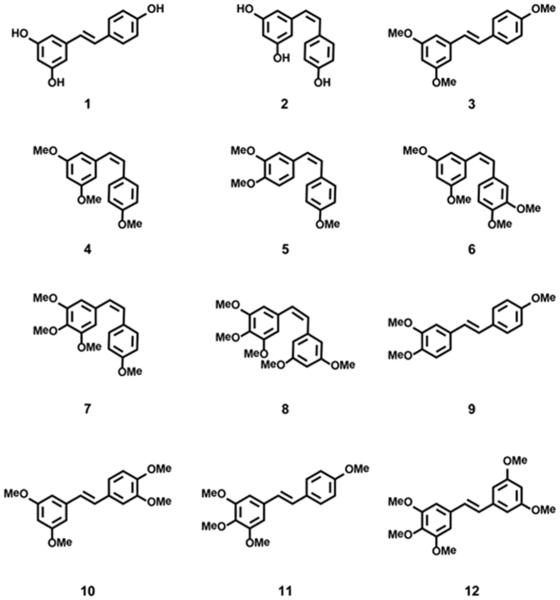
Abstract
Resveratrol (E-3,5,4’-trihydroxystilbene) is a polyphenol found in red wine that has been shown to have multiple anti-cancer properties. Although cis (Z) and trans (E) isomers of resveratrol occur in nature, the cis form is not biologically active. However, methylation at key positions of the cis form results in more potent anti-cancer properties. This study determined that synthetic cis-polymethoxystilbenes (methylated analogues of cis-resveratrol) inhibited cancer-related phenotypes of metastatic B16 F10 and non-metastatic B16 F1 mouse melanoma cells. In contrast with cis or trans-resveratrol and trans-polymethoxystilbene which were ineffective at 10 μM, cis-polymethoxystilbenes inhibited motility and proliferation of melanoma cells with low micromolar specificity (IC50 <10 μM). Inhibitory effects by cis-polymethoxystilbenes were significantly stronger with B16 F10 cells and were accompanied by decreased expression of β-tubulin and pleckstrin homology domain-interacting protein, a marker of metastatic B16 cells. Thus, cis-polymethoxystilbenes have potential as chemotherapeutic agents for metastatic melanoma.
Keywords: polymethoxystilbenes, cell motility, proliferation, β-tubulin, PHIP, anti-cancer
Introduction
Melanoma is a deadly form of skin cancer that is often related to excessive exposure to ultraviolet light. In view of the rising incidence of melanoma over the past decade, research has focused on approaches using drugs that would prevent metastasis of the primary lesion (1). In recent years, activating mutations of B-raf and N-ras were implicated in mechanisms that drive proliferation and metastasis of 50% of human melanomas (2). These enzymes are now being used successfully in the clinic as targets for small molecule inhibitors and immunotherapies. However, for melanomas that express wildtype B-raf and N-ras and therefore arise from other, undefined mechanisms, there is a need for alternate strategies. In particular, trans-resveratrol (E-3,5,4’-trihydroxystilbene, 1; Chart 1), a polyphenol found in red wine, and its related analogues are being investigated as potential drugs for melanoma and other cancers (3-6).
Chart 1.
Chemical structures of cis- and trans-resveratrol (1-2) and their cis- (4 - 9) and trans- (3, 10-13) polymethoxystilbene analogues.
Although 1 was once thought to act exclusively as a free radical scavenger and thus as an anti-oxidant, its pro-oxidant effects (resulting in oxidative breakage of DNA) are apparent at low micromolar concentrations. In its pro-oxidant role, resveratrol (4-8 μM) treatment of human leukemia cells leads to increased superoxide levels (7). Resveratrol can cause pro-oxidant effects in cultured tumor cells (8), and a protective effect in neuronal cells by inducing pro-oxidant heme oxygenase (9). It has been argued that its pro-oxidant effects may be part of a general anti-cancer and anti-aging mechanism of plant polyphenolic compounds. The dual nature of resveratrol as anti-oxidant or pro-oxidant is thought to be dependent on hydroxylation pattern, concentration and cell type, as previously discussed (10).
As an anti-cancer agent, resveratrol (1) has been studied extensively for its anti-inflammatory and anti-proliferative effects (11-13). Multiple cellular targets include the estrogen and androgen receptors (14), Akt/PKB (4), protein kinase C (15,16), ribonucleotide reductase (12,17), mTOR (13,18), and tubulin (19). In addition, 1 was found to be a radiation sensitizer for melanoma (20). In view of its multifocal effects, resveratrol and its synthetic analogues have potential therapeutic value for the treatment of certain cancers (16).
Resveratrol was shown to be selectively inhibitory of drug-resistant human melanoma cells DM738 and DM443 (at 50 µM) in comparison with normal dermal cells (5), and to inhibit highly invasive B16 F10 and B16 BL6 mouse melanoma cells (at 100 µM) (4). With regard to other cancer cells, resveratrol (at 50 µM) was demonstrated to have anti-estrogenic and anti-migration effects with metastatic human breast cells (14), and (at 1 µM) to promote apoptosis of human prostate cells via the MAPK pathway (21). Efforts to use resveratrol (1) in a mouse xenograft model were compromised by the difficulty in maintaining a high serum level of this drug over time due to its rapid clearance and metabolism (5). Consequently, analogues of resveratrol that have enhanced bioavailability are needed (22,23).
