Abstract
Respiratory syncytial virus (RSV) infection remains a significant global health burden disproportionately affecting infants and leading to long-term lung disease. IL-17A has been shown to be involved in regulating viral and allergic lung inflammatory responses, which has led to a more recent interest in its role in RSV infection. Using a neonatal mouse model of RSV, we demonstrate that neonates fail to develop IL-17A responses compared to adult mice; the main immediate IL-17A contributor in adults were γδ T cells. Antibody neutralization of IL-17A in adult mice caused increased lung inflammation and airway mucus from RSV, while exogenous IL-17A administration to RSV-infected neonates caused decreased inflammation but no change in airway mucus. We also observed a lack of pro-inflammatory cytokine production (IL-1β, IL-6) from infected neonates. Using human cord blood mononuclear cells (CBMCs) and adult peripheral blood mononuclear cells (PBMCs), we compared inflammasome activation by direct retinoic acid-inducible gene I (RIG-I) agonism; CBMCs failed to induce pro-inflammatory cytokines or IL-17A+ γδ T cells compared to PBMCs. Our results indicate that RSV disease severity is in part mediated by a lack of inflammasome activation and IL-17A production in neonates.
Keywords: gamma delta T cell, inflammasome, infant RSV
Introduction
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infection in infants and young children worldwide, causing an estimated 64,000,000 cases and 160,000 deaths each year1. Clinically, severe RSV in infants is characterized by cough (with/without wheezing), increased airway mucus production, and increased pulmonary inflammation2. Alarmingly, severe RSV infection during infancy is associated with the development of long term airways diseases such as asthma3, 4. Data from ours and others’ laboratories have demonstrated that infant humans (and neonatal rodents) respond differently to RSV compared to adults5–7, though the precise mechanisms underlying these immunological differences remain largely unknown.
IL-17A belongs to the IL-17 family of cytokines, which includes six members: IL17A-F. All members of this family are involved in inflammatory responses; however, only IL-17A, IL-17F, and IL-17E (IL-25) are produced by hematopoietic cells. Though IL-17A and IL-17F share 50% homology and both bind the IL-17 receptor (IL-17R), IL-17A has much higher affinity for IL-17R and induces stronger intracellular signaling than IL-17F8. IL-17A is associated with a range of inflammatory diseases including rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and asthma. The role(s) of IL-17 during RSV infection, in infants and adults, has not yet been fully elucidated.
CD4+ T cells are regarded as the main resource of IL-17A, but CD8+ T cells, gamma delta (γδ) T cells, NK cells, and neutrophils are also notable IL-17A producers9. Under certain circumstances such as viral hepatitis or allergic airway inflammation, γδ T cells have been reported to be the main source of IL-17A10, 11. Additionally, IL-17 production by both γδ T cells and CD4+ T cells is promoted by IL-1β12 – a product of inflammasome activation (typically via retinoic acid-inducible gene I protein [RIG-I] recognizing dsRNA during RSV infection13).
Recently, it has been reported that local IL-17A levels are increased during RSV infection in infants14. In RSV-infected adult mice, IL-17A has been shown to play a pathogenic role15 and a protective role16 with respect to exacerbated allergic airway responses; these differences are possibly due to infectious dose or viral strain. While the aforementioned studies have helped to gain some insight into a role for IL-17A during RSV infection, they utilized only adult mice. Here we seek to understand the role of IL-17A in early RSV infections using our neonatal mouse model of RSV.
The present study explores and compares the role(s) of IL-17A in neonatal vs. adult RSV infection. Compared to infected adult mice, RSV-infected neonatal mice fail to produce IL-17A and activated-inflammasome markers such as IL-1β and IL-6. Flow cytometric analyses of IL-17A producing cells confirm γδ T cells as the main source of early IL-17A, and modulation of IL-17A during acute RSV infection significantly alters disease outcomes. RIG-I-dependent activation of the inflammasome in human infant cord blood mononuclear cells (CBMCs) and adult peripheral blood mononuclear cells (PBMCs) revealed vastly attenuated cytokine production in CBMCs which may explain the lack of IL-17A during infant RSV infection.
Results
IL-17A and cytokines involved in its induction are not induced following RSV infection in neonatal mice
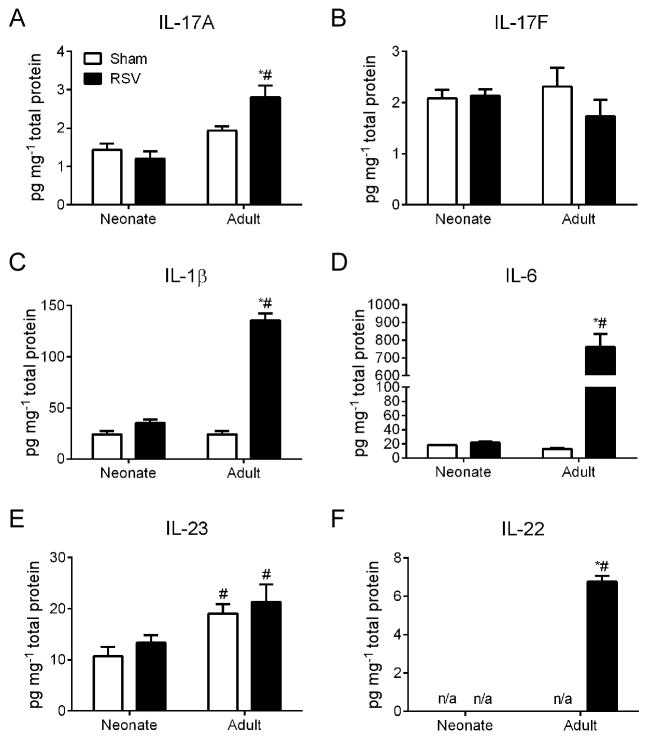
To determine the association between IL-17A and RSV infection, we infected neonatal and adult mice with RSV and compared cytokine levels in whole lung homogenates across a time-course from 0.25 to 10 days post infection (dpi) looking at IL-17A protein in the lungs of neonatal and adult mice infected with RSV. We observed an initial significant increase in IL17-A in adults as compared to neonates as early as 0.5 dpi and no significant change in IL17-A was observed in the neonates up to 10 dpi (data not shown). The adult response peaked at 1 dpi. Therefore, we chose to focus our later experiments at this optimal timepoint (1 dpi). RSV infection significantly increased expression of IL-17A but not IL-17F in the lungs of adult mice (Fig. 1A, B). Cytokines involved in the induction of IL-17A including IL-1β and IL-6 were also elevated in the lungs of RSV infected adult mice but not RSV infected neonatal mice (Fig. 1C, D) compared to uninfected controls of the same age. There was no change in IL-23 production in response to RSV infection; however its expression was increased in adult lungs compared to neonatal lungs (Fig. 1E). Furthermore, expression of IL-22, an IL-17-induced cytokine17, 18 was observed only in the lungs of RSV infected adult mice (Fig. 1F).
