Abstract
Long-term administration of L-3,4-dihydroxyphenylalanine (levodopa), the mainstay treatment for Parkinson’s disease (PD), is accompanied by fluctuations in its duration of action and motor complications (dyskinesia) that dramatically affect the quality of life of patients. Levodopa-induced dyskinesias (LID) can be modeled in rats with unilateral 6-OHDA lesions via chronic administration of levodopa, which causes increasingly severe axial, limb and oro-facial abnormal involuntary movements (AIMs) over time. In previous studies, we showed that direct activation of CB1 cannabinoid receptors alleviated rat AIMs. Interestingly, elevation of the endocannabinoid anandamide by URB597 (URB), an inhibitor of endocannabinoid catabolism, produced an anti-dyskinetic response that was only partially mediated via CB1 receptors and required the concomitant blockade of transient receptor potential vanilloid type-1 (TRPV1) channels by capsazepine (CPZ) [1]. In this study, we showed that stimulation of peroxisome proliferator-activated receptors (PPAR), a family of transcription factors activated by anandamide, contributes to the anti-dyskinetic effects of URB+CPZ, and that direct activation of the PPARγ subtype by rosiglitazone (RGZ) alleviates levodopa-induced AIMs in 6-OHDA rats. AIM reduction was associated with an attenuation of levodopa-induced increase of dynorphin, zif-268 and of ERK phosphorylation in the denervated striatum. RGZ treatment did not decrease striatal levodopa and dopamine bioavailability, nor did it affect levodopa antiparkinsonian activity. Collectively, these data indicate that PPARγ may represent a new pharmacological target for the treatment of LID.
Keywords: Dyskinesia, Parkinson’s disease, cannabinoid, PPARγ, levodopa, rosiglitazone
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by loss of dopaminergic neurons projecting from the substantia nigra pars compacta to the striatum, a brain region controlling the execution and planning of motor behaviors [2]. The dopamine precursor L-3,4-dihydroxyphenylalanine (levodopa) is still considered the mainstay therapy for PD motor symptoms. However, its long-term use causes disabling complications, collectively known as levodopa-induced dyskinesias (LID) [3, 4], consisting of uncontrolled choreiform movements of the body that profoundly affect the patients’ quality of life. Clinically, LID management remains challenging due to the relatively few pharmacological treatments available, such as the NMDA antagonists amantadine and dextromethorphan [5–7], which are limited by the development of tolerance [8], abuse liability and toxic side effects [9]. On the other hand, the 5-HT1A agonist sarizotan, which has shown anti-dyskinetic activity in preclinical models [10], has failed in clinical trials [11] as it worsens parkinsonian symptoms. Therefore, there is an urgent need to develop new pharmacotherapies to control LID without interfering with the beneficial (pro-motor) effects of levodopa.
LID can be modeled in rats with 6-hydroxydopamine (6-OHDA) unilateral nigro-striatal lesions via chronic administration of low doses of levodopa. In these rats, chronic levodopa induces increasingly severe axial, limb and oro-facial abnormal involuntary movements (AIMs), which have been extensively characterized by different research groups and validated pharmacologically [1, 12–15].
In previous studies, we showed that direct activation of cannabinoid CB1 receptors alleviated rat AIMs [1, 16, 17], whereas elevation of the endocannabinoid anandamide via inhibition of its degradation by URB597 (URB) [18, 19] ameliorated AIMs only when URB was co-administered with the TRPV1 antagonist capsazepine (CPZ) [1]. As the anti-dyskinetic effect of URB+CPZ was only partially reversed by the CB1 antagonist AM251 [1], these studies suggest the involvement of alternative endocannabinoid-sensitive targets.
In addition to CB receptors, anandamide can activate specific subtypes of peroxisome proliferator-activated receptors (PPAR), a family of transcription factors [20, 21] that are expressed throughout the body [22, 23] and regulate the transcription of target genes primarily involved in metabolism, energy homeostasis, cell differentiation and inflammation [24–26]. Among the three major PPAR subtypes identified so far, PPAR alpha (α), beta/delta (b/δ) and gamma (γ), PPARγ are highly expressed in the basal ganglia [23, 27].
Activation of PPARα and β/δ has been shown to play a role in neurogenesis [28, 29] and to produce neuroprotective effects in models of ischemic brain injury [30, 31]. The PPARγ agonists rosiglitazone (RGZ) and pioglitazone (PGZ), which are used in the clinic as insulin sensitizers for the treatment of type-2 diabetes, have also shown neuroprotective actions in animal models of PD [32, 33] and other neurodegenerative disorders [31, 34–36]. However, little is known about the ability of PPAR to modulate motor function and/or possibly play a role in the motor complications induced by repeated levodopa administration.
Several studies have reported molecular changes following chronic levodopa, including modification of the extracellular signal-regulated kinase (ERK) [37], upregulation of mRNA encoding the neuropeptide prodynorphin (pDyn) [38, 39], increased expression of immediate early genes (IEGs) associated with dopamine D1 receptor supersensitivity, such as zif-268 [40, 41], and changes in striatal synaptic plasticity [42]. Interestingly, pharmacotherapies that attenuate levodopa-induced AIMs have been shown to affect these biochemical markers of dyskinesia to some degree [43, 44].
In this study, we showed that elevation of endocannabinoid tone via systemic administration of URB+CPZ ameliorated levodopa-induced AIMs in 6-OHDA rats through a PPAR-mediated mechanism. We also showed that direct stimulation of PPARγ by RGZ attenuated levodopa-induced AIMs and associated striatal markers without affecting levodopa bioavailability or its anti-parkinsonian action.
MATERIALS AND METHODS
Drugs
Desipramine hydrochloride, levodopa methyl ester, 6-OHDA hydrochloride, benserazide and amphetamine were purchased from Sigma Chemicals Co. (St. Louis, MO); Rosiglitazone potassium salt, BADGE, GW9662, oleylethanolamide (OEA), WY14643 and URB597 were from Cayman Chemicals (Ann Arbor, MI); Capsazepine was from Tocris bioscience (Ellisville, MI), GW7647 was from Sigma (St. Louis, MO, USA) and Pioglitazone was from Biomol (Plymouth Meeting, PA).
Animals
Animal care and all experiments were conducted in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and approved by the Institutional Animal Care and Use Committees of the University of Texas Health Science Center at San Antonio.
Male Wistar rats (270–280 g; Charles River Laboratories, Wilmington, MA) were housed on a 12-h dark-light cycle at 22±1 °C and habituated to housing conditions for 1 week before experiments. Food and water were provided ad libitum.
6-OHDA lesion
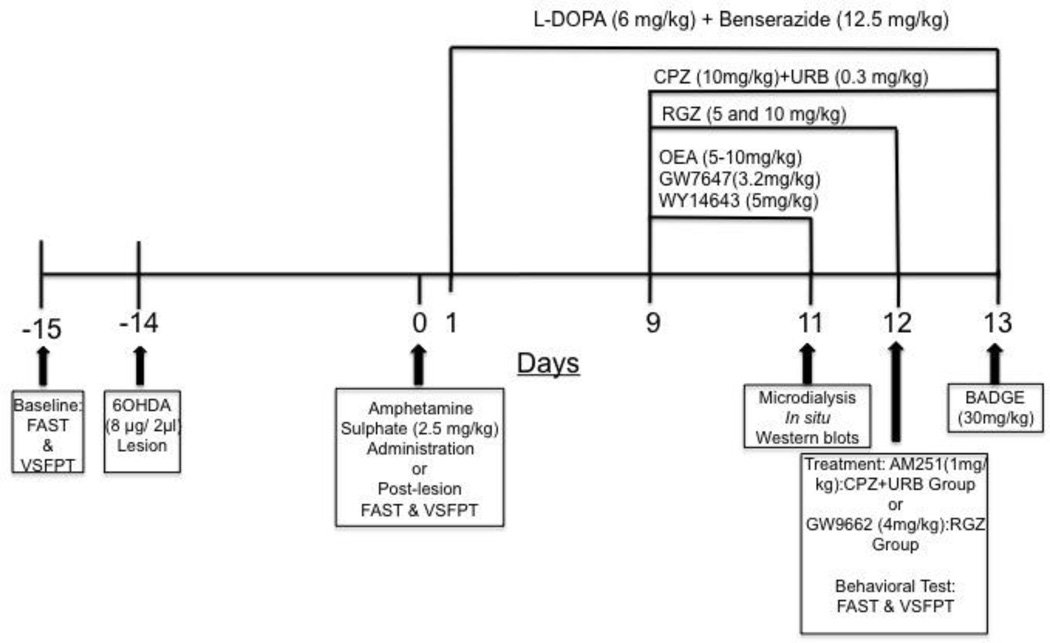
Dopamine-denervating lesions were performed by unilateral injection of 6-OHDA into the left medial forebrain bundle (MFB) as previously reported [45]. Briefly, rats were treated with desipramine hydrochloride (25 mg/kg, i.p., 30 minutes before surgery) and anesthetized with an intraperitoneal (i.p.) injection of a cocktail (0.85 ml/kg, i.p.) containing ketamine (100 mg/ml), xylazine (100 mg/ml) and acepromazine (10 mg/ml) in saline, then positioned on a stereotaxic frame (Kopf Instruments, Tujunga, CA). Two microliters of 6-OHDA dissolved in 0.1% ascorbate saline (4 µg/µl) or a corresponding volume of 0.1% ascorbate saline (SHAM-operated rats) was injected into the left MFB [relative to bregma and midline, in mm: anteroposterior (AP) −4.3, mediolateral (ML) +1.6, dorsoventral (DV) −8.3] [46] at a flow rate of 0.5 µl/min using a 10 µl Hamilton microsyringe with a 32-gauge needle. Two weeks post-surgery, 6-OHDA-lesioned rats received an acute injection of amphetamine (2.5 mg/kg, i.p.) to assess the efficacy of the lesion by measuring amphetamine-induced rotational behavior (Scheme 1). Only rats displaying more than 5 full ipsilateral rotations per min (corresponding to about 90% depletion of dopaminergic terminals [15] were included in the study.
