Abstract
Anti-angiogenic therapy is commonly used for the treatment of CRC. Although patients derive some clinical benefit, treatment resistance inevitably occurs. The MET signaling pathway has been proposed to be a major contributor of resistance to anti-angiogenic therapy. MET is upregulated in response to VEGF pathway inhibition and plays an essential role in tumorigenesis and progression of tumors. In this study we set out to determine the efficacy of cabozantinib in a preclinical CRC PDTX model. We demonstrate potent inhibitory effects on tumor growth in 80% of tumors treated. The greatest antitumor effects were observed in tumors that possess a mutation in the PIK3CA gene. The underlying antitumor mechanisms of cabozantinib consisted of inhibition of angiogenesis and Akt activation and significantly decreased expression of genes involved in the PI3K pathway. These findings support further evaluation of cabozantinib in patients with CRC. PIK3CA mutation as a predictive biomarker of sensitivity is intriguing and warrants further elucidation. A clinical trial of cabozantinib in refractory metastatic CRC is being activated.
Keywords: Colorectal cancer, Angiogenesis, c-MET, RET, VEGFR2, PIK3CA
Introduction
Induction of angiogenesis with resultant tumor neovascularization is a well-recognized hallmark of cancer. A balance of pro-angiogenic and inhibitory factors governs angiogenesis.1, 2 Central among the pro-angiogenic factors is the vascular endothelial growth factor (VEGF) pathway that promotes the proliferation of endothelial cells and contributes to key aspects of the growth and metastasis of cancer cells.1, 2 Targeting either VEGF or its cognate receptor, VEGFR2 has been a focus of drug development in oncology.
Modulating tumor vasculature with anti-angiogenic agents is clearly a viable strategy in the treatment paradigm of advanced colorectal cancer (CRC). Maintenance of VEGF inhibition with bevacizumab added to standard second line chemotherapy beyond disease progression portends a survival benefit compared to chemotherapy alone.3 Treatment with regorafenib, an oral multikinase inhibitor of VEGFR1-3, Tie2, Kit, RET, RAF, PDGFR and FGFR in treatment refractory patients resulted in a median survival benefit of 1.4 months compared to placebo.4 Nevertheless, the benefits of these compounds have been modest secondary to both intrinsic and acquired resistance.
The MET signaling pathway has been identified as a mechanism of resistance to VEGF therapy.5–8 MET is widely expressed in many cancers and is activated by hepatocyte growth factor (HGF), a protein that is secreted by cells of mesenchymal lineage.9 MET overexpression occurs in up to 70% of CRC and is associated with progression, metastasis and a poor prognosis.10, 11 Intriguingly, Bardelli et al 12 attributed amplification of the MET locus as a cause of resistance to cetuximab. Treatment of CRC patient-derived xenografts displaying MET amplification with a MET inhibitor led to encouraging antitumor activity.12 Accordingly, there is great interest in targeting the VEGF/MET axis in many different malignancies.
Cabozantinib is an oral multikinase inhibitor. The principal targets are receptor tyrosine kinases central to cancer cell growth and tumor angiogenesis including MET, RET, AXL and VEGFR2. Treatment with cabozantinib in preclinical noncolorectal models has shown a reduction in angiogenesis, tumor cell proliferation and survival.13, 14 The effects of cabozantinib in preclinical models of CRC have yet to be investigated; in this study, we evaluated the antitumor effects of cabozantinib in our CRC patient-derived tumor xenograft (PDTX) model.
Materials and Methods
CRC explant xenograft model
Fresh colorectal tumor tissue was obtained from consenting patients at the University of Colorado Hospital in accordance with protocols approved by the Colorado Multiple Institutional Review Board (COMIRB). Female athymic nude mice aged four-to-six weeks were purchased from Harlan laboratories (Washington DC) under an approved protocol by the Institutional Animal Care and Use Committee (IACUC). Tumor specimens were cut into 3mm3 pieces and injected using a trochar into the right and left flank of mice. A CRC patient derived tumor xenograft (PDTX) bank was maintained by passaging into subsequent generations when tumor volumes reached ~ 1000–1500 mm3 until expansion for treatment studies.15, 16 For treatment studies, tumors were expanded in the left and right flanks of 5–6 mice (10 evaluable tumors per group). Mice were randomized into vehicle or cabozantinib groups when tumor volumes reached ~200 mm3. Mice were treated daily with cabozantinib (30 mg/kg- daily- weekdays) by oral gavage for 28 days. Mice were monitored daily for signs of toxicity and tumor size was evaluated twice per week by caliper measurements using the following formula: tumor volume = [length × width2] * 0.52. Tumor growth inhibition index (TGII), a standardized measure of tumor growth, was calculated for each CRC explant using the following formula: TGII = (tumor volume of treated on Day 28 − tumor volume of treated on Day 0)/(tumor volume of control on Day 28 − tumor volume of control on Day 0) × 100. Cases with a TGII of ≤ 20% were considered sensitive, TGII of > 20% were considered resistant to cabozantinib.