Although both cis (Z) and trans (E) isomers of resveratrol occur in nature, the cis form (Z-3,5,4’-trihydroxystilbene; 2) exhibits little biological activity. However, recent reports identified several methylated derivatives of both 1 and 2 that display greatly improved bio-activity with cultured cancer cells. The trimethylated cognate of trans-resveratrol (E-3,5,4’-trimethoxy stilbene; 3) proved to be a potent inhibitor of cell motility and invasion of hepatocarcinoma cells (IC50 ≤ 1 μM) (24). In other studies, the trimethylated cognate of cis-resveratrol (Z-3,5,4’-trimethoxystilbene; 4) was tested for anti-tumor activity. With IC50 = 300 nM, 4 was nearly 100-fold more effective than 3 in inhibiting proliferation of colon carcinoma cells (25, 26). Other modifications of the aromatic rings of resveratrol have been developed, notably addition of more hydroxy groups or acyloxy groups as substituents were found to be effective. In this regard, E-3,4,5,4’-tetrahydroxystilbene (at 500 nM) activated apoptosis in transformed fibroblasts with 100-fold higher potency than in non-transformed fibroblasts (27), while trans-resveratrol analogues bearing various acyl residues at C-4’ were effective in the 10-50 µM range with human melanoma and pancreatic cancer cells (28).
Since resveratrol was shown to interact directly with tubulin protein monomers and microtubules, the structure of tubulin has been used as a template to correlate with the cellular potency of an analogue with its docking characteristics at the colchicine binding site in β-tubulin (26). This site was previously established to be a target for mitotic inhibitors of microtubule polymerization and depolymerization. Unlike the resveratrol-related trans-polymethoxystilbenes, most of the cis-polymethoxystilbenes, such as 4, produced potent anti-proliferative effects with human colorectal SW480 cells. Interestingly, in docking studies, 4 and the other cis-analogues largely overlapped the docked configuration of combretastatin A-4 at the colchicine binding site, implicating this region in β-tubulin as a potential target.
The development of analogues of resveratrol with improved biological activity continues to be a focus of current research (29). The present study explores the effects of several resveratrol-related cis- and trans-polymethoxystilbenes on metastatic mouse melanoma B16 F10 cells, non-metastatic B16 F1 cells, and immortalized melanocytes all which were isolated from C57BL/6 mice (30, 31). Because both B16 cell lines express wildtype B-raf and wildtype N-ras (32, 33), they offer a model system for studying alternative mechanisms that give rise to malignant melanoma. For the studies presented here, cis (Z) and trans (E) isomers of resveratrol are compared with their polymethylated analogues for differential effects on motile behavior and proliferation of melanoma cells.
Materials and Methods
Materials
Cell culture media, fetal bovine serum, antibiotics, trypsin, and Alamar Blue were purchased from Life Technologies (Carlsbad, CA). Rabbit polyclonal antibodies for β-tubulin and β-actin were obtained from Cell Signaling Technology (Beverly, MA) and rabbit polyclonal anti-PHIP was purchased from Bethyl Laboratories (Montgomery, TX). Secondary antisera were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Protein dye reagent and protein markers were from Bio-Rad (Hercules, California). Chemiluminescence reagents (West Pico Super Signal) were purchased from Pierce (Rockford, Il). Trans-resveratrol (1), protease inhibitors, phosphatase inhibitors, phenylthiourea, and tetradecanoyl phorbol acetate were purchased from Sigma-Aldrich (St. Louis, MO). Trans-resveratrol (1), cis-resveratrol (2), trans-3,5,4’-trimethoxystilbene (3) and cis-3,5,4’-trimethoxystilbene (4) were purchased from Cayman Chemical Co. (Ann Arbor, MI). Immortalized mouse melanocytes (melan-a cells) were obtained from the Wellcome Trust Functional Genomics Cell Bank and the laboratory of Dr. Dorothy Bennett (St. George’s, University of London, UK).
Chemistry
The cis-resveratrol methylated analogues 5-8 were synthesized through an Arbuzov rearrangement followed by a Horner-Emmons-Wadsworth reaction, according to a procedure previously employed by some of us (26). The main product of this reaction was a trans stilbenoid (compounds 9-12) that was subsequently converted to its cis isomer (compounds 5-8) by photo-isomerization. Namely, compound 5 was obtained by photo-isomerization of 9 in turn obtained by reaction of the diethyl (4-methoxybenzyl)-phosphonate (prepared from 4-methoxybenzylchloride) with 3,4-dimethoxybenzaldehyde. Compound 6 was prepared by photo-isomerization of 10 in turn obtained by reaction of diethyl (3,5-dimethoxybenzyl)-phosphonate (prepared from 3,5-dimethoxybenzylbromide) with 3,4-dimethoxybenzaldehyde. Compound 7 was obtained by photo-isomerization of 11 in turn obtained by reaction of the diethyl (4-methoxybenzyl)-phosphonate (prepared from 4-methoxybenzylchloride) with 3,4,5-trimethoxybenzaldehyde. Compound 8 was obtained by photo-isomerization of 12 in turn obtained by reaction of diethyl (3,5-di-methoxybenzyl)-phosphonate (prepared from 3,5-dimethoxybenzylbromide) with the 3,4,5-trimethoxybenzaldehyde. Irradiation experiments were performed using a Rayonet photochemical reactor, as previously reported (34). All photo-isomerizations were obtained with 80-82% conversion to the Z isomer. Each mixture was resolved by silica gel PLC column chromatography using a gradient of EtOAc-n-hexane to separate the Z product from the residual E isomer. Spectral data of compounds 5 - 8 are in perfect agreement with those reported in the literature (35-37).