Figure 1. IL-17A and cytokines involved in its induction are not induced following RSV infection in neonatal mice.
Neonates (5 days old) and adults (6–8 weeks old) were infected with RSV and cytokine protein levels in whole lung homogenates was determined at 1 dpi by ELISA (IL-17A, IL-1β) or multiplex assay (IL-17F, IL-6, IL-23, IL-22). Levels of IL-17A (A), IL-17F (B), IL-1β (C), IL-6 (D), IL-23 (E), and IL-22 (F) were normalized to pg/mg of total lung protein. Control mice were age-matched and sham (serum-free media) infected. Data are representative of at least two independent experiments. N = 4–6 mice per group per experiment, n/a = levels below detection. Data plotted as means ± SEM. *P<0.05 vs. age-matched control group, #P<0.05 vs. opposite age group. Two-way ANOVA with Bonferroni post-test.
γδ T cells are the main source of IL-17A during early RSV infection
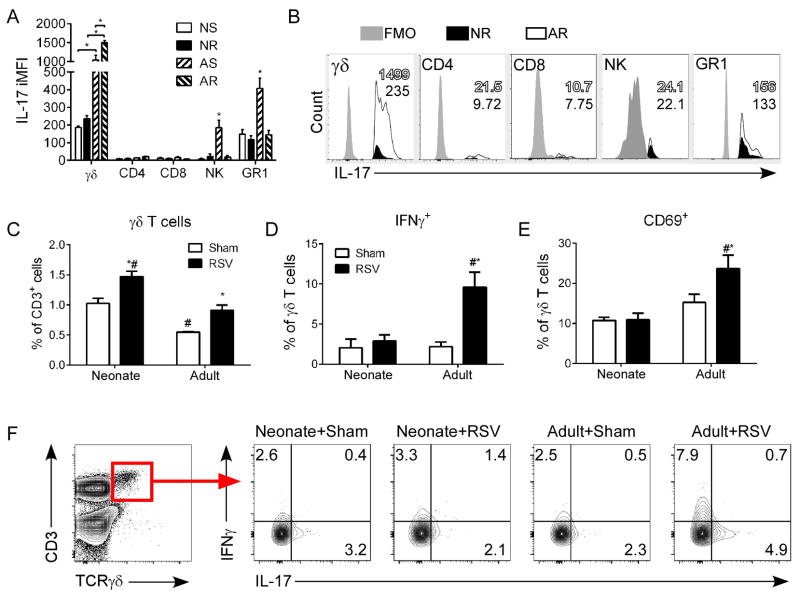
To determine the cellular source of IL-17A in RSV-infected mice, we analyzed all IL-17A+ cells in the lungs by flow cytometry using surface marker expression to attribute production to known IL-17-producing cell populations (i.e., γδ T cells, CD4/8 T cells, NK cells, neutrophils) at 1 dpi. Using the percentage of these populations making up all IL-17A+ cells and the intensity of IL-17A expression within that respective population (i.e., iMFI), we were able to deduce the source of IL-17A in RSV-infected neonatal (NR) and adult (AR) mice compared to sham controls (NS or AS, respectively) (Fig. 2). Most notably, adult mice either RSV or sham infected produced the greatest amounts of IL-17A. In adults, γδ T cells were the main source of IL-17A during early RSV infection (Fig. 2A,B), although neutrophils also produced a significant amount of IL-17A following infection (~15 fold induction versus ~4 fold induction). In neonates, both γδ T cells and neutrophils were the major sources of IL-17A; however, the IL-17A iMFI was not significantly different between neonatal RSV or sham infected mice. The levels of γδ T cells in the lungs increased during RSV infection in both adults and neonates (Fig. 2C); surprisingly, the overall levels of γδ T cells were significantly higher in neonates. Because γδ T cells also produce IFNγ, which is important in anti-viral responses, we also looked at IFNγ expressing γδ T cells. The percentage of IFNγ producing γδ T cells were elevated only in the lungs of RSV-infected adult mice (Fig. 2D,F). The percentage of activated γδ T cells followed a similar trend as evidenced by CD69 expression (Fig. 2E).
Figure 2. γδ T cells are the main source of IL-17A during early RSV infection.
Neonates and adults were infected with RSV and at 1 dpi lung cells were isolated. Cells were stimulated in vitro with PMA and ionomycin, stained with dead cell-excluding dye and antibodies to IL-17A, IFNγ, CD3, CD4, CD8, CD69, TCRγδ, panNK, and GR1. The cells were then analyzed by flow cytometry. (A) Integrated median fluorescent intensity (iMFI) of IL-17A for γδ T cells, CD4 T cells, CD8 T cells, NK cells, and neutrophils. (B) Representative flow cytometry plots demonstrating IL-17A contribution by cell population in RSV-infected neonates (black fill) and adults (open) compared with IL-17A FMO controls (grey fill). The iMFI for neonates (black fill text) and adults (black outline with open fill text) is shown as an inset in each histogram. (C) Levels of γδ T cells in the lungs. (D) Percent of γδ T cells expressing IFNγ. (E) Percent of γδ T cells expressing CD69. (F) Representative flow cytometry plots demonstrating IFNγ and IL-17A expression by γδ T cells. Control mice were age-matched and sham (serum-free media) infected. Data are representative of three independent experiments. N = 4–6 mice per group per experiment. Data plotted as means ± SEM. *P<0.05 vs. age-matched control group, #P<0.05 vs. opposite age group. Two-way ANOVA with Bonferroni post-test.