Isogenic cell lines
HCT116 PIK3CA isogenic cell lines (123 wild type and 125 mutant) were kindly provided to us by Bert Vogelstein at Johns Hopkins. The 123 wild type and 125 mutant isogenic cell lines were injected in the left and right flanks of athymic nude mice at a concentration of 1×106 cells/injection. When tumor volumes reached ~150 mm3, the mice were randomized into control or cabozantinib treatment. Mice were treated daily with cabozantinib (30 mg/kg- daily- weekdays) by oral gavage for 14 days. Mice were monitored daily for signs of toxicity and tumor size was evaluated twice per week by caliper measurements using the following formula: tumor volume = [length × width2] * 0.52.
Immunoblotting
Tumor tissues (50 mg/mouse) were minced on ice and placed in a tube containing 1/4-inch ceramic spheres and homogenized using a FastPrep-24 instrument for 20 seconds (MP bio). After homogenization, tubes were centrifuged at 1,000 g at 4°C for 1 minute to reduce the foam. The resulting supernatant was placed into a clean microfuge tube and centrifuged at 14,000 g at 4°C for 15 minutes. Protein concentration was determined in the supernatant using the Pierce™ 660nm Protein Assay. Fifty micrograms of sample were electrophoresed on 4–12% Bis-Tris precast gels (Life Technologies). Protein was electrotransfered to nitrocellulose membranes using the iBlot® - Western Blotting System (Life Technologies). After transfer, membranes were blocked at room temperature with TBST [10 mmol/L Tris-HCl (pH 7.5), 0.5 mol/L NaCl, and 0.1% (v/v) Tween 20] containing 5% nonfat milk (BioRad) for 1 hr. Cleaved caspase 3, p/t Akt, p/t ribosomal S6, p/t MET, p/t RET, and actin primary antibodies (Cell Signaling Technologies) were diluted at 1:1,000 in TBST containing 5% protease-free bovine serum albumin (Sigma-Aldrich), and the membranes were incubated overnight at 4°C with rocking. After washing three times with TBST, the membranes were incubated for 1 h at room temperature with anti-mouse IgG horseradish peroxidase–conjugated antibody at a final dilution of 1:50,000 in TBST. After washing three times with TBST, bound antibodies were detected by enhanced chemiluminescence (Millipore).
Receptor tyrosine kinase array
Tumor tissue was homogenized, lysed and protein concentration was determined as described above (immunoblotting). The slides containing 39 antibodies/well (RTK array, Cell Signaling Technologies) were blocked for 15 minutes. After blocking, 75 μg of diluted lysate was added to each well of the slide and incubated overnight at 4°C with gentle rocking. The slides were then washed and detection antibody was added to the slide and followed by DyLight 680®-linked Streptavidin. Slide images were captured using the Odyssey Infrared Imaging System (Li-Cor) and the spots were quantified using the Odyssey system software.
Gene Pathway Analysis by RNA Seq
Total RNA from CRC explants were extracted using RNAeasy kit (Qiagen) and profiled using RNA Seq. Raw expression values were extracted and normalized by the Affymetrix Power Tools based on Robust Multiarray Average (RMA) approach. Multiple probe sets representing the same gene were collapsed by the maximum value. To analyze the pathway enriched in the control versus cabozantinib treated explants, we used the GSEA (gene set enrichment analysis) software version 2.0.13 obtained from the Broad Institute (http://www.broad.mit.edu/gsea).17 We used the pathways defined by the Kyoto Encyclopedia of Genes and Genomes (KEGG) as the gene set in this study.18 Gene set permutations were performed 1000 times for each analysis. We used the nominal p-value and Normalized Enrichment Score (NES) obtained from GSEA to sort the pathways up and down regulated in the cabozantinib treated groups.
Immunohistochemistry
Tumor tissues from control and cabozantinib treated mice were placed in formalin immediately after surgical excision and processed into paraffin wax blocks. Sections were deparaffinized using standard histologic procedures, and an antigen retrieval method was used to ensure optimal antigen integrity and expression. Human cleaved caspase 3 (Cell Signaling Technology), and mouse CD34 (Becton Dickinson), antibodies were used for IHC. Cleaved caspase 3 was assessed for overall staining of human cells and CD34 was evaluated for staining of mouse endothelial cells. CD34 staining was scored by counting the number of CD34 vessels/mm2 by a blinded gastrointestinal pathologist.
Statistical analysis
An unpaired Student t-test was used to determine whether the means between control and cabozantinib treated groups were significant at end of treatment (~28 days) and to determine differences in tumor growth inhibitory index (TGII) between PIK3CA wild type and mutant. The differences were considered significant when the P value was <0.05. All error bars are represented as the standard error of the mean (SEM).