Cell-based Assays
Cell culture
B16 cells (F1 and F10) were cultured in 60-mm or 100-mm Falcon dishes containing RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 1% penicillin/streptomycin and 0.1% fungizone. Cells were grown at 37°C in a 5% CO2 atmosphere and sub-cultured twice per week. In the same growth medium, immortalized mouse melanocytes (melan-a cells) were cultured at 37°C with 10% CO2 in the presence of 200 nM tetradecanoyl phorbol acetate (TPA). Prior to the experiment, cells were plated and incubated overnight. The next day, the cells were washed with PBS, and fresh medium was restored. Each compound was added to cells at the indicated concentration and incubated at 37°C and 5% CO2 prior to the experiment.
Preparation of cell lysates
Cells were lysed in 0.25 mL lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100) containing phosphatase inhibitors (2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/μl leupeptin) and protease inhibitors. Cells were sonicated three times for 10 s and centrifuged at 10000 × g for 10 min to remove insoluble material. The resulting lysate was analyzed for protein content using the Bio-Rad reagent and bovine serum albumin as standard.
Western blot analysis
Cell lysates were denatured by heating in sample buffer at 95 °C for 5 min. Samples were resolved by 7.5% SDS-PAGE gel and transferred to a PVDF membrane (Millipore; Billerica, MA). The membrane was incubated overnight with antibodies to β-tubulin. After washing the membrane with TBST (150 mM NaCl, 20 mM Tris pH 7.4, 0.1% Tween 20, v/v), the appropriate horseradish-peroxidase conjugated secondary antibody was added and the membrane was incubated at 4°C for 1-2 h. After washing three times with TBST, the membrane was visualized with reagents for chemiluminescence.
Motility assay
Measurements were carried out with a 10-well glass slide and a pre-chilled cell sedimentation manifold (Creative Scientific Methods Inc., Phoenix, AZ). Cells (4 × 103/well) were pipetted into the manifold in triplicate enabling their sedimentation onto the slide as a small concentric circle. After incubation for approximately 16 h at 37 °C and 5% CO2, the manifold was removed (t=0) allowing the cells to radiate outwardly. At this time the media in each well was replaced with media containing 10 μM of a resveratrol analogue or DMSO (0.1%, v/v). Both at t=0 and following incubation with the analogues for 18 h at 37 °C, images of the cells were recorded and analyzed using Motic Image 2.0 software. The motility of each cell sample was judged by the increase in area occupied by the cells and averaging the values for triplicate wells.
Wound healing assay
Cells were plated in a 6-well plate (Falcon) and grown for 24-48 hours until a dense monolayer was achieved. Two parallel lines were drawn with colored markers on the undersurface of the plate to identify the area to be analyzed. A “scratch wound” of uniform width was created in this region by drawing a sterile micropipette tip across the monolayer using a ruler as a guide. Triplicate wells were washed twice with serum-free media before adding 1 ml serum-free medium containing 10 μM of the resveratrol analogue. By use of a camera (Motic Image) attached to a phase contrast Nikon microscope, the area of the wound was measured in 4-8 fields/well at t=0 and t=5 h and the difference in the occupied area was measured with Motic Image 2.0 software.
Cell proliferation assay
Proliferation of cells treated with selected analogues was measured by using Alamar Blue (Life Technologies) in a fluorescence-based assay (38). B16 F10 cells or B16 F1 cells were added to a 96-well plate (3×103 cells/200 μl) and incubated for 4 h at 37°C and 5% CO2. Melan-a mouse melanocytes (9 × 103 cells/200 μl) were added to a 96-well plate and incubated for 2 h at 37°C and 10% CO2 in complete growth medium. The growth medium was replaced with medium containing 10 μM resveratrol or resveratrol analogue or the vehicle control (0.1%, v/v) which was added to six replicate wells. Wells containing growth medium without cells served as the blank. Unless otherwise specified, cells were incubated for 40 h at 37°C, followed by addition of Alamar Blue to a final concentration of 5% (v/v). After 3 h at 37°C, the fluorescence was measured using a plate reader (SpectraMax M5 Microplate Reader) set at λex = 544 nm and λem = 590 nm. The number of cells measured was within the range of cells that gave a linear response to Alamar Blue, and therefore was directly proportional to the number of actively metabolizing cells.
Statistical analysis
Statistical significance was judged by applying the Welch’s unpaired two sample t-test.
Results
Comparison of trimethoxystilbenes 3 and 4, analogues of cis-(Z)- and trans-(E)-resveratrol, on motility of B16 F10 cells
The replacement of the hydroxy groups with methoxy groups on the aromatic rings in resveratrol structure can engender increased biological activity, as previously shown. In the initial experiment, the effect of 50 µM trans-resveratrol (1) and cis-resveratrol (2) and their corresponding trimethylated analogues (namely E-3,5,4’-trimethoxystilbene 3 and Z-3,5,4’-trimethoxystilbene 4) (Chart 1) was tested in an assay of cell motility. As shown in Figure 1A, 50 µM of both 1 and 2 were without effect on the motility of F10 cells. However, 50 µM of 3 and 4 produced significant decreases in motility, where 4 was the more potent inhibitor. Compared to the control, the effect by 4 was statistically significant. A dose-response curve, shown in Figure 1B, indicated that 4 inhibited F10 cell motility with IC50 = 10 µM. This curve was biphasic in nature as it consisted of a high affinity component produced by the initially steep slope at 1 µM (the lowest concentration of 4 that was tested), and a lower affinity component as shown by the shallow slope of the curve at concentrations above 1 μM.
Figure 1.