Scheme 1.
Drug Treatment and Behavioral tests
The timeline of the pharmacological treatments is summarized in Scheme 1
AIM Scoring
6-OHDA-lesioned and sham-operated rats (n=8/group) received levodopa (6 mg/kg + benserazide 12.5 mg/kg, s.c) once a day up to 13 days. Animals were placed individually in a plexiglas box and their axial, limb and oro-facial AIMs were monitored daily between 10:00 a.m. and 04:00 p.m. for 5 min per animal at 60, 90 and 120 minutes after levodopa injection by two trained researchers blind to the treatment. AIMs were scored on a severity scale ranging from 0 to 4 as previously described [1, 45]. From day 9 to 11, independent groups of animals treated with levodopa (n=8/group) received an i.p. injection (15 min before levodopa) of one (or a combination of) the following drugs: the TRPV1 antagonist capsazepine (CPZ, 10mg/kg, i.p.); the FAAH inhibitor URB597 (URB, 0.3 mg/kg, i.p.); the PPARγ agonist rosiglitazone (RGZ, 5 and 10 mg/kg, i.p.); and the PPARα agonists oleylethanolamide (OEA, 5–10 mg/kg, s.c.), or GW7647 (3.2 mg/kg, i.p.) or WY14643 (5 mg/kg, i.p.). Animals treated with levodopa and injected with URB+CPZ from day 9 to 13 (n=8) received the CB1 receptor antagonist, AM251 (1mg/kg, i.p.;15 min before URB+CPZ) on day 12, and the non-selective PPAR antagonist BADGE (30 mg/kg, i.p., 15 min before URB+CPZ) on day 13. Animals treated with levodopa and injected with RGZ from day 9 to 12 (n=8) received the selective PPARγ antagonist GW9662 (4 mg/kg, i.p., 15 min before RGZ) on day 12. Control groups (n=8) received chronic levodopa plus vehicle (PEG/Tween-80/saline 5/5/90%: URB597, RGZ, OEA, GW7647, WY14643, BADGE, GW9662; or PEG/Tween-80/saline 10/10/80%: CPZ & AM251; i.p.).
Forepaw Adjusting Steps Test (FAST)
The FAST was carried out in rats at 3 time points: prior to the 6-OHDA lesion (baseline), 2 weeks after lesion (prior to levodopa treatment), and on day 12 (after the last injection of either vehicle or RGZ). Rats were tested 15 minutes after levodopa administration as previously described by Chang et al. [47]. Briefly, rats were held at a 45° angle by restraining both hind paws and one of their forepaws, so that they could bear their own weight with the free forepaw, and then were moved across a table-top at a speed of 90 cm/12 s, alternating the restraining of the right and left forepaw. The number of forehand and backhand adjusting steps for each weight-bearing forepaw was counted in 3 trials for each direction.
Vibrissae-Stimulated Forepaw Placement Test (VSFPT)
The VSFPT was performed at the same time points reported above for the FAST (15 minutes after levodopa administration) as previously described by Lindner et al. [48]. Briefly, rats were held parallel to the floor while restraining both hind paws and one of their forepaws, then were moved vertically, allowing the ipsilateral vibrissae to touch the edge of a table top. The number of forepaw (right and left) placements on the edge of the tabletop resulting from stimulation of the ipsilateral vibrissae were recorded over 10 trials.
Locomotor Activity
In a separate cohort of intact rats (n=6–14/group), locomotor activity (total distance traveled and total number of rearings) was recorded using the ActiMot Activity Measuring System v 6.07 (TSE Systems GmbH; Bad Homburg, Germany). Rats were either treated with vehicle (PEG:Tween-80:saline, 5:5:90), RGZ (5 and 10 mg/kg, i.p.) and immediately placed in the ActiMot system for 1 hr to assess their locomotor activity in response to a novel environment.
Microdialysis
6-OHDA-lesioned rats (n=5/group) received levodopa (6 mg/kg + benserazide 12.5 mg/kg, s.c) once a day up to 11 days. RGZ (10 mg/kg, i.p., 15 minutes prior to levodopa) was co-administered from day 9 to 11. On day 11, rats were anaesthetized with chloral hydrate (400 mg/4 ml/kg/ip) and placed in a stereotaxic apparatus. The skull was exposed, and a small hole was drilled in the ipsilateral side. The microdialysis probe was implanted vertically in the striatum (anterior 1.55; lateral 3.2 from bregma; vertical 5.4 from dura) as previously described [49], and then fixed on the skull with dental cement. Rats were housed in Plexiglas hemispheric bowls with food and water available ad libitum. Experiments were performed on freely moving rats 24 h after probe implant. A Ringer’s solution (147 mM, NaCl; 2.2 mM, CaCl2; 4 mM, KCl) was pumped through the dialysis probe at a constant rate of 1 µl/min. Dialysates were collected every 20 minutes (starting 90 min before and finishing 180 min after drug administration), and injected without purification into an HPLC apparatus equipped with reverse-phase column (C8 3.5 mm, Waters, Mildford Massachusetts) and a coulometric detector (ESA Coulochem II, Bedford, MA) to quantify simultaneously dopamine and levodopa methyl ester. The first electrode was set at −90 mV and the second electrode at +280 mV. The following solution was used as mobile phase: 75 mM NaH2PO4, 20 mM EDTA, 1 mM sodiumdecanesulphonate, 0.01% triethylamine and 15% methanol, pH 5.70 and pumped with a Jasco pump at a flow rate of 0.6 ml/min. Following the experiment, all rats were sacrificed to verify probe position.
In situ hybridization
An independent group of rats treated with either RGZ (5 or 10 mg/kg, i.p., 15 minutes before levodopa) or vehicle (n=5–6/experimental group) were anesthetized with isofluorane on day 11 of levodopa treatment, 90 min after the last injection of levodopa, to mimic the same conditions of the animals used for the behavioral studies (see above). After decapitation, brains were rapidly removed, frozen in dry ice-cooled isopentane and stored at −20 C°. Cryostat coronal sections (12 µm) mounted on glass slides were postfixed in 4% paraformaldehyde solution and processes as described [50]. Antisense [35S]-labelled or digoxigenin-labelled (for double-labeling experiments) riboprobes were generated by in vitro transcription from plasmids containing the zif-268, dynorphin or enkephalin cDNA as previously described [50, 51]. For radioactive in situ hybridization, each slide was hybridized with 100 µl of buffer, containing 2×106 cpm of radioactive probe complementary to zif-268 or dynorphin mRNA. For combined fluorescence in situ hybridization/immunohistochemistry, each slide was hybridized with 3 µl of digoxigenin probe complementary to enkephalin or dynorphin mRNA, as markers of D1R-containing and D2R-containing neurons respectively, and hybridization was carried out at 55 °C overnight. After washing, slides were incubated in Tris-buffered saline containing 3% normal goat serum (Sigma-Aldrich), 0.3% Triton X-100 and rabbit IgG directed against digoxigenin coupled to rhodamine (1:200; Roche) for 4 h. Sections were then rinsed 3×10 min in Tris-buffered saline and immunoreacted with PPARγ antibodies (monoclonal mouse anti-PPARγ, 1:100, Santa Cruz Biotechnology Cat. sc-7196, USA) and proper secondary antibody. To enhance PPARγ signal, a three-step indirect labeling was performed combining biotinylated donkey IgG (1:500, Jackson Immuno-Research, Europe, UK) and streptavidin fluorescein conjugate [52](1:400, Vector, USA). 5µM Hoechst 33258 solution (Sigma, Italy) was used as nuclear counterstaining.
For autoradiographic analysis, slides were air-dried and exposed to X-ray films for 10 days. A section through the medial striatal level (10 mm rostral to the interaural line) from each rat was analyzed for zif-268 and dynorphin mRNA (n=5–6/experimental group). Quantitative analysis of labelling was performed using the Scion Image analysis program. The average grey value of white matter was subtracted from the striatal value to correct for background labeling [50].
