Abstract
Although viral emergence is commonly associated with cross-species transmission, the processes and determinants of viral evolution in a novel host environment are poorly understood. We address key questions in virus emergence and evolution using data generated from two unique natural experiments: the deliberate release of myxoma virus (MYXV) and rabbit hemorrhagic disease virus (RHDV) as biological control (biocontrol) agents against the European rabbit in Australia, and which have been of enormous benefit to Australia’s ecosystem and agricultural industries. Notably, although virulence evolution in MYXV and RHDV followed different trajectories, a strongly parallel evolutionary process was observed in Australia and Europe. These biocontrol agents were also characterised by a lack of transmission to non-target host species, suggesting that there are major barriers to successful emergence.
Keywords: virus, emergence, evolution, biocontrol, rabbit
The evolution of viral emergence
Emerging viral infections are increasing in number and a major concern to human, animal and plant health [1]. There is a strong association between viral emergence and cross-species transmission (host jumping), and most emerging viral infections in humans have a zoonotic origin. Although cross-species transmission events often result in spill-over (i.e., dead-end) infections, species jumps occasionally lead to the successful establishment of endemic infections [1–3], with the human immunodeficiency virus (HIV) and a number of subtypes of influenza A virus serving as high-profile examples. However, there are relatively few cases in which the precise evolutionary and ecological events that underpin emergence and successful spread in a novel host species are known, and the factors responsible for emergence can often only be inferred retrospectively. As a consequence, cases in which viral emergence has been deliberately human mediated may be especially informative, providing a valuable ‘natural experiment’.
Here we show how two instances of the human release of viruses as biocontrol agents against European rabbits – myxoma virus (MYXV) and rabbit hemorrhagic disease virus (RHDV) – can shed important light on key aspects of viral emergence and evolution. We focus on (i) the nature of viral evolution in a novel environment, (ii) the evolution of host resistance, (iii) parallel viral evolution on a continental scale, and (iv) the evolution of host range.
Viruses as biocontrol agents
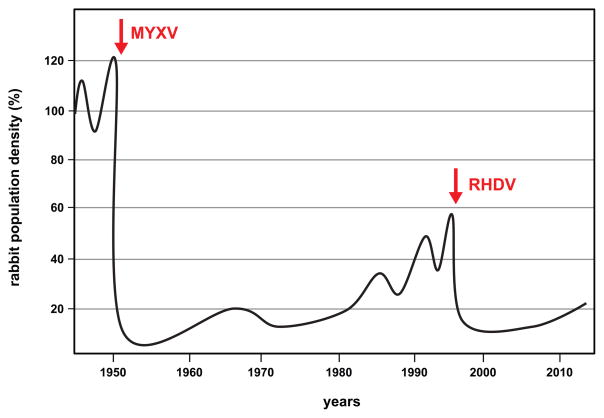
Two viruses – MYXV and RHDV – have been released in Australia to control the abundance and spread of the European rabbit (Oryctolagus cuniculus). The release of MYXV, starting in the 1950s (Box 1), and of RHDV which began the mid-1990s (Box 2), are the most successful accomplishments in the biological control of vertebrate populations, and together have reduced the size of the invasive rabbit population by approximately 85% [4] (Figure 1). Because of understandable concerns over safety, the only other instance of a virus being successfully used as a vertebrate biocontrol was the deliberate deployment of feline parvovirus (also known as feline panleukopenia virus) against cats on Marion Island [5]. This small and remote island in the Southern Indian Ocean had an estimated feral cat population of over 2,000 in 1975, and which posed a major threat to the island’s bird population, with 450,000 killed each year. Following the release of parvovirus the cat population declined to approximately 600 animals within a few years, which were then successfully eradicated using traps and hunting [6]. Other attempts to control vertebrate species with viruses have proven less successful. Classical swine fever virus was intentionally released several times in the 1950s to control the wild swine population on small islands in California. While an initial reduction in population size of approximately 80% was observed, on each occasion the virus disappeared after a few years, and was not able to persist in the wild swine population [7, 8]. In contrast, viruses have been used widely as biological pesticides in insect control, and are considered environmentally friendly compared to chemical pesticides. For example, the use of baculoviruses has had a substantial positive impact on agriculture, including protection of soybean crops against the velvet bean caterpillar in Brazil [9], and several baculoviruses are commercially available as pesticides [10, 11].