IL-17A reduces RSV disease severity in neonatal mice
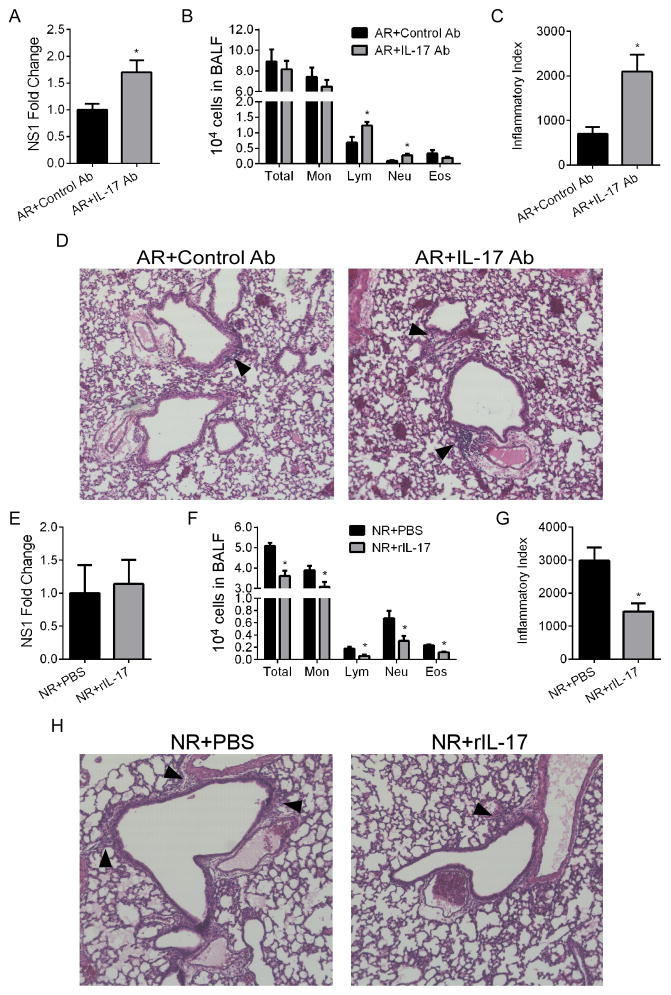
To investigate the contribution of IL-17A to RSV induced pathogenesis, we decided to reverse the age-specific effect of IL-17A induction by γδ T cells by neutralizing IL-17A in adults and supplementing IL-17A in neonates (Fig. 3). In RSV-infected adults, IL-17A neutralization resulted in increased lung viral copy number (Fig. 3A), BALF lymphocytes and neutrophils (Fig. 3B), and pulmonary pathology (Fig. 3C,D). Administration of recombinant IL-17A (rIL-17) during RSV infection in neonatal mice did not alter lung viral copy number (Fig. 3E), resulted in significant decreases in total BALF cellularity (Fig. 3F), and significantly improved lung pathology (Fig. 3G,H). None of these groups displayed measurable airway mucus production (data not shown).
Figure 3. IL-17A reduces RSV disease severity in neonatal mice.
Neonates and adults were pre-treated with rIL-17 or anti-IL-17A antibody, respectively, and infected with RSV. At 4 dpi, pulmonary RSV levels, BALF cellularity, or lung histopathology were determined. (A, E) Pulmonary RSV levels determined by fold expression of NS1 mRNA in adults and neonates, respectively. (B, F) Total BALF counts and differentials from treated and infected adults and neonates, respectively. (C, G) Inflammatory index of lung histopathology from treated and infected adults and neonates, respectively. (D, H) Representative 20x images of H&E stained lung sections showing peribronchiolar/perivascular inflammation. The arrowheads indicate cellular infiltrates. Data are representative of at least two independent experiments. N=4–6 mice per group per experiment. Data plotted as means ± SEM. *P<0.05 vs. control groups. Student’s t-test.
IL-17A levels during initial RSV infection dictates pathology following RSV reinfection
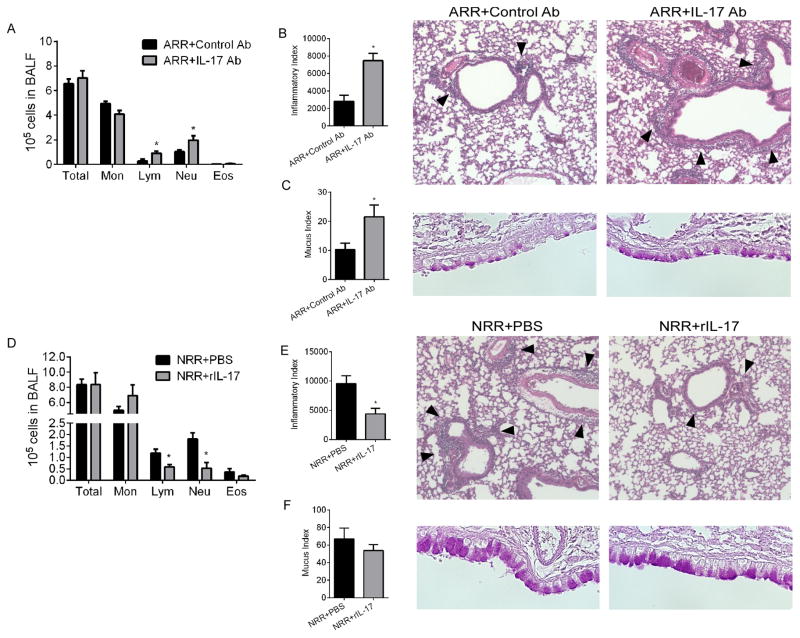
We have previously demonstrated that RSV reinfection of mice initially infected as neonates (but, importantly, not as adults) results in substantial immunopathophysiology which closely mimics what is seen clinically in human infants with severe RSV6, 19. In order to observe the impacts of respectively decreasing or increasing IL-17A during initial RSV infection on reinfection responses in adults and neonates, mice treated as in Figure 3 were reinfected with RSV 28 days after initial infection (Fig. 4). Following reinfection, mice initially infected as adults and treated with IL-17A-neutralizing antibody (ARR+IL-17Ab) during the initial infection had significantly elevated lymphocyte and neutrophil numbers in the BALF (Fig. 4A). Compared to controls (ARR+Control Ab), these mice exhibited significantly worse lung pathology with increased peribronchiolar/perivascular infiltrates (Fig. 4B) and increased mucus-producing cells in the airways (Fig. 4C). Following reinfection, mice initially infected as neonates and treated with rIL-17A (NRR+rIL-17) during the initial infection displayed decreased lymphocyte and neutrophil numbers in the BALF compared to controls (NRR+PBS) (Fig. 4D). Though lung immunopathology in both of these groups were expectedly worse compared to that following initial infection, the NRR+rIL-17 group had significantly less peribronchiolar/perivascular inflammation (Fig. 4E). Quantitatively, airway mucus was not affected by rIL-17 administration during initial infection in the neonate (Fig. 4F).