Western Blotting
An independent group of rats treated with either RGZ (10 mg/kg, i.p., 15 minutes before levodopa) or vehicle (n=8/group) were anesthetized with isofluorane on day 11 of levodopa treatment, 90 min after the last injection of levodopa, and sacrificed by decapitation. Brains were rapidly removed and frozen in cold 2-methylbutane (−50 °C). Brain coronal sections (1 mm) were cut in an ice-cold stainless steel mold using razor blades and the striatum dissected out and homogenized in a glass Potter-Elvehjem, homogenized in ice-cold lysis buffer (50 mM Tris-HCl, 100 mM NaCl, 0.1% Triton X-100, 0.1% SDS, 1 mM Na3VO4, 10 mM NaF, 1 mM EDTA) and 1% protease inhibitor cocktail (Sigma Chemical, St. Louis, USA) and centrifuged at 16000 g for 30 min at 4 °C. Equal amounts of protein (20 µg) were resolved by SDS-PAGE (10%), transferred onto PVDF membranes (0.2 µm), and incubated for 1 h in 5% fat-free milk in Tris buffer saline + 0.05% Tween-20 (TBS-T buffer) at room temperature. Membranes were then incubated overnight at 4°C using the following primary antibodies: PPARα (Biovision, 1:1000), PPARγ, p-ERK1/2 and ERK 1 (Santa Cruz, 1:1000), and beta-Actin (Sigma, 1:10,000). After three 5-min washes in TBS-T, membranes were incubated with the appropriate secondary horseradish peroxidase-linked antibodies (Santa Cruz, 1:2000;) for 60 min at room temperature. Protein bands were visualized using the ECL kit (Amersham, GE Healthcare, Buckinghamshire, England) followed by exposure to X-ray film. Band immunoreactivity was quantified by densitometry using NIH image software.
Statistical Analysis
AIM data were expressed as medians ± interquartile ranges and analyzed using the Kruskal-Wallis test followed by Dunn’s multiple comparison test. The AIM data collected after administration of AM251 and BDG were also expressed as median ± interquartile ranges and were analyzed by Friedman test followed by Dunn’s multiple comparison test. AST and VSFPT data were expressed as percent of adjusting steps ± S.E.M. with respect to the forepaw ipsilateral to the 6-OHDA lesion (intact) and as percent of successful placements of each forepaw ± S.E.M. with respect to the intact limb, respectively. FAST, VSFPT and locomotor activity data were analyzed by one-way ANOVA, followed by Newman-Keuls post hoc. Microdialysis data were expressed as means ± S.E.M. and analyzed by two-way ANOVA for repeated measures with group and time as independent factors, followed by Tukey post hoc. All other data were expressed as means ± S.E.M. and analyzed by t-test (PPAR protein) or ANOVA followed by Tukey (in situ hybridization) or Newman-Keuls (P-ERK western blots) post-hocs. Significance was set at p<0.05.
RESULTS
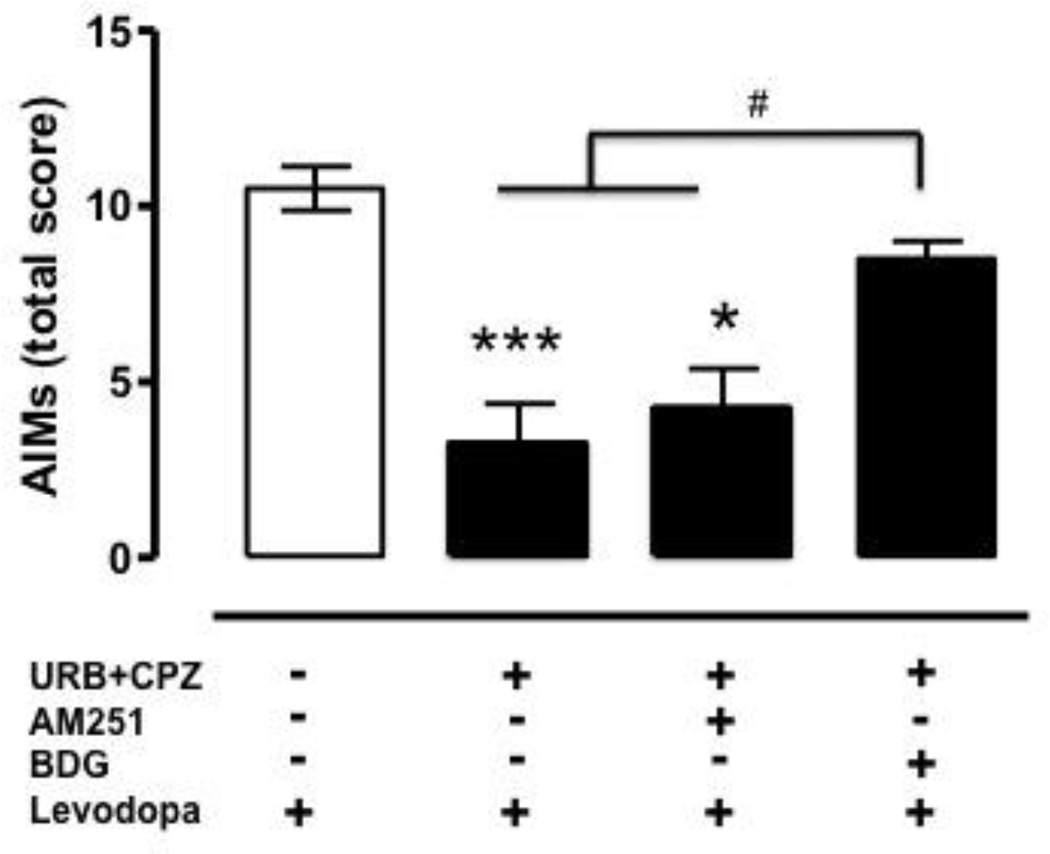
Chronic administration of levodopa (6 mg/kg, plus benserazide, 12.5 mg/kg, s.c., 1 injection/day for 13 days) produced severe AIMs in 6-OHDA-lesioned at 60 (data not shown) and 90 (Fig. 1) minutes after levodopa injection (Kruskal-Wallis, H=17.15, 3 d.f., p=0.0001), whereas no AIMs were observed at 120 min due to the wearing off of levodopa effect. As expected, sham-operated rats did not show AIMs at any time point (data not shown). As previously reported [1], sub-chronic co-administration (day 9–11) of the FAAH inhibitor URB597 (URB, 0.3 mg/kg, 15 min before levodopa) and the TRPV1 antagonist capsazepine (CPZ, 10 mg/kg, 15 min before URB) had no significant effect during the first 60 min (data not shown), but markedly reduced total AIMs 90 min after the last levodopa injection (Fig. 1). The selective CB1 antagonist AM251, when given at a dose known to fully antagonize CB1 receptors (1 mg/kg, i.p, 15 min before levodopa+URB+CPZ, day 12), partially reversed total AIMs (Fig. 1). As URB administration has been shown to elevate brain anandamide in dyskinetic rats [1], which in turn may activate non-cannabinoid targets, such as PPARα and γ[21], the same animals received the non-selective PPAR antagonist BADGE (BDG, 30 mg/kg i.p., 15 min before URB+CPZ) on day 13 of levodopa treatment. BDG had no effect on total AIMs when given alone (data not shown), but reversed the URB+CPZ anti-dyskinetic effect (Fig. 1), revealing the involvement of PPAR receptors in this response.
Figure 1.
Effect of systemic administration of vehicle (5% PEG + 5% Tween-80 + 90% saline, i.p., 15 min before levodopa; open bar) on total levodopa-induced AIMs (axial+orofacial+limb) in 6-OHDA-lesioned rats (n=8/group). The FAAH inhibitor URB597 (URB 0.3 mg/kg, i.p., day 11, 15 min before levodopa) was co-administered with the TRPV1 antagonist capsazepine (CPZ, 10 mg/kg, i.p., 15 min before URB). The CB1 antagonist AM251 (1 mg/kg, i.p.) or the non-selective PPAR antagonist BADGE (BDG, 30 mg/kg, i.p.) were administered 15 min before CPZ (followed by URB) on day 12 and 13 of levodopa treatment, respectively. Levodopa effects were analyzed by Kruskal-Wallis followed by Dunn’s multiple comparison test (H=17.15, 3 d.f., p=0.0001; ***p<0.001 and *p<0.05 vs. levodopa+veh; #p<0.05 vs. URB+CPZ+BDG). AM251 and BDG effects were analyzed by Friedman test followed by Dunn’s multiple comparison test [Friedman statistic (chi-square)=9.33, 2 d.f., p=0.0055]. Data are expressed as median ± interquartile range.
