in gene expression induced by ATP was found at 16 hours post treatment. Additionally, samples from three independent experiments were cultured for 21 days with and without ATP (100 µM) to examine whether agarose culture influences gene expression and maintains cell phenotype. Total RNA from each sample was obtained using a modified version of the trizol (Tri-Reagent, Molecular Research Center, Cincinnati, OH) protocol. To improve the yield of RNA, 2 ml of trizol were added to the samples to facilitate agarose homogenization. After homogenization, vortexing and incubation for 5 minutes at room temperature, the samples were centrifuged for 10 minutes at 5000 rpm. The supernatants were collected and the trizol protocol was followed starting from the phase separation step. At the end of the procedure, the RNA pellets were left to dry 5 minutes at room temperature and 20 µl of DNase/RNase free water were added. The RNA pellets were left to swell for 5 minutes at room temperature and then stored at −80°C overnight. The following day, the pellets were homogenized and centrifuged at 12000 rpm for 20 minutes at 4°C to collect the supernatant containing the RNA. RNA was quantified using the Qubit RNA BR assay kit (Life Technologies, Carlsbad, CA) and reverse transcribed to cDNA using the High capacity cDNA reverse transcription kit (Applied Biosystems, Foster, CA), according to the manufacturers’ specifications. The levels of mRNA of the anabolic genes aggrecan and type II collagen were measured using real-time PCR (One step Plus, Applied Biosystems) and normalized by that of the endogenous control (18s) and the average of the internal controls. The2−ΔΔCT method was applied assuming that the amplification efficiencies of the target and the reference genes were approximately equal (Livak and Schmittgen, 2001). Student’s t-tests were performed to compare relative changes in gene expression between the Control and the treatment group of the same cell type as well as between different time points. The primer sequences were as follows: aggrecan forward primer: AGACAGTGACCTGGCCTGAC; aggrecan reverse primer: CCAGGGGCAAATGTAAAGG; type II collagen forward primer: TGAGAGGTCTTCCTGGCAAA; type II collagen reverse primer: ATCACCTGGTTTCCCACCTT; 18S forward primer: CGGCTACCACATCCAAGGA; 18S reverse primer: AGCTGGAATTACCGCGGCT. The sizes of PCR products for aggrecan, type II collagen and 18S were 151, 161 and 188 bp, respectively.
Intracellular ATP measurements
Samples were cultured for 2 hours with 100 µM ATP (NP: n = 9; AF: n = 9 for Control and ATP treatment group). The time point was selected based on a previous study of endothelial cells, which reported a maximal increase of intracellular ATP generation after 2 hours of ATP treatment (Andreoli, et al., 1990). After incubation with ATP, the samples were dissolved in lysis buffer consisting of 15% 1.5 M NaCl, 15% 50 mM EDTA, 1% Triton-X 100 and 10% 100 mM Tris–Cl at pH 7.4 by heating at 65 °C. The lysates were centrifuged for 10 minutes at 9000 rpm and the supernatants were collected for intracellular ATP and DNA content measurements. Intracellular ATP was measured using the Luciferin-luciferase method (Sigma-Aldrich) and a plate reader (DTX880, Beckman Coulter, Brea, CA). Values of intracellular ATP were quantified and normalized by DNA content. To compare variations of intracellular ATP on each cell type, a student t-test was performed between the Control and the treatment group. To evaluate differences between NP and AF cells, values of NP cells were normalized by the average of AF Control groups and student t-tests were performed.