Results
Potent antitumor activity of cabozantinib on tumor growth in a CRC PDTX explant model
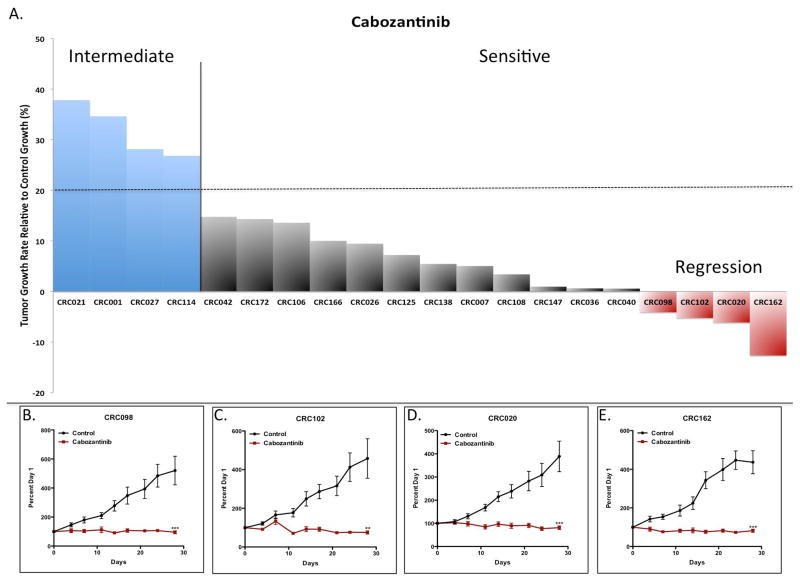
We assessed the antitumor activity of cabozantinib on 20 distinct CRC PDTX explants. Supplementary table 1 shows the patient characteristics, mutations in KRAS, TP53, PIK3CA (common genes mutated in CRC) and mutations in MET, VEGFR2, RET and AXL (targets of cabozantinib). Mutations in KRAS (60%) were slightly overrepresented compared to CRC patients overall, while no mutations were identified in MET, VEGFR2, RET, or AXL. As shown in figure 1A, 16 out 20 (80%) CRC explants exhibited sensitivity (TGII: tumor growth of treated compared to control ≤ 20%) to cabozantinib. A TGII of > 20% (< 80% tumor growth inhibition) was chosen as a cut-off to be more stringent on the classification between sensitive and resistant. The 4 CRC explants CRC021, 001, 027 and 114 were considered more resistant (TGII > 20%) to cabozantinib. The 4 most sensitive tumors were CRC098, 102, 020 and 162 which all showed tumor regression in response to cabozantinib in the PDTX model (figure 1 BE).
Figure 1.
The effects of cabozantinib on tumor growth in CRC explants. (A) Twenty CRC explants were treated with cabozantinib 30 mg/kg daily for 28 days. (A) There were 4 resistant (TGII > 20%) CRC explants (CRC021, CRC001, CRC027 and CRC114) and 16 sensitive (TGII ≤ 20%) CRC explants. Columns, mean (n = 10 tumors per group). (B–E) Growth curves of the 4 CRC explants that demonstrated tumor regression: (B) CRC098, (C) CRC020, (D) CRC102, and (E) CRC162. Significance **, p < 0.01; ***, p < 0.001.
Cabozantinib reduces receptor tyrosine kinase activation in CRC explants
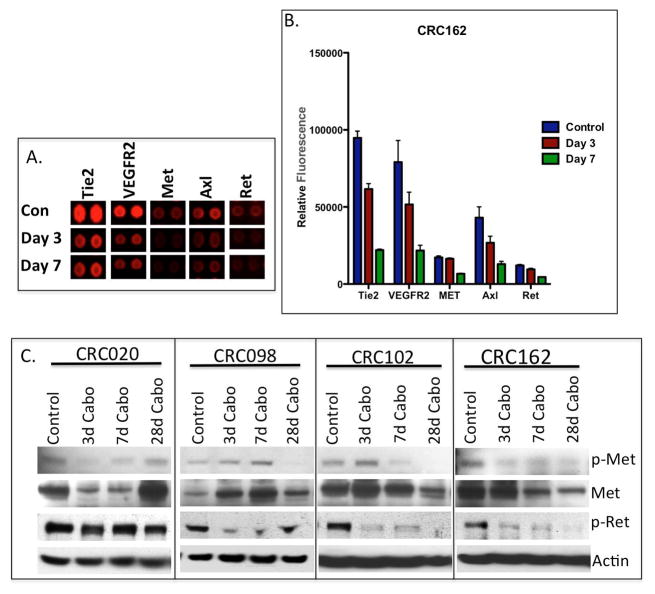
Cabozantinib is a small molecule inhibitor that targets multiple receptor tyrosine kinases. Therefore, we used a receptor tyrosine kinase array to evaluate the effects of cabozantinib treatment on the sensitive CRC162 explant. As displayed in figure 2A and B, cabozantinib reduced the phosphorylation of Tie2, VEGFR2, MET, AXL and RET at 3 and 7 days post treatment. To confirm these results, we investigated the treatment effects on the inhibition of MET and RET by immunoblotting in 4 sensitive explants CRC020, CRC098, CRC102, and CRC162. As shown in figure 2C, cabozantinib inhibited the phosphorylation of MET at day 28 in CRC098, day 7 in CRC102, and day 3 in CRC020 and CRC162. Interestingly, we observed an increase in protein expression of the MET receptor in CRC098 and CRC102 after cabozantinib treatment. RET phosphorylation was diminished in the 3 CRC explants CRC098, 102 and 162 as early as 3 days post treatment. In the CRC intermediate explants CRC021 and 027, the phosphorylation of MET and RET were undetectable (supplemental figure 1).