Chronic levodopa does not alter striatal PPAR expression in dyskinetic rats
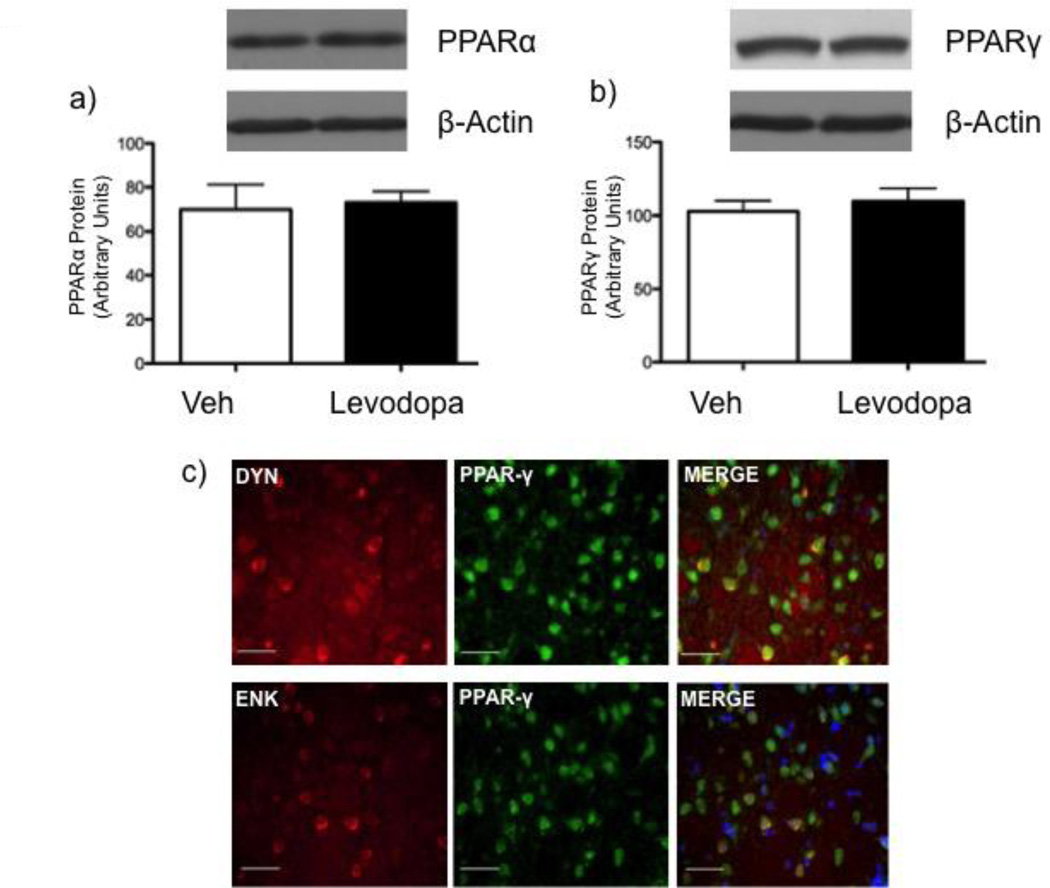
Levels of PPARs as quantified by western blotting revealed that chronic levodopa had no effect on striatal PPARα and PPARγ expression (Fig. 2a and b) in 6-OHDA-lesioned groups (PPARα: t=0.2586, 6 d.f., p=0.8046; PPARγ: t=0.6088, 5 d.f., p=0.5692).
Figure 2.
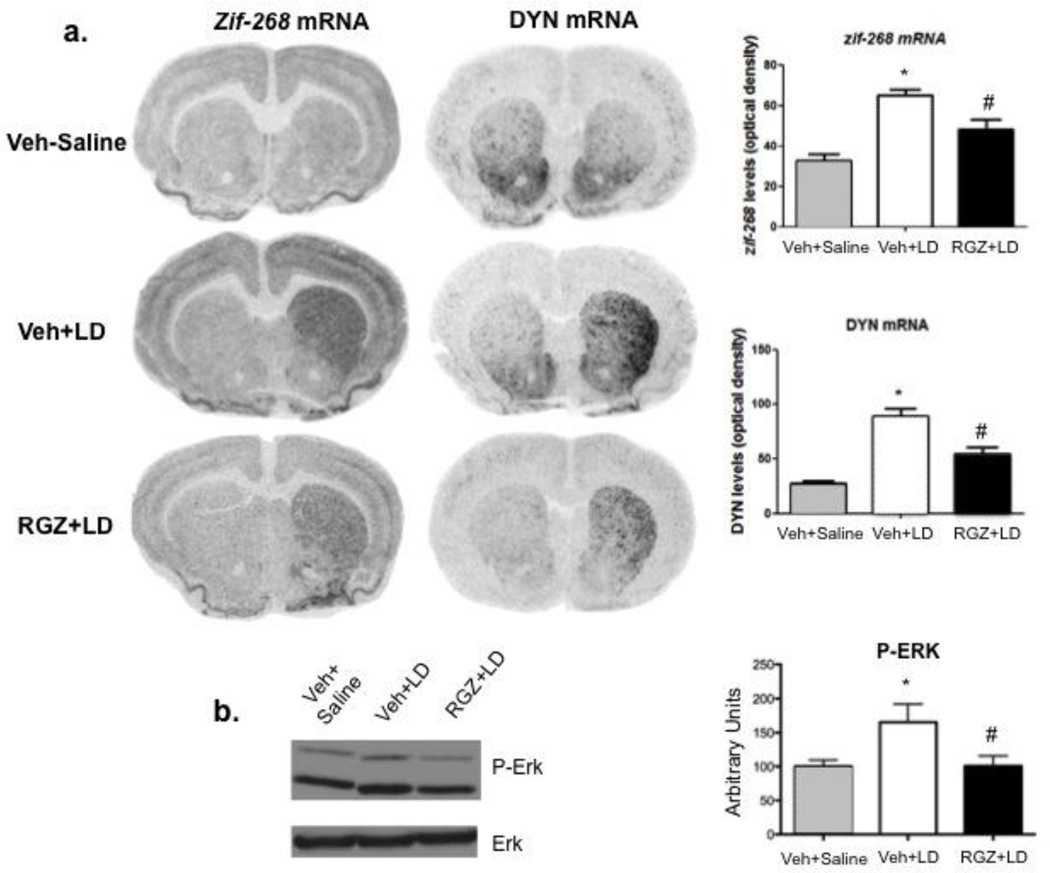
Effect of chronic vehicle (saline, s.c.; open bars) or levodopa (6 mg/kg, s.c.; filled bars) on PPARα (a) and PPARγ (b) protein in the striatum of 6-OHDA-lesioned rats 1 h after last drug injection. (PPARα: t=0.2586, 6 d.f., p=0.8046; PPARγ: t=0.6088, 5 d.f., p=0.5692). Data are expressed as mean ± S.E.M. (C) PPARγ (green), dynorphin (red) or enkephalin (red) expression and respective colocalization (yellow) in striatal neurons of 6-OHDA-lesioned rats. Nuclei are visualized by Hoechst (blue). Scale bar, 50 µm.
Fluorescence in situ hybridization/immunohistochemistry showed that PPARγ were expressed in both dynorphinergic and enkephalinergic neurons, indicating a colocalization with both D1R- and D2R-containing medium spiny neurons (Fig 2c).
Direct activation of PPARγ receptors alleviates AIMs
As FAAH inhibition elevates both oleylethanolamide (OEA) and anandamide, which in turn can bind to the PPARα and γ subtypes, respectively[53, 54], we investigated if direct activation of these receptors produced an anti-dyskinetic effect similar to that observed after URB+CPZ administration.
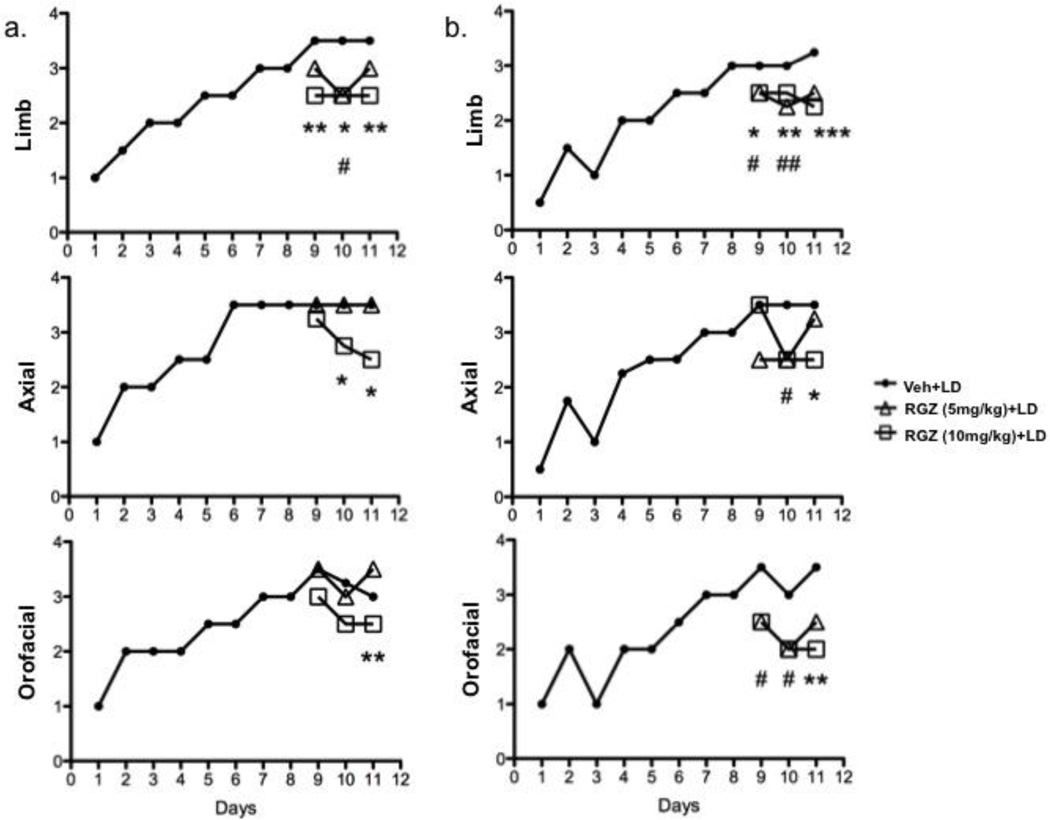
None of the PPARα agonists investigated (OEA, 5–10 mg/kg, s.c.; GW7647, 3.2 mg/kg, i.p.; WY14643, 5 mg/kg, i.p.; day 9–11, 15 min before levodopa) affected levodopa-induced AIMs (data not shown). By contrast, the selective PPARγ agonist RGZ reduced AIMs at 60 min (RGZ 5 mg/kg: Limb day 10, H=9.948, 25 d.f., p=0.0069. RGZ 10 mg/kg: Limb day 9, H=10.97, 23 d.f., p=0.0042; Limb day 10, H=9.948, 25 d.f., p=0.0069; Limb day 11, H=11.08, 23 d.f., p=0.0039; Axial day 10, H=9.986, 19 d.f., p=0.0065; Axial day 11, H=8.933, 20 d.f., p=0.0115; Orofacial day 11, H=13.24, 24 d.f., p=0.0013) and 90 min (RGZ 5mg/kg: Limb day 9, H=9.869, 17 d.f., p=0.0072; Limb day 10, H=15.27, 19 d.f., p=0.0005; Axial day 10, H=8.932, 14 d.f., p=0.0135; Orofacial day 9, H=13.24, 11 d.f., p=0.0399; Orofacial day 10, H=7.169, 11 d.f., p=0.0278. RGZ 10mg/kg: Limb day 9, H=9.869, 17 d.f., p=0.0072; Limb day 10, H=15.27, 19 d.f., p=0.0005; Limb day 11, H=13.98, 19 d.f., p=0.0009; Axial day 11, H=7.918, 13 d.f., p=0.0191; Orofacial day 11, H=10.97, 11 d.f., p=0.0088) after levodopa administration (Fig. 3a and b, respectively).