RESULTS
The effects of ATP treatment on the accumulation of PG and collagen
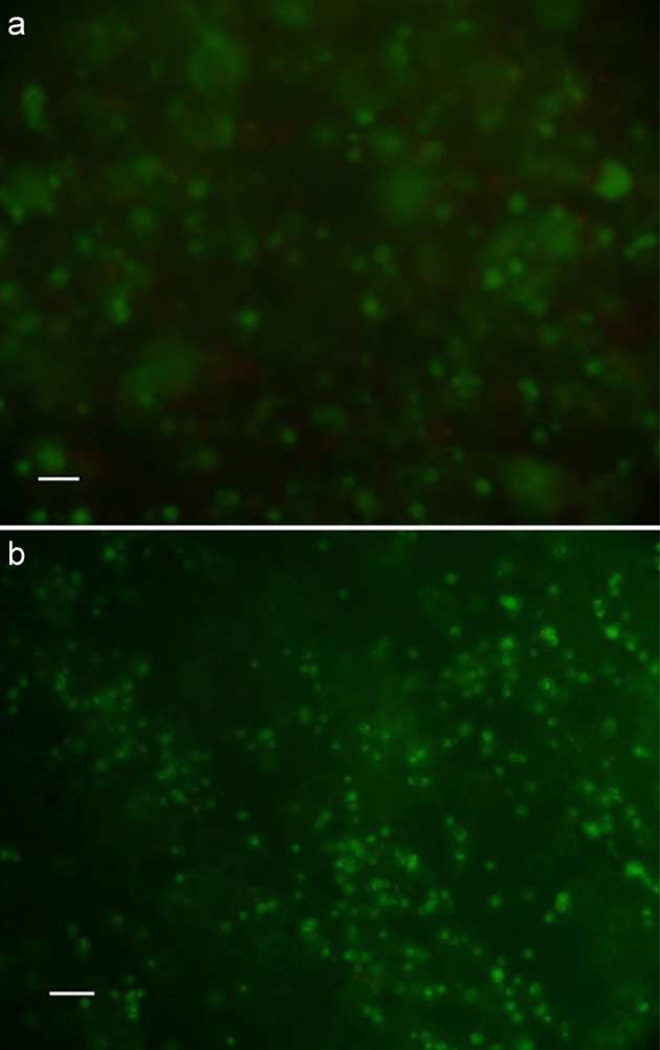
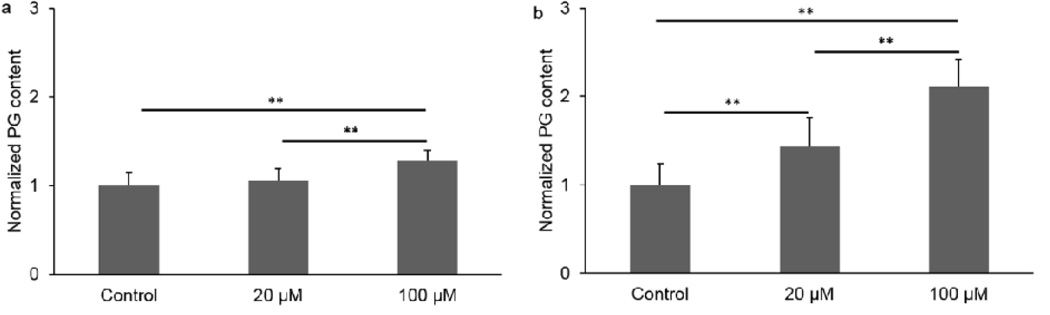
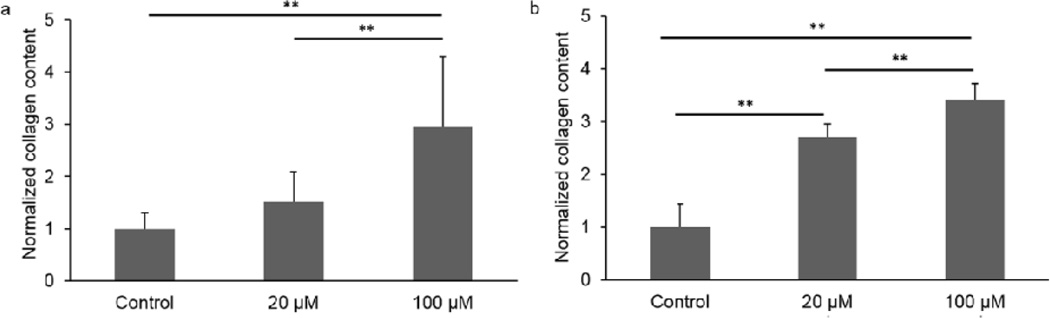
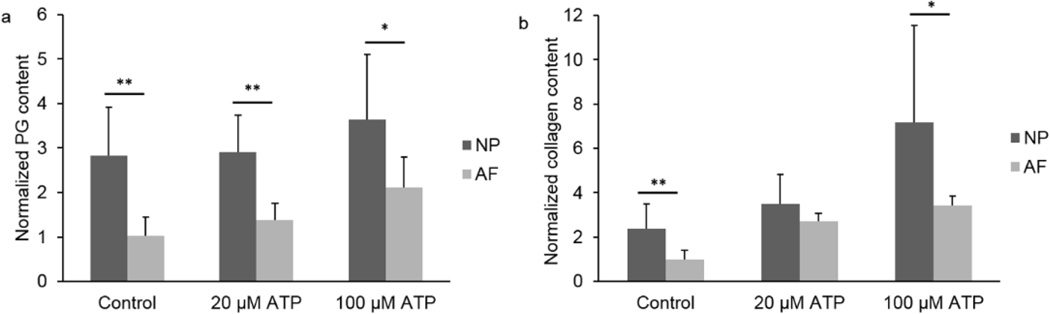
Cell viability staining confirmed that there were no detrimental effects on cells after 21 days of 100 µM ATP treatment (Fig. 1). In NP cells, 100 µM ATP treatment significantly increased PG and collagen as compared to both the 20 µM ATP and Control groups after 21 days of treatment (Fig. 2a and 3a). No significant difference was found in ECM deposition between the Control and the 20 µM ATP groups. In AF cells, both ATP treatment groups exhibited significantly higher PG and collagen levels than the Control group, while the 100 µM ATP group showed a significant higher increase in the contents of PG and collagen than the 20 µM ATP group after 21 days of treatment (Fig. 2b and 3b). The PG content deposited by NP cells was significantly higher than AF cells under all treatment conditions (Fig. 4a). The collagen content accumulated by NP cells was significantly higher in the Control and the 100 µM ATP groups compared to their respective AF group. No significant difference in collagen content was found between NP and AF cells treated with 20 µM ATP (Fig. 4b).