Figure 4. IL-17A levels during initial RSV infection dictates pathology following RSV reinfection.
Neonates and adults were infected and reinfected with RSV at 4 weeks postprimary infection. BALF cellularity and lung histopathology were determined at 6 days postsecondary infection. (A, D) Total BALF counts and differentials from reinfected mice that were initially treated and infected as adults or neonates, respectively. (B, E) Inflammatory index and representative 20x images of H&E stained lung sections showing peribronchiolar/perivascular inflammation. The arrowheads indicate cellular infiltrates. (C, F) Mucus index and representative 40x images of airway epithelium. Data are representative of at least two independent experiments. N=4–6 mice per group per experiment. Data plotted as means ± SEM. *P<0.05 vs. control groups. Student’s t-test.
Adoptive transfer of adult γδ T cells is sufficient to protect against RSV-induced pathology in neonates
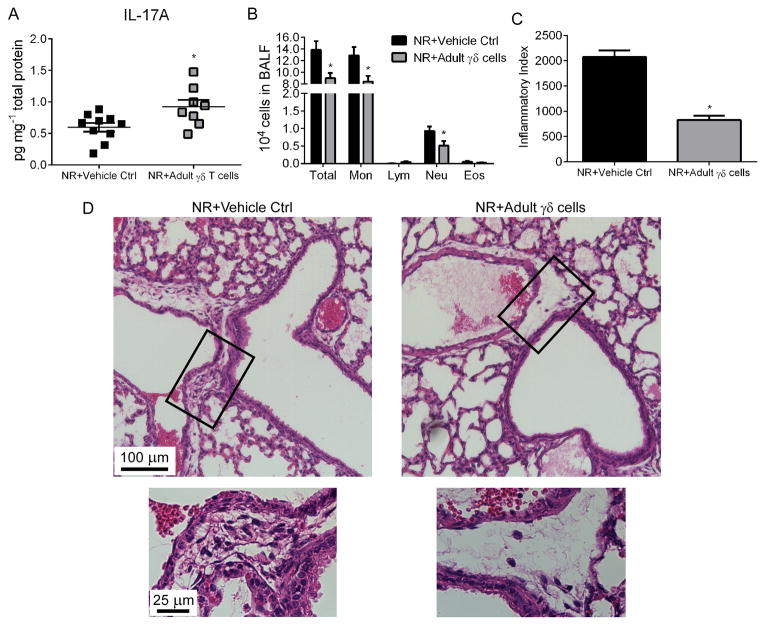
Our data indicated that neonatal mice have impaired γδ T cell function with respect to RSV-induced IL-17A induction, and that in neonatal mice disease severity was reduced with rIL-17 treatment during initial infection. Since adult γδ T cells appeared to be more functionally competent, we isolated these cells from the lungs of naïve adult mice and adoptively transferred them intranasally into the lungs of neonatal mice prior to initial RSV infection (Fig. 5). Following RSV infection, γδ T cell-recipient neonates (NR+Adult γδ T cells) displayed significantly more IL-17A in the lungs (Fig. 5A), less total cells in the BALF (mainly due to decreased monocyte and neutrophil numbers) (Fig. 5B), and significantly decreased peribronchiolar/perivascular infiltrates (Fig. 5C,D).
Figure 5. Adoptive transfer of adult γδ T cells is sufficient to protect against RSV-induced pathology in neonates.
γδ T cells from adults were adoptively transferred into the lungs of neonates prior to RSV infection. (A) Whole lung IL-17A levels from RSV-infected neonatal mice pre-treated with adult γδ T cells or vehicle control, determined at 1 dpi. (B) Total BALF counts and differentials determined at 4dpi. (C, D) Inflammatory index and representative 20x (top) images with enhanced 60x inset (bottom) images of H&E stained lung sections showing peribronchiolar/perivascular inflammation. Data are representative of at least two independent experiments. N=5 mice per group per experiment. Data plotted as means ± SEM. *P<0.05 vs. control groups. Student’s t-test.