Box 1. MYXV.
MYXV is a double-strand DNA virus of the family Poxviridae, which seemingly uses the tapeti (Sylvilagus brasiliensis), a South American leporid species, as its natural host. Tapetis are found from Mexico throughout Central America, and as far south as northern Argentina (http://www.iucnredlist.org/details/41298/0). A divergent strain of MYXV (the Californian MSW strain) infects the brush rabbit (Sylvilagus bachmani) in the Western USA and Mexico [72]. Although few studies have been undertaken, MYXV infection in tapetis and brush rabbits leads to skin lesions but rarely causes severe disease. In contrast, MYXV is very virulent in the European rabbit, resulting in myxomatosis [68]. Hence, the cross-species transmission of MYXV from its natural hosts to European rabbits famously resulted in a marked increase in virulence, although the size of the host barrier to this emergence event is unknown. The European rabbit (Oryctolagus cuniculus) was introduced multiple times into Australia for hunting during the 19th century [25]. Rabbits spread quickly across the country and became a major vertebrate pest, severely impacting both indigenous species and Australian agriculture. In 1950 a highly virulent strain of MYXV (SLS) was successfully released into wild rabbits in Australia as a form of biocontrol [73], causing an immediate reduction in the size of the rabbit population. However, within a few years of its release more attenuated strains of MYXV were observed in the field, and shortly after the rabbit population began to recover [73]. In 1952 a second strain of MYXV (the Lausanne strain, LU, also of grade 1 virulence) was released in France, although this was a private release and not part of an organised biocontrol program, for which the perpetrator was fined one franc (as a reduction in rabbit numbers was deemed beneficial) [26, 73]. MYXV spread rapidly across France and other parts of Europe, including the UK [15], reducing the size of the rabbit population by 90–99%. A major negative ecological consequence of both MYXV and RHDV occurred in Spain, where the decline in rabbit populations directly affected predators that fed on rabbits, including the endangered Iberian lynx [15, 74]. Several attempts were made to inhibit viral spread, including vaccination, but success was limited [75]. Strikingly, as in Australia, MYXV rapidly attenuated in Europe.
Box 2. RHDV.
RHDV is a single-stranded positive-sense RNA virus (family Caliciviridae, genus Lagovirus) [76]. In 1984 the first outbreak of severe rabbit hemorrhagic disease (RHD) was reported in China [77], where it killed a remarkable 140 million rabbits within a year [30]. This new disease spread rapidly to Europe, most likely with the export of rabbit fur and meat. RHD was reported in Italy in 1986 and in Spain shortly afterwards [78]. The associated fatality rate was again very high, ranging from 55–75% [79], and the rabbit population experienced a dramatic reduction in size, although death rates varied among regions. In 1991 RHDV was imported into Australia for testing as a possible biocontol agent, which began in March 1995 on Wardang Island off the coast of South Australia [25]. Despite strict quarantine conditions, the virus reached the mainland in the same year and spread rapidly, with unanticipated transmission via blow flies (which fed on the carcasses of RHDV-infected animals) being the most likely source [26, 80]. In September 1996 RHDV was officially registered as a pest control agent. As with the previous release of MYXV, a remarkable loss of up to 95% of the rabbit population was documented in the first months of the RHDV epidemic [26].
Figure 1. Temporal impact of MYXV and RHDV on rabbit populations in Australia.
The relative rabbit abundance in Australia since 1945. Rabbit population density is nominally set to 100% in 1945. The arrows indicate the timing of the release of MYXV in 1950 and RHDV in 1995. Adapted from [4].
While the success of MYXV and RHDV viruses as biocontrol agents in rabbits in Australia is well documented, saving billions of dollars for the agricultural industries and helping to protect indigenous ecosystems, these viruses have also revealed fundamental aspects of virus evolution and emergence. In particular, they represent a unique opportunity to determine the patterns and processes of evolution as a virus establishes itself in a novel and naïve host population, and because MYXV and RHDV resulted in rabbit epidemics in both Australia and Europe, key aspects of viral emergence can be studied in parallel. While MYXV has been useful in revealing how virulence may evolve following a species jump, the processes responsible for the emergence of RHDV are less clear, although current data suggests that it emerged from a previously non-pathogenic group of rabbit viruses by mutation (or recombination) in the absence of a species jump and is still associated with high virulence [12]. In addition, both MYXV and RHDV provide insights into the evolution of host resistance [13], including interactions with co-circulating viruses. Finally, an important aspect of these case studies is that the same mammalian host species – the European rabbit – can be studied in both the laboratory and in nature, which will greatly assist the identification of virulence determinants.