Fig. 1.
Viability of IVD cells treated with 100 µM ATP for 21 days (green/red: alive/dead cells). Bar- 100µm. a. NP cells. b. AF cells
Fig. 2.
Proteoglycan content of IVD cells treated with ATP at different concentrations for 21 days. a. NP cells. b. AF cells (n = 9; **p < 0.01 indicates statistically significant differences between groups)
Fig. 3.
Collagen content of IVD cells treated with ATP at different concentrations for 21 days. a. NP cells. b. AF cells. (n = 9; **p < 0.01 indicates statistically significant differences between groups)
Fig. 4.
Comparative ECM macromolecule content of NP and AF cells treated with ATP at different concentrations for 21 days. NP values were normalized by the average of the Control groups of AF cells. a. PG content. b. Collagen content. (n = 9; *p < 0.05 and **p < 0.01 indicate statistically significant differences between groups)
The effects of ATP treatment on the gene expression of aggrecan and type II collagen
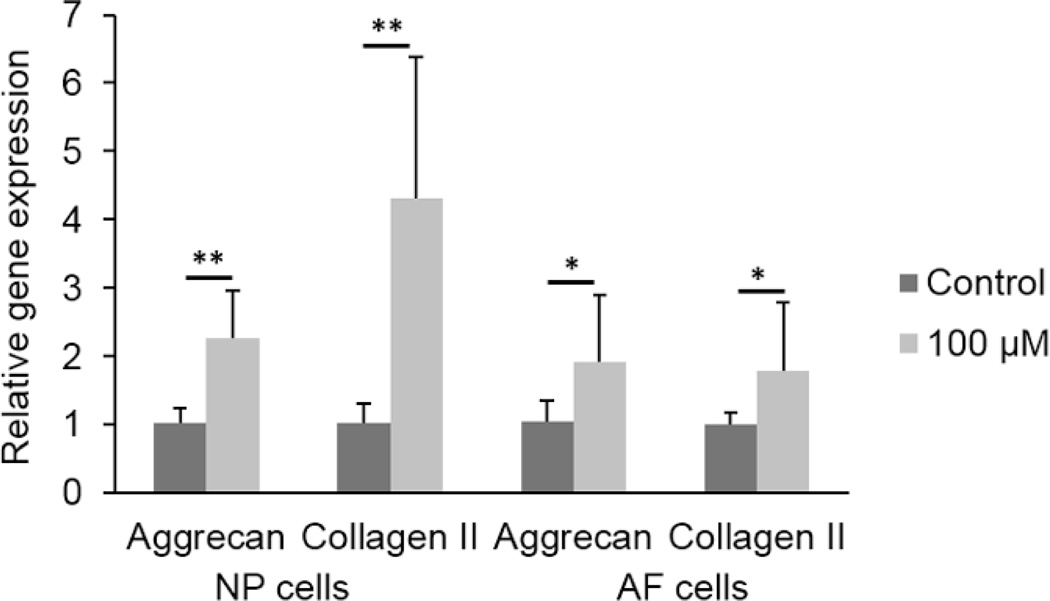
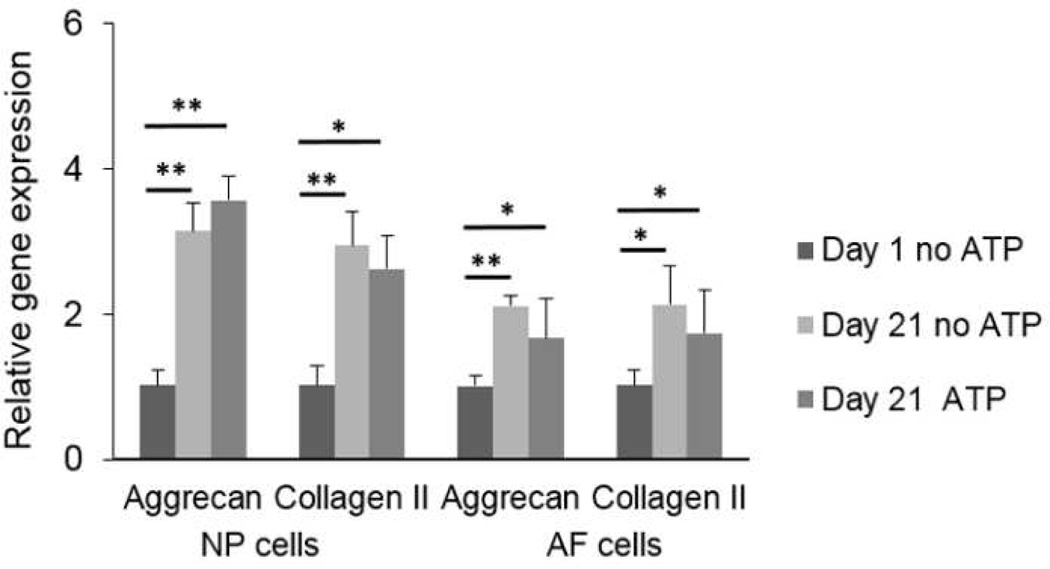
The gene expressions of aggrecan and type II collagen in NP and AF cells were significantly higher after 16 hours of 100 µM ATP treatment compared to control conditions (Fig. 5). In addition, long-term culture in agarose upregulated the gene expression of aggrecan and type II collagen in both cell types. However, no significant differences were found in gene expression between the 100 µM ATP and Control groups at 21 days of culture (Fig. 6).