Figure 3.
Time course of the effects of systemic administration of levodopa (6 mg/kg, s.c., 1 injection per day; filled circles) measured at 60 (a) and 90 (b) minutes after levodopa injection. The selective PPARγ agonist, rosiglitazone (RGZ, 5 and 10 mg/kg, i.p.; open triangles and open squares, respectively) was administered 15 minutes before levodopa from day 9 through day 11. Panel a: RGZ 5 mg/kg: Limb day 10, H=9.948, 25 d.f., p=0.0069. RGZ 10 mg/kg: Limb day 9, H=10.97, 23 d.f., p=0.0042; Limb day 10, H=9.948, 25 d.f., p=0.0069; Limb day 11, H=11.08, 23 d.f., p=0.0039; Axial day 10, H=9.986, 19 d.f., p=0.0065; Axial day 11, H=8.933, 20 d.f., p=0.0115; Orofacial day 11, H=13.24, 24 d.f., p=0.0013. Panel b: RGZ 5mg/kg: Limb day 9, H=9.869, 17 d.f., p=0.0072; Limb day 10, H=15.27, 19 d.f., p=0.0005; Axial day 10, H=8.932, 14 d.f., p=0.0135; Orofacial day 9, H=13.24, 11 d.f., p=0.0399; Orofacial day 10, H=7.169, 11 d.f., p=0.0278. RGZ 10mg/kg: Limb day 9, H=9.869, 17 d.f., p=0.0072; Limb day 10, H=15.27, 19 d.f., p=0.0005; Limb day 11, H=13.98, 19 d.f., p=0.0009; Axial day 11, H=7.918, 13 d.f., p=0.0191; Orofacial day 11, H=10.97, 11 d.f., p=0.0088. # and * refer to RGZ 5mg/kg and RGZ 10mg/kg respectively. #*p<0.05, ##, **p<0.01, ***p<0.001, Kruskal-Wallis followed by Dunn’s multiple comparison test. Data are expressed as median. The interquartile ranges were removed for clarity.
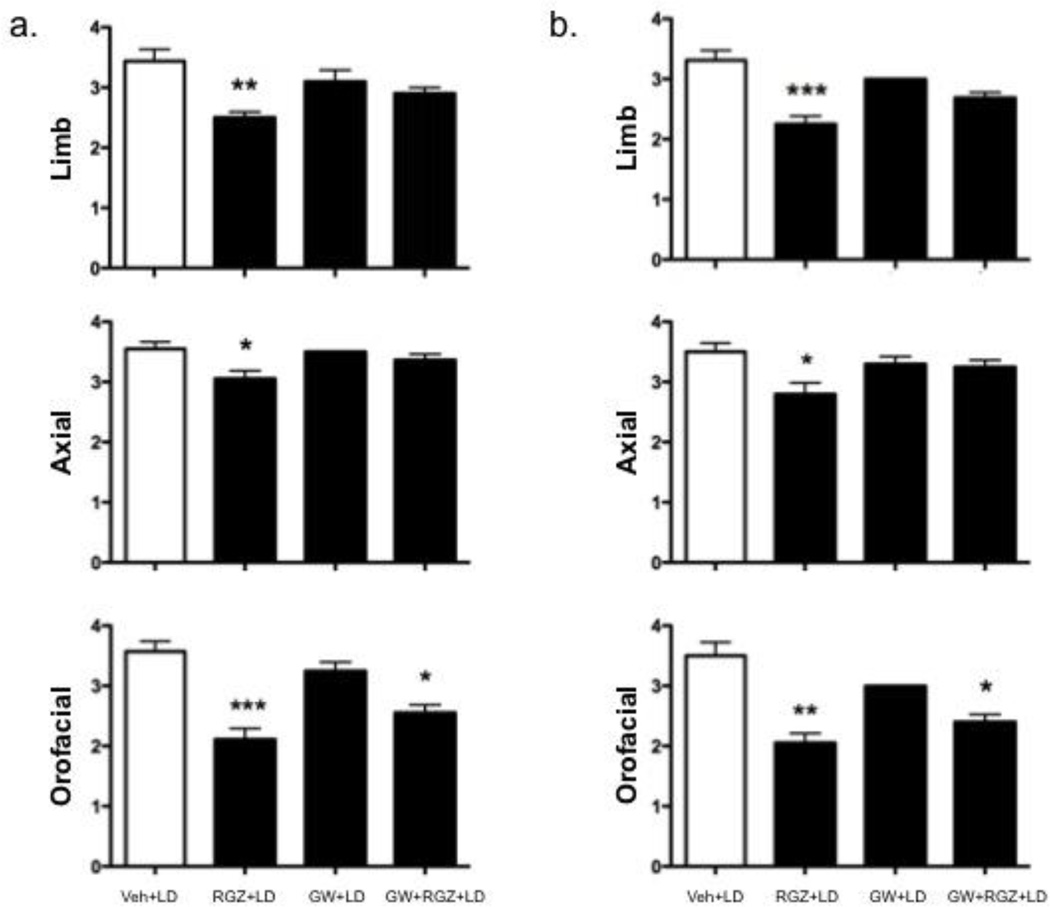
The selective PPARγ antagonist, GW9662 (4 mg/kg, i.p.) reversed the beneficial effect of RGZ (10 mg/kg, i.p.) on limb (60 min: H=15.02, 34 d.f., p=0.0018; 90 min: H=18.69, 28 d.f., p=0.0003) and axial (60 min: H=8.507, 36 d.f., p=0.04; 90 min: H=7.675, 34 d.f., p=0.05) AIMs, whereas it had no effect on orofacial AIMs (Fig. 4a and b).
Figure 4.
The selective PPARγ antagonist GW9662 (4 mg/kg, i.p.) reversed the anti-dyskinetic effect of rosiglitazone (RGZ, 10 mg/kg, i.p.) at 60 (a) and 90 (b) min after levodopa administration on day 12. GW9662 was administered 15 min before RGZ. Panel a: Limb, H=15.02, 34 d.f., p=0.0018; Axial, H=8.507, 36 d.f., p=0.04. Panel b: Limb, H=18.69, 28 d.f., p=0.0003; Axial, H=7.675, 34 d.f., p=0.05. *p<0.05; **p<0.01 and ***p<0.001 vs. veh+LD, Kruskal-Wallis followed by Dunn’s multiple comparison test. Data are expressed as median ± interquartile range.
Treatment with RGZ in intact rats did not affect general locomotor activity, as measured by total distance traveled (Vehicle=727.59 m, RGZ 5mg/kg=880.68 m, RGZ 10mg/kg=785.03 m; Fgroup (2,25)=2.796, p=0.0802) and total number of rearings (Vehicle=390.07, RGZ 5mg/kg=304.63, RGZ 10mg/kg=332.17; Fgroup (2, 25)=0.7833, p=0.4678) (data not shown).
RGZ reduces L-DOPA-induced increase of zif-268, dynorphin and p-ERK
We next investigated the effects of RGZ treatment on biochemical markers of levodopa-induced AIMs, such as striatal zif-268, dynorphin and p-ERK 1/2. Dyskinetic rats receiving chronic levodopa as reported above (n=7–8/group) showed elevated zif-268 and dynorphin mRNA levels in the denervated striatum. Moreover, RGZ treatment (10 mg/kg, i.p., days 9–11 of levodopa) reverted both markers to control levels (Fig. 5a), in contrast no effect of RGZ was detected in the contralateral (intact) striatum (data not shown).
Figure 5.
