Abstract
Transplantation of neural progenitor cells (NPCs) may be a potential treatment strategy for traumatic brain injury (TBI) due to their intrinsic advantages, including the secretion of neurotrophins. Neurotrophins are critical for neuronal survival and repair, but their clinical use is limited. In this study, we hypothesized that pericontusional transplantation of NPCs genetically modified to secrete a synthetic, human multineurotrophin (MNTS1) would overcome some of the limitations of traditional neurotrophin therapy. MNTS1 is a multifunctional neurotrophin that binds all three tropomyosin-related kinase (Trk) receptors, recapitulating the prosurvival activity of 3 endogenous mature neurotrophins. NPCs obtained from rat fetuses at E15 were transduced with lentiviral vectors containing MNTS1 and GFP constructs (MNTS1-NPCs) or fluorescent constructs alone (control GFP-NPCs). Adult rats received fluid percussion-induced TBI or sham surgery. Animals were transplanted 1 week later with control GFP-NPCs, MNTS1-NPCs, or injected with saline (vehicle). At five weeks, animals were evaluated for hippocampal-dependent spatial memory. Six weeks post surgery, we observed significant survival and neuronal differentiation of MNTS1-NPCs and injury-activated tropism towards contused regions. NPCs displayed processes that extended into several remote structures, including the hippocampus and contralateral cortex. Both GFP- and MNTS1-NPCs conferred significant preservation of pericontusional host tissues and enhanced hippocampal neurogenesis. NPC transplantation improved spatial memory capacity on the Morris water maze (MWM) task. Transplant recipients exhibited escape latencies approximately half that of injured vehicle controls. While we observed greater transplant survival and neuronal differentiation of MNTS1-NPCs, our collective findings suggest that MNTS1 may be superfluous in terms of preserving the cytoarchitecture and rescuing behavioral deficits given the lack of significant difference between MNTS1- and GFP-control transplanted groups. Nevertheless, our overall findings support the potential of syngeneic NPC transplantation to enhance endogenous neuroreparative responses and may therefore be an effective treatment for TBI.
Keywords: Traumatic brain injury, neural progenitor cell transplantation, multineurotrophin, hippocampal neurogenesis, neuroprotection, spatial memory
Introduction
Traumatic brain injury (TBI) is a significant global health problem that often results in long-lasting impairment. Functional deficits result from a combination of pathological events that include selective neuronal loss, damage to the microenvironment, as well as decreased levels of hippocampal neurogenesis, which is crucial for hippocampal-dependent memory (Yu et al., 2008; Zhao et al., 2008).
Neurotrophins contribute to the functional integrity of the CNS through regulation of neuronal survival, differentiation, repair, neurite outgrowth, synaptic plasticity, and apoptosis (Chao, 2003). Each mature neurotrophin has a cognate Trk receptor. Through these specific interactions, neurotrophin-Trk signaling increases the expression of survival-promoting genes, prodifferentiation genes, and other substrates involved in synaptic plasticity (Reichardt, 2006). Neurotrophin-Trk interactions have clinical potential due to intrinsic neurorestorative activity. However, there are some limitations to using neurotrophins therapeutically, such as short half-lives, negligible blood brain barrier permeability, and limited diffusion in CNS parenchyma (Lessman et al., 2003). Furthermore, cells express Trk receptors differentially and thus may only be responsive to cognate neurotrophins. The generation of multitargeting neurotrophins with broader binding specificities may therefore be an effective treatment for promoting protection and recovery after TBI.
NPCs possess tropic properties, maintain multipotency, and can be genetically modified to deliver potentially therapeutic molecules (Gage and Temple, 2013). Transplanted NPCs can integrate within existing host circuitry, provide and provoke trophic support, and modulate host immune responses (Cossetti et al., 2012). Important to this study, NPC-mediated trophic secretion can mobilize endogenous stem cells and enhance neuroregenerative responses, such as hippocampal neurogenesis, within the injured milieu (Shetty et al., 2014).
Hippocampal neurogenesis occurs continually throughout the life of most mammals (Ming and Song, 2011). Active neural stem cells (NSCs) residing in the subgranular zone (SGZ) of the adult dentate gyrus (DG) give rise to doublecortin (DCX)-positive immature neurons, which make unique contributions to specific aspects of hippocampal-dependent function, including spatial memory (Deng et al., 2010). CNS injury induces acute neurogenic responses, which have been shown to contribute to some degree of recovery after TBI (Blaiss et al., 2011). However, this endogenous neuroreparative response is insufficient as residual cognitive deficits persist. Therapeutic strategies that enhance endogenous neuroreparative responses may augment recovery processes and remain a critical area of study.
We sought to enhance the inherent salutary effects of NPCs through genetic modification. We investigated the potential benefits of transplanting NPCs that were transduced to continually secrete MNTS1, a multineurotrophin with multiple neurotrophic specificities. Through the exchange and mutation of 8 amino acid residues on mature human NT-3, Urfer et al. (1994) generated a human multifunctional, multitargeting molecule that retains the capacity to bind all Trk receptors and supports the survival of NGF-, BDNF-, and NT-3-responsive neurons.
The objective of this study was to assess histopathological and functional outcomes with transplantation of control NPCs and MNTS1-expressing NPCs in an experimental model of TBI. We hypothesized that engaging all Trk signaling cascades via MNTS1, together with the intrinsic advantages of NPC transplantation, would result in significant rescue of neuropathological outcomes and augmentation of endogenous reparative responses after moderate TBI.
Materials and Methods
Construction of the MNTS1 Lentivirus
The MNTS1 molecule was generated via selective point mutations and amino acid residue exchanges on a mature human NT-3 backbone as discussed previously (Urfer et al., 1994). MNTS1 complementary DNA was produced synthetically by GeneArt® Gene Synthesis (Life Technologies, Carlsbad, CA) and subcloned into the lentiviral vector pRRLsinPPT-CMV-MCS-WPRE as previously described (Dull et al., 1998). The lentiviral particles were generated by The Miami Project Viral Vector Core of University of Miami Miller School of Medicine. Lentiviruses were produced using the four-plasmid method (Follenzi and Naldini, 2002). Viral titers, shown as transducing units from 107 to 108, were determined by a p24 enzyme-linked immunosorbent assay (ELISA) assay (Perkin Elmer, Waltham, MA) used to quantify p24 core protein concentrations. Purified lentiviral particles were stored at −80°C until use.
Isolation and Preparation of Neural Progenitor Cells
NPCs were isolated from Sprague Dawley rat fetuses at embryonic stage E15. Frontal cortical tissue was microdissected in Lebovitz’s L-15 medium (GIBCO/Life Technologies, Carlsbad, CA). Cortical tissue was transferred to a conical tube, titrated to obtain a single-cell solution, and counted using trypan blue (Life Technologies). Between 600,000 to 700,000 cells were placed on sterile, polystyrene 10 cm tissue culture dishes (Corning, Corning, NY) in NeuralCult proliferation medium solution (StemCell Technologies, Vancouver, BC) that was selective for neurospheres and supplemented with penicillin-streptomycin, 10 μg/mL of basic fibroblast growth factor (bFGF), 10 μg/mL epidermal growth factor (EGF), and 0.2% Heparin. Two days later, neurospheres were infected with lentiviral vectors containing MNTS1 and GFP (pLV-eGFP) constructs, or GFP and blue fluorescent protein (BFP; pLV-EBFP2-nuc) constructs, and 2–3 mL of fresh medium was added to dishes. After two days of proliferation, NPCs were assessed for positive lentiviral transduction and cell viability.
If infection was positive, cells were plated on 10 cm fibronectin (Sigma-Aldrich, St. Louis, MO)-coated tissue culture dishes and allowed to adhere and colonize for an additional 1–2 days. On day of transplantation, NPCs were trypsinized with Hank’s Balanced Salt Solution (HBSS; GIBCO/Life Technologies, Carlsbad, CA) and centrifuged at 2000 rpm for 4.5 min. Medium was carefully removed and pellet was resuspended in fresh medium. Cells were assessed for viability and counted using trypan blue in a hemacytometer, followed by microcentrifugation at 6500 rpm for 1.5 min. One million cells were resuspended in 10 μl of fresh medium (for a final volume of 100,000/μl) and kept on ice until transplantation (< 30 minutes).
Animals
Adult male Sprague Dawley rats (n = 58) were randomly assigned to 1 of 6 groups: sham/vehicle (n = 12), sham/GFP-NPCs (n = 8), sham/MNTS1-NPCs (n = 7), TBI/vehicle (n = 15), TBI/GFP-NPCs (n = 8), or TBI/MNTS1-NPCs (n = 8). Animal care was in accordance with the guidelines set forth by the University of Miami Animal Care and Use Committee and the NIH Guide for the Care and Use of Laboratory Animals. Animals were housed in a temperature-controlled room (22°C) with a 12-h light/dark cycle and allowed at least 7 days of acclimation before undergoing any experimentation. All animals had access to food and water ad libitum, except for a 24 h fast before the surgical procedure in order to maintain consistent glucose levels.