Fig. 5.
Aggrecan and type II collagen gene expressions of NP and AF cells treated with 100 µM ATP for 16 hours. (n = 12 for NP, n = 9 for AF; *p < 0.05 and **p < 0.01 indicate statistically significant differences between groups)
Fig. 6.
Effect of long-term agarose culture on gene expression in IVD cells with and without ATP treatment (100 µM). (n = 3; *p < 0.05 and **p < 0.01 indicate statistically significant differences between groups)
The effects of ATP treatment on the intracellular ATP content
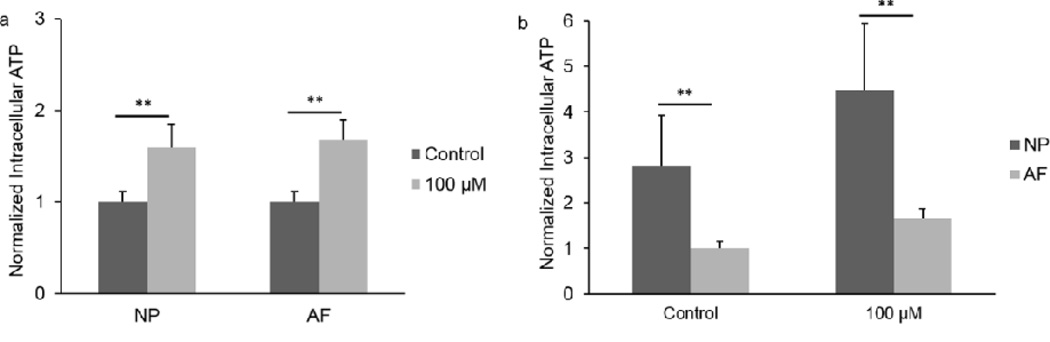
Intracellular ATP content significantly increased in NP and AF cells after 2 hours of 100 µM ATP treatment compared to their Control groups (Fig. 7a). In addition, a comparison between NP and AF cells showed that NP cells have a significant higher intracellular ATP content than AF cells under all conditions (Fig. 7b).
Fig. 7.
Intracellular ATP content of IVD cells treated with 100 µM ATP for 2 hours. a. Comparison between experimental groups of the same cell type. b. Comparison between NP and AF cells. (n = 9; **p < 0.01 indicates statistically significant differences between groups)
DISCUSSION
Increased matrix breakdown, altered matrix synthesis (reduced synthesis of aggrecan and synthesis of type I collagen instead of type II collagen) and apoptosis are among the metabolic changes that contribute to IVD degeneration (Adams and Roughley, 1976, Freemont, 2009). Moreover, aggrecan has shown to inhibit nerve growth in vitro, suggesting that loss of aggrecan is associated with in growth of nerves that may cause low back pain in degenerated IVDs (Johnson, et al., 2002). Proper biosynthesis of ECM in the IVD is a complex process that requires an extensive amount of ATP to be accomplished, especially PG biosynthesis which uses ATP as an energy source and building block (Hirschberg, et al., 1998). The findings that high levels of extracellular ATP promoted ECM biosynthesis and intracellular ATP production in IVD cells suggest that the high accumulation of extracellular ATP found in the NP (Wang, et al., 2013) may play an important role in maintaining a healthy ECM structure of the IVD. Furthermore, to our knowledge, this is the first study to demonstrate that extracellular ATP influences ECM biosynthesis and intracellular ATP content in IVD cells.
