Abstract
Caspases are a protein family of cellular executors, initiating cell death. A recent report by Shi et al. discovered that caspases 4/5/11 are novel cytosolic lipopolysaccharide receptors. These caspases activate through oligomerization and direct interaction with lipopolysaccharide. This has implications for the mechanism of caspase-mediated pyroptosis and sepsis drug development.
Lipopolysaccharide (LPS), a major structural element of the outer membrane of Gram-negative bacteria, triggers an innate immune response through Toll-like Receptor 4 (TLR4). It has recently come to light that in addition to its canonical TLR4-mediated behaviors, intracellular LPS activates pro-inflammatory caspase-11 and induces an immune response independent of TLR4 [1, 2]. This non-canonical response allows caspase-11 to serve as an innate immune sensor for cytosolic bacteria such as Burkholderia thailandensis. Hypotheses regarding the mechanism of LPS-induced caspase-11 activation were generated by examining other members of the caspase family, particularly caspase-1. Briefly, an inflammasome scaffold recruits and activates caspase-1 through conserved caspase activation recruitment domain (CARD) interactions between the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 [3]. Based on this existing paradigm, the “mysterious” non-canonical intracellular LPS receptor was suspected to contain a CARD domain.
In a recent report, Shi et al. successfully identified the intracellular receptor of LPS [4]. Focusing on CARD-containing proteins, the authors screened 18 proteins for caspase-11 activation. However, none was able to induce pyroptosis. Instead, it was observed that during caspase-4/5/11 preparation, auto-oligomerization occurred with bacterially purified caspases, but not insect cell purified caspases. Thus, it was hypothesized that caspase-4/5/11 may be oligomerized by a bacterial component, such as LPS. LPS was introduced to human monocytes using the Neon Transfection System, and the expected pyroptotic response was observed. Interestingly, these results also expanded to non-monocyte cells, such as HeLa and HL60. Pyroptosis observed in these human cell lines required caspase-4, but was independent of caspase-1 or ASC. This model is disparate from existing caspase activation mechanisms, as it does not require canonical inflammasome components. Surface plasmon resonance showed the direct binding of LPS to caspase, nailing down caspase-4/5/11 as an intracellular LPS receptor. Additional mutagenesis studies showed that several lysine residues in the CARD domain mediate LPS binding, conjecturing a critical charge interaction. These results not only expose LPS as the activator of caspase-4, but also widen LPS sensing to non-immune cells, expanding known causes of septic activation. Further, LPS recognition is highly conserved, as horseshoe crab factor C is also activated by LPS.
LPS binds directly to caspase-4/5/11, presenting a novel activation mechanism for pyroptosis and sepsis. TLR4 activation has a previously established role in septic shock, which is caused by a rampant inflammatory response to LPS during infection. However, advanced drug candidates targeting TLR4 (e.g. TAK242, Eritoran) have met with little success in late stage clinical trials as anti-septics, despite their high potency and specificity [5]. It was speculated that these drugs failed because TLR4 inhibition alone is not sufficient. The presence of high doses of LPS during septic shock, some of which may be aberrantly located in the cytoplasm during infection, may also necessitate caspase inhibition. The identification of caspase-4 as a functional human homolog of capsase-11 expands murine sepsis models to human applications, and caspase-4 expression in non-macrophage cells enhances the scope of septic inflammation.
Most intriguingly, LPS binding-induced oligomerization of caspases is a prerequisite for activation. These results are consistent with the importance of polymeric assembly in other inflammasomes [3, 6]. It may be rational to speculate that self-assembly and aggregation is a potential unifying mechanism for caspase activation and signaling propagation. When adaptor proteins or caspase-activating molecules (e.g. LPS) aggregate, caspases may be recruited to the complex, placing individual caspases in close proximity. Under these conditions, when a single caspase molecule becomes activated, a self-propagating signaling cascade may result from the induced proximity, allowing robust signal amplification and rapid signal transduction (Figure 1). This potential mechanism would explain ASC polymerization-induced caspase-1 activation [3], small molecule aggregate-induced caspase-3 activation [7], death-inducing signaling complex (DISC) induced caspase-8 activation [8], and apoptosome-induced caspase-9 activation [9]. For example, recent studies by the Wells group showed that a small molecule agent, 1541, forms fibrils, which bind capsase-3 and allow activation [6].
Figure 1.