Fluid-Percussion Traumatic Brain Injury
Rats were anesthetized with 3% isoflurane in 30% O2/70% N2O and placed in a stereotaxic frame. Animals received a 4.8 mm craniotomy over the right parietal cortex, 3.8 mm posterior to bregma and 2.5 mm lateral to midline. A modified plastic injury hub (3.5 mm inner diameter) was bonded to the skull over the exposed dura with cyanoacrylic adhesive. Twenty-four h after the craniotomy, animals were reanesthetized, intubated, and mechanically ventilated with 0.5–0.75% isoflurane. Pancuronium bromide (1.0 mg/kg) was administered intravenously to facilitate ventilation. The tail artery was cannulated to ensure blood gases, pH, glucose, and mean arterial blood pressure (MABP) measurements were consistent among animals. Rectal and temporalis muscle thermometers measured body and brain temperatures using self-adjusting feedback warming lamps. All variables were maintained within physiological ranges from 15 min prior to TBI and for up to 30 min post injury. A moderate (1.8–2.2 Atmospheres) fluid percussion-induced TBI was produced over the right parietal cortex. Sham animals underwent identical procedures minus the fluid percussion insult.
Physiology
All physiological variables including pH, pO2, pCO2 and MABP were within normal ranges before and after the fluid percussion insult. Animals displayed normal activity within 24 h after recovery from anesthesia as detected by visual inspection of grooming behavior, posture, and locomotion. There was no significant weight loss in any experimental group 7 days post surgery or at the time of perfusion-fixation (6 weeks post surgery).
NPC Transplantation
One week after TBI or sham surgery, animals were returned to a stereotaxic frame and reanesthetized with 3% isoflurane in 30% O2/70% N2O. Staples were removed and craniotomy was identified. Stereotaxic injection coordinates were as follows: −3.5 and −3.0 mm below dura, −4.3 mm posterior to bregma, and −5.0 mm lateral to midline. Four μl of medium containing 400,000 NPCs (100,000 cells per μl) were aspirated into a Gastight® 26 gauge microsyringe (Hamilton Co., Reno, NV). Using a motorized stereotaxic injector (Stoelting Co., Wood Dale, Illinois), medium + cells were slowly injected into the right parietal cortex at a rate of 1 μl per minute. 200,000 cells were transplanted at each depth. Before and after each injection, the syringe was allowed to remain in the parenchyma for 2 min for tissue acclimation. Cells were transplanted over the course of 12 min. Vehicle groups were injected with 4 μl of 0.9% sterile saline using identical transplantation procedures and coordinates. After the final injection, animals were restapled and returned to their home cages. All animals displayed normal activity within 24 h after recovery from anesthesia. All animals received 10 mg/kg of the immunosuppressant, cyclosporine A (CsA; Novartis Pharmaceuticals Corp., East Hanover, NJ). Intraperitoneal (IP) injections of CsA began 2 days prior to transplantation procedure and continued daily for 14 days post transplantation. A subset of animals (total n = 25; sham/vehicle = 5; sham/GFP = 4; sham/MNTS = 3; TBI/vehicle = 5; TBI/GFP = 3; TBI/MNTS1 =5) received injections of a concentrated aqueous solution of 5-bromo-2′-deoxyuridine (BrdU) and 5-fluoro-2′-deoxyuridine (FdUrd) labeling reagent (10:1; 1 mL/100g of rat bodyweight administered IP; Invitrogen Life Technologies, Grand Island, NY) beginning day of transplantation and daily thereafter for 7 days.
Cognitive Assessment
Five weeks after TBI or sham surgery, spatial memory was evaluated via the Morris water maze (MWM), which consisted of a circular pool (122 cm diameter, 60 cm deep) filled with water rendered opaque by white, non-toxic paint. Submerged heaters maintained the water temperature between 22–25°C. A round platform (10 cm diameter) was submerged 5 cm beneath the water surface. The pool was divided into 4 equal-sized quadrants, one of which included the hidden platform. Prominent extramaze cues served as visual reference points. Swim patterns were recorded and analyzed via Ethovision (Noldus Information Technology, Leesburg, VA). MWM parameters evaluated included escape latency (time to locate platform), percentage of total time spent in each quadrant, and swim speed.
Animals were tested for 3 consecutive days, each day consisting of 4 trials at 5-min intervals. For each trial, animals were released from the edge of the pool into a randomly selected quadrant. Release point order was identical for all animal groups. During each trial, the animals were allowed 60 sec to find the hidden platform. If they failed to locate it, they were guided to the platform. Animals were required to remain on the platform for an additional 30 sec for memory acquisition training.
Immunohistochemistry
At 6 weeks post TBI or sham procedures, animals were anesthetized with 3% isoflurane in 30% O2/70% N2O and transcardially perfused with saline (80 mL for 2 min) followed by 4% paraformaldehyde (4°C, 350 mL at a pressure of 100–120 mmHg for 28 min). Brains were immediately removed, placed in 4% paraformaldehyde for 48 h and then cryoprotected in 20% sucrose in phosphate-buffered saline. Brains sections (45 μm thickness) were cut with a frozen sliding microtome (LEICA SM200R, Leica Microsystems, Inc., Buffalo Grove, IL). Serial sections at 270 μm intervals were immunostained with hematoxylin and eosin (H&E), rat anti-BrdU (when applicable, 1:150), mouse anti-neuronal nuclei (NeuN; 1:400), chicken anti-GFP (1:2000), mouse anti-glial fibrillary acidic protein (GFAP; 1:2000), rabbit anti-Olig2 (1:250), rabbit anti-NG2 (1:500), rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1; 1:1000), or mouse anti-aldehyde dehydrogenase 1 (Aldh1; 1:800) as previously described (Atkins et al., 2010; Bregy et al., 2012). Development of immunofluorescent staining was conducted with goat anti-rat Alexa Fluor 488 (1:200), goat anti-chicken Alexa Fluor 488 (1:200), goat anti-mouse Alexa Fluor 568/594/647 (1:200), or goat anti-rabbit Alexa Fluor 568/594/647 (1:200) depending on the outcome measure.
Volumetric and Stereological Analyses
Antibody penetration was verified in all sections using 100x magnification. Cortical contusion volumes were determined by tracing the contused areas in serial H&E sections with a 5x objective on an Axiophot 200 M microscope (Zeiss Microscopy, LLC, Thornwood, NY) using Neurolucida software (MicroBrightfield, Inc., Williston, VT). Cortical contusion boundaries were well demarcated by hemorrhage and shearing at the gray/white matter interface between the cortex and external capsule in the ipsilateral hemisphere. Serial bregma levels were observed beginning at −1.0 mm posterior to bregma. At first indication of contusion, all subsequent bregma levels were included for volumetric analysis up until −6.8 mm posterior to bregma. Separate serial sections from −3.0 to −5.8 mm posterior to bregma were chosen to determine neuron survival within parietal cortical regions. NeuN-immunoreactive cells were quantified by a blinded observer in an unbiased manner using Stereoinvestigator software (MicroBrightfield, Inc; Gundersen et al., 1988). The parietal cortex overlying the contused area was contoured at 4x. The epicenter of neuronal loss was identified and a border was defined 1000 μm to the right and to the left of the epicenter to give a total rectangular contour width of 2000 μm. A counting grid of 250 × 250 μm was placed over the contoured parietal cortex. Using a 70 × 70 μm counting frame, NeuN-positive cells were counted in 30–40 randomly-placed sampling sites with a 60x 1.4 NA objective to obtain an estimated population of cells per μm3.
Stereoinvestigator software was also used to conduct quantitative assessment of BrdU/NeuN-immunoreactive cells in the ipsilateral hippocampus. The DG cell layer was contoured using a BX51TRF Olympus microscope (Olympus America, Center Valley, PA, USA) at 4x magnification. Using a PlanApo N 60x/1.42 oil objective, a blinded observer analyzed 5 sections between bregma levels −3.8 mm and −5.0 mm posterior to bregma with a 100 × 100 μm counting frame and 115 × 115 μm counting grid in 40–55 randomly-placed sampling sites. To determine numbers of internalized GFP- and GFP/NeuN-positive cells in transplanted animals, all sections positive for GFP-positive cell bodies in a single series were quantified.