Impaired IL-17A and IFNγ responses in γδ T cells following inflammasome activation in human cord blood mononuclear cells
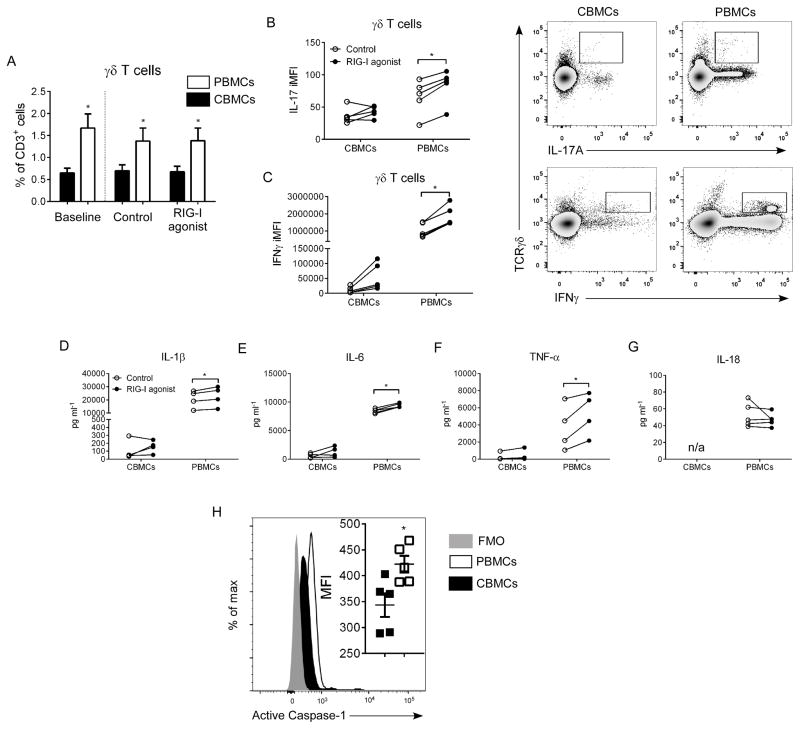
Our initial cytokine data demonstrated that neonates infected with RSV failed to induce IL1β and IL-6. This indicated a defect in inflammasome activation during neonatal RSV infection. To explore this effect in vitro, we treated infant human cord blood mononuclear cells (CBMCs) and adult peripheral blood mononuclear cells (PBMCs) with a RIG-I agonist (Fig. 6). The amount of γδ T cells remained significantly higher in adult PBMCs, independent of culture time or treatment (Fig. 6A). Additionally, γδ T cells in adult PBMCs had elevated IL-17A and IFNγ responses compared to CBMCs (Fig. 6B,C). Cytokine production was measured in culture supernatants; RIG-I treatment resulted in significantly higher production of IL-1β, IL-6, and TNFα in PBMCs but not in CBMCs (Fig. 6D–F). IL-18 was observed only in PBMC supernatants; no difference was observed between control and RIG-I agonist-treated PBMC samples (Fig. 6G). Moreover, activated caspase-1 expression was significantly elevated in γδ T cells from adult PBMCs (Fig. 6H).
Figure 6. Impaired IL-17A and IFNγ responses in γδ T cells following inflammasome activation in human cord blood mononuclear cells.
Human CBMCs and adult PBMCs (2 × 105 cells/well) were treated in vitro with a RIG-I agonist (1 μg/ml, 48 hr). (A) γδ T cells were quantified in pre- and post-treatment samples by flow cytometry. (B,C) Following incubation, IL-17A and IFNγ iMFI was determined in γδ T cells by flow cytometry. Production of IL-1β (D), IL-6 (E), TNFα (F), and IL-18 (G) were measured in cell culture supernatants by multiplex or ELISA assay. (H) Expression of active caspase-1in CBMCs (black fill) vs. adult PBMCs (open) compared to FMO control (grey fill). Data are representative of four independent experiments. N=5 samples per group from individual donors per experiment. Data plotted as means ± SEM. *P<0.05 vs. control groups. Paired student’s t-test.
Discussion
The relatively recent research focus on infant immunity has helped to gain mechanistic insight as to how and why this population is immunologically distinct from adults. Severe RSV infection remains an important public health issue due to its prevalence in infants, association with long-term airway disease, and lack of effective prophylactic treatment1–3. Our neonatal mouse model of RSV infection recapitulates many of the symptoms observed in RSV-infected human infants, making it an appropriate translational tool5–7; typically, mice infected initially as adults fail to develop long-term airways dysfunction and do not develop significant pulmonary inflammation or airway mucus to the same degree as mice initially infected as neonates.
Though less numerous than conventional T cells, γδ T cells are critical regulators of inflammation and virally-exacerbated allergic disease20, 21. Here, we demonstrated that early (1 dpi) γδ T cell-mediated IL-17A responses are attenuated in neonates infected with RSV compared to adults. Surprisingly, infected neonates have significantly higher numbers of γδ T cells in the lung at this time point, though they are less activated. Antibody-mediated neutralization of IL-17A in adult mice during initial infection resulted in enhanced pulmonary inflammation and mucus, while treatment with rIL-17 in neonatal mice during initial infection resulted in an attenuation of pulmonary inflammation and airway mucus. These data support a protective role for IL-17A during RSV infection. Interestingly, one study comparing plasma cytokine levels in infants found significantly elevated IL-17A (~40 fold) in infants with moderate RSV disease compared to those with severe RSV disease22. Additionally, lower IL-17A concentrations have also been observed in BALF from infant patients requiring ventilation for severe RSV compared to non-ventilated RSV patients14. Both of these clinical studies support a protective role for IL-17A during infant RSV infection, and indicate that varied IL-17A responses could be partly responsible for moderate vs. severe disease. Unfortunately, there are no comparative IL-17A data in RSV-infected adult humans, although our mouse data suggest fully competent inflammasome activation and IL-17A production play a major role.