Figure 2.
Inhibition of receptor tyrosine kinase activation with cabozantinib treatment. (A) A representative image and (B) densitometry of the receptor tyrosine kinase for the evaluation of cabozantinib treatment on the phosphorylation of Tie2, VEGFR2, MET, AXL and RET in CRC162, n=2 mice/tumour per group for control and cabozantinib treated. Reduced phosphorylation of all kinase targets was observed at day 3 and 7 of cabozantinib treatment. (C) A representative western blot of total and phosphorylated MET and RET in 4 sensitive CRC explants at treatment days 3, 7 and 28.
Tumors most sensitive to cabozantinib harbor a mutation in the PIK3CA gene
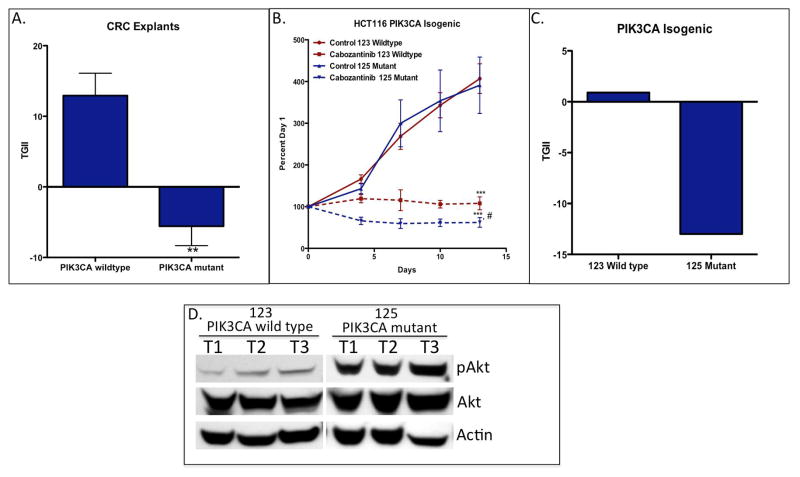
In this study, we assessed whether a particular gene mutation was associated with sensitivity or resistance to cabozantinib. Interestingly, comparison of the TGII between PIK3CA wild type and mutant CRC explants showed a statistically significant difference in tumor response to cabozantinib; tumors that possess a mutation in the PIK3CA gene exhibited enhanced sensitivity to cabozantinib (figure 3A). In order to confirm the importance of the PIK3CA mutation and response to cabozantinib, we assessed treatment effects on the PIK3CA isogenic (wild type and mutant) HCT116 cell line in a xenograft model. The only difference genetically between these two cells lines is PIK3CA status. As shown in figure 3B, both the wild type and mutant cell line-derived tumor xenografts demonstrated significant (p < 0.001) sensitivity to cabozantinib. However, the PIK3CA mutant cell line-derived tumor xenograft showed a significantly (p < 0.05) greater sensitivity to treatment in comparison to the PIK3CA wild type cell line. In particular, tumor regression was observed in the PIK3CA mutant cell line while static effects were seen in the PIK3CA wild type tumors (figure 3C). Baseline Akt activation was significantly greater in the PI3KCA mutant cell line-derived tumor xenograft compared to wild type demonstrating that this mutation is functionally more active (figure 3D). Of note, there were no baseline differences observed between sensitive and resistant CRC explants with respect to MET or MACC1 gene expression.
Figure 3.
Tumors harbouring a PIK3CA mutation exhibit enhanced sensitivity to cabozantinib. (A) Comparison of tumor growth (TGII) in PIK3CA wild type and mutant CRC explants treated with cabozantinib at end of study. ** p < 0.01, TGII comparison between PIK3CA mutant (CRC020, 040, 098 and 162) vs. PIK3CA wild type explants. (B) The isogenic 123 PIK3CA wild type and 125 PIK3CA mutant cell line-derived tumor xenografts were treated with cabozantinib 30 mg/kg daily for 14 days. Tumors with a PIK3CA mutation had greater sensitivity to cabozantinib when compared to PIK3CA wild type. Mean n = 10 tumours per group; s.e.m ***, significance (*P<0.001) compared with vehicle-treated tumours; #, p < 0.05, comparison between PIK3CA wild type vs. mutated treated mice. (C) Graph comparing the TGII of the 123 PIK3CA wild type and 125 PIK3CA mutant cell line-derived tumor xenografts at the end of study. (D) Akt phosphorylation in 123 PIK3CA wild type and 125 PIK3CA mutant cell line-derived tumor xenografts. Baseline levels of Akt were increased in the mutant tumors when compared with wild type.