Effect of 6-OHDA vehicle (Veh) plus saline (gray bars), or vehicle plus levodopa (Veh+LD, empty bars) or levodopa plus rosiglitazone (LD+RGZ, 10 mg/kg, i.p.; filled bars) on zif-268 and dynorphin mRNA levels (a), and p-ERK 1/2 protein levels (b) in the ipsilateral striatum of 6-OHDA-lesioned rats 90 min after last levodopa injection (day 12). Panel a: one-way ANOVA [zif-268: Fgroup(1,12)=15.55, p=0.0005; dynorphin: Fgroup (1,12)=16.19, p=0.0004] followed by Tukey post hoc test (*p<0.01 vs. veh+saline; #p<0.05 vs. veh+LD). Panel b: one-way ANOVA [Fgroup (1,22)=4.6418, p=0.042415] followed by Newman-Keuls post hoc (*p<0.05 vs. veh+saline; #p<0.05 vs. veh+LD). Data are expressed as mean ± S.E.M.
In the same animals, chronic levodopa significantly increased striatal p-ERK1/2 without affecting overall ERK expression. This elevation was reversed by RGZ treatment (10 mg/kg, i.p.) [Fgroup (1,22)=3.2631, p<0.05] (Fig. 5b). We also observed that chronic L-DOPA increased the levels of the transcription factor deltaFosB, an event that has been associated with AIMs development [55]. This elevation, however, was not altered by RGZ (10 mg/kg, i.p.) (Data not shown).
RGZ does not affect striatal levodopa and dopamine bioavailability
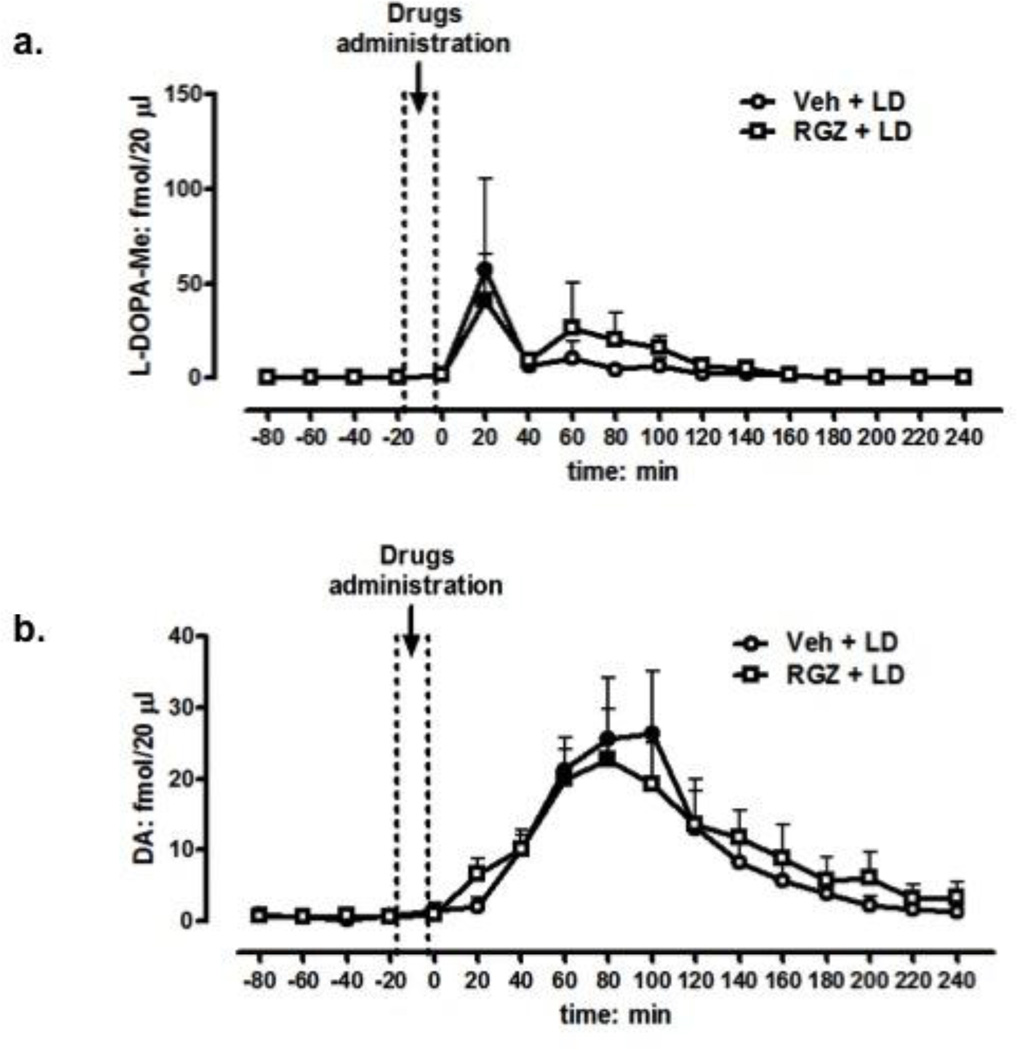
To assess whether RGZ could affect levodopa/dopamine bioavailability in the denervated striatum, we measured extracellular levodopa and dopamine levels by microdialysis in 6-OHDA treated rats (n = 5–7/group) receiving the same levodopa regimen described above. As expected, levodopa administration produced a significant elevation of intrastriatal levodopa (peak at 20 min post administration) and dopamine (peak at 80 min post administration), which returned to baseline levels within 120 or 180 min post-injection, respectively (ANOVA: Ftime(17,170)=3.22, p=0.00005; Fig 6a and b, respectively). Systemic co-administration of an effective dose of RGZ (10 mg/kg, i.p.) did not change striatal levodopa or dopamine outputs (ANOVA: Fgroup(1,10)=0.07, p=0.7; Fig. 6a and b, respectively).
Figure 6.
Effect of vehicle (5% PEG + 5% Tween-80 + 90% saline; open circles) or RGZ (10 mg/kg, i.p.; open squares) on levodopa (a) and dopamine (b) output in the ipsilateral striatum of 6-OHDA-lesioned rats (n = 5–7/group) 90 min after the last levodopa injection on day 12. ANOVA, Ftime(17,170)=3.22, p=0.00005; Fgroup(1,10)=0.07, P=0.7 (a and b, respectively). Data are expressed as mean ±S.E.M.
RGZ does not reduce levodopa anti-parkinsonian activity
We finally performed a series of behavioral tests to assess sensorimotor asymmetries in 6-OHDA-lesioned rats and the effect of RGZ on levodopa-mediated improvement of these asymmetries.
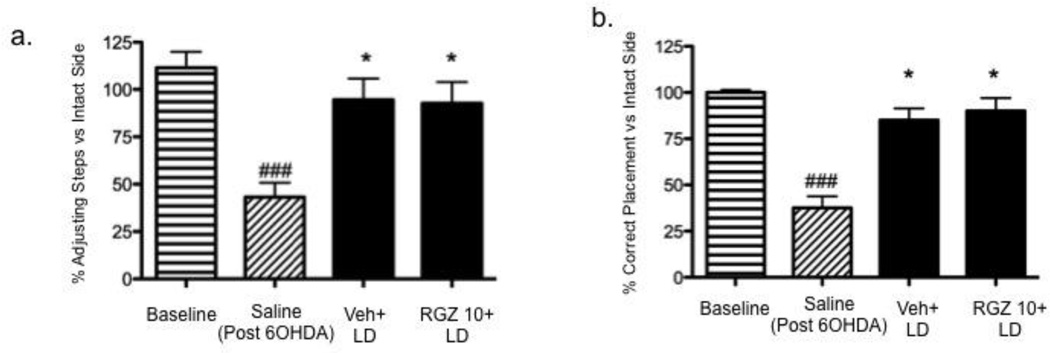
Using the FAST, we found that all rats used both forepaws equally before the 6-OHDA surgery (Fig. 7a); however, following 6-OHDA denervation, rats displayed a significant impairment of the forepaw ipsilateral to the lesion that was reversed by an acute injection of levodopa (6 mg/kg + 12.5 benserazide, s.c.). The beneficial effect of levodopa was not altered by co-administration of RGZ (10 mg/kg, 15 min before levodopa) [Fgroup (3, 20)=7.188, p=0.0018; baseline vs. Saline, *p<0.05, Saline vs. Veh+LD and RGZ10+LD] (Fig. 7a).
Figure 7.
Effect of vehicle (5% PEG + 5% Tween-80 + 90% saline; dark grey bar) or RGZ (10 mg/kg, i.p.; filled bar) on FAST (a) and VSFPT (b). Drugs were administered 15 min before levodopa (6 mg/kg, s.c). Panel a: one-way ANOVA [Fgroup (3, 20)=7.188, p=0.0018] followed by Newman-Keuls post hoc (###p<0.001 vs. baseline; *p<0.05 vs. saline). Panel b: one-way ANOVA [Fgroup (3, 20)=43.94, p<0.0001] followed by Newman-Keuls post hoc (###p<0.0001 vs. baseline; *p<0.05, vs. saline). Data are expressed as mean ± S.E.M
In the VSFPT, all rats displayed an equal number of successful forepaw placements before the 6-OHDA surgery (Fig. 7b); following DA denervation, the number of successful placements of the contralateral limb decreased significantly, and this deficiency was reversed by an acute injection of levodopa (6 mg/kg + 12.5 benserazide, s.c.). As in the FAST, the effect of levodopa was not altered by co-administration of RGZ (10 mg/kg, 15 min before levodopa, i.p.) [Fgroup (3,20)=43.94, p<0.0001; saline vs. baseline, p<0.05; saline vs. veh+LD and RGZ10+LD, p<0.05] (Fig. 7b).