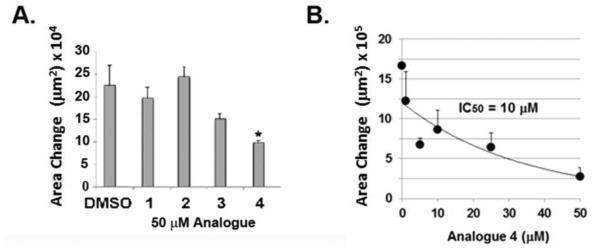
Impact on motility of B16 F10 melanoma cells by trans- and cis-resveratrol and their methylated analogues. A. Cell motility was measured by the cell sedimentation assay. Triplicate samples of B16 F10 cells were treated with 50 μM trans-resveratrol (1), cis-resveratrol (2), or their corresponding methylated analogues 3 and 4. The vehicle control was DMSO (0.1%, v/v). This experiment was performed three times with similar results. (*, P < 0.05) B. Dose dependence of 4 was tested in triplicate at increasing concentrations (0 - 50 μM) and the effect on cell motility was measured.
It was further observed that when F10 cells were treated with 4 at 10 μM, there was substantial loss of β-tubulin protein, as demonstrated by the Western blot in Figure 2. However, the parent resveratrol isomers 1 and 2, as well as the trans-trimethoxystilbene 3 had no detectable effect on β-tubulin expression. It was noted that treatment of non-metastatic F1 cells with 4 also led to decreased expression of β-tubulin, whereas 1-3 had no effect (Figure 2). Analyzed in the same blot was pleckstrin homology interacting protein (PHIP), a recently identified marker of metastatic B16 cells (39). In F10 cells, PHIP was observed to be down-regulated slightly by 1 and 2, but a stronger inhibitory effect was observed with trimethoxystilbenes 3 or 4, irrespective of their geometry. However with F1 cells, PHIP was seen to be more resistant to the effects of 1-4.
Figure 2.
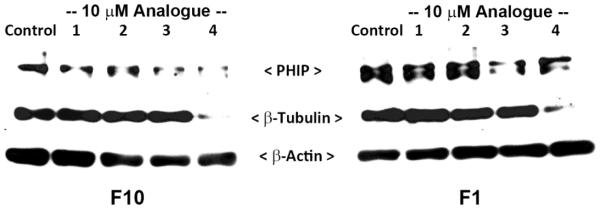
Effects of resveratrol and cis (Z)- and trans (E)-trimethoxystilbene analogues (1-4) on β-tubulin and PHIP expression. Following treatment of cells with the indicated compound at 10 µM or ethanol (0.1%, v/v) for 18 h, cell lysates were prepared and an aliquot of each sample (10 μg per lane) was resolved by 8% SDS-PAGE, transferred to a PDVF membrane, and probed with anti-β-tubulin (1:2000), anti-PHIP (1:2000), or anti-β-actin (1:5000) (loading control), followed by HRP-conjugated anti-rabbit secondary antibody and chemiluminescence detection. The results are representative of three independent experiments.
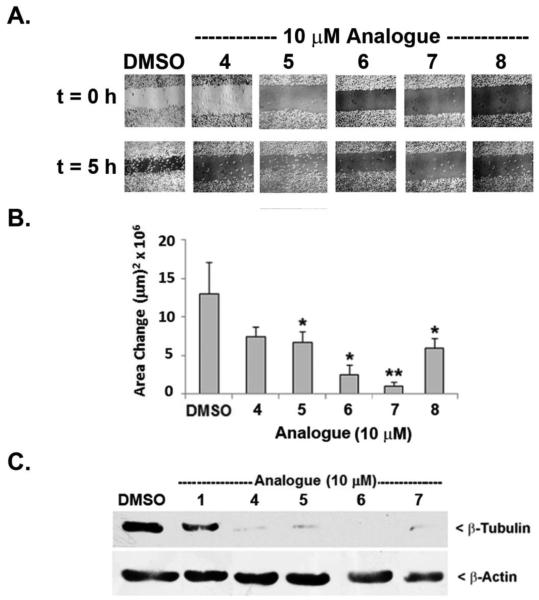
Testing of additional polymethoxystilbene analogues of cis-(Z)-resveratrol 5 – 8 on motility of F10 cells
In view of the impact of trimethoxystilbene 4 on F10 cell movement at low micromolar concentrations, additional polymethoxystilbene analogues of cis-resveratrol were synthetized, namely cis-3,4,4’-trimethoxystilbene (5), cis-3,5,3’,4’-tetramethoxystilbene (6), cis-3,4,5,4’-tetramethoxystilbene (7) and cis-3,4,5,3’,5’-pentamethoxystilbene (8). These compounds were evaluated for their effect on the motility of B16 F10 cells to establish the substitution pattern that improves upon the properties of 4. As can be observed in a wound healing assay (Figure 3A), compounds 5 - 8 (at 10 μM) substantially inhibited cell motility relative to the control (Figure 3B). Trimethoxy- (5) and pentamethoxy- (8) analogues were similar to 4 in that they produced inhibition in the 50-60% range. However, tetramethoxy analogues 6 and 7 showed a somewhat higher potency of inhibition (80-90%). These findings suggest that a tetramethoxylated cis-stilbenoid is more effective in inhibiting motility than either a tri- or pentamethoxy cis-analogue.
Figure 3.
Testing of additional cis (Z)-polymethoxystilbenes on cell motility and β-tubulin expression. A,B. B16 F10 cells that had been treated with 10 µM of the indicated analogue or DMSO (0.1% v/v) were tested for cell motility over a period of 18 h in a wound healing assay, images were taken at t=0 and t=5h. In comparing the two time points, motility was determined by the change in the wound area (A) for 4-8 microscope fields per well and averaged for each treatment. Statistical significance was determined by comparing analogue-treated cells with the DMSO control (*, P < 0.1; **, P < 0.01). C. Western blot analysis of lysates (50 µg protein per lane) that had been prepared from cells treated for 18 h with 10 µM of the indicated analogue. The blot was probed with anti-β-tubulin (1:2000) or anti-β-actin (1:5000) followed by HRP-conjugated secondary antibody and detected by chemiluminescence. The results are representative of three independent experiments.