Decrease in PI3K pathway gene expression and Akt activation in cabozantinib treated tumors
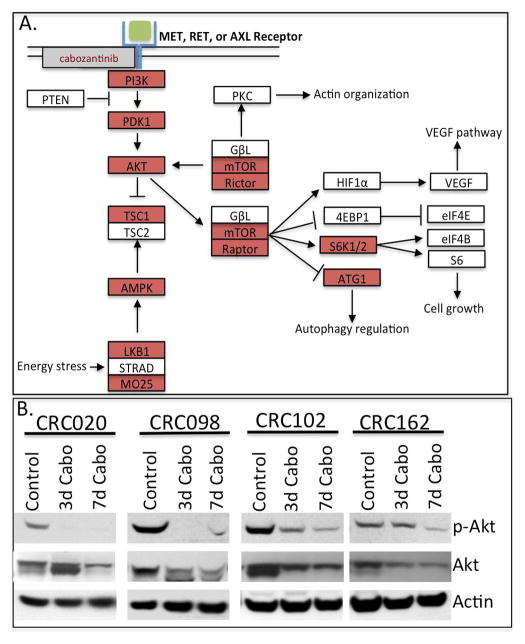
We investigated the effects of cabozantinib on gene expression profiles after 3 days of treatment on CRC020, CRC098, and CRC102 by RNA Seq and pathway analysis. Cabozantinib significantly decreased expression of genes involved in the phosphatidylinositol (PI3K) and mTOR signaling pathways (supplemental Table 2 and figure 4A). Further investigation of the effects of cabozantinib on the PI3K pathway at the protein level revealed potent inhibition of phosphorylation of Akt protein in CRC020, CRC098, CRC102 and CRC162 (figure 4B). Other pathways noted to be significantly down regulated after cabozantinib treatment included genes involved in cell cycle, DNA replication, TGF-beta and p53 signaling (supplemental table 2).
Figure 4.
Downregulation of the PI3K pathway and Akt activity after cabozantinib treatment. (A) A depiction of the PI3K pathway after 3 days of treatment with cabozantinib: red shows genes that are downregulated. (B) Evaluation of Akt activation by western blot in 4 CRC sensitive explants. Cabozantinib inhibited Akt activation in CRC explants.
Cabozantinib treatment significantly reduces angiogenesis and induces apoptosis
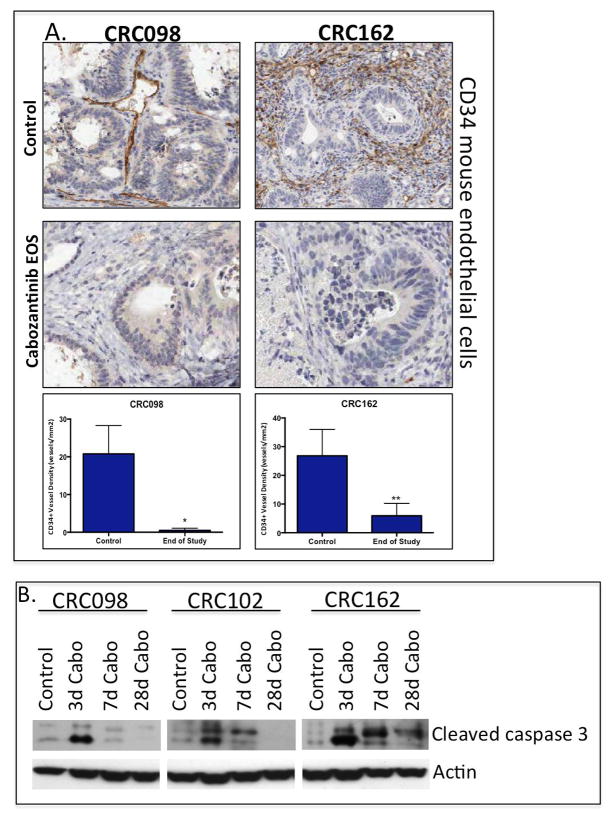
Since cabozantinib also targets Tie2 and VEGFR2, we assessed the treatment effects on angiogenesis by CD34 staining of mouse endothelial cells by immunohistochemistry at the end of study in 2 sensitive CRC explants. As shown in figure 5A, there was a profound decrease in CD34 positive cells after 28 days of cabozantinib treatment. Furthermore, cabozantinib demonstrated a significant increase in cleaved caspase 3 in the sensitive CRC explants (figure 5B). The increase in cleaved caspase 3 was observed as early as day 3 of treatment.
Figure 5.
Cabozantinib inhibits tumor angiogenesis and induces apoptosis. (A) A significant reduction of CD34 staining in mouse endothelial cells was observed at 28 days of treatment in the CRC098 and CRC162 sensitive tumors. (B) An increase in cleaved caspase 3 activity was seen as early as treatment day 3.
Discussion
Our study reveals potent activity of the c-VEGFR2 and c-Met inhibitor, cabozantinib, in a PDTX model of CRC. The reliance on intratumoral blood vessels in enhancing the growth and progression of tumors makes targeting angiogenesis an attractive treatment for patients with CRC. Although such drugs are successful at attenuating angiogenesis, the clinical benefit is limited.3, 4 Given that the MET receptor has been identified to facilitate resistance to VEGF inhibition by promoting the growth, survival and metastasis of tumor cells 5–8, dual targeting of VEGF/Met signaling with cabozantinib warrants further clinical evaluation against current anti-angiogenic therapies for CRC patients.