In skin cells, galactosyltransferase-I, an enzyme that synthesizes the linkage region between the core protein and the glycosaminoglycan chains of PGs, enhanced its activity after incubation with ATP (Higuchi, et al., 2001). In addition, chondrocytes cultured with ATP demonstrated to increase proteoglycan and collagen deposition (Croucher, et al., 2000, Waldman, et al., 2010). Those previous studies support our finding, suggesting that extracellular ATP can mediate cellular ECM biosynthesis. In addition, it was also found that upregulation of ECM synthesis by exogenous ATP was diminished by antagonist of P2 receptors, suggesting the involvement of a purinergic signaling pathway (Waldman, et al., 2010).
This study found that a lower ATP concentration (i.e., 20 µM) induced a significant increase in accumulation of both ECM molecules by AF cells compared to NP cells. This finding suggests that AF cells may be more sensitive to low concentrations of extracellular ATP than NP cells. This difference in cellular responses to ATP between NP and AF cells could be explained by our previous study which found that NP cells reside in an environment with a higher level of extracellular ATP (~165 µM) than AF cells (<10 µM) (Wang, et al., 2013). Furthermore, our findings of higher PG and collagen accumulations and intracellular ATP content by the NP groups compared to the AF counterpart groups are consistent with our previous studies, which suggested that NP cells are more metabolically active than AF cells (Czamanski, et al., 2011, Fernando, et al., 2011). The differences in the metabolic activities between AF and NP cells may be explained by differences in cell phenotypes in which AF cells are elongated and resemble fibroblasts, whereas NP cells are spheroidal and chondrocyte-like (Buckwalter, 1995). Also, both cell types have distinct embryonic origins. NP cells are derived from the notochord and AF cells are derived from the mesenchyme (Roughley, 1976).
The IVD is subjected to static and dynamic loading at different magnitudes and frequencies during daily activities. Mechanical loading activates different mechanotransduction pathways, which can lead to modification of cell function, metabolism and gene expression (Chowdhury and Knight, 2006, Maclean, et al., 2004). Previous studies showed that mechanical loading mediates ECM biosynthesis of IVD cells (Kasra, et al., 2006, Korecki, et al., 2009, Maclean, et al., 2004, Ohshima, et al., 1995, Walsh and Lotz, 2004). It was also found that mRNA expressions of aggrecan and collagens in NP and AF regions were altered by specific mechanical loading regimens (Hutton, et al., 1999, Maclean, et al., 2004, Neidlinger-Wilke, et al., 2006), while similar effects of mechanical loading were observed on the incorporation of [35S]-sulfate and [3H]-proline (measures of protein synthesis) into collagens and PGs, respectively (Hutton, et al., 1999). In our study, mRNA levels of aggrecan and type II collagen were upregulated by extracellular ATP, which also correlated with their corresponding protein synthesis. Since our previous studies have shown that static and dynamic loading alter ATP production and release in IVD cells (Czamanski, et al., 2011, Fernando, et al., 2011) and in-situ energy metabolism in the IVD (Wang, et al., 2013), the finding of this study suggests that mechanical loading may affect ECM production of IVD cells via an extracellular ATP pathway.
Upregulation of ECM gene expression observed in both cell types without ATP treatment after 21 days of culture indicates that the agarose culture is capable of maintaining cellular phenotypes, which is consistent with the findings in a previous study of 3-dimensional alginate culture (Baer, et al., 2001). Increased gene expression in agarose culture over time may be due to changes in nutrimental condition (i.e., higher level of nutrients in the culture media compared to the avascular in-vivo condition of the IVD) and ECM environment in agarose culture (i.e., more ECM deposition around cells). Moreover, a previous study showed that a single dose of ATP on the first day of culture promoted ECM biosynthesis in bovine chondrocyte pellets cultured over 7 and 21 days (Croucher, et al., 2000). Hence, in our study, ATP did not upregulate gene expression after 21 days of culture, suggesting that a short term of ATP treatment may be adequate to elicit significant effects on IVD cells. In addition, it was reported that high content of proteoglycans has a potential role as an inhibitor of ATP hydrolysis (Vieira, et al., 2001). Therefore, no effects of ATP seen on gene expression after 21 days of culture may be due to accumulation of ATP resulting from an overall increase in ECM deposition over time.
In this study, it was also found that extracellular ATP treatment promoted intracellular ATP production in IVD cells. This finding is consistent with previous studies which reported that treatment with extracellular nucleotides or adenosine increased the concentration of intracellular ATP (Andreoli, et al., 1990, Lasso de la Vega, et al., 1994). In cancer cells, the action of exogenous ATP appeared to be mediated by the hydrolysis of extracellular ATP and subsequently uptake of adenosine into cells, which increased intracellular ATP contents (Lasso de la Vega, et al., 1994). In human umbilical vein endothelial cells, treatment with 25 µM of ATP, ADP, AMP or adenosine significantly raised intracellular ATP levels through the same mechanism (i.e, adenosine uptake) (Andreoli, et al., 1990). In addition, previous studies have also reported that extracellular ATP signaling via P2X4 receptor mediates intracellular ATP oscillations, which are involved in prechondrogenic condensation in chondrogenesis (Kwon, 2012, Kwon, et al., 2012). Hence, these evidence suggest that intracellular ATP production could be mediated by hydrolysis of extracellular ATP, subsequent uptake of adenosine into cells, and/or activation of purinergic receptors on the cell membrane.