Tubulin protein content was evaluated following treatment with 10 μM concentration of the cis-polymethoxystilbenes 4–7. Compared to the control, dramatic decreases in intracellular β-tubulin protein were observed with all tested cis-polymethoxystilbenes and in the case of 6, the level of expression fell below detectable limits (Figure 3C).
Effects of resveratrol compounds on proliferation of B16 melanoma cells and melanocytes
Compound 4 had previously been described as anti-proliferative towards colon carcinoma cells and colorectal cells (25, 26, 35, 36). Resveratrol compounds (1,2) and their trimethoxystilbenes (3,4) were tested at 10 μM for 40 h to determine their effects on proliferation of F10 and F1 melanoma cells, and melanocytes. In a comparison of the four compounds (Figure 4), 4 was the most effective in decreasing proliferation of F10 cells (hatched bars), F1 cells (dark grey bars), and melanocytes (light grey bars). Relative to control-treated cells, 4 produced 75% inhibition of proliferation with F10 cells, whereas it produced 50-60% inhibition with F1 cells, and only 20-25% inhibition of melanocytes. These results suggest that these cells exhibit differential sensitivity to the anti-proliferative effects of 4.
Figure 4.
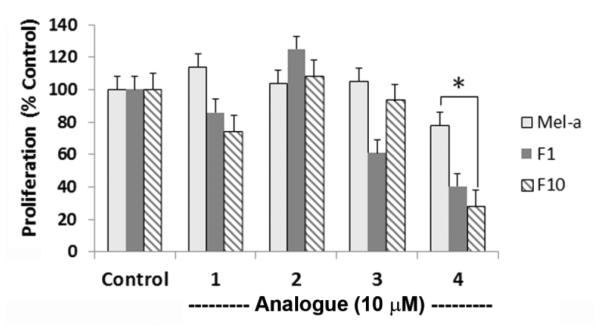
The effect of resveratrol compounds (1-4) on proliferation of melanoma cells and melanocytes. Metastatic F10 cells, non-metastatic F1 cells (each plated at 3×103 cells per well), or melanocytes (9×103 cells per well) were treated for 40 h with 10 μM of the indicated analogue or an equivalent volume of ethanol (0.1%, v/v). The fluorescent signal was read after a 3-h treatment with Alamar Blue (5%, v/v). For each condition, six replicate measurements were averaged and reported as the percentage ± s.d. of the value for control-treated cells (*, P < 0.0001). The results are representative of two or more independent experiments.
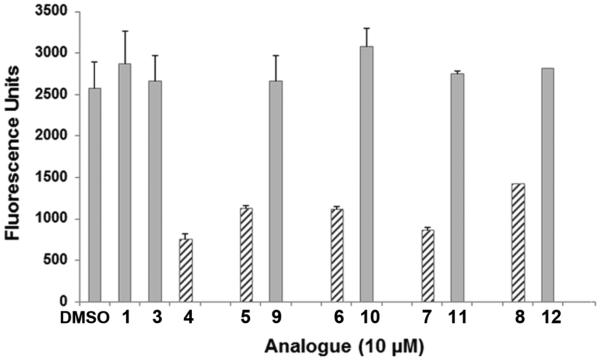
Differential effects of cis- and trans-polymethoxystilbene analogues on proliferation of F10 melanoma cells
In a proliferation assay, cis/trans pairs of compounds (Chart 1) were compared at 10 μM. The cis-polymethoxy analogues (hatched bars) were generally found to decrease cell proliferation by 55-70% relative to control-treated cells (Figure 5), of which 4 was the strongest inhibitor of cell proliferation (71% inhibition) followed by 7 (66% inhibition), whereas analogues 5, 6, and 8 were significantly less potent (45% inhibition). It is notable that neither trans-resveratrol (1) nor any of the counterpart trans-polymethoxystilbenes (3, 9-12) (grey bars) had a significant effect on the proliferation of F10 cells.
Figure 5.
Comparison of the effect of cis (Z)- and trans (E)-polymethoxystilbene isomers on the proliferation of F10 cells. Comparison of cis analogues (hatched bars) (4 - 8) and their counterpart trans analogues (grey bars) (3 and 9 - 12), where each compound was tested at 10 μM (or 0.1% DMSO, v/v) with 3×103 cells. After 48 h, the number of cells was analyzed by adding Alamar Blue for 3 h, followed by fluorescence detection and quantitation. Triplicate measurements were averaged and the standard deviation was calculated. The results are representative of three independent experiments.
Discussion
The findings of this investigation identified a group of synthetic polymethoxystilbenes, analogues of cis-resveratrol (2), as inhibitory to both motility (an aspect of metastasis) and proliferation of metastatic melanoma cells with effective potencies in the low micromolar range (IC50 = 1 - 10 μM). Our findings indicate that these methylated analogues of cis-resveratrol are 5-fold more effective at inhibiting motility of B16 F10 cells than previously measured with trans-resveratrol (1) (4). The impact on melanoma cells by the cis-analogues contrasted sharply with the lower or absent inhibitory activity observed with trans-resveratrol or the trans-trimethoxy analogue 3 (E-3,5,4’-trimethoxystilbene) at 10 µM. When compared against the trimethylated cognate of cis-resveratrol (Z-3,5,4’-trimethoxystilbene, 4) as a reference compound, the present work established the potency of 4 for inhibiting motility of metastatic melanoma cells. Furthermore, we identified additional cis-resveratrol analogues (Chart 1) obtained by synthesis, namely Z-3,4,4’-trimethoxystilbene (5), Z-3,5,3’,4’-tetramethoxystilbene (6), Z-3,4,5,4’-tetramethoxystilbene (7) and Z-3,4,5,3’,5’-pentamethoxystilbene (8), as having similar potency in the low micromolar range. Notably, at 10 μM, inhibition of motility by (6) and (7) was reproducibly stronger than that observed for (4), (5), and (8). These results confirm the observation by others (26, 40, 41) that subtle modification of the substitution pattern in resveratrol-related structure may have significant effects on their anti-tumor properties.