Cabozantinib, which is FDA approved for progressive, metastatic medullary thyroid cancer, has activity in preclinical models of different malignancies. In particular, treatment with cabozantinib reduced tumor proliferation and angiogenesis that resulted in an induction of apoptosis in a breast cancer xenograft model.19 In addition, cabozantinib significantly diminished the growth of breast cancer cells in the lung in comparison to sunitinib (a VEGFR2 inhibitor) in an experimental model of metastasis.19 In a transgenic RIP-Tag2 pancreas neuroendocrine tumor (PNET) mouse model, cabozantinib treatment resulted in a significant reduction in liver metastases, which was associated with an increase in overall survival when compared to anti-VEGF therapy alone 20. In other preclinical studies, cabozantinib (30 mg/kg) demonstrated antitumor activity in lung, glioma, pancreas, thyroid and prostate cancers.19, 21, 22 Our data demonstrates that cabozantinib exhibits antitumor activity in a preclinical CRC PDTX model. There were 16 CRC explants that were sensitive to treatment; twelve CRC explants displaying static effects on tumor growth and 4 explants showing tumor regression. The dose of 30 mg/kg used in this study is well within the pharmacodynamic range as previously described in other studies using this compound 14, 20, 21, 23. The main effect of cabozantinib on tumor growth was through a reduction of angiogenesis in our sensitive tumors, accompanied by induction of apoptosis. This preclinical study demonstrates that cabozantinb has excellent antitumor activity in a more clinically relevant CRC explant model and may be a viable candidate for evaluation in patients with CRC.
Given that cabozantinib inhibits different receptor tyrosine kinases that play a role at inducing tumorigenic growth 24–28, we evaluated treatment effects on several key targets of this compound. As expected, we observed a decrease in the phosphorylation of Tie2, VEGFR2 (pro-angiogenic factors) and the MET, RET and AXL receptors (oncogenic pathways). In addition, upregulation of the MET receptor was seen after treatment with cabozantinib indicating a possible compensatory mechanism from the inhibition of MET and VEGFR2. Similar findings have been described where blockade of VEGF signaling results in hypoxia induced expression and activation of the MET receptor.5, 8, 20 This is likely mediated through the binding of HIF1α to the MET promoter and MET gene transcription.29 This increase in MET expression and activation is postulated to be responsible for tumor cell survival under these conditions ultimately triggering a more aggressive phenotype.30–33 Together, these findings suggest the value of targeting the MET receptor in conjunction with VEGF pathway inhibition with the goal of preventing drug resistance to VEGF targeted therapy.
Unlike the MET receptor, the contribution of AXL and RET tyrosine kinase activation to tumor growth and drug resistance is not well characterized in CRC. While AXL is recognized as a poor prognostic marker in CRC and is important for invasion and migration of tumor cells 34, the influence of this pathway on CRC tumor growth is less understood.28 RET alterations have been described in thyroid (mutations) and lung cancers (fusions) and potentiate growth of tumors cells 35, 36; inactivating mutations in RET may instead have a tumor suppressor function in CRC. Of note, we did not identify any fusions or mutations in the RET or AXL receptors in our CRC explants. Further studies are needed to delineate the importance of these pathways on tumorigenic growth in CRC.
The PI3K signaling pathway plays a central role in the dissemination of signals to downstream targets that are important for proliferation and survival of tumor cells.37 In CRC, activating mutations in the PIK3CA gene have been reported in ~10–20% of patients 38, 39 and PI3K is one of the major signaling pathways dysregulated in this disease.40 Interestingly, in this study we discovered that tumors that possess a mutation in the PIK3CA gene had significantly greater sensitivity to cabozantinib when compared to PIK3CA wild type tumors. CRC020, CRC040 and CRC098 contained a mutation in the helical domain at amino acid position 542, while a mutation was identified in the C2 domain in CRC162 at amino acid position 478. In contrast to helical mutations, D478Y has not been described in CRC. However, other mutations in the C2 domain have been proposed to increase membrane binding of the p110 subunit subsequently leading to an increase in lipid kinase activity.41 In order to confirm the association between a PIK3CA mutation and sensitivity to cabozantinib, we used a PIK3CA isogenic cell line xenograft model where we compared the effects of cabozantinib between PIK3CA wild type vs. mutant tumors. This experiment produced similar results to the CRC explants, whereby tumors with a PIK3CA mutation exhibited enhanced sensitivity to cabozantinib when compared to PIK3CA wild type tumors. Further investigation of the treatment effects on the PI3K pathway showed a significant reduction in gene expression of many components of this pathway as well as activation of Akt, a focal point of the PI3K pathway. Therefore, in addition to altering tumor angiogenesis, in these studies cabozantinib significantly inhibited the PI3K signaling pathway further facilitating the death to tumor cells that are dependent on PI3K signaling. There may be several different explanations for enhanced activity in PIK3CA mutant tumors to cabozantinib in this study. First, cabozantinib inhibited gene expression of many components of the PI3K/mTOR signaling pathway and this reduction may have overall disrupted the robust downstream signaling in PIK3CA mutant tumors that are reliant on the PI3K pathway for survival. A second possibility is that off-target effects of cabozantinib on PI3K or AKT activation cannot be ruled out. Finally, a study by Corcoran et al. 42 showed that BRAF mutant CRCs exhibit elevated levels of EGFR phosphorylation, suggesting that this particular mutation may enhance tumor growth by leading to the upregulation of the EGF receptor. It is possible that a PIK3CA mutation in CRC tumors have increased upregulation and activation of RTKs such as MET or RET, and as a result we see greater efficacy with RTK inhibiton with cabozantinib. Although these are all potential reasons, further studies are needed to understand this relationship between increased sensitivity to cabozantinib in PIK3CA mutant tumors. These findings indicate that a mutation in the PIK3CA gene (C2 domain, helical domain and kinase domain) may be a potential biomarker for sensitivity to cabozantinib in CRC.