Statistics
In all quantification and analysis procedures, observers were blinded to the nature of the experimental manipulation. Data are expressed as mean ± standard error of the mean (SEM). GFP-positive cell survival and neuronal differentiation quantifications were analyzed using the Mann-Whitney Rank Sum test. Contusion volumes and cortical neuron protection were analyzed using one-way analysis of variance (ANOVA). Double-labeled BrdU/NeuN-positive cell quantifications were analyzed using the Student’s t-test. Significance of MWM outcomes was determined using two-way repeated measures ANOVA or Student’s t-test depending on the outcome measure. Post-hoc analysis was performed using Student-Newman-Keuls (SNK) corrections. Significance level was p < 0.05 using two-tailed testing.
Results
Increased Survival and Neuronal Differentiation of MNTS1-Secreteing NPCs
One week after TBI or sham surgery, animals were transplanted with either GFP-control NPCs, MNTS1-transduced NPCs, or injected with saline (vehicle). NPCs were transplanted pericontusionally into the right parietal cortex of the traumatized hemisphere at two different depths. The transplantation coordinates utilized in the present study were selected after a series of optimization experiments and deemed most conducive for graft migration. Five weeks after transplantation, animals were sacrificed for immunohistochemical analysis.
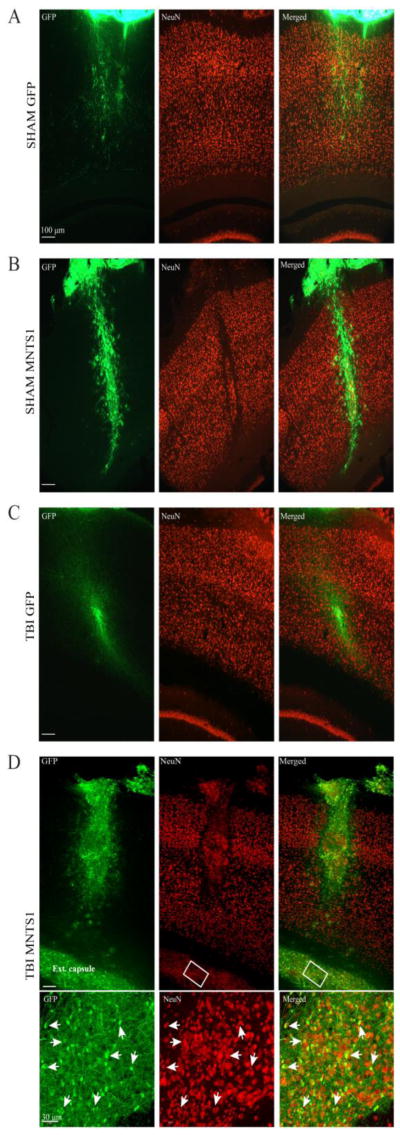
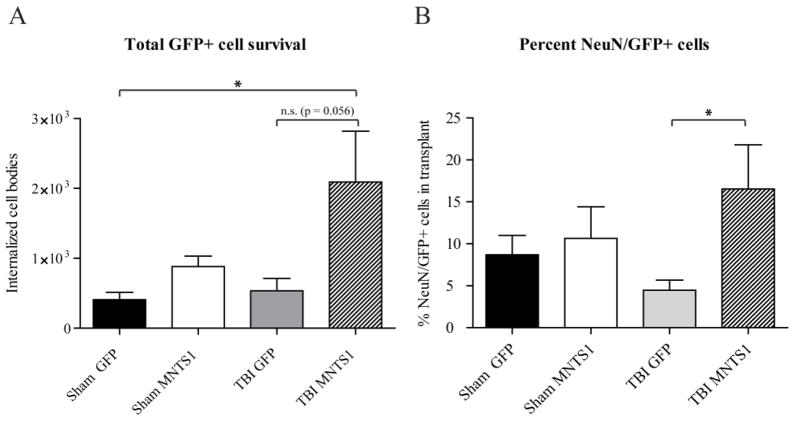
NPCs express Trk receptors (Barnabe-Heider and Miller, 2003). Thus, based on our hypothesis that MNTS1 would engage a greater number of Trk-responsive neurons in prosurvival signaling pathways, we sought to determine whether transplanted NPCs secreteing MNTS1 themselves displayed greater survival rates relative to control NPCs 6 weeks post TBI or sham surgery. Internalized (defined as cells ventral to dura mater) GFP-positive cell bodies were compared across all transplanted groups (Figure 1A–D). Non-biased stereological analyses revealed significantly greater levels of MNTS1-transduced NPCs in the traumatized brain relative to GFP-control NPCs in sham animals (p = 0.03; Figure 2A). We did not detect a statistically significant difference between GFP-positive NPC survival in TBI/MNTS1 animals compared to cells in TBI/GFP animals, however a trend towards significance was evident (p = 0.056). Consistent with our hypothesis, MNTS1-NPCs in sham animals also exhibited slightly elevated survival rates compared to GFP-control NPCs, albeit not significantly so.
Figure 1.
GFP-positive cell survival and neuronal differentiation 6 weeks post surgery. A-D) Representative images showing GFP-positive transplanted NPCs from a sham/GFP, sham/MNTS1, TBI/GFP, and a TBI/MNTS1 animal, respectively. B, D) Several MNTS1-transduced NPCs were also immunoreactive for NeuN, indicating neuronal differentiation. D) After TBI, numerous MNTS1-transduced NPCs were observed ventrolateral from needle tract and within the external capsule. Boxed region in D demarcates area of high magnification in bottom panel. Arrows indicate transplanted NPCs with GFP/NeuN double-labeled immunoreactivity. Green, GFP; red, NeuN; ext. capsule, external capsule.
Figure 2.
Stereological assessment of transplanted GFP-positive cell survival and neuronal differentiation. A) TBI/MNTS1 animals had greater GFP-positive cell survival compared to control GFP-NPCs transplanted into sham animals. B) The percentage of NeuN/GFP-double labeled cells was significantly increased in TBI/MNTS1 animals relative to the TBI/GFP group. Data are expressed as mean ± SEM. *p < 0.005.
Mature neurotrophin-Trk interactions also mediate neuronal differentiation (Reichardt, 2006). Thus, we sought to quantify levels of grafted NPCs that exhibited a neuronal phenotype. NeuN is a reliable marker to identify mature neurons. Quantification of double-labeled GFP/NeuN-positive cells revealed significant neuronal differentiation in TBI/MNTS1-NPC grafts compared to the GFP-control TBI group at 6 weeks post injury (p = 0.016; Figure 2B). Although brain trauma coupled with MNTS1-transduction induced greater neuronal differentiation of transplanted NPC, levels of grafted NeuN-positive NPCs in the sham/MNTS1 group trended toward significance relative to the injured GFP control transplanted group.
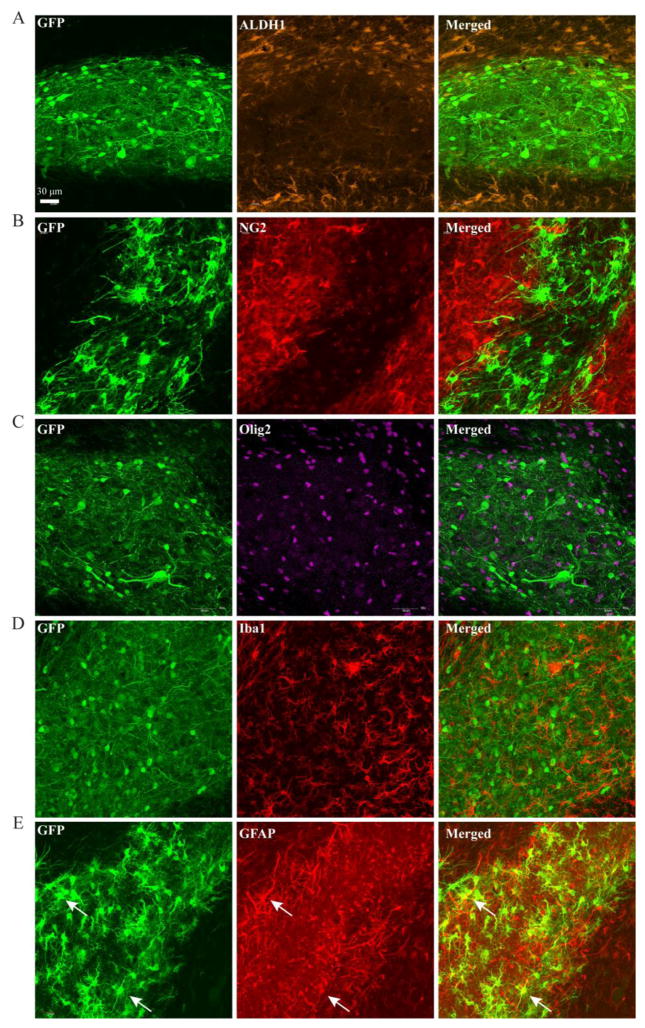
Additional cell lineage markers were utilized to detect non-neuronal phenotypes. We assessed MNTS1-transduced NPCs in both sham and injured animals. As demonstrated in Figure 2A, there was significantly less survival of GFP-control NPCs and, of those that were present, the majority appeared undifferentiated (data not shown). Immunohistochemical findings demonstrated that transplanted NPCs did not co-express markers for mature astrocytes (Aldh1), oligodendrocytes or oligodendrocyte progenitor cells (Olig2 and NG2, respectively), or activated microglia/macrophages (Iba1; Figure 3A–D). Some grafted NPCs did express GFAP, a lineage marker indicative of astrocytes, however this expression is consistent with the immature glial-like NPC phenotype (Figure 3E).