DISCUSSION
In this study, we showed that the anti-dyskinetic effects produced by co-administration of the FAAH inhibitor URB597 and the TRPV1 receptor antagonist CPZ in 6-OHDA-lesioned rats required the recruitment of PPAR. In line with these findings, we also showed that direct stimulation of PPARγ by RGZ produced a dose-dependent reduction of levodopa-induced AIMs without producing motor suppression or affecting the anti-parkinsonian activity of levodopa.
Our study confirms previous investigations showing that URB597 ameliorates AIMs in denervated rats when co-administered with CPZ [1]. This anti-dyskinetic effect is only partially CB1-dependent, as the CB1 antagonist AM251 did not completely reverse the URB+CPZ-induced reduction of levodopa-induced AIMs. The dose of AM251 used in this study has been shown to fully antagonize CB1 receptors and to block the anti-dyskinetic response of the CB agonist WIN55,212-2 in the same rat model [1]. Thus, the lack of AM251 action on AIMs indicates that the elevation of endocannabinoids or other signaling lipids following FAAH pharmacological blockade by URB may lead to the recruitment of non-CB1 targets.
Systemic administration of URB is known to enhance brain anandamide as well as other FAAH substrates such as OEA [1], which in turn can stimulate non-CB receptors, including the nuclear transcription factors PPAR [20, 21]. In support of this hypothesis, we found that AIM alleviation by URB+CPZ co-treatment was fully reversed by the non-selective PPAR antagonist BADGE. While we did not observe any anti-dyskinetic effect following systemic administration of various PPARα agonists, we cannot rule out that the lack of effect might be due to the short half-life of these drugs and/or the use of a single dose. On the other hand, direct activation of PPARγ by the thiazolidinedione (TZD) RGZ ameliorated levodopa-induced AIMs in a dose- and time-dependent manner.
Moreover, we found that chronic levodopa did not affect the levels of PPARγ protein in the striatum. Although western blotting data do not rule out different outcomes on the variety of PPAR-γ-expressing cell types, such as microglia or neurons, our data suggest that changes in receptor levels are not involved in the anti-dyskinetic activity of RGZ.
Interestingly, RGZ did not produce hypomotility, nor did it prolong the bioavailability of levodopa and/or its conversion into dopamine, as indicated by the lack of changes in the concentration of both compounds in microdialysates from the denervated striatum. In line with these observations, RGZ administration did not affect the ability of levodopa to correct sensorimotor asymmetries in 6-OHDA rats, as revealed by the FAST and VSFPT. Thus, these results indicate that RGZ might be co-administered with levodopa as an effective adjunct therapy to control dyskinesias.
The molecular mechanisms underlying the beneficial action of RGZ are currently unknown. Several reports have shown neuroprotective effects following PPARγ activation by TZD in pre-clinical models of various neurological disorders including PD [52, 56, 57], as well as cognitive enhancement in Alzheimer’s disease [58] and cerebral ischemia [30]. Nevertheless, it is unlikely that the anti-dyskinetic effect of RGZ observed in our study results from a neuroprotective action on degenerating dopaminergic neurons. Indeed, RGZ was administered to denervated animals three weeks after the 6-OHDA lesion, a time point when approximately 95% of dopamine neurons have already been depleted. On the other hand, RGZ treatment attenuated levodopa-induced increases in striatal zif-268 and dynorphin mRNA, which are both upregulated in dyskinetic 6-OHDA rats and correlated with AIM severity [40, 50]. Moreover, RGZ normalized striatal p-ERK, a key regulator of cytosolic signaling cascades linked to the development and maintenance of levodopa-induced AIMs [59–61]. As inhibition of ERK phosphorylation has been shown to decrease levodopa-induced AIMs in both rodent and primate models of PD [59], our data suggest that activated PPARγ reduce levodopa-associated motor disturbances by promoting changes in gene expression and signaling cascades that in turn may counteract the maladaptive synaptic plasticity occurring in dyskinetic states [42]. Although deltaFosb is a downstream target of p-ERK and a biochemical marker of levodopa-induced AIMs [55], RGZ did not alter its levels in dyskinetic animals, suggesting that RGZ decreased AIMs via a deltaFosb independent mechanism.
There is evidence that the MAPK/ERK signaling pathway can inhibit the transcription activity as well as cellular localization (nucleus to cytosol) of PPARγ via phosphorylation and protein-protein interaction, respectively [62]. Nevertheless, further studies are necessary to determine whether levodopa-induced phosphorylation of ERK may affect the phosphorylation status or cellular distribution of PPARγ. As the TZD, troglitazone, has been shown to increase the activity of protein-tryosine phosphatase-1B [63], which in turn can regulate ERK signaling, we could speculate that the RGZ-mediated decrease of p-ERK may result from RGZ ability to increase phosphatase activity.
It is noteworthy that activated PPARγ can form heterodimers with the retinoid X receptor (RXR) and repress genes regulated by other transcription factors via protein-protein interactions or by sequestering co-activators necessary for their transcription [64]. In particular, the RXR/PPARγ complex has been shown to repress activated protein-1 (AP-1) [65], an important modulator of basal ganglia function that binds to “AP-1 sites” of several genes relevant to PD and dyskinesia, including the catecholamine rate-limiting enzyme tyrosine hydroxylase [66], dopamine D1 receptors [67], protein kinase A [68], pro-dynorphin, deltaFosB and zif-268 [69]. Nevertheless, as the anti-dyskinetic effect of RGZ was not completely reversed by the selective PPARγ antagonist GW9662, it is plausible that other targets than PPARγ might be involved, at least in part, in this beneficial action. In this regard, RGZ has shown off-target binding to ion channels and modulators, which might be implicated in its cardiotoxic effects [70].
A recent study by Gonzalez-Aparicio et al. [71] showed that administration of the anandamide analog OEA, an endogenous ligand of PPARα reduced levodopa-induced dyskinesia by blockade of TRPV1 receptors. This observation is in agreement with our previous studies showing that co-administration of the TRPV1 antagonist capsazepine is required to unmask the anti-dyskinetic properties of URB597 [1]. In our study, however, we did not observe an anti-dyskinetic effect following OEA administration. This discrepancy may be attributed to the use of a different species (rats vs. mice) or to differences in the 6-OHDA lesion target (medial forebrain bundle vs. dorsolateral striatum) or the extent of lesion, which may have affected the efficacy of OEA in our experiments versus those reported by Gonzalez-Aparicio et al. [71].
In conclusion, our data suggest that PPARγ is a promising new target for the treatment of L-DOPA-induced motor disturbances. Given the mild anti-dyskinetic effect observed after RGZ administration in rats, it remains to be determined whether this pharmacological approach might significantly affect dyskinetic symptoms in patients.
Although the repurposing of RGZ for dyskinesia pharmacotherapy would be facilitated by the fact that this drug is already available on the market for the treatment of type-II diabetes, the severe side effects associated with its administration [72] have discouraged the pharmaceutical industry from exploring new indications for RGZ. Nevertheless, the FDA has recently removed several restrictions on the use of RGZ based on new data from the RECORD study sponsored by GSK, [http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm376516.htm]. In addition, new insights on the role of PPARγ in human dyskinesias may emerge from an ongoing phase 2 clinical trial carried out in early PD patients receiving pioglitazone, another PPARγ agonist with a more benign pharmacological profile (clinicaltrial.gov identifier: NCT01280123). Although this study focuses on the neuroprotective effects of pioglitazone, one of the secondary outcomes is the assessment of therapy-induced complications, which should clarify whether TZD administration may not only prevent neuronal degeneration, but also ameliorate LID.
Highlights.
Administration of the PPARγ agonist, rosiglitazone, decreases L-dopa induced dyskinesia.
Rosiglitazone treatment attenuates L-DOPA-induced increase of zif268, dynorphin and p-ERK.
Rosiglitazone does not affect levodopa bioavailability nor its antiparkinsonian activity.
ACKNOWLEDGMENTS
Supported by NS050401-07 (to A. Giuffrida), M.J.Fox Foundation (to A. Giuffrida and A. Carta), and Ruth L. Kirschstein National Research Service Award 5F31-NS073411-03 (to A. Martinez).
