Abstract
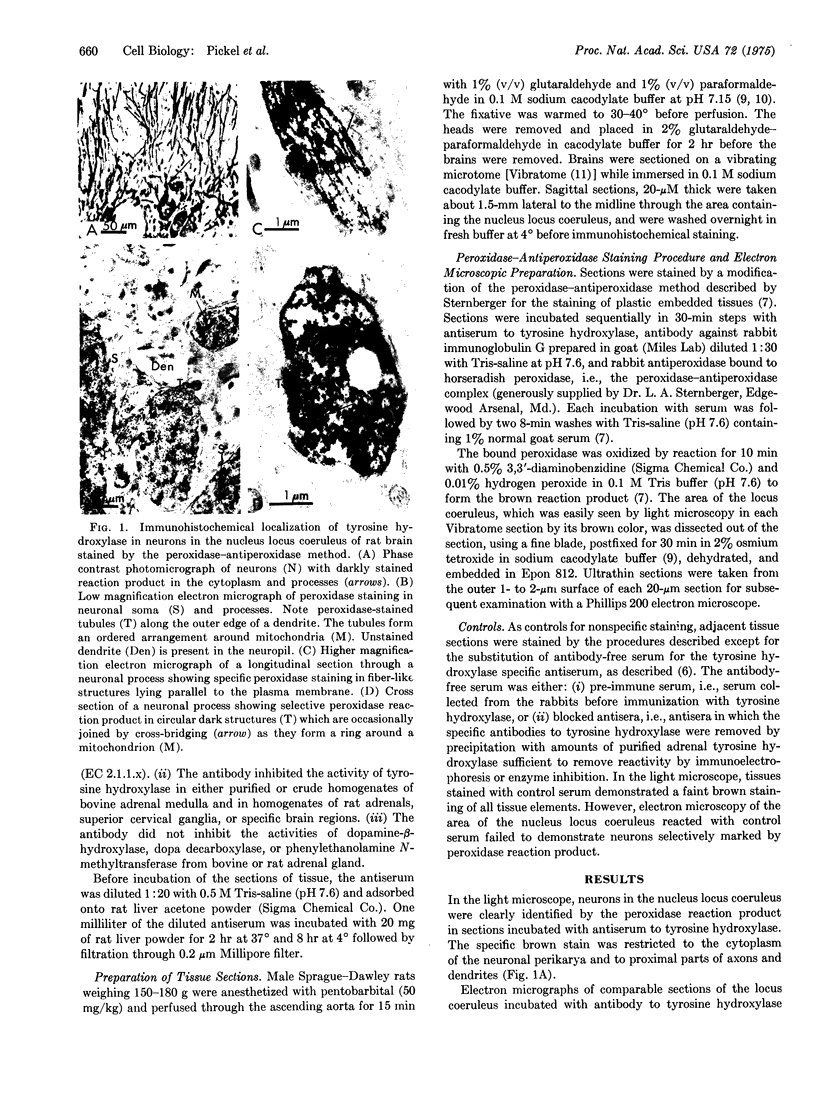
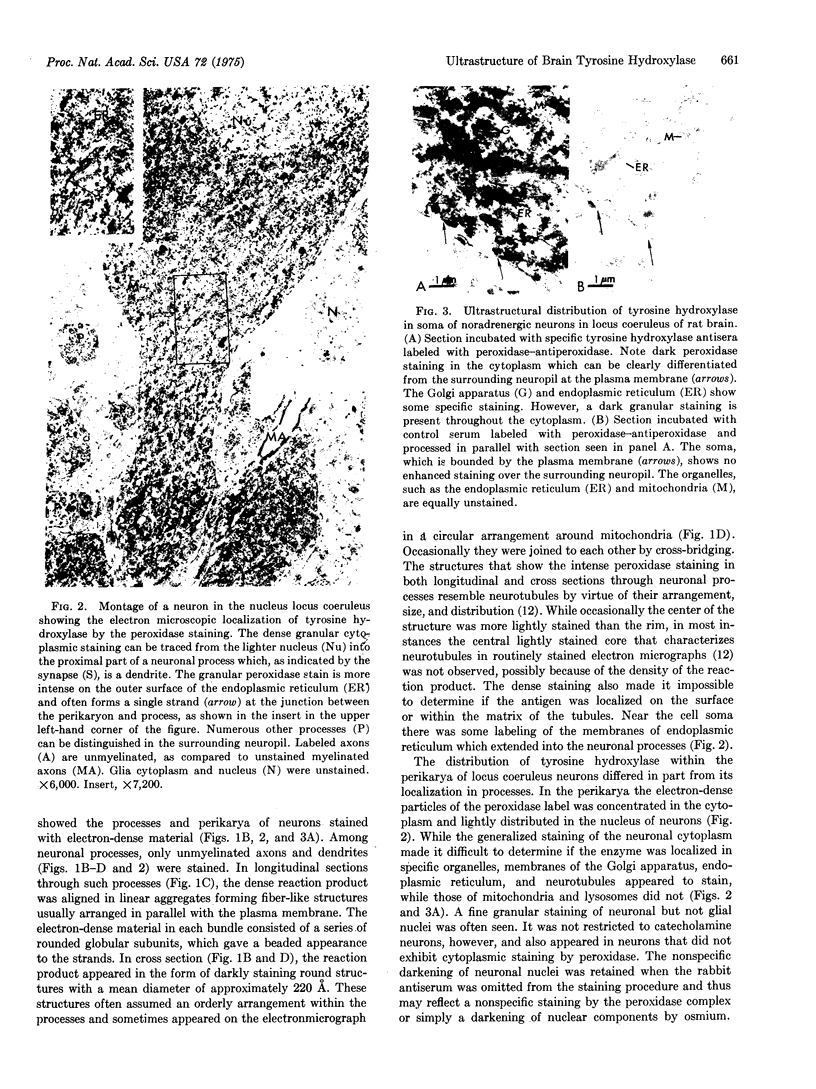
Tyrosine hydroxylase (EC 1.14.16.2), the enzyme catalyzing the rate-limiting step in catecholamine biosynthesis, was localized by electron microscopy within noradrenergic neurons of the nucleus locus coeruleus of the rat brain with a specific antibody to tyrosine hydroxylase labeled by the peroxidase-antiperoxidase immunohistochemical method. Labeled cell bodies and their processes were easily distinguished from unstained neuronal elements in the neuropil. The hydroxylase was only seen in the neuronal cytoplasm. Its distribution in processes was different from that in cell soma. In longitudinal sections of axons and dendrites, the peroxides reaction product appeared as fiber-like strands aligned parallel with the plasma membrane. In cross section, the labeled structures had a diameter of 220 A and exhibited an orderly distribution within the processes. The size and distribution of the peroxidase-stained structures suggest that they are neurotubules. In the perikarya, the cytoplasm was diffusely stained; the reaction was most intense on membranes of endoplasmic reticulum and Golgi apparatus, whereas lysosomes and mitochondria did not stain. The ultrastructural localization of tyrosine hydroxylase is consistent with biochemical data suggesting that the enzyme exists in different states in cell body and processes. The ultrastructural identification of enzymes subserving synthesis of neurotransmitters in central neurons and their processes may provide a useful tool in mapping the distribution of chemically specific synapses on identifiable neurons in brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calissano P., Cozzari C. Interaction of nerve growth factor with the mouse-brain neurotubule protein(s). Proc Natl Acad Sci U S A. 1974 May;71(5):2131–2135. doi: 10.1073/pnas.71.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T. Tyrosine hydroxylase in rat brain--cofactor requirements, regional and subcellular distribution. Biochem Pharmacol. 1972 Jul 15;21(14):1935–1944. doi: 10.1016/0006-2952(72)90006-8. [DOI] [PubMed] [Google Scholar]