Existing studies that examine IL-17A and RSV in mice demonstrate the importance of IL-17A in disease outcomes. In an RSV/OVA model of allergic lung inflammation, RSV-induced IL-17A decreased airway inflammation, eosinophilia, neutrophilia, and airway hyperresponsiveness16, which agrees with our own data demonstrating IL-17A as a negative regulator. However, another group demonstrated that IL-17A neutralization during RSV infection causes decreased airway mucus and neutrophilia15. In addition to being primarily focused on allergic airways disease clinically associated with RSV, these studies differ from the present one by RSV strain used and, most importantly, in the age of the mouse at initial RSV infection – adults. The pathogenesis of RSV strain A2, used in the present study, has been directly compared to strain Line 19 and has proven to induce less morbidity with respect to mucus induction and airway dysfunction23, 24. Indeed, this study and our previous studies with strain A2 have shown mild disease upon initial infection, but greater disease upon reinfection which has high clinical correlativity5, 6, 25. In an IL-13-induced airway inflammation model, exogenous IL-17A treatment had dose-dependent effects; while a lower dose of IL-17A decreased γδ T cell influx but greatly increased eosinophilia, a higher dose of IL-17A paradoxically decreased all pulmonary inflammation26. Furthermore, this same study demonstrated the inflammation-inhibiting effects of IL-17A-producing γδ T cells adoptively transferred into naïve mice. In our study, neonatal mice infected with RSV had higher numbers of lung γδ T cells compared to adults, however, the IL-17A production and overall activation status of these cells were significantly attenuated. In humans, similar lymphocyte functional deficits have been demonstrated in infant populations27, 28. Despite the well-established role of CD8+ T cells in mediating RSV in adult mice29, 30, recent evidence in human infants suggests that innate immune cells (e.g., macrophages, neutrophils, γδ T cells) may be more important for viral clearance due to the absence of an efficient lymphocyte response31.
We observed a large disparity in pro-inflammatory cytokine production (i.e., IL-1β, IL-6; IL17A) in RSV-infected neonates compared to adults. Because IL-1β is a direct product of inflammasome activation and IL-17A is a product of inflammasome-activated γδ T cells, we hypothesized that this was due to impaired activation of the inflammasome. IL-17A is essential for lung inflammation caused by viral-based synthetic dsRNA32, and has been shown to act in vitro as a super-inducer of RSV- and RIG-I agonist-induced pro-inflammatory responses33. We compared the ability of a known inflammasome signaling mediator involved in early anti-RSV responses34, 35, RIG-I, to activate the inflammasome and signal pro-inflammatory cytokine production in CBMCs vs. adult PBMCs. We unexpectedly observed a higher total number of γδ T cells in adult PBMCs, which contrasts what we observed in mice. We attribute this difference to measuring two different biological compartments (lung vs. periphery) and experimental methods (in vivo vs. in vitro) in which cell recruitment to the lungs may/may not take place. This effect has also been observed by other groups36. However, similar to the RSV-infected neonatal mice, the RIG-I agonist-stimulated CBMCs failed to exhibit increases in IL-1β, IL-6, IL-18, and TNFα compared with the adult PBMCs. Additionally, RIG-I activation caused significantly elevated IL-17A and IFNγ responses in γδ T cells from adult PBMCs but not CBMCs. Cleavage and activation of caspase-1 is the hallmark of inflammasome activation37, and γδ T cells from CBMCs displayed a relative lack of active caspase-1 compared to that of adult PBMCs. To summarize, in response to RSV infection we observed that 1) there are more γδ T cells in the neonatal lung; 2) neonatal mice produce less IL-1β, a product of inflammasome activation, than adult mice; and 3) although there are more γδ T cells in the lungs of the neonate, these cells are less capable of producing IL-17A on a per cell basis or in bulk in response to RSV infection. If you combine this with our data from CBMCs demonstrating a decreased ability of these cells to cleave and activate caspase-1 or to produce IL-1β, IL-18, or IL-17A following stimulation with RIG-I agonist, then our data cumulatively suggest that neonatal γδ T cells are inherently defective in inflammasome activation.
Our findings have demonstrated further differences between neonatal and adult immunity, and strengthened the argument that age-appropriate animal models are vital for understanding/ successful translation of infant disease. Admittedly, more extensive studies on IL-17A in human infant RSV disease are necessary to better understand its role(s), and whether it is a suitable target for therapy.
Methods
Mice
BALB/c mice were purchased as breeders from Harlan Laboratories (Indianapolis, IN, USA). All mice were housed in the vivarium at the University of Tennessee Health Science Center, were maintained in ventilated microisolator cages, and housed in a specific pathogen-free animal facility. Breeders were time mated, and age-matched 5 day old pups were used for neonatal experiments and 6–8 week old female mice were used for adult experiments. All animal protocols were prepared in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center.
RSV infection
Human RSV strain A2 was purchased as a sucrose-gradient purified virus from Advanced Biotechnologies (Columbia, MD, USA). The virus preparation was determined to be free of bacteria, yeast, and fungi. For all mouse studies, the virus was propagated in Vero cells (ATCC; Manassas, VA, USA) using serum free medium (SFM4MegaVir™; HyClone, Logan, UT, USA) and stored at −80°C until use. For neonatal infections, 5 day old pups were anesthetized with 5% isofluorane and infected intranasally with 2×105 median tissue culture infective dose (TCID50) per gram of body weight in 10 μl total volume. Similarly, adult mice were isofluorane-anesthetized and intranasally infected with 2×105 TCID50 per gram of body weight in 50 μl total volume. Sham control mice were given the same volume of vehicle instilled intranasally. All reinfections occurred 28 days post-initial infection with 2×105 TCID50 per gram of body weight in 50 μl total volume.
Protein detection
The levels of IL-17A and IL-1β in homogenized whole lungs were detected by ELISA (R&D; Minneapolis, MN, USA) according to manufacturer’s instructions. The levels of IL-6, IL-23a, IL-22, and IL-17F in homogenized whole lungs were detected by cytokine multiplex (Millipore; Billerica, MA, USA according to manufacturer’s instructions. Assay sensitivity for each cytokine is 5 pg/ml, 4.8 pg/ml, 7.8 pg/ml, 342 pg/ml, 2.4 pg/ml, and 10 pg/ml, respectively. Values below the detection limit of the assay were excluded. Values were then plotted per mg of total lung protein.