In addition to the treatment of progressive, metastatic medullary thyroid cancer 36, cabozantinib is currently being evaluated in the clinic for the treatment of other solid malignancies. Cabozantinib potently inhibits MET activation, which may be critical for potentiating resistance to VEGF inhibitors. We illustrated that cabozantinib has growth inhibitory properties in our preclinical CRC PDTX model and that tumors harbouring a mutation in the PIK3CA gene may have enhanced sensitivity to this compound. These findings support further evaluation of cabozantinib in patients with CRC. PIK3CA mutation as a predictive biomarker of sensitivity is intriguing and warrants further elucidation. A clinical trial of cabozantinib in refractory metastatic CRC is being initiated.
Supplementary Material
Novelty and Impact.
MET is upregulated in response to VEGF pathway inhibition and plays an essential role in tumorigenesis and progression of tumors. Herein we demonstrated that cabozantinib (VEGFR2 and MET inhibitor) exhibited potent antitumor in vivo activity in our preclinical colorectal cancer patient-derived tumor xenograft model. Interestingly, tumors possessing a PIK3CA mutation displayed the greatest sensitivity to cabozantinib. These findings support further investigation of cabozantinib in patients with CRC and PIK3CA mutation as a predictive biomarker.
Acknowledgments
This preclinical work was supported with research funds from Exelixis.
This paper was supported by research funds of Chonbuk National University in 2011.
CEA gratefully acknowledges support from American Cancer Society Postdoctoral Fellowship 11-183-01-TBG; the Alliance for Clinical Trials in Oncology Foundation; the Mt. Zion Health Fund, Gump Cancer Fund from the Resource Evaluation and Allocation Committee, and Helen Diller Family Comprehensive Cancer Center; and National Cancer Institute under the National Institutes of Health Award K08CA17515301.
Dr. Arcaroli received research funds from Exelixis for support of this preclinical work.
Dr. Robin Kelley received research funding as a site principal investigator for a clinical trial of cabozantinb in liver cancer. Also, she is a steering committee member for the clinical trial.
References
- 1.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69 (Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 2.Hubbard J, Grothey A. Antiangiogenesis agents in colorectal cancer. Current opinion in oncology. 2010;22:374–80. doi: 10.1097/CCO.0b013e328339524e. [DOI] [PubMed] [Google Scholar]
- 3.Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, Vieitez JM, Bouche O, Borg C, Steffens CC, Alonso-Orduna V, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. The lancet oncology. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 5.di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK, Fazlollahi L, Andronesi OC, Frosch MP, Wen PY, Plotkin SR, Hedley-Whyte ET, Sorensen AG, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer research. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD, Christensen JG. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer research. 2010;70:10090–100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 9.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nature reviews Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi H, Bilchik A, Saha S, Turner R, Wiese D, Tanaka M, Kuo C, Wang HJ, Hoon DS. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:1480–8. [PubMed] [Google Scholar]
- 11.Pratap Kanwal, Singh Raghav HMA, Wang Wenting, Manyam Ganiraju C, Broom Bradley, Eng Cathy, Overman Michael J, Kopetz Scott. MET overexpression as a hallmark of the epithelial-mesenchymal transition (EMT) phenotype in colorectal cancer. Journal of Clinical Oncology. 2013;31:Abstract 3529. [Google Scholar]
- 12.Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, Sartore-Bianchi A, Scala E, Cassingena A, Zecchin D, Apicella M, Migliardi G, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer discovery. 2013;3:658–73. doi: 10.1158/2159-8290.CD-12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navis AC, Bourgonje A, Wesseling P, Wright A, Hendriks W, Verrijp K, van der Laak JA, Heerschap A, Leenders WP. Effects of dual targeting of tumor cells and stroma in human glioblastoma xenografts with a tyrosine kinase inhibitor against c-MET and VEGFR2. PloS one. 2013;8:e58262. doi: 10.1371/journal.pone.0058262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen HM, Ruppender N, Zhang X, Brown LG, Gross TS, Morrissey C, Gulati R, Vessella RL, Schimmoller F, Aftab DT, Corey E. Cabozantinib inhibits growth of androgen-sensitive and castration-resistant prostate cancer and affects bone remodeling. PloS one. 2013;8:e78881. doi: 10.1371/journal.pone.0078881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg PV, Kuramochi H, Tanaka K, Singh S, et al. An in vivo platform for translational drug development in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:4652–61. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 16.Dangles-Marie V, Pocard M, Richon S, Weiswald LB, Assayag F, Saulnier P, Judde JG, Janneau JL, Auger N, Validire P, Dutrillaux B, Praz F, et al. Establishment of human colon cancer cell lines from fresh tumors versus xenografts: comparison of success rate and cell line features. Cancer research. 