Viral emergence following a species jump: the case of MYXV
The release of MYXV in European rabbits is a classic example of viral emergence following a species jump (Box 1). This evolutionary process was famously documented by Frank Fenner and colleagues who classified MYXV infections into five virulence grades (denoted 1–5) depending on survival time following infection and the case fatality rate in laboratory rabbits [14]. Accordingly, the original Standard Laboratory Strain (SLS) virus that was first successfully released in 1950 is of grade 1 virulence, such that rabbits die 10–15 days after infection and with a case fatality rate of over 99%. In contrast, the case fatality rate of the most attenuated strains (grade 5) is below 50% [14], but still far more virulent than in the natural reservoir hosts (Box 1). In Australia, MYXV virulence declined rapidly after its release and viruses of all five virulence grades were isolated in the field, although those of ‘intermediate’ virulence (grade 3; mean case fatality rate of 88% and a mean survival time of 21.5 days) were the most commonly observed [15].
The importance of MYXV with respect to the study of virulence evolution is that the apparent dominance of viruses of intermediate virulence grade was interpreted to mean that those of higher virulence were selected against because they killed the host too quickly, while viruses of lower virulence did not produce sufficiently high viral loads to insure transmission. In contrast, viruses of intermediate virulence might have high viral loads while keeping the host alive long enough to enable transmission [16] (Figure 2). As a consequence, the example of MYXV offered support to the theory that virulence levels reflect an evolutionary trade-off with transmissibility [17]. Although still a viable explanation for the observed data, phylogenetic studies have revealed that there have been both decreases and increases in virulence among those MYXV lineages circulating in Australia, such that high virulence may be favoured in some localities [18, 19]. Accordingly, the evolution of virulence in MYXV is likely to be more nuanced than previously anticipated, and that it is in part mediated by local differences in rabbit ecology (including population size and density) and resistance.
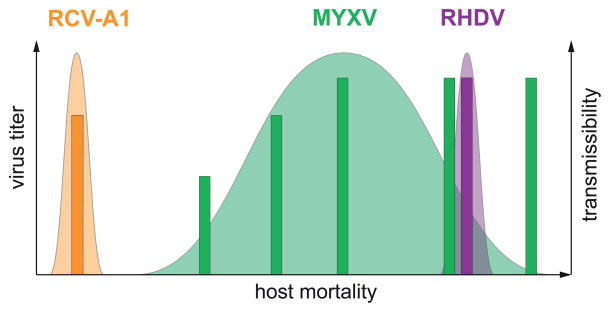
Figure 2. Possible relationship between virulence and transmission in rabbit viruses.
The x-axis depicts approximate host mortality rates (a measure of virulence) for the three rabbit viruses (colored accordingly) ranging from 0% (benign) to 100% (always lethal), including the different virulence grades of MYXV. The likely relationship between mortality and hypothetical values for viral titer (bars) and transmissibility (bell-shaped distributions) is also shown. Transmission via the fecal-oral route does not require host mortality so that RCV-A1 can evolve to low virulence. In contrast, RHDV can also be transmitted via flies that feed on animal carcasses, such that host death increases transmission rate. In the case of MYXV predominant mosquito and flea transmission occurs via diseased (live) animals, leading to a trade-off between virulence and transmission. Importantly, despite the markedly difference virulence levels in the three viruses, each can be considered as maximizing transmissibility.
Viral emergence without a species jump: the case of RHDV
In contrast to MYXV, highly virulent RHDV seemingly emerged from a non-virulent strain of this virus likely in the absence of a host jump before its first description in China in 1984 (Box 2). However, the exact processes surrounding this event (including the possible role of other host species), as well as its timing, are still the source of considerable uncertainty and debate [20, 21], particularly given the growing number of caliciviruses identified in lagomorphs with differing levels of virulence [22, 23].