A surprising observation was the dramatic decrease in detectable β-tubulin protein levels in cells treated with the cis-polymethoxystilbenes (Figures 2 and 3B). Importantly, the loss of β-tubulin correlated with the effect on motile behavior by a given compound (Figures 1 and 3), as well as with extensive truncation of microtubule structure (unpublished observations). These findings suggest a mechanistic model for the substantial decreases observed in cell motility and proliferation. Microtubules are central to cell locomotion, and their contribution to B16 F10 cell movement was previously documented (42). The impact on microtubules by resveratrol analogues can be understood by their known binding activity with the colchicine binding site in β-tubulin. This interaction was shown to be strengthened with cis-polymethoxystilbenes (26), consequently preventing formation of polymers (or destabilizing existing polymers) of α/β-tubulin heterodimers. In this regard, 4 inhibited tubulin polymerization in colon carcinoma cells (CaCo-2 and SW480) with IC50 = 4 μM (19, 25), and 7 inhibited tubulin polymerization comparable to that of the known cytotoxic agent combretastatin A4 (36). The unexpected loss of β-tubulin expression in melanoma cells treated with polymethoxy cis-analogues 4 – 7 suggests that these analogues may also activate the proteasome, thereby leading to tubulin degradation. This outcome was previously attributed to agents that destabilize microtubules, particularly in neural cells (43). Since melanocytes derive from neural crest cells during development, the intrinsic nature of melanoma cells may render tubulin protein particularly susceptible to degradation by polymethoxy cis-resveratrol analogues. Alternatively, transcriptional down-regulation of tubulin mRNA may occur as proposed by others who tested trimethoxy- and tetramethoxystilbene analogues of trans-resveratrol in hepatoma cells (24, 44). These possible modes of action provide avenues for further investigation with polymethoxy cis-resveratrol compounds.
To explore additional targets of the cis-methoxystilbenes, we compared 1-4 for their effects on a molecular marker of metastatic melanoma. In this regard, 10 μM 3 or 4 led to substantial decreases in the expression of PHIP (Figure 2). Down-regulation of this protein by cis- trimethoxystilbene 4 correlated with the observed inhibition of motility, an aspect of metastatic behavior. However, the observation that both cis (4) and trans (3) trimethoxystilbenes were similarly effective with F10 cells implied that PHIP down-regulation was independent of the geometry of the aromatic rings in contrast with β-tubulin down-regulation which was produced exclusively by the cis-polymethoxystilbenes.
Additional analysis showed that ROS production was not altered in F10 or F1 cells that had been treated with 10 μM of 1-4 (unpublished observations). Thus, at low micromolar concentrations, neither pro-oxidant nor anti-oxidant activities of resveratrol compounds were evident in melanoma cells (unpublished observations).
Acknowledgements
We thank Shatarupa De and Xin Zhao (Graduate Center of C.U.N.Y.) for technical assistance. This work was supported by funding (to VLM) from PSC-CUNY, URME small grant programs sponsored by The City University of New York, and a FIR grant (to CT) by the University of Catania.
References
- 1.Muthusamy V, Piva TJ. Melanoma cell signaling: looking beyond RAS-RAF-MEK skin cancers. In: La Porta C, editor. Risk Factors, Prevention, and Therapy. InTech; 2011. [Google Scholar]
- 2.Conde-Perez A, Larue L. Human relevance of NRAS/BRAF mouse melanoma models. Eur J Cell Biol. 2013;93:82–86. doi: 10.1016/j.ejcb.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Chillemi R, Sciuto S, Spatafora C, Tringali C. Anti-tumor properties of stilbene-based resveratrol analogues: recent results. Nat Prod Comm. 2007;2:499–513. [Google Scholar]
- 4.Bhattacharya S, Darjatmoko SR, Polans AS. Resveratrol modulates the malignant properties of cutaneous melanoma via changes in the activation and attenuation of the anti-apoptotic proto-oncogenic protein Akt/PKB. Melanoma Res. 2011;21:180–187. doi: 10.1097/CMR.0b013e3283456dfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osmond GW, Augustine CK, Zipfel PA, Padussis J, Tyler DS. Enhancing melanoma treatment with resveratrol. J Surg Res. 2012;172:109–115. doi: 10.1016/j.jss.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 6.Spatafora C, Tringali C. Natural-derived polyphenols as potential anticancer agents. Anticancer Agents Med Chem. 2012;12:902–918. doi: 10.2174/187152012802649996. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad KA, Clement M-V, Pervaiz S. Pro-oxidant activity of low doses of resveratrol inhibits hyfrogen peroxide-induced apoptosis. Ann NY Acad Sci. 2003;1010:365–373. doi: 10.1196/annals.1299.067. [DOI] [PubMed] [Google Scholar]