Flow cytometry
Lung single cell suspensions were prepared using a standardized protocol as previously described38. Cells were stained with the following antibodies from BD Biosciences and eBioscience: eFluor450-CD3 (17A2), PerCP-CD4 (RM4-5), FITC-CD8a (53-6.7), Biotin-TCRγδ (GL3), PE-Streptavidin, APC-IL-17A (eBio17B7), APC-IFNγ (XMG1.2), FITC-CD69 (H1.2F3), PerCP-Cy5.5-Gr1 (RB6-8C5), and PECy7-panNK (DX5). For intracellular staining, cells were stimulated for 5 h with 5 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich; St. Louis, MO, USA) in the presence of a protein-transport inhibitor (GolgiPlug, BD Biosciences; San Jose, CA, USA). Following stimulation, cells were collected, stained with a fixable viability dye (eBioscience), fixed, permeabilized (Fixation and Permeabilization Buffer; eBioscience), then intracellularly stained. Samples were read using a FACSCanto II (BD Biosciences) flow cytometer and analyzed with FlowJo software (version 7.6.5 for Windows; Tree Star; Ashland, OR, USA). To simultaneously compare both quantity (% of cells) and quality (intensity of staining) of IL-17-producing populations, integrated median fluorescent intensity (iMFI) was calculated by multiplying percent of x cell type (i.e., CD4, CD8, γδ, Gr1, NK) of total IL-17A+ cells multiplied by the IL-17A MFI of x cell type.
BALF cellularity
Bronchoalveolar lavage fluid was isolated by flushing mouse lungs with PBS containing 2% BSA (1.0 ml for adults and 0.1 ml for neonates). Collected cells were spun onto glass slides and stained with a Hema-3 staining kit (Thermo Fisher Scientific; Logan, UT, USA). Differential cell counts were determined by two unbiased observers in a blinded manner using standard morphological criteria.
Lung histopathology
Mice were euthanized and retrograde perfusion performed to remove blood from the lungs. The lungs were then gravity inflated with zinc formalin. Following fixation, lungs were paraffin embedded, sectioned, mounted on slides, and stained with either hematoxylin and eosin or Periodic Acid Schiff (PAS) to quantify cellular infiltrates or airway mucus production, respectively. Images were acquired and analyzed with the EVOS imaging system (Life Technologies; Carlsbad, CA, USA). Inflammation index was quantified by manually counting the number of peribronchiolar/perivascular inflammatory cells divided by the peribronchiolar/perivascular area (mm2). Mucus expression was quantified as the number of mucus positive cells per total circumference length of airway (mm).
Viral burden
RSV viral burden in the lungs was assessed using a well-established protocol, which has been shown to strongly correlate with viral load as determined by plaque assay25, 39, 40. Briefly, real-time PCR was performed on normalized total lung RNA as previously described. NS1 primers, forward: 5′-CACAACAATGCCAGTGCTACAA-3′, reverse: 5′-TTAGACCATTAGGTTGAGAGCAATGT-3′ (IDT; Coralville, IA).
In vivo IL-17 modulation
Neonatal mice were administered recombinant IL-17A intranasally (Peprotech; 20 ng in 10 μl PBS 0.1% BSA) 2 hr post RSV infection and again 48 hr post RSV infection. Control animals were administered vehicle. Adult mice were injected i.p. with 300 μg anti-IL-17A antibody (Amgen; Thousand Oaks, CA, USA) 2 hr prior to RSV infection, then again 48 hr and 96 hr post RSV infection. Controls were administered IgG isotype control antibody (Southern Biotech; Birmingham, AL, USA).
Gamma delta T cell adoptive transfer
Lung single cell suspensions from adult mice were prepared using a standardized protocol as previously described38. Cells were centrifuged on a Ficoll-gradient to isolate mononuclear cells, and γδ T cells were isolated using a positive selection kit (Miltenyi Biotech; Germany). Cells were adoptively transferred intranasally (6.2×104 cells, based on number of γδ T cells in RSV-infected adult) into neonates 2 hr prior to RSV infection. Sample isolation purity and adoptive transfer efficiency were verified by flow cytometry.
CBMC/PBMC RIG-I activation
Purified CBMCs and PBMCs from individual donors (AllCells.com) were thawed and counted according to supplier’s recommendations. Cells were cultured in a 96-well, flat bottom plate (USA Scientific; Ocala, FL, USA) in RPMI 1640 medium containing 2 mM L-glutamine, 1 mM sodium pyruvate, and 10% FBS (HyClone; Logan, UT, USA). 2 × 105 total cells/well were treated with 1 μg/ml RIG-I agonist (5′ppp-dsRNA complexed with LyoVec; Invivogen, San Diego, CA, USA) or media control for 48 hr. For intracellular cytokine staining, a protein transport inhibitor was added during the final 5 hr of incubation (GolgiPlug; BD Bioscience, San Jose, CA, USA). Cells were stained with the following antibodies from eBioscience or BD Biosciences: eFluor450-CD3 (OKT3), FITC/PE-TCRγδ (B1.1), APC-IL-17A (8D4-8), PE-IFNγ (4S.B3). For detection of activated caspase-1, live cells were incubated with FAM-FLICA Caspase-1 Kit (ImmunoTechnologies; Bloomington, IN) during the final hour of incubation. Culture supernatants were flash frozen and stored at −80C° until cytokine multiplex (Millipore; Billerica, MA, USA) or ELISA (eBioscience) analysis according to manufacturer’s instructions; concentrations below the detection limit of the assay were excluded.
Statistics
Data are presented as means ± SEM using Prism5 (Graphpad; La Jolla, CA, USA). Statistics were analyzed with Prism5 and InStat3 (Graphpad; La Jolla, CA, USA). Two-way ANOVA with Bonferroni post-tests or student’s t-tests (paired or unpaired) were used to compare groups, where appropriate. P values <0.05 were considered significant.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants (R01AI090059, R01ES015050, and P42ES013648) to SAC. We thank Amgen for providing the IL-17A neutralizing antibody used in this study. We also thank Dr. Jay Kolls, University of Pittsburgh, for insightful discussions about this data.