Figure 3.
Cell lineage markers to determine transplanted NPC fate in MNTS1 animals. A–D) Transplanted NPCs were negative for Aldh1 (a mature astrocyte marker), negative for NG2 and Olig2 expression (markers for oligodendrocyte precursor cells and mature oligodendrocytes, respectively), and negative for Iba1 expression (marker of activated microglia/macrophages). E) There was some co-localization of NPCs and GFAP (astrocytic marker), which is consistent with immature glial-like progenitor cells. Images were taken from transplant grafts residing in the external capsule 5 weeks post transplantation.
Injury-Induced Tropism and Long-Distance Projections of Transplanted NPCs
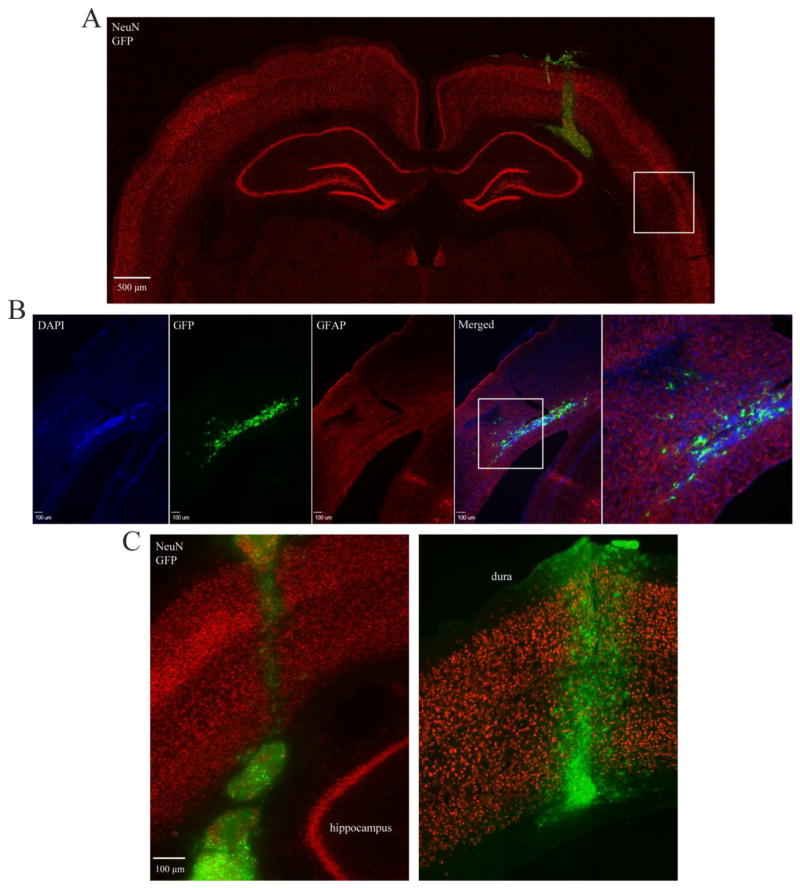
Five weeks following transplantation, exogenous NPCs in TBI animals were observed ventrolateral from the cortical needle tract, within the external capsule and oriented towards areas of injury (Figure 4A). Several NPCs were observed directly ventral to the contused region within the parietal cortex (Figure 4B). We observed injury-induced tropism of transplanted NPCs in both GFP-control and MNTS1-transduced NPC groups (Figure 4C, left panel), however cell body translocation appeared more robust in some MNTS1 animals (data not shown). Exogenous NPCs in sham uninjured animals had negligible tropism with the majority of neural progenitor cell bodies remaining within or directly proximal to the needle tract (Figure 4C, right panel).
Figure 4.
Injury-induced tropism of transplanted NPCs 5 weeks post transplantation. A) Montage showing MNTS1-transplant location within injured rat brain 6 weeks post TBI. Box demarcates area of neuronal fallout. Green, GFP; red, NeuN. B) Tropic MNTS1-NPCs appeared to reenter cortex at contused cortical regions. Box demarcates magnified image in last panel. Blue, dapi; green, GFP; red, GFAP. C, left) Representative image showing tropism of MNTS1-transduced cells 6 weeks after TBI. Numerous NPCs were no longer evident at transplantation coordinates. Cells were observed in the lateral external capsule, ventral to parietal cortex and oriented towards lesions. C, right) Representative image of limited NPC tropism in a sham/MNTS1 animal 6 weeks post surgery. Transplant cell survival was evident, which we attributed to MNTS1-transduction. Green, GFP; red, NeuN.
All transplanted groups, irrespective of injury or transduction profile, displayed long neurites projecting from NPC grafts. Projections were extensive and infiltrated several remote cortical and subcortical brain structures, including the hippocampus (Figure 5, 6A). GFP-positive processes were observed from bregma levels −2.8 to −5.3 and in both hemispheres, with several processes crossing the corpus callosum (Figure 6B). In injured animals, several processes appeared to target areas of tissue pathology, including gray/white matter interface lesions and cortical contusions (Figure 6C). All transplanted groups displayed extensive processes, however they appeared more prominent in MNTS1-transduced animals (data not shown). This observation supports our previous finding that MNTS1 transduction significantly augments NPC graft survival. Furthermore, the majority of GFP-positive neurites exhibited spine-like formations throughout the length of the process (Figure 6A, 7A). Additional qualitative observations revealed that several transplanted NPCs appeared directly proximal to host neurons (GFP-negative; NeuN-positive; Figure 7B).
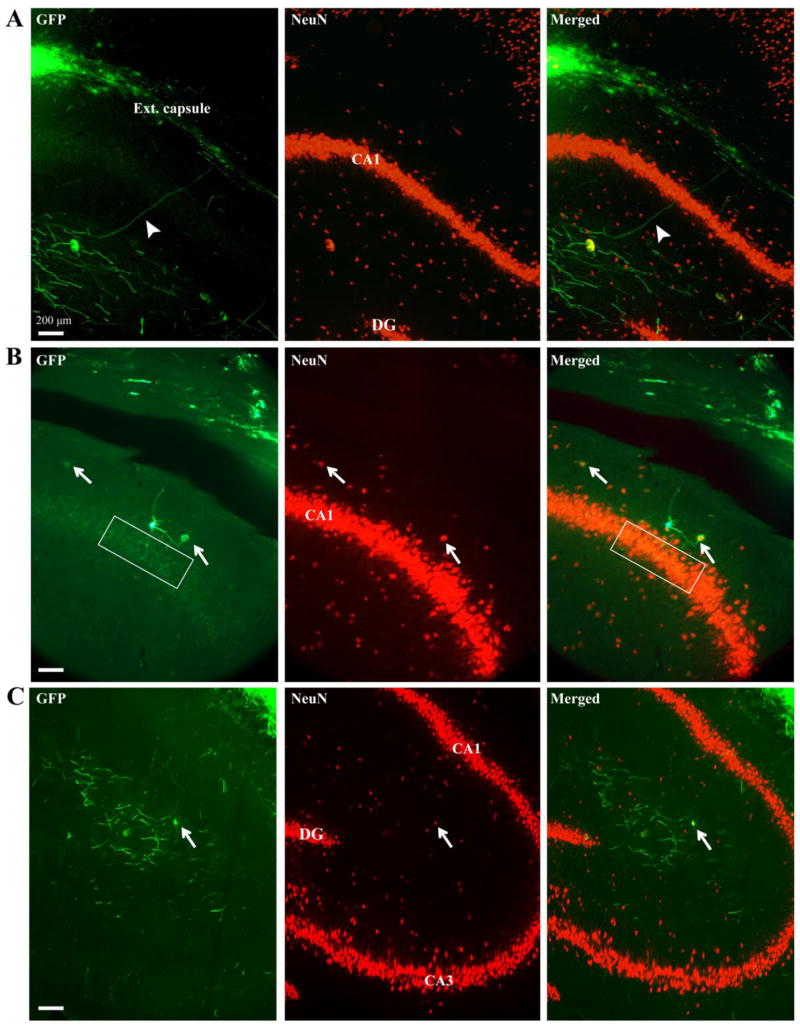
Figure 5.