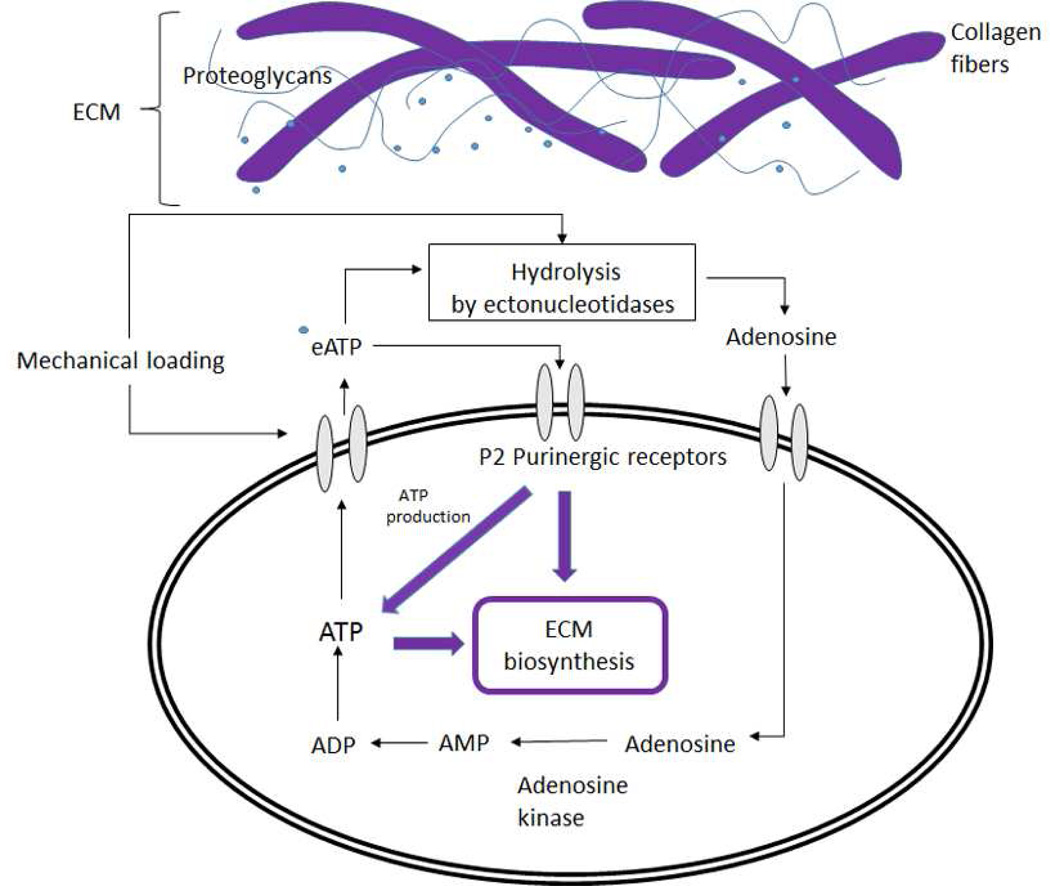
Due to the avascular nature of the IVD, delivery of nutrients to IVD cells relies on diffusion. In humans, about 25% of water is extruded from the disc due to high loads during daily activities (Paesold, et al.). A decrease in disc hydration reduces supply (diffusion) of oxygen and glucose for cellular ATP production, which is essential for maintaining cell viability and normal ECM production especially in the center of the disc (i.e., NP region). Since mechanical loading could promote hydrolysis of extracellular ATP which is highly accumulated in the NP (Wang, et al., 2013), intracellular ATP levels in IVD cells could be increased via the adenosine uptake mechanism described in the previous section, compensating the effects of mechanical loading on nutrient supply. When disc hydration is recovered during rest at night (Boos, et al., 1993), cells could produce more ATP which could be released and accummulated in the ECM. Therefore, high accumulation of extracellular ATP in the NP region (Wang, et al., 2013) could play an important role in maintaining normal activities of IVD cells. It also suggests a mechanobiological pathway for regulating ECM biosynthesis via ATP metabolism (Fig. 8). Since the porcine model may not exactly simulate the conditions of human discs, future studies are required to confirm our findings on human IVD cells.
Fig. 8.