- Geffen L. B., Livett B. G. Synaptic vesicles in sympathetic neurons. Physiol Rev. 1971 Jan;51(1):98–157. doi: 10.1152/physrev.1971.51.1.98. [DOI] [PubMed] [Google Scholar]
- Hartman B. K. Immunofluorescence of dopamine- -hydroxylase. Application of improved methodology to the localization of the peripheral and central noradrenergic nervous system. J Histochem Cytochem. 1973 Apr;21(4):312–332. doi: 10.1177/21.4.312. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Fuxe K., Goldstein M. Immunohistochemical studies on monoamine-containing cell systems. Brain Res. 1973 Nov 23;62(2):461–469. doi: 10.1016/0006-8993(73)90709-9. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A. Modification of the Falck-Hillarp formaldehyde fluorescence method using the Vibratome: simple, rapid and sensitive localization of catecholamines in sections of unfixed or formalin fixed brain tissue. Histochemie. 1972;29(4):325–339. doi: 10.1007/BF00279815. [DOI] [PubMed] [Google Scholar]
- Jarrott B., Geffen L. B. Rapid axoplasmic transport of tyrosine hydroxylase in relation to other cytoplasmic constituents. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3440–3442. doi: 10.1073/pnas.69.11.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R. T., Mandell A. J. Regulatory properties of soluble and particulate rat brain tyrosine hydroxylase. J Biol Chem. 1972 May 25;247(10):3114–3122. [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]
- Pohorecky L. A., Zigmond M., Karten H., Wurtman R. J. Enzymatic conversion of norepinephrine to epinephrine by the brain. J Pharmacol Exp Ther. 1969 Feb;165(2):190–195. [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert P., Kreutzberg G. W., Lux H. D. Neuroplasmic transport in dendrites: effect of colchicine on morphology and physiology of motoneurones in the cat. Brain Res. 1972 Dec 12;47(2):331–343. doi: 10.1016/0006-8993(72)90643-9. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Wuerker R. B., Kirkpatrick J. B. Neuronal microtubules, neurofilaments, and microfilaments. Int Rev Cytol. 1972;33:45–75. doi: 10.1016/s0074-7696(08)61448-5. [DOI] [PubMed] [Google Scholar]