GFP-positive processes and some GFP/NeuN-positive cell bodies infiltrated the hippocampus in both injured and non-injured animals 6 weeks post surgery. A) NPC grafts residing in external capsule extended GFP-positive processes (arrowhead) through CA1 of the hippocampus and into the stratum radiatum. B) GFP-positive cell bodies, one of which was also NeuN-positive (arrow), extended processes into CA1 of the hippocampus (boxed region). C) GFP-positive processes and GFP/NeuN-positive cell body (arrow) were observed within the stratum radiatum. Green, GFP; red, NeuN; ext. capsule, external capsule; DG, dentate gyrus; CA, cornu Ammonis.
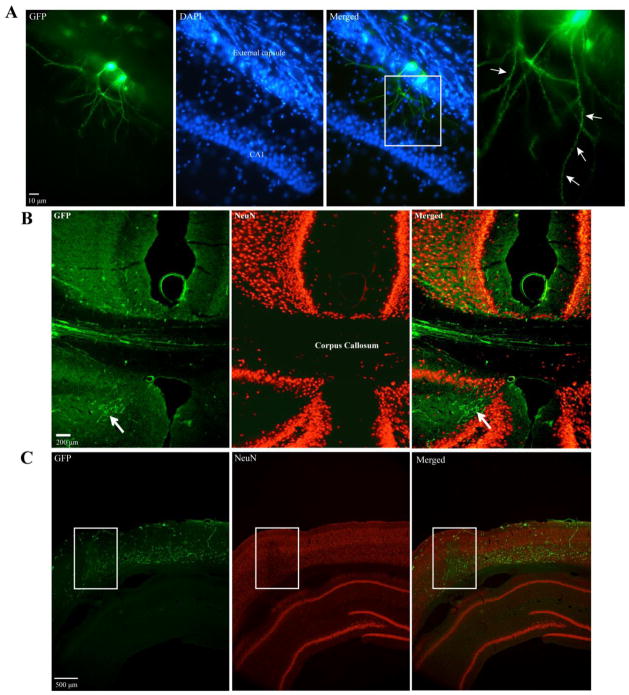
Figure 6.
Neurite extension from transplanted NPCs was observed in all transplanted groups 5 weeks post transplantation. A) GFP-positive cell bodies in ventral external capsule extended processes into CA1 of ipsilateral hippocampus. Box demarcates magnified image in last panel. Arrows indicate spine-like formations on GFP-positive processes. Representative image from a sham/GFP animal. B) GFP-positive processes originating from transplanted NPCs extended into the contralateral cortex via the corpus callosum. GFP-positive projections were observed in all transplanted animals irrespective of injury or transduction profile. Arrow indicates NPC processes in subcortical regions. Representative image from a sham/MNTS1 animal. C) GFP-positive processes infiltrated areas of pericontusional neuronal fallout in parietal cortex. Box demarcates region of NeuN loss 6 weeks after TBI. Green, GFP; blue, dapi; red, NeuN.
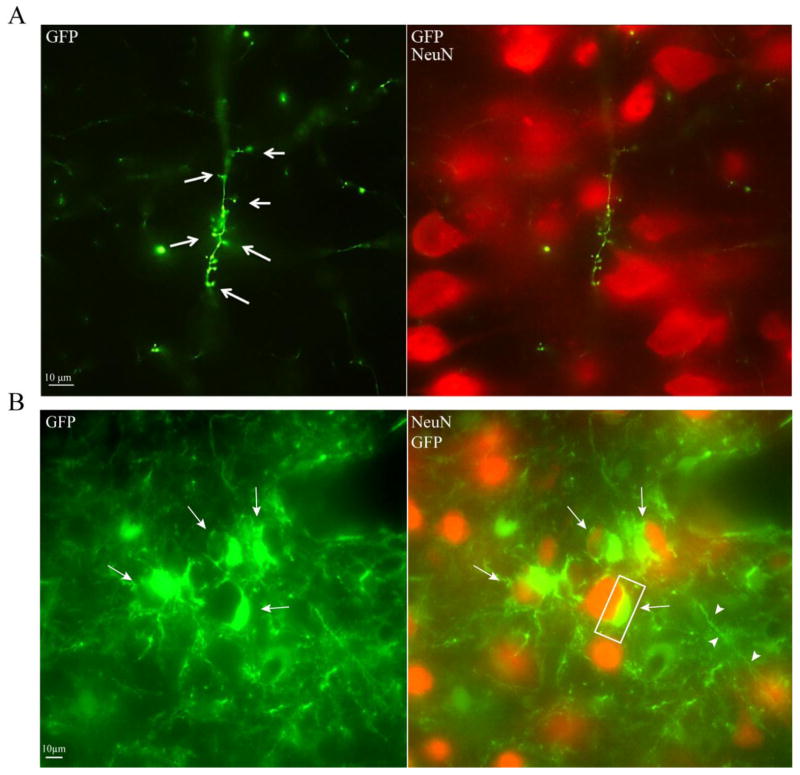
Figure 7.
Spine-like formations and host-donor proximity. A) Spine-like formations observed on a GFP-positive process extending from NPC transplant. B) Representative image of sham/MNTS1 animal showing close proximity between GFP-positive transplanted NPCs and host neurons. Arrows indicate transplanted cell bodies that appear directly adjacent to host neurons. Box demarcates area of possible interaction (yellow). Green, GFP; red, NeuN.
NPC Transplantation Significantly Improved Histopathological Outcomes after TBI
Consistent with this rodent model of moderate fluid percussion TBI, we observed well-demarcated lesions along the ipsilateral gray/white matter interface between the parietal cortex and external capsule, as well as selective fallout of vulnerable parietal neurons adjacent to the cortical contusion. Although exogenous NPC survival and neuronal differentiation was significantly greater in MNTS1 animals, we found that both transplanted groups demonstrated significant histological improvement 6 weeks after TBI (Figure 8, 9).
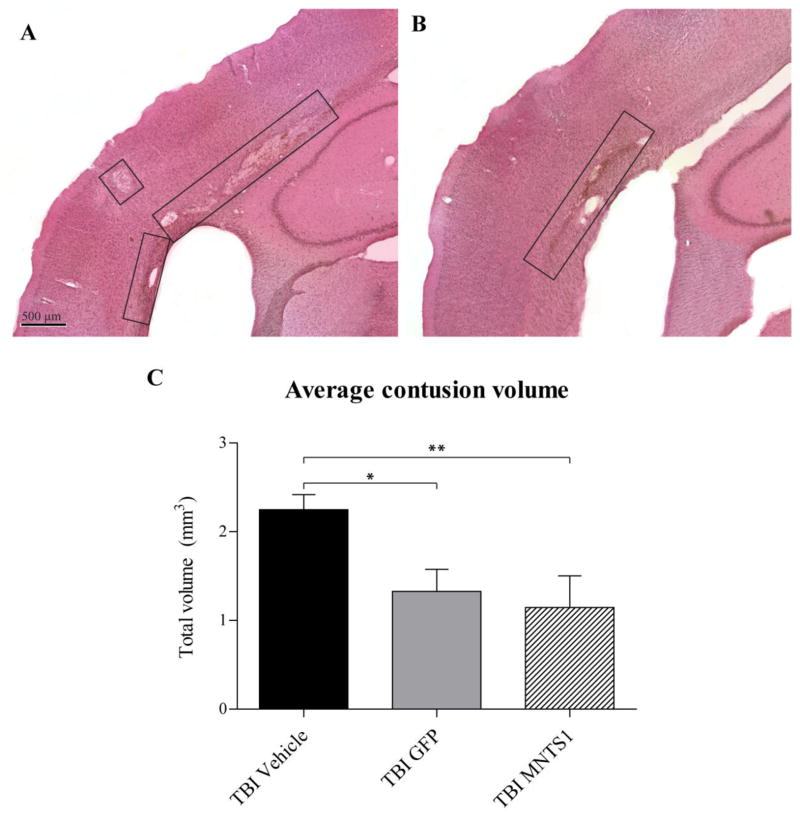
Figure 8.
Transplanted NPCs were cytoprotective at contusion site. Representative images of lesions (boxes) from a TBI/vehicle (A) and TBI/MNTS1 (B) animal 6 weeks post TBI. (C) Volumetric analyses demonstrated significantly reduced contusion volumes in transplanted TBI animals compared to vehicle-injected TBI animals (*p < 0.05; **p < 0.01).
Figure 9.