- 8.Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22:1111–1117. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang H, Kim YS, Koehler RC, Dore S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann NY Acad Sci. 2003;993:276–286. doi: 10.1111/j.1749-6632.2003.tb07534.x. [DOI] [PubMed] [Google Scholar]
- 10.Qian YP, Cai YJ, Fan GJ, Wei QY, Yang J, Zheng LF, Li XZ, Fang JG, Zhou B. Antioxidant-based lead discovery for cancer chemoprevention: The case of resveratrol. J Med Chem. 2009;52:1963–1974. doi: 10.1021/jm8015415. [DOI] [PubMed] [Google Scholar]
- 11.Vitale N, Kisslinger A, Paladino S, Procaccini C, Matarese G, Pierantoni GM, Mancini FP, Tramontano D. Resveratrol couples apoptosis with autophagy in UVB-irradiated HaCaT cells. PLoS One. 2013;8(11):e80728. doi: 10.1371/journal.pone.0080728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madlener S, Saiko P, Vonach C, Viola K, Huttary N, Stark N, Popescu R, Gridling M, Vo NT, Herbacek I, Davidovits A, Giessrigl B, Venkateswarlu S, Geleff S, Jäger W, Grusch M, Kerjaschki D, Mikulits W, Golakoti T, Fritzer-Szekeres M, Szekere T, Krupitza G. Multifactorial anticancer effects of digalloyl-resveratrol encompass apoptosis, cell-cycle arrest, and inhibition of lymphendothelial gap formation in vitro. Brit J Cancer. 2010;102:1361–1370. doi: 10.1038/sj.bjc.6605656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Liu F. Targeting mTOR: evaluating the therapeutic potential of resveratrol for cancer treatment. Anticancer Agents Med Chem. 2013;13:1032–1038. doi: 10.2174/18715206113139990113. [DOI] [PubMed] [Google Scholar]
- 14.Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005;7:128–140. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slater SJ, Seiz JL, Cook AC, Stagliano BA, Buzas CJ. Inhibition of protein kinase C by resveratrol. Biochim Biophys Acta. 2003;1637:59–69. doi: 10.1016/s0925-4439(02)00214-4. [DOI] [PubMed] [Google Scholar]
- 16.Das J, Pany S, Majhi A. Chemical modifications of resveratrol for improved protein kinase C alpha activity. Bioorg Med Chem. 2011;19:5321–5333. doi: 10.1016/j.bmc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Saiko P, Graser G, Giessrigl B, Steinmann MT, Schuster H, Lackner A, Grusch M, Krupitza G, Jaeger W, Somepalli V, Golakoti T, Fritzer-Szekeres M, Szekeres T. Digalloylresveratrol, a novel resveratrol analog inhibits the growth of human pancreatic cancer cells. Invest New Drugs. 2013;31:1115–1124. doi: 10.1007/s10637-013-0009-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Yu T, Zhu C, Sun H, Qiu Y, Zhu X, Li J. Resveratrol triggers protective autophagy through the ceramide/Akt/mTOR pathway in melanoma B16 cells. Nutr Cancer. 2014;66:435–440. doi: 10.1080/01635581.2013.878738. [DOI] [PubMed] [Google Scholar]
- 19.Chabert P, Fougerousse A, Brouillard R. Anti-mitotic properties of resveratrol analog (Z)-3,5,4’-trimethoxystilbene. Biofactors. 2006;27:37–46. doi: 10.1002/biof.5520270104. [DOI] [PubMed] [Google Scholar]
- 20.Fang Y, Bradley MJ, Cook KM, Herrick EJ, Nicholl MB. A potential role for resveratrol as a radiation sensitizer for melanoma treatment. J Surg Res. 2013;183:645–653. doi: 10.1016/j.jss.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Shih A, Zhang S, Cao HJ, Boswell S, Wu Y-H, Tang H-Y, Lennartz MR, Davis FB, Davis P, Lin H-Y. Inhibitory effect of epidermal growth factor on resveratrol-induced apoptosis in prostate cancer cells is mediated by protein kinase C-α. Mol Cancer Ther. 2004;3:1355–1363. [PubMed] [Google Scholar]
- 22.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 23.Walle T. Bioavailability of resveratrol. Ann NY Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 24.Weng C-J, Wu C-F, Huang H-W, Wu C-H, Ho CT, Yen G-C. Evaluation of anti-invasion effect of resveratrol and related methoxy analogues on human hepatocarcinoma cells. J Agric Food Chem. 2010;58:2886–2894. doi: 10.1021/jf904182y. [DOI] [PubMed] [Google Scholar]
- 25.Schneider Y, Chabert P, Stutzmann J, Coelho D, Fougerousse A, Gosse F, Launay J-F, Brouillard R, Raul F. Resveratrol analog (Z)-3,5,4’-trimethoxystilbene is a potent anti-mitotic drug inhibiting tubulin polymerization. Int J Cancer. 2003;107:189–196. doi: 10.1002/ijc.11344. [DOI] [PubMed] [Google Scholar]