References
- 1.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–19. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 3.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 4.Regnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:820–6. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 5.You D, Becnel D, Wang K, Ripple M, Daly M, Cormier SA. Exposure of neonates to respiratory syncytial virus is critical in determining subsequent airway response in adults. Respir Res. 2006;7:107. doi: 10.1186/1465-9921-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cormier SA, You D, Honnegowda S. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert Rev Anti Infect Ther. 2010;8:1371–80. doi: 10.1586/eri.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tregoning JS, Yamaguchi Y, Harker J, Wang B, Openshaw PJ. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008;82:4115–24. doi: 10.1128/JVI.02313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–75. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou L, Jie Z, Desai M, Liang Y, Soong L, Wang T, et al. Early IL-17 production by intrahepatic T cells is important for adaptive immune responses in viral hepatitis. J Immunol. 2013;190:621–9. doi: 10.4049/jimmunol.1201970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murdoch JR, Lloyd CM. Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing {gamma}{delta}T cells. Am J Respir Crit Care Med. 2010;182:464–76. doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–48. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 13.Yoboua F, Martel A, Duval A, Mukawera E, Grandvaux N. Respiratory syncytial virus-mediated NF-kappa B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK beta. J Virol. 2010;84:7267–77. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faber TE, Groen H, Welfing M, Jansen KJ, Bont LJ. Specific increase in local IL-17 production during recovery from primary RSV bronchiolitis. J Med Virol. 2012;84:1084–8. doi: 10.1002/jmv.23291. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, et al. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011;179:248–58. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcomb DC, Boswell MG, Reiss S, Zhou W, Goleniewska K, Toki S, et al. IL-17A inhibits airway reactivity induced by respiratory syncytial virus infection during allergic airway inflammation. Thorax. 2013;68:717–23. doi: 10.1136/thoraxjnl-2012-202404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 18.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dakhama A, Lee YM, Gelfand EW. Virus-induced airway dysfunction: pathogenesis and biomechanisms. Pediatr Infect Dis J. 2005;24:S159–69. doi: 10.1097/01.inf.0000188155.46381.15. discussion S166–7. [DOI] [PubMed] [Google Scholar]
- 20.Glanville N, Message SD, Walton RP, Pearson RM, Parker HL, Laza-Stanca V, et al. gammadeltaT cells suppress inflammation and disease during rhinovirus-induced asthma exacerbations. Mucosal Immunol. 2013;6:1091–100. doi: 10.1038/mi.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodd J, Riffault S, Kodituwakku JS, Hayday AC, Openshaw PJ. Pulmonary V gamma 4+ gamma delta T cells have proinflammatory and antiviral effects in viral lung disease. J Immunol. 2009;182:1174–81. doi: 10.4049/jimmunol.182.2.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larranaga CL, Ampuero SL, Luchsinger VF, Carrion FA, Aguilar NV, Morales PR, et al. Impaired immune response in severe human lower tract respiratory infection by respiratory syncytial virus. Pediatr Infect Dis J. 2009;28:867–73. doi: 10.1097/INF.0b013e3181a3ea71. [DOI] [PubMed] [Google Scholar]
- 23.Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, et al. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am J Pathol. 2006;169:977–86. doi: 10.2353/ajpath.2006.051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol. 2011;85:5782–93. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You D, Marr N, Saravia J, Shrestha B, Lee GI, Turvey SE, et al. IL-4Ralpha on CD4+ T cells plays a pathogenic role in respiratory syncytial virus reinfection in mice infected initially as neonates. J Leukoc Biol. 2013;93:933–42. doi: 10.1189/jlb.1012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinyanjui MW, Shan J, Nakada EM, Qureshi ST, Fixman ED. Dose-dependent effects of IL-17 on IL-13-induced airway inflammatory responses and airway hyperresponsiveness. J Immunol. 2013;190:3859–68. doi: 10.4049/jimmunol.1200506. [DOI] [PubMed] [Google Scholar]
- 27.Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human gamma delta T cells at birth. J Immunol. 1994;153:3979–88. [PubMed] [Google Scholar]
- 28.Engelmann I, Moeller U, Santamaria A, Kremsner PG, Luty AJ. Differing activation status and immune effector molecule expression profiles of neonatal and maternal lymphocytes in an African population. Immunology. 2006;119:515–21. doi: 10.1111/j.1365-2567.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–33. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–8. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speer ME. Respiratory syncytial virus pathophysiology and affect of palivizumab in special populations: cystic fibrosis and immunosuppression. J Pediatr Pharmacol Ther. 2011;16:74–6. doi: 10.5863/1551-6776-16.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JP, Kim YS, Tae YM, Choi EJ, Hong BS, Jeon SG, et al. A viral PAMP double-stranded RNA induces allergen-specific Th17 cell response in the airways which is dependent on VEGF and IL-6. Allergy. 2010;65:1322–30. doi: 10.1111/j.1398-9995.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 33.Ryzhakov G, Lai CC, Blazek K, To KW, Hussell T, Udalova I. IL-17 boosts proinflammatory outcome of antiviral response in human cells. J Immunol. 2011;187:5357–62. doi: 10.4049/jimmunol.1100917. [DOI] [PubMed] [Google Scholar]
- 34.Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–45. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–11. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campos Alberto EJ, Shimojo N, Aoyagi M, Kohno Y. Differential effects of tumour necrosis factor-alpha and interleukin-12 on isopentenyl pyrophosphate-stimulated interferon-gamma production by cord blood Vgamma9 T cells. Immunology. 2009;127:171–7. doi: 10.1111/j.1365-2567.2008.02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saravia J, You D, Thevenot P, Lee GI, Shrestha B, Lomnicki S, et al. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014;7:694–704. doi: 10.1038/mi.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boukhvalova MS, Yim KC, Prince GA, Blanco JC. Methods for monitoring dynamics of pulmonary RSV replication by viral culture and by real-time reverse transcription-PCR in vivo: Detection of abortive viral replication. Curr Protoc Cell Biol. 2010;Chapter 26(Unit26):6. doi: 10.1002/0471143030.cb2606s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cormier SA, Shrestha B, Saravia J, Lee GI, Shen L, DeVincenzo JP, et al. Limited Type I Interferons and Plasmacytoid Dendritic Cells during Neonatal Respiratory Syncytial Virus Infection Permit Immunopathogenesis upon Reinfection. J Virol. 2014;88:9350–60. doi: 10.1128/JVI.00818-14. [DOI] [PMC free article] [PubMed] [Google Scholar]