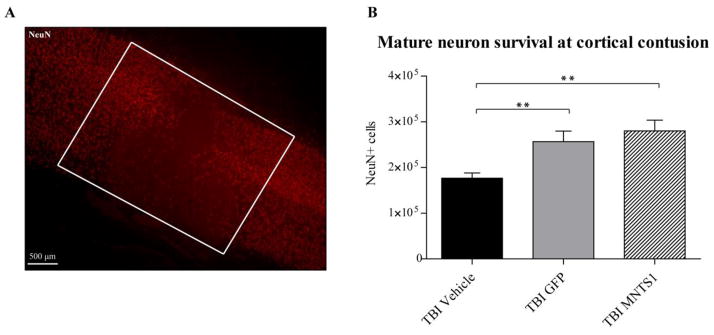
Transplanted NPCs protected vulnerable parietal neurons at cortical contusion. A) Representative image showing NeuN-positive cell loss at cortical contusion 6 weeks post TBI (boxed region). B) Quantification of neuronal survival within the cortical contusion showed that animals receiving NPCs had significantly increased levels of NeuN-positive cells compared to TBI vehicle animals (**p < 0.001).
Volumetric analysis of brain contusions is a reliable measure of injury severity and corresponding neurological impairment. Six weeks after TBI, we observed a significant reduction in total average contusion volume in both MNTS1- and GFP-control transplanted groups compared to their TBI vehicle counterparts (p = 0.004; SNK: TBI/vehicle vs. TBI/GFP: p = 0.014; TBI/vehicle vs. TBI/MNTS1: p = 0.008; Figure 8). Quantification of mature host neurons at parietal contusions revealed similar findings. NPC transplantation, with or without MNTS1 transduction, was associated with significantly greater levels of NeuN-positive cell survival at the cortical contusion relative to vehicle-injected animals 6 weeks post TBI (p < 0.001; SNK: TBI/vehicle vs. TBI/GFP: p = 0.002; TBI/vehicle vs. TBI/MNTS1: p = 0.002; Figure 9).
Our findings suggest that both transplanted groups, receiving either GFP-control or MNTS1-transduced NPCs, exhibited significant neuroprotection and preservation of surrounding cytoarchitecture 6 weeks after injury. Contrary to our original hypothesis, MNTS1 was not superior in improving histopathological outcomes.
NPC Transplantation Increased Endogenous DG Neurogenesis
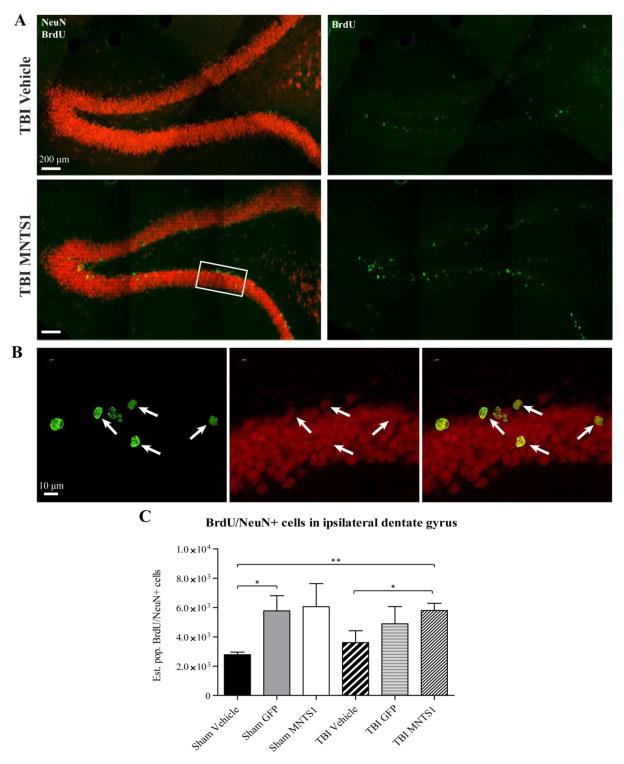
To determine levels of newly-generated neurons in the hippocampal DG, a subset of animals was injected with BrdU, a thymidine analog marker for dividing cells that is incorporated into newly replicated DNA during the S-phase of the cell cycle. Quantification of the number of BrdU/NeuN-positive cells in the ipsilateral DG across groups demonstrated considerable differences between NPC-receiving animals and vehicle-injected control groups 6 weeks post TBI (Figure 10). Sham/GFP animals had significantly more BrdU/NeuN-positive cells in the ipsilateral DG compared to their sham/vehicle counterparts (sham/vehicle vs. sham/GFP: p = 0.032). After TBI, MNTS1 animals had significantly greater levels of endogenous hippocampal neurogenesis compared to both injured and uninjured vehicle groups 6 weeks post TBI (sham/vehicle vs. TBI/MNTS1: p = 0.007; TBI/vehicle vs. TBI/MNTS1: 0.048). These results suggest a greater magnitude of endogenous hippocampal neurogenesis with NPC transplantation irrespective of transduction profile.
Figure 10.
NPC transplantation increased levels of endogenous DG neurogenesis. Representative images of BrdU/NeuN double-labeled cells in the ipsilateral DG from a TBI/vehicle (A) and a TBI/MNTS1 (B) animal 6 weeks post TBI. Green, BrdU; red, NeuN. C) Nonbiased stereological assessment of double-labeled cells showed that transplantation of GFP-control NPCs increased levels of BrdU/NeuN-positive cells in the sham group relative to sham/vehicle animals. Injured animals transplanted with MNTS1-expressing NPCs exhibited a greater magnitude of hippocampal neurogenesis compared to injured and uninjured vehicle-injected control animals (*p < 0.05; **p < 0.01).
Cell Transplantation Improved Hippocampal-Dependent Cognitive Outcomes
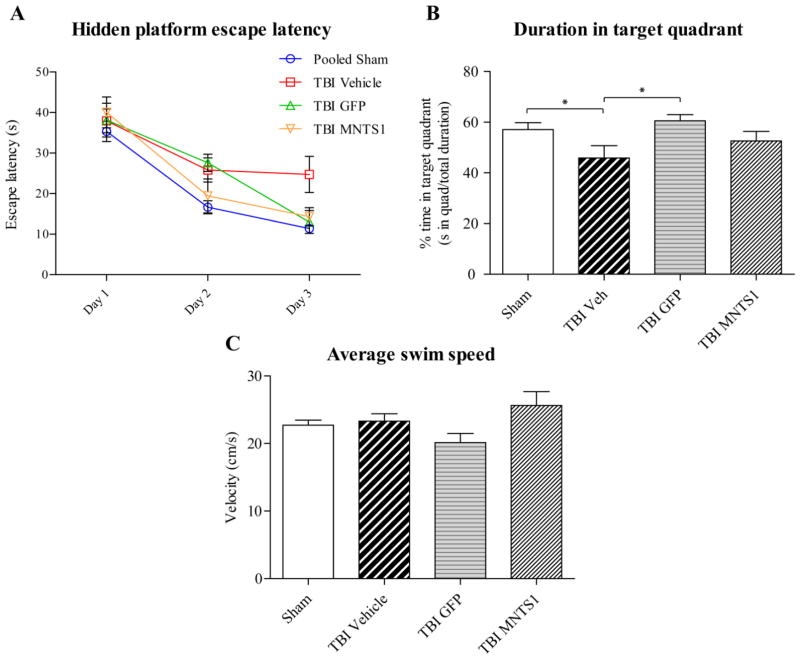
Whether these histological changes resulted in any functional differences was evaluated next. Hippocampal-dependent spatial memory capacity was evaluated using the MWM paradigm 5 weeks after surgery. We did not detect any significant differences between sham/vehicle and sham-transplanted groups, so they were collapsed into a single sham group for data analysis. After 12 trials over a period of 3 days, TBI/vehicle control animals exhibited significant spatial memory deficits, consistent with this injury model. In contrast, both NPC-transplanted TBI groups exhibited no deficits, performing on par with sham animals and significantly better than their TBI control counterparts on the MWM hidden platform task (Figure 11). By the third day of testing, escape latency was significantly reduced in sham and both transplanted groups relative to TBI/vehicle controls (TBI/vehicle vs. sham: p < 0.001; TBI/vehicle vs. TBI/GFP: p = 0.031; TBI/vehicle vs. TBI/MNTS1: p = 0.025; Figure 11A). Furthermore, sham and TBI/GFP animals spent a significantly greater percentage of total swim time in the target quadrant (where platform was submerged) relative to their TBI/vehicle control counterparts on Day 3 (TBI/vehicle vs. sham: p = 0.037; TBI/vehicle vs. TBI/GFP: p = 0.047; Figure 11B). Swim speed was not significantly different among groups, indicating that the observed differences were not a result of an inability to execute the swim task (p = 0.25; Figure 11C). Improvement in hippocampal-dependent spatial memory as assessed by MWM performance in both MNTS1-NPC and GFP-NPC transplanted animals is consistent with the finding that both transplanted groups had histological improvement and greater levels of endogenous hippocampal neurogenesis relative to TBI vehicle control animals.
Figure 11.