- 26.Mazue F, Colin D, Gobbo J, Wegner M, Rescifina A, Spatafora C, Fasseur D, Delmas D, Meunier P, Tringali C, Latruffe N. Structural determinants of resveratrol for cell proliferation inhibition potency: experimental and docking studies of new analogs. Eur J Med Chem. 2010;45:2972–2980. doi: 10.1016/j.ejmech.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Gosslau A, Chen M, Ho CT, Chen KY. A methoxy derivative of resveratrol analogue selectively induced activation of the mitochondrial apoptotic pathway in transformed fibroblasts. Brit J Cancer. 2005;92:513–521. doi: 10.1038/sj.bjc.6602300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong Y, Osmond G, Brewer KI, Tyler DS, Andrus MB. Synthesis of 4’-ester analogs of resveratrol and their evaluation in malignant melanoma and pancreatic cell lines. Bioorg Med Chem Lett. 2010;20:1198–1201. doi: 10.1016/j.bmcl.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Roman BI, De Coen LM, Mortier STFC, De Ryck T, Vanhoecke BWA, Katritzky AR, Bracke ME, Stevens CV. Design, synthesis, and structure-activity relationships of some novel, highly potent anti-invasive (E)- and (Z)-stilbenes. Bioorg Med Chem. 2013;21:5054–5063. doi: 10.1016/j.bmc.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 30.Raz A, Mclellan WL, Hart IR, Bucana CD, Hoyer LC, Sela B-A, Dragsten P, Fidler IJ. Cell surface properties of B16 melanoma variants with differing metastatic potential. Cancer Res. 1980;40:1645–1651. [PubMed] [Google Scholar]
- 31.Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- 32.Melnikova VO, Bolshakov SV, Walker C, Ananthaswamy HN. Genomic alterations in spontaneous and carcinogen-induced murine melanoma cell lines. Oncogene. 2004;23:2347–2356. doi: 10.1038/sj.onc.1207405. [DOI] [PubMed] [Google Scholar]
- 33.Marone R, Erhart D, Mertz AC, Bohnacker T, Schnell C, Cmiljanovic V, Stauffer F, Garcia-Echeverria C, Giese B, Maira S-M, Wymann MP. Targeting melanoma with dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors. Mol Cancer Res. 2009;7:601–613. doi: 10.1158/1541-7786.MCR-08-0366. [DOI] [PubMed] [Google Scholar]
- 34.Cardile V, Chillemi R, Lombardo L, Sciuto S, Spatafora C, Tringali C. Antiporliferative activity of methylated analogues of E- and Z-resveratrol. Naturforsch. 2007;62 c:189–195. doi: 10.1515/znc-2007-3-406. [DOI] [PubMed] [Google Scholar]
- 35.Cushman M, Nagarathnam D, Gopal D, Chakraborti AK, Lin CM, Hamel E. Synthesis and evaluation of stilbene and dihydrostilbene derivatives as potential anticancer agents that inhibit tubulin polymerization. J Med Chem. 1991;34:2579–2588. doi: 10.1021/jm00112a036. [DOI] [PubMed] [Google Scholar]
- 36.Cushman M, Nagarathnam D, Gopal D, He HM, Lin CM, Hamel E. Synthesis and evaluation of analogues of (Z)-1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)etheanes potential cytotoxic and antimitotic agents. J Med Chem. 1992;35:2293–2306. doi: 10.1021/jm00090a021. [DOI] [PubMed] [Google Scholar]
- 37.Zaki MA, Balachandran P, Khan S, Wang M, Mohammed R, Hetta MH, Pasco DS, Muhammad I. Cytotoxicity and modulation of cancer-related signaling by (Z)- and (E)-3,4, 3',5'-tetramethoxystilbene isolated from Eugenia rigida. J Nat Prod. 2013;76:679–684. doi: 10.1021/np300893n. [DOI] [PubMed] [Google Scholar]
- 38.Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R. The use of Alamar Blue assay for quantitative analysis of viability, migration, and invasion of choriocarcinoma cells. Human Reprod. 2007;22:1304–1309. doi: 10.1093/humrep/dem011. [DOI] [PubMed] [Google Scholar]
- 39.De Semir D, Nosrati M, Bezrookove V, Dar AA, Federman S, Bienvenu G, Venna S, Rangel J, Climent J, Tamguney TMM, Thummala S, Yong S, Leong SPL, Hagg C, Billings P, Miller JR, Sagebiel RW, Debs R, Kashani-Sabet M. Pleckstrin homology domain-interacting protein (PHIP) as a marker and mediator of melanoma metastasis. Proc Natl Acad Sci. 2012;109:7067–7072. doi: 10.1073/pnas.1119949109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardile V, Lombardo L, Spatafora C, Tringali C. Chemo-enzymatic synthesis and growth-inhibition activity of resveratrol analogues. Bioorg Chem. 2005;33:22–33. doi: 10.1016/j.bioorg.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Belleri M, Ribatti D, Nicoli S, Cotelli F, Forti L, Vannini V, Stivala LA, Presta M. Antiangiogenic and vascular-targeting activity of the microtubule-destabilizing trans-resveratrol derivative 3,5,4′-trimethoxystilbene. Mol Pharm. 2005;67:1451–1459. doi: 10.1124/mol.104.009043. [DOI] [PubMed] [Google Scholar]
- 42.Ballestrem C, Wehrle-Haller B, Hinz B, Imhof BA. Actin-dependent lamellipodia formation and microtubule-dependent tail retraction control-directed cell migration. Mol Biol Cell. 2000;11:2999–3012. doi: 10.1091/mbc.11.9.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huff LM, Sackett DL, Poruchynsky MS, Fojo T. Microtubule disrupting chemotherapeutics result in enhanced proteasome-mediated degradation and disappearance of tubulin in neural cells. Cancer Res. 2010;70:5870–5879. doi: 10.1158/0008-5472.CAN-09-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cichocki M, Baer-Dubowska W, Wierzchowski M, Murias M, Jodynis-Liebert J. 3,4,5,4'-trans-tetramethoxystilbene (DMU-212) modulates the activation of NF-κB, AP-1, and STAT3 transcription factors in rat liver carcinogenesis induced by initiation-promotion regimen. Mol Cell Biochem. 2014;391:27–35. doi: 10.1007/s11010-014-1983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]