A postulated mechanobiological pathway regulates EMC biosynthesis in IVD cells via ATP metabolism. Mechanical loading stimulates ATP release (Czamanski, et al., 2011, Fernando, et al., 2011) via a transport mechanism, through a membrane channel, or by leakage through a damaged cellular membrane (Graff, et al., 2000). Extracellular ATP (eATP) activates P2 purinergic receptors on the cell membrane, which are involved in the ECM biosynthesis process (Chowdhury and Knight, 2006) and also in the production of ATP (Kwon, 2012, Kwon, et al., 2012). Mechanical loading may promote eATP hydrolysis (Wang, et al., 2013). Adenosine, which results from the hydrolysis of eATP, is uptaken into the cell and adenosine kinase rephosphorylates adenosine to AMP that is subsequently rephosphorylated into ATP (Andreoli, et al., 1990, Lasso de la Vega, et al., 1994), which serves as an energy source and building block for ECM biosynthesis
In summary, this study demonstrated that extracellular ATP promotes the biosynthesis of ECM and intracellular ATP production in IVD cells. Gene expression of aggrecan and type II collagen in NP and AF cells was also upregulated by extracellular ATP. In addition, NP cells were found to be less sensitive to low concentrations of extracellular ATP than AF cells while NP cells exhibited higher accumulation of PG, collagen, and intracellular ATP compared to AF cells.
ACKNOWLEDGEMENT
This study was supported by the grant AR056101 from the NIH and the VA Merit Review Grant. The authors thank Carlos Barrera and Brittany Rodriguez for their assistance in the IVD cells isolation.
REFERENCES
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 1976;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- Andreoli SP, Liechty EA, Mallett C. Exogenous adenine nucleotides replete endothelial cell adenosine triphosphate after oxidant injury by adenosine uptake. The Journal of laboratory and clinical medicine. 1990;115:304–313. [PubMed] [Google Scholar]
- Baer AE, Wang JY, Kraus VB, Setton LA. Collagen gene expression and mechanical properties of intervertebral disc cell-alginate cultures. J Orthop Res. 2001;19:2–10. doi: 10.1016/S0736-0266(00)00003-6. [DOI] [PubMed] [Google Scholar]
- Boos N, Wallin A, Gbedegbegnon T, Aebi M, Boesch C. Quantitative MR imaging of lumbar intervertebral disks and vertebral bodies: influence of diurnal water content variations. Radiology. 1993;188:351–354. doi: 10.1148/radiology.188.2.8327677. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. Journal of Cell Science. 1995;108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- Chou AI, Bansal A, Miller GJ, Nicoll SB. The effect of serial monolayer passaging on the collagen expression profile of outer and inner anulus fibrosus cells. Spine (Phila Pa 1976) 2006;31:1875–1881. doi: 10.1097/01.brs.0000229222.98051.9a. [DOI] [PubMed] [Google Scholar]
- Chowdhury TT, Knight MM. Purinergic pathway suppresses the release of .NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. Journal of cellular physiology. 2006;209:845–853. doi: 10.1002/jcp.20768. [DOI] [PubMed] [Google Scholar]
- Croucher LJ, Crawford A, Hatton PV, Russell RG, Buttle DJ. Extracellular ATP and UTP stimulate cartilage proteoglycan and collagen accumulation in bovine articular chondrocyte pellet cultures. Biochimica et Biophysica Acta. 2000;18:297–306. doi: 10.1016/s0925-4439(00)00055-7. [DOI] [PubMed] [Google Scholar]
- Czamanski J, Yuan TY, Fernando H, Castillo A, Gu WY, Cheung HS, Huang CY. Difference in energy metabolism of annulus fibrosus and nucleus pulposus cells of the intervertebral disc. Cellular and Molecular Bioengineering. 2011;4:302–310. doi: 10.1007/s12195-011-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fernando HN, Czamanski J, Yuan T-Y, Gu W, Salahadin A, Huang C-YC. Mechanical loading affects the energy metabolism of intervertebral disc cells. Journal of Orthopaedic Research. 2011;29:1634–1641. doi: 10.1002/jor.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- Graff RD, Lazarowski ER, Banes AJ, Lee GM. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis and Rheumatology. 2000;43:1571–1579. doi: 10.1002/1529-0131(200007)43:7<1571::AID-ANR22>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- Grunhagen T, Shirazi-Adl A, Fairbank JCT, Urban JPG. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthopedic Clinics of North America. 2011;42:465–477. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Tamura S, Tanaka K, Takagaki K, Saito Y, Endo M. Effects of ATP on regulation of galactosyltransferase-I activity responsible for synthesis of the linkage region between the core protein and glycosaminoglycan chains of proteoglycans. Biochemistry and Cell Biology. 2001;79:159–164. [PubMed] [Google Scholar]
- Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annual Review of Biochemistry. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- How-Ran G, Tanaka S, Halperin WE, Cameron LL. Back pain prevalence in US industry and estimates of lost workdays. American Journal of Public Health. 1999;89:1029–1035. doi: 10.2105/ajph.89.7.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton WC, Elmer WA, Boden SD, Hyon S, Toribatake Y, Tomita K, Hair GA. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine. 1999;24:1507. doi: 10.1097/00007632-199908010-00002. [DOI] [PubMed] [Google Scholar]
- Johnson K, Svensson CI, Etten DV, Ghosh SS, Murphy AN, Powell HC, Terkeltaub R. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis and Rheumatism. 2004;50:1216–1225. doi: 10.1002/art.20149. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis and Rheumatology. 2002;46:2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- Kasra M, Merryman WD, Loveless KN, Goel VK, Martin JD, Buckwalter JA. Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. Journal of Orthopaedic Research. 2006;24:1967–1973. doi: 10.1002/jor.20253. [DOI] [PubMed] [Google Scholar]
- Knight MM, Ghori SA, Lee DA, Bader DL. Measurement of the deformation of isolated chondrocytes in agarose subjected to cyclic compression. Medical Engineering & Physics. 1998;20:684–688. doi: 10.1016/s1350-4533(98)00080-0. [DOI] [PubMed] [Google Scholar]
- Korecki CL, Kuo CK, Tuan RS, Iatridis JC. Intervertebral disc cell response to dynamic compression is age and frequency dependent. Journal of Orthopaedic Research. 2009;27:800–806. doi: 10.1002/jor.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ. Extracellular ATP signaling via P2X (4) receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. Journal of Endocrinology. 2012;214:337–348. doi: 10.1530/JOE-12-0131. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Ohmiya Y, Honma Ki, Honma S, Nagai T, Saito K, Yasuda K. Synchronized ATP oscillations have a critical role in prechondrogenic condensation during chondrogenesis. Cell Death and Disease. 2012;3:e278. doi: 10.1038/cddis.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasso de la Vega MC, Terradez P, Obrador E, Navarro J, Pellicer JA, Estrela JM. Inhibition of cancer growth and selective glutathione depletion in Ehrlich tumour cells in vivo by extracellular ATP. Biochemical Journal. 1994;298(Pt 1):99–105. doi: 10.1042/bj2980099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Knight MMF, Bolton J, Idowu BD, Kayser MV, Bader DL. Chondrocyte deformation within compressed agarose constructs at the cellular and sub-cellular levels. Journal of Biomechanics. 2000;33:81–95. doi: 10.1016/s0021-9290(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. Journal of Orthopaedic Research. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Neidlinger-Wilke C, Wurtz K, Urban JP, Borm W, Arand M, Ignatius A, Wilke HJ, Claes LE. Regulation of gene expression in intervertebral disc cells by low and high hydrostatic pressure. Eur Spine J. 2006;15:6. doi: 10.1007/s00586-006-0112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman RE, Logan MA. The determination of hydroxyproline. Journal of Biological Chemistry. 1950;184:299–306. [PubMed] [Google Scholar]
- Ohshima H, Urban JPG, Bergel DH. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. Journal of Orthopaedic Research. 1995;13:22–29. doi: 10.1002/jor.1100130106. [DOI] [PubMed] [Google Scholar]
- Paesold G, Nerlich A, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J. 2007;16:447–468. doi: 10.1007/s00586-006-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. Journal of Orthopaedic Research. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 1976;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- Urban JPG, Smith S, Fairbank JCT. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. 2710.1097/2701.brs.0000146499.0000197948.0000146452. [DOI] [PubMed] [Google Scholar]
- Usprech J, Chu G, Giardini-Rosa R, Martin K, Waldman SD. The Therapeutic Potential of Exogenous Adenosine Triphosphate (ATP) for Cartilage Tissue Engineering. Cartilage. 2012;3:364–373. doi: 10.1177/1947603512444723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira VP, Rocha JB, Stefanello FM, Balz D, Morsch VM, Schetinger MR. Heparin and chondroitin sulfate inhibit adenine nucleotide hydrolysis in liver and kidney membrane enriched fractions. The international journal of biochemistry & cell biology. 2001;33:1193–1201. doi: 10.1016/s1357-2725(01)00083-8. [DOI] [PubMed] [Google Scholar]
- Waldman SD, Usprech J, Flynn LE, Khan AA. Harnessing the purinergic receptor pathway to develop functional engineered cartilage constructs. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:864–872. doi: 10.1016/j.joca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. Journal of Biomechanics. 2004;37:329–337. doi: 10.1016/s0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- Wang C, Gonzales S, Levene H, Gu W, Huang CY. Energy metabolism of intervertebral disc under mechanical loading. Journal of Orthopaedic Research. 2013;31:1733–1738. doi: 10.1002/jor.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]