Cell transplantation improved hippocampal-dependent cognitive outcomes. Five weeks post surgery, all groups underwent the MWM hidden platform task. A) After 3 days of testing, post-hoc analysis revealed that both NPC-transplanted TBI groups and sham animals had significantly reduced escape latencies on the hidden platform task relative to TBI/vehicle controls (TBI/vehicle vs. sham: ##p < 0.001; TBI/vehicle vs. TBI/GFP, *p = 0.031; TBI/vehicle vs. TBI/MNTS1: +p = 0.025). Two-way repeated measures ANOVA was significant for trial day (p < 0.001), group (p = 0.005), but not for trial day × group interaction (p = 0.088). B) On Day 3, sham and TBI/GFP groups spent a greater percentage of time in the target quadrant compared to vehicle control animals. C) There were no significant differences in swim speeds among groups suggesting that the injury did not affect swimming skills.
Discussion
Our findings suggest that syngeneic NPC transplantation, with or without multineurotrophin release, promoted significant rescue of vulnerable cortical neurons and perilesional cytoarchitecture after experimental TBI. Furthermore, NPC transplantation resulted in a greater magnitude of endogenous hippocampal neurogenesis relative to vehicle-treated animals. Transplanted NPC-mediated changes manifested in significant improvement in hippocampal-dependent spatial memory after TBI, with NPC-receiving animals exhibiting MWM performances comparable to sham levels, and significantly better than injured vehicle controls. MNTS1 expression in particular had significant effects on survival and neuronal differentiation of transplanted NPCs, while the injured brain milieu influenced NPC tropism.
We hypothesized that genetic modification of NPCs with a prosurvival multineurotrophin would significantly augment behavioral and histological recovery relative to GFP-control NPCs. However, we observed similar beneficial effects with both control- and MNTS1-NPC transplantation 6 weeks after TBI. These observations suggest that the transplanted NPCs themselves have remedial intrinsic properties that contributed to the protective, proneurogenic, and restorative outcomes observed in the present studies.
Our findings are consistent with published reports demonstrating that NPC transplantation is a viable treatment option for TBI (Harting, et al., 2008). Ex vivo genetic manipulation prior to transplantation may provide additional support in terms of long-term survival and neuronal differentiation, which has also been described in the literature (Bakshi et al., 2006). Previous data have characterized the prosurvival capacity of MNTS1 in vitro, including dorsal root ganglion survival and extensive neurite outgrowth with application of MNTS1-rich medium (Urfer et al., 1994; unpublished data). MNTS1 recapitulated the prosurvival activity as effectively as a “cocktail” of neurotrophins comprised of NGF, BDNF, and NT-3 (Urfer et al., 1994). Additional in vitro experiments established consistent, reliable levels of secreted MNTS1 from NPCs as measured by enzyme-linked immunosorbent assay (ELISA) and that NPCs transduced with MNTS1 remained indistinguishable from control NPCs with multipotentiality properties intact and unaffected by transduction (unpublished data). Recent in vivo work demonstrated that transduction of mesenchymal stem cells with MNTS1 enhanced axonal growth and prevented cutaneous hypersensitivity in an experimental model of spinal cord injury (Kumagai et al., 2013).
Quantitative assessments revealed greater survival of MNTS1-transduced NPCs. NSCs and NPCs secrete neurotrophins and express Trk receptors (Barnabe-Heider and Miller, 2003). Thus, neurotrophin-Trk binding can affect cells in both a paracrine and an autocrine manner. We hypothesize that greater transplant survival in MNTS1-receiving animals resulted from the multineurotrophin acting in an autocrine/paracrine manner to engage exogenous NPCs in prosurvival signaling pathways.
After injury, the brain rapidly undergoes a series of neurochemical and proinflammatory alterations that may significantly affect the viability of transplanted cells (Bullock et al., 1995; Bramlett and Dietrich, 2004). Thus, in this study, we expected transplant survival to be reduced in TBI groups relative to sham animals. However, TBI/MNTS1-transplanted animals had greater internalized cell body survival compared to transplanted sham groups. We speculate that in addition to hostile posttraumatic events, the injury-induced upregulation of neuroreparative events, such as growth factors, acute hippocampal neurogenesis, angiogenesis, and release of anti-inflammatory cytokines may have beneficial effects on transplanted cell survival after TBI (Morganti-Kossman et al., 1997; Yoshimura et al., 2001; Sköld et al., 2005). The greater magnitude of NPC transplant survival in TBI/MNTS1 animals relative to sham/MNTS1-transplanted groups may therefore be attributed to endogenous protective modifications that allow the inflammatory environment to be more permissive for neurorestorative strategies, such as NPC transplantation, which in turn may confer reciprocal remedial effects via immunomodulation and trophic support.
In addition to increased MNTS1-NPC survival, we also found a greater proportion of NeuN-positive MNTS1-NPCs compared to GFP-control NPCs. While significantly increased levels of neuronal differentiation in transplants were observed only in the TBI/MNTS1 group, sham/MNTS1 animals also exhibited higher levels of NeuN-positive NPCs that trended towards significance relative to GFP-control NPCs in injured animals. We conclude that neuronal differentiation occurred as a result of enhanced Trk signaling mediated by MNTS1. Whether MNTS1 was acting in an autocrine/paracrine manner to stimulate a neuronal phenotype in transplanted cells or if the presence of MNTS1 created a favorable host milieu conducive to neuronal differentiation has yet to be clarified
We also found that after TBI, transplanted NPCs, with or without MNTS1 transduction, exhibited considerable tropism towards cortical contusions. After TBI, there is an upregulation of various cytokines and chemokines that are secreted by reactive astrocytes at neuroinflammatory lesions. Their upregulation creates a chemoattractant gradient to which tropic stem cells are recruited (Xu et al., 2007; Wu et al., 2012). NPCs constitutively express cell adhesion molecules, integrins, and chemokine receptors, which are important cell surface molecules for stem cell homing towards the injured CNS (Martino and Pluchino, 2006). Our observation of greater NPC tropism in the inflamed brain is consistent with the permissibility of the injured host milieu for migration. We suggest that trauma-induced upregulation of chemokines generated chemoattractant gradients and inflamed endothelial cells mediated “molecular tethering” of NPCs towards neuroinflammatory lesions. Consistent with this notion, migration was negligible in sham, uninjured animals.
We observed extensive GFP-positive processes that infiltrated several cortical and subcortical structures, including the hippocampus, contralateral cortex, and contused regions. The majority of these processes exhibited spine-like formations, which may be indicative of host-donor cell interactions. Several groups have reported on the role of BDNF and TrkB signaling in spine formation, particularly in the SGZ of the hippocampus (Alonso et al., 2004). Dendritic spinogenesis is frequently correlated with learning and strengthening of synapses (Zhou et al., 2004). The present studies did not determine whether spine-like formations were forming synaptic contacts with neighboring cells. If GFP-positive processes from transplanted NPCs were participating in the local circuitry, this may have increased cell survival in an activity-dependent manner.
After NPC transplantation, we found that both transplanted groups had significantly reduced contusion volumes and greater cortical neuron survival compared to TBI/vehicle controls 6 weeks post TBI. Because both MNTS1- and GFP-control transduced NPCs displayed significant cytoprotective effects after TBI, we hypothesized that tissue preservation was attributed to the inherent remedial properties of transplanted NPCs and not solely to MNTS1 secretion. In addition to prosurvival and neuroprotective growth factor secretion, NPCs can also modulate neuroinflammatory responses in the traumatized brain (Plucino et al., 2005; Walker et al., 2012). NPC-mediated neuroprotection after TBI may also be due in part to compensatory cellular replacement and integration within compromised host circuitry, which may protect vulnerable cells from dying. This speculation is further corroborated by the presence of numerous spine-like formations throughout the length of most GFP-positive processes and direct adjacency of grafted cells to host neurons, two observations that may indicate functional interactions with the host environment.
Several studies have reported significant increases in SGZ progenitor cell proliferation and maturation in the traumatized hemisphere after experimental TBI (Dash et al., 2001; Urrea et al., 2007). However whether the acute upregulation of hippocampal neurogenesis post injury is sustained and functionally-relevant remains unclear. Previous work has demonstrated a persistent depression of DCX-positive immature neurons at chronic time points after TBI (Rola et al., 2006; Gao et al., 2008; Atkins et al., 2010). Prolonged depletion of immature neurons results in impaired hippocampal neurogenesis (Blaiss et al., 2011). We found that both MNTS1- and GFP-control NPCs exerted robust endogenous neurogenic effects in the hippocampal DG 6 weeks post surgery compared to vehicle-treated groups. We suspect that NPC trophic secretion may have contributed to this endogenous proneurogenic effect. Although NPCs were transplanted into the right parietal cortex, we observed GFP-positive processes infiltrating the hippocampal formation, including CA1 and stratum radiatum regions. Endogenous hippocampal neurogenesis may have been elicited by the release of trophic factors from distal neurites of transplanted NPCs or due to transplanted NPCs evoking trophic support from neighboring hippocampal host cells. These NPC-mediated events could therefore result in enhanced trophic signaling and a subsequent increase in the magnitude of endogenous hippocampal neurogenesis. Another possible explanation for increased SGZ neurogenesis with cell transplantation is NPC-mediated immune modulation, which would allow for a more permissive environment conducive to greater SGZ neurogenesis after TBI. Given that hippocampal neurogenesis is closely correlated with hippocampal-dependent memory (Snyder et al., 2005; Zhao et al., 2008), therapeutic interventions, such as neurotrophic factor support and immunomodulation, that may augment the survival of newly-generated hippocampal neurons are critical.