Glossary
- 6-OHDA
6-hydroxydopamine
- FAAH
fatty acid amide hydrolase
- PPAR
peroxisome proliferator activated receptor
- URB
URB597
- CPZ
capsazepine
- RGZ
rosiglitazone
- BDG
BADGE
- TZD
thiazolidinedione
- AIMs
abnormal involuntary movements
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Morgese MG, et al. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson's disease: role of CB(1) and TRPV1 receptors. Exp Neurol. 2007;208(1):110–119. doi: 10.1016/j.expneurol.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graybiel AM, et al. The basal ganglia and adaptive motor control. Science. 1994;265(5180):1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 3.Rascol O, et al. Limitations of current Parkinson's disease therapy. Ann Neurol. 2003;53(Suppl 3):S3–S12. doi: 10.1002/ana.10513. discussion S12–5. [DOI] [PubMed] [Google Scholar]
- 4.Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease. A community-based study. Brain. 2000;123(11):2297–2305. doi: 10.1093/brain/123.11.2297. [DOI] [PubMed] [Google Scholar]
- 5.Bonifati V, et al. Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol. 1994;17(1):73–82. doi: 10.1097/00002826-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Gottwald MD, Aminoff MJ. Therapies for dopaminergic-induced dyskinesias in Parkinson disease. Ann Neurol. 2011;69(6):919–927. doi: 10.1002/ana.22423. [DOI] [PubMed] [Google Scholar]
- 7.Verhagen Metman L, et al. A trial of dextromethorphan in parkinsonian patients with motor response complications. Mov Disord. 1998;13(3):414–417. doi: 10.1002/mds.870130307. [DOI] [PubMed] [Google Scholar]
- 8.Thomas A, et al. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75(1):141–143. [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz RH. Adolescent abuse of dextromethorphan. Clin Pediatr (Phila) 2005;44(7):565–568. doi: 10.1177/000992280504400702. [DOI] [PubMed] [Google Scholar]
- 10.Gregoire L, et al. Low doses of sarizotan reduce dyskinesias and maintain antiparkinsonian efficacy of L-Dopa in parkinsonian monkeys. Parkinsonism Relat Disord. 2009;15(6):445–452. doi: 10.1016/j.parkreldis.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Goetz CG, et al. Placebo influences on dyskinesia in Parkinson's disease. Mov Disord. 2008;23(5):700–707. doi: 10.1002/mds.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dekundy A, et al. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179(1):76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Lundblad M, et al. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16(1):110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Lundblad M, et al. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol. 2005;194(1):66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Winkler C, et al. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10(2):165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer B, et al. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18(6):1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- 17.Martinez A, et al. The cannabinoid agonist WIN55212-2 decreases L-DOPA-induced PKA activation and dyskinetic behavior in 6-OHDA-treated rats. Neurosci Res. 2012;72(3):236–242. doi: 10.1016/j.neures.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fegley D, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313(1):352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 19.Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9(1):76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 20.LoVerme J, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319(3):1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- 21.O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152(5):576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cimini A, et al. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005;130(2):325–337. doi: 10.1016/j.neuroscience.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123(1):131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 24.Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. Br J Pharmacol. 2000;129(5):823–834. doi: 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Espejo E, et al. Experimental parkinsonism alters anandamide precursor synthesis, and functional deficits are improved by AM404: a modulator of endocannabinoid function. Neuropsychopharmacology. 2004;29(6):1134–1142. doi: 10.1038/sj.npp.1300407. [DOI] [PubMed] [Google Scholar]
- 26.Stienstra R, et al. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148(6):2753–2763. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]
- 27.Cullingford TE, et al. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem. 1998;70(4):1366–1375. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramanan S, et al. The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys. 2009;75(3):870–877. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu S, et al. Retinoic acid induces neurogenesis by activating both retinoic acid receptors (RARs) and peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) J Biol Chem. 2012;287(50):42195–42205. doi: 10.1074/jbc.M112.410381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collino M, Patel NS, Thiemermann C. PPARs as new therapeutic targets for the treatment of cerebral ischemia/reperfusion injury. Ther Adv Cardiovasc Dis. 2008;2(3):179–197. doi: 10.1177/1753944708090924. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, et al. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20(8):1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]
- 32.Dehmer T, et al. Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88(2):494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 33.Schintu N, et al. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009;29(5):954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- 34.Inestrosa NC, Toledo EM. The role of Wnt signaling in neuronal dysfunction in Alzheimer's Disease. Mol Neurodegener. 2008;3:9. doi: 10.1186/1750-1326-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiaei M, et al. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2005;191(2):331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Klotz L, et al. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J Immunol. 2005;175(8):4948–4955. doi: 10.4049/jimmunol.175.8.4948. [DOI] [PubMed] [Google Scholar]
- 37.Fasano S, et al. Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with L-dopa-induced dyskinesia. Proc Natl Acad Sci U S A. 2010;107(50):21824–21829. doi: 10.1073/pnas.1012071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10(8):2694–2706. [PubMed] [Google Scholar]
- 39.Guan Q, et al. Changes in the prodynorphin gene and DARPP-32 state in 6-OHDA-lesioned rats following long-term treatment with l-dopa. Neurosci Lett. 2007;426(1):64–68. doi: 10.1016/j.neulet.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 40.Bastide MF, et al. Immediate-early gene expression in structures outside the basal ganglia is associated to l-DOPA-induced dyskinesia. Neurobiol Dis. 2013;62C:179–192. doi: 10.1016/j.nbd.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Santini E, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27(26):6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cenci MA, Konradi C. Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res. 2010;183:209–233. doi: 10.1016/S0079-6123(10)83011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Y, et al. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci U S A. 2011;108(2):840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rylander D, et al. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther. 2009;330(1):227–235. doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundblad M, et al. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci. 2002;15(1):120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. tThe rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998. xxvi p., 237 of plates. [Google Scholar]
- 47.Chang JW, et al. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88(2):617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- 48.Lindner MD, et al. Validation of a rodent model of Parkinson's Disease: evidence of a therapeutic window for oral Sinemet. Brain Res Bull. 1996;39(6):367–372. doi: 10.1016/0361-9230(96)00027-5. [DOI] [PubMed] [Google Scholar]
- 49.Pinna A, et al. Modification of adenosine extracellular levels and adenosine A(2A) receptor mRNA by dopamine denervation. Eur J Pharmacol. 2002;446(1–3):75–82. doi: 10.1016/s0014-2999(02)01818-6. [DOI] [PubMed] [Google Scholar]
- 50.Carta AR, et al. Dyskinetic potential of dopamine agonists is associated with different striatonigral/striatopallidal zif-268 expression. Exp Neurol. 2010;224(2):395–402. doi: 10.1016/j.expneurol.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Carta AR. Differential regulation of GAD67, enkephalin and dynorphin mRNAs by chronic-intermittent L-dopa and A2A receptor blockade plus L-dopa in dopamine-denervated rats. Synapse. 2002;44(3):166–174. doi: 10.1002/syn.10066. [DOI] [PubMed] [Google Scholar]
- 52.Carta AR, et al. Rosiglitazone decreases peroxisome proliferator receptor-gamma levels in microglia and inhibits TNF-alpha production: new evidences on neuroprotection in a progressive Parkinson's disease model. Neuroscience. 2011;194:250–261. doi: 10.1016/j.neuroscience.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 53.Bouaboula M, et al. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517(3):174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 54.Fu J, et al. Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005;48(8):1147–1153. doi: 10.1016/j.neuropharm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6(6):461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- 56.Breidert T, et al. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J Neurochem. 2002;82(3):615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- 57.Carta AR. PPAR-gamma: therapeutic prospects in Parkinson's disease. Curr Drug Targets. 2013;14(7):743–751. doi: 10.2174/1389450111314070004. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Rivera J, Denner L, Dineley KT. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav Brain Res. 2011;216(1):255–261. doi: 10.1016/j.bbr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feyder M, Bonito-Oliva A, Fisone G. L-DOPA-Induced Dyskinesia and Abnormal Signaling in Striatal Medium Spiny Neurons: Focus on Dopamine D1 Receptor-Mediated Transmission. Front Behav Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santini E, et al. Dopamine- and cAMP-regulated phosphoprotein of 32-kDa (DARPP-32)-dependent activation of extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin complex 1 (mTORC1) signaling in experimental parkinsonism. J Biol Chem. 2012;287(33):27806–27812. doi: 10.1074/jbc.M112.388413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westin JE, et al. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62(7):800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burgermeister E, et al. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2007;27(3):803–817. doi: 10.1128/MCB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akasaki Y, et al. A peroxisome proliferator-activated receptor-gamma agonist, troglitazone, facilitates caspase-8 and −9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1B on human glioma cells. J Biol Chem. 2006;281(10):6165–6174. doi: 10.1074/jbc.M505266200. [DOI] [PubMed] [Google Scholar]
- 64.Feige JN, et al. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. 2006;45(2):120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Ricote M, et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 66.Yoon SO, Chikaraishi DM. Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron. 1992;9(1):55–67. doi: 10.1016/0896-6273(92)90220-8. [DOI] [PubMed] [Google Scholar]
- 67.Zhou QY, Li C, Civelli O. Characterization of gene organization and promoter region of the rat dopamine D1 receptor gene. J Neurochem. 1992;59(5):1875–1883. doi: 10.1111/j.1471-4159.1992.tb11023.x. [DOI] [PubMed] [Google Scholar]
- 68.Singh IS, et al. Molecular cloning and characterization of the promoter region of the mouse regulatory subunit RII beta of type II cAMP-dependent protein kinase. Biochem Biophys Res Commun. 1991;178(1):221–226. doi: 10.1016/0006-291x(91)91802-j. [DOI] [PubMed] [Google Scholar]
- 69.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28(3):370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann BR, et al. Chemical proteomics-based analysis of off-target binding profiles for rosiglitazone and pioglitazone: clues for assessing potential for cardiotoxicity. J Med Chem. 2012;55(19):8260–8271. doi: 10.1021/jm301204r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez-Aparicio R, Moratalla R. Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson s disease. Neurobiol Dis. 2013;62C:416–425. doi: 10.1016/j.nbd.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]