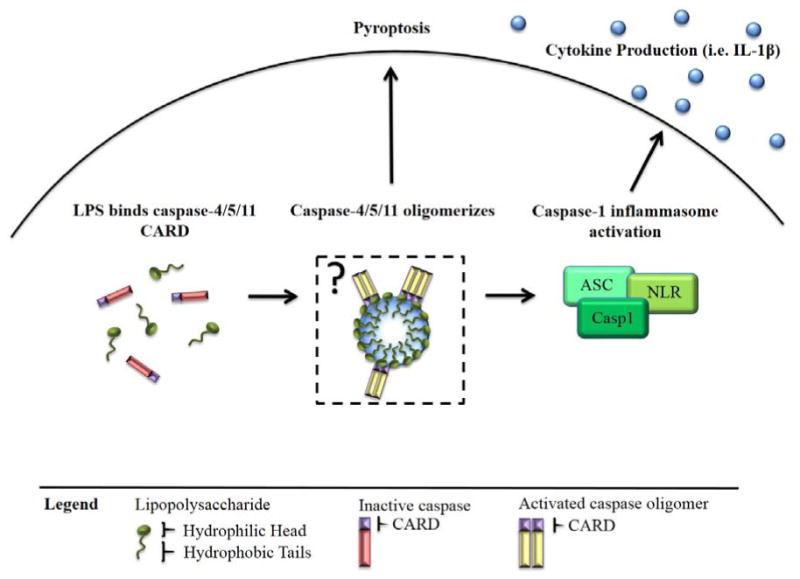
A schematic overview of LPS-induced caspase-4/5/11 activation. Shi et al. have demonstrated that LPS (green) binds to the CARD domain (purple) of caspase-4/5/11 (red), inducing its oligomerization and consequential proteolytic cleavage. Upon activation, caspase-4/5/11 (yellow) may cause pyroptosis and cell death, or inflammation through caspase-1. In the box, we speculate that the positively charged residues present in the caspase CARD domain may interact with the negatively charged LPS lipid head group. Once multiple caspase molecules are localized to the micelle, their induced proximity may result in auto-activation of the caspase to its active, oligomeric state.
It is conceivable that LPS can form a similar structure for capsase-4/5/11 localization and oligomerization. LPS is an amphipathic molecule that can self-aggregate into supramolecular complexes with an apparent critical micelle concentration (CMC) of 10-20 μg/ml [10]. In previously reported intracellular LPS shock models, cells are treated with high doses (1-2 μg/ml) of LPS [1, 2]. Nonetheless, the intracellular environment is highly crowded, which means local LPS concentrations might reach the CMC and facilitate association with caspase-4/5/11. Mutagenesis studies demonstrated the essential role of the positive residues in the CARD domain for LPS binding. Caspase-4/5/11 therefore may bind to the negatively charged polar heads of LPS micelle aggregates via these positively charged residues. In turn, this may result in assembly of caspase-4/5/11 and proximity-induced auto-activation. Indeed, disruption of LPS aggregation by the detergents Triton-100 and Tween-20 prevents caspase-4/5/11 activation, consistent with an aggregation hypothesis.
While the identification of caspases as intracellular LPS receptors is a major step forward, the mode of LPS binding to caspase-4/5/11 remains unclear. As LPS activation is not specific for an individual caspase, binding appears to occur through the conserved CARD domain. Caspase activation by aggregation and self-assembly may offer a unifying hypothesis. Together with previous research regarding other members of the caspase family, this new finding supports aggregation as a conserved signal transduction mechanism in innate immunity and inflammation [3, 4, 6, 8, 9]. Future work may examine the higher order structures of LPS during caspase binding, and determine the minimum concentrations required for induction of oligomerization. It also remains to be seen how the acylation state of LPS contributes to binding [2]. Additional attention should be paid to the therapeutic applications of this research, particularly for septic shock. A small-molecule caspase inhibitor could fill the void left by TLR4 inhibitors; alternatively, these two groups of inhibitors may complement each other as anti-septics. These results will certainly have long-term implications for inflammasome studies and drug design.
Acknowledgments
We thank the National Institutes of Health (R01GM103843 and R01GM101279) and the National Science Foundation (CHE0954819) for financial support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 2.Hagar JA, et al. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai X, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 5.Rice TW, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 6.Lu A, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolan DW, et al. Small-molecule activators of a proenzyme. Science. 2009;326:853–858. doi: 10.1126/science.1177585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Z, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Pop C, et al. The apoptosome activates caspase-9 by dimerization. Mol Cell. 2006;22:269–275. doi: 10.1016/j.molcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Santos NC, et al. Evaluation of lipopolysaccharide aggregation by light scattering spectroscopy. ChemBioChem. 2003;4:96–100. doi: 10.1002/cbic.200390020. [DOI] [PubMed] [Google Scholar]