Consistent with our immunohistochemical findings, we observed improved hippocampal-dependent cognition on the MWM spatial memory task in both GFP- and MNTS1-NPC transplanted animals relative to TBI/vehicle control groups. We cannot definitively conclude whether spatial memory rescue in transplanted animals was attributed to cytoprotective effects and/or elevated endogenous hippocampal neurogenesis, or to some other parameter that was not fully evaluated, such as long-distance processes and spine-like morphologies.
It is important to ascertain whether exogenous cell transplantation leads to prolonged immune responses or tumorigenicity. Studies conducted to assess long-term effects and temporal profiles of cell transplants are required to fully interpret the implications of these findings. Cellular dose-response studies and the therapeutic window for cellular transplantation will also have to be critically assessed to further clarify translational relevance. In the present studies, we transplanted NPCs 7 days post injury. This time point was selected for two principle reasons. First, we feel that it is relevant in terms of when cellular transplant interventions are initiated after injury from a clinical standpoint. Second, in the acute period after TBI, the injured brain milieu is hostile and unfavorable for the survival and integration of exogenous cells. At a seven day time point however, proinflammatory cascades begin to attenuate, allowing for a more permissive environment for NPC survival, migration, differentiation, and integration. If transplantation is delayed until more chronic phases of TBI, NPC-mediated cytoprotection and neurogenic responses may be highly dependent on multineurotrophin transduction.
Collectively, our findings support the therapeutic potential of NPC transplantation in an experimental model of TBI. Because there were no significant histological or behavioral differences between MNTS1- and GFP-control NPCs, we propose that the inherent remedial properties of NPCs were sufficient to overcome some of the histopathology and cognitive deficits observed in this model of moderate brain injury. Future investigations as to the nature and relevance of long-distance neurites and spine-like formations may reveal additional important properties of NPCs to target and to optimize the therapeutic potential of cell transplantation strategies for the treatment of TBI.
Highlights.
NPC transplantation preserved cytoarchitecture and spatial memory after TBI
NPC transplantation stimulated hippocampal neurogenesis with or without injury
Multineurotrophin secretion enhanced graft survival and neuronal differentiation
Acknowledgments
The studies were supported by NIH NS030291 to WDD and W81XWH-06-1-0187 awarded to WDD. The authors wish to thank Mrs. Ofelia Furones-Alonso, Mr. Pingping Jia and Mrs. Yunfang Wang for their technical contributions to this study, and to thank Dr. Coleen Atkins for proofreading the manuscript.
Abbreviations
- BrdU
5-bromo-2′-deoxyuridine
- FdUrd
5-fluoro-2′-deoxyuridine
- Aldh1
Aldehyde dehydrogenase 1
- bFGF
Basic fibroblast growth factor
- BFP
Blue fluorescent protein
- CsA
Cyclosporine A
- DG
Dentate gyrus
- DCX
Doublecortin
- ELISA
Enzyme-linked immunosorbent assay
- EGF
Epidermal growth factor
- GFAP
Glial fibrillary acidic protein
- GFP
Green fluorescent protein
- HBSS
Hank’s Balanced Salt Solution
- H&E
Hematoxylin and eosin
- IP
Intraperitoneal
- Iba1
Ionized calcium-binding adapter molecule 1
- MABP
Mean arterial blood pressure
- MWM
Morris water maze
- MNTS1
Multineurotrophin
- NPCs
Neural progenitor cells
- NeuN
Neuronal nuclei (for immunohistochemistry)
- SGZ
Subgranular zone
- TBI
Traumatic brain injury
- Trk
Tropomyosin-related kinase
- ANOVA
Analysis of variance
- SEM
Standard error of the mean
- SNK
Student-Newman-Keuls
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Truettner JS, Lotocki G, Sanchez-Molano J, Kang Y, Alonso OF, Sick TJ, Dietrich WD, Bramlett HM. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci. 2010;32:1912–1920. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi A, Shimizu S, Keck CA, Cho S, LeBold DG, Morales D, Arenas E, Snyder EY, Watson DJ, McIntosh TK. Neural progenitor cells engineered to secrete GDNF show enhanced survival, neuronal differentiation and improve cognitive function following traumatic brain injury. Eur J Neurosci. 2006;23:2119–2134. doi: 10.1111/j.1460-9568.2006.04743.x. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003;23:5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci. 2011;31:4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Bregy A, Nixon R, Lotocki G, Alonso OF, Atkins CM, Tsoulfas P, Bramlett HM, Dietrich WD. Posttraumatic hypothermia increases doublecortin expressing neurons in the dentate gyrus after traumatic brain injury in the rat. Exp Neurol. 2012;233:821–828. doi: 10.1016/j.expneurol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Myseros JS, Marmarou A, Woodward JJ, Young HF. Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann N Y Acad Sci. 1995;765:290–297. doi: 10.1111/j.1749-6632.1995.tb16586.x. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Cossetti C, Alfaro-Cervello C, Donega M, Tyzack G, Pluchino S. New perspectives of tissue remodelling with neural stem and progenitor cell-based therapies. Cell Tissue Res. 2012;349:321–329. doi: 10.1007/s00441-012-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Gao X, Deng-Bryant Y, Cho W, Carrico KM, Hall ED, Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J Neurosci Res. 2008;86:2258–2270. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garga N, Lowenstein DH. Posttraumatic epilepsy: a major problem in desperate need of major advances. Epilepsy Curr. 2006;6:1–5. doi: 10.1111/j.1535-7511.2005.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, MØLler A, Nielsen K, Nyengaard JR, Pakkenberg B, SØRensen FB, Vesterby A, West MJ. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Harting MT, Baumgartner JE, Worth LL, Ewing-Cobbs L, Gee AP, Day MC, Cox CS., Jr Cell therapies for traumatic brain injury. Neurosurg Focus. 2008;24:3–4. doi: 10.3171/FOC/2008/24/3-4/E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai G, Tsoulfas P, Toh S, McNiece I, Bramlett HM, Dietrich WD. Genetically modified mesenchymal stem cells (MSCs) promote axonal regeneration and prevent hypersensitivity after spinal cord injury. Exp Neurol. 2013;248:369–380. doi: 10.1016/j.expneurol.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–136. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Contantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;36:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202:189–199. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Shetty AK. Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: Can early neural stem cell grafting intervention provide protection? Epilepsy Behav. 2014;13:631–638. doi: 10.1016/j.yebeh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld MK, von Gertten C, Sandberg-Nordqvist AC, Mathiesen T, Holmin S. VEGF and VEGF receptor expression after experimental brain contusion in rat. J Neurotrauma. 2005;22:353–367. doi: 10.1089/neu.2005.22.353. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Urfer R, Tsoulfas P, Soppet D, Escandon E, Parada LF, Presta LG. The binding epitopes of neurotrophin-3 to its receptors trkC and gp75 and the design of a multifunctional human neurotrophin. Embo J. 1994;13:5896–5909. doi: 10.1002/j.1460-2075.1994.tb06935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- Walker PA, Bedi SS, Shah SK, Jimenez F, Xue H, Hamilton JA, Smith P, Thomas CP, Mays RW, Pati S, Cox CS., Jr Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population. J Neuroinflammation. 2012;9:1742–2094. doi: 10.1186/1742-2094-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Chen Q, Peng H, Dou H, Zhou Y, Huang Y, Zheng JC. Directed migration of human neural progenitor cells to interleukin-1beta is promoted by chemokines stromal cell-derived factor-1 and monocyte chemotactic factor-1 in mouse brains. Transl Neurodegener. 2012;1:2047–9158. doi: 10.1186/2047-9158-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang S, Jiang X, Zhao Y, Gao M, Zhang Y, Wang X, Tano K, Kanehara M, Zhang W, Ishida T. Hypoxia-induced astrocytes promote the migration of neural progenitor cells via vascular endothelial factor, stem cell factor, stromal-derived factor-1alpha and monocyte chemoattractant protein-1 upregulation in vitro. Clin Exp Pharmacol Physiol. 2007;34:624–631. doi: 10.1111/j.1440-1681.2007.04619.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci (USA) 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]