2007;67:398–407. doi: 10.1158/0008-5472.CAN-06-0594. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–4. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, Orf J, You A, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Molecular cancer therapeutics. 2011;10:2298–308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 20.Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You WK, Chapman HA, Christensen JG, Aftab DT, McDonald DM. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer discovery. 2012;2:270–87. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentzien F, Zuzow M, Heald N, Gibson A, Shi Y, Goon L, Yu P, Engst S, Zhang W, Huang D, Zhao L, Vysotskaia V, et al. In Vitro and In Vivo Activity of Cabozantinib (XL184), an Inhibitor of RET, MET, and VEGFR2, in a Model of Medullary Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2013;23:1569–77. doi: 10.1089/thy.2013.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hage C, Rausch V, Giese N, Giese T, Schonsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell death & disease. 2013;4:e627. doi: 10.1038/cddis.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham TJ, Box G, Tunariu N, Crespo M, Spinks TJ, Miranda S, Attard G, de Bono J, Eccles SA, Davies FE, Robinson SP. Preclinical evaluation of imaging biomarkers for prostate cancer bone metastasis and response to cabozantinib. Journal of the National Cancer Institute. 2014;106:dju033. doi: 10.1093/jnci/dju033. [DOI] [PubMed] [Google Scholar]
- 24.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature reviews Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nature reviews Cancer. 2010;10:575–85. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 26.Moserle L, Jimenez-Valerio G, Casanovas O. Antiangiogenic therapies: going beyond their limits. Cancer discovery. 2014;4:31–41. doi: 10.1158/2159-8290.CD-13-0199. [DOI] [PubMed] [Google Scholar]
- 27.Phay JE, Shah MH. Targeting RET receptor tyrosine kinase activation in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:5936–41. doi: 10.1158/1078-0432.CCR-09-0786. [DOI] [PubMed] [Google Scholar]
- 28.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Molecular cancer therapeutics. 2011;10:1763–73. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 29.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 30.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nature reviews Molecular cell biology. 2010;11:834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 31.Lengyel E, Prechtel D, Resau JH, Gauger K, Welk A, Lindemann K, Salanti G, Richter T, Knudsen B, Vande Woude GF, Harbeck N. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. International journal of cancer Journal international du cancer. 2005;113:678–82. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. The Journal of cell biology. 2001;153:1023–34. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y, Merlino G. Constitutive c-Met signaling through a nonautocrine mechanism promotes metastasis in a transgenic transplantation model. Cancer research. 2002;62:2951–6. [PubMed] [Google Scholar]
- 34.Dunne PD, McArt DG, Blayney JK, Kalimutho M, Greer S, Wang T, Srivastava S, Ong CW, Arthur K, Loughrey M, Redmond K, Longley DB, et al. AXL is a key regulator of inherent and chemotherapy-induced invasion and predicts a poor clinical outcome in early-stage colon cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:164–75. doi: 10.1158/1078-0432.CCR-13-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drilon A, Wang L, Hasanovic A, Suehara Y, Lipson D, Stephens P, Ross J, Miller V, Ginsberg M, Zakowski MF, Kris MG, Ladanyi M, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer discovery. 2013;3:630–5. doi: 10.1158/2159-8290.CD-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EE, Janisch L, Nauling F, Hong DS, Ng CS, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2660–6. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature reviews Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 38.Velho S, Oliveira C, Ferreira A, Ferreira AC, Suriano G, Schwartz S, Jr, Duval A, Carneiro F, Machado JC, Hamelin R, Seruca R. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur J Cancer. 2005;41:1649–54. doi: 10.1016/j.ejca.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Barault L, Veyrie N, Jooste V, Lecorre D, Chapusot C, Ferraz JM, Lievre A, Cortet M, Bouvier AM, Rat P, Roignot P, Faivre J, et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. International journal of cancer Journal international du cancer. 2008;122:2255–9. doi: 10.1002/ijc.23388. [DOI] [PubMed] [Google Scholar]
- 40.Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikenoue T, Kanai F, Hikiba Y, Obata T, Tanaka Y, Imamura J, Ohta M, Jazag A, Guleng B, Tateishi K, Asaoka Y, Matsumura M, et al. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer research. 2005;65:4562–7. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 42.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, Brown RD, Della Pelle P, Dias-Santagata D, Hung KE, Flaherty KT, Piris A, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer discovery. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.