The release of RHDV in Australia in the mid-1990s paints a very different picture of virulence evolution to that observed in MYXV. In South Australia, where RHDV escaped from quarantine in 1995, the virus was highly effective and reduced the rabbit population down to 5% of its initial size [24]. However, a marked variability was observed among localities, as the rabbit decline was only 53% in Queensland and almost no reduction was observed in some coastal parts of New South Wales. These differences in rabbit mortality (and virus transmission) may in part reflect differences in climate, as a greater rabbit loss was seen in more arid areas (such as South Australia) whereas little effect was observed in high-rainfall sites including parts of New South Wales [25]. It is possible that the impact of climate reflects the fact that flies, which are likely a major mode of RHDV transmission, are less active at cooler temperatures [26]. In addition, the presence of a second, benign, calicivirus (termed Australian rabbit calicivirus, RCV-A1) that circulates in rabbit populations in some parts of Australia including the wetter parts of New South Wales, seemingly provides some protection against RHDV [4, 26, 27]. Although only a limited number of RCV-A1 viruses are currently available for study, it is possible that this virus first entered Australia with imported rabbits in the 19th century [28]. More important is that the RCV-A1 is associated with only very mild disease, which may reflect its exclusively fecal-oral transmission, such that there is no selective cost in retaining a healthy rabbit as virus will always be excreted [29] (Figure 2). In contrast, the high virulence of RHDV is associated with high viral loads in the liver, in which lesions are observed post-mortem [30]. Future genomic comparisons to identify candidate virulence mutations followed by in vivo studies to systematically exchange the components of the pathogenic (RHDV) and non-pathogenic caliciviruses (RCV-A1) will be central to revealing how and why these closely related viruses differ so profoundly in virulence.
More striking, particularly given the earlier experience with MYXV, is that there is currently no evidence that RHDV has become attenuated as virulence appears to be consistently high in the field [31]. Indeed, there is even evidence that RHDV virulence has increased in some localities [12]. Importantly, that highly virulent RHDV likely evolved from a lower virulence ancestor in the same host species strongly suggests that increased rabbit mortality was selectively beneficial. However, the potency of RHDV as a biocontrol agent has been diminished by the evolution of resistance in the rabbit (see below) [25] as well as by cross-protection from RCV-A1.
Viral evolution in a naïve environment
The strong selection for attenuation in MYXV led to extensive research on the viral genetic mutations that might control virulence. While a number of candidate mutations have been identified, the functions of the genes involved have not been fully determined [18, 32, 33], such that their role in controlling virulence in the field remains unclear [34, 35]. However, one strong conclusion is that there is no single genetic ‘switch’ that determines virulence in MYXV, as there are multiple phylogenetic pathways to both high and low virulence grades [18]. Such adaptive flexibility might reflect the relatively large (161.8 kb) genome of MYXV that allows phenotypic adaptation through mutational accumulation in multiple genes [36]. In contrast, RNA viruses such as RHDV are characterised by major constraints on genome size, such that gene contents are always limited, resulting in frequent convergent evolution and pleiotropy [37, 38]. In these circumstances it is expected that the same virulence-determining mutations will arise multiple times. Indeed, there are other instances in RNA viruses in which a single mutation has led to a marked change in virulence. For example, a major outbreak of Venezuelan equine encephalitis virus (VEEV) in the mid-1990s resulted in more than 75,000 human infections in Latin America [39, 40]. VEEV has avirulent and virulent serotypes, and a single mutation in the envelope gene can change the former to the latter [41]. Similarly, a single mutation has been associated with an increase of virulence in West Nile virus in birds [42]. Whether a limited number of mutations are responsible for the emergence of a high virulence strain of RHDV is unknown [43–45], and recombination has also been invoked [46]. Also of note is that an antigenic variant of RHDV (RHDVa) has been documented, first in Italy (in 1997), and subsequently in diverse geographic localities [47]. Importantly, RHDVa appears to be replacing the originally circulating strains of RDHV in locations such as the Iberian Peninsula [48] and Italy [49], and has emerged a number of times in the USA [50]. Accordingly, there are plans to use RHDVa as an additional agent of rabbit biocontrol in Australia (http://www.feral.org.au/pestsmart-rhd-boost/). More recently, a new and phylogenetically distinct lagomorph calicivirus, denoted RHDV2 or RHDVb, has been documented in Europe, causing mortality in both vaccinated adult rabbits [22] and young rabbits [51, 52], although its origin and genomic and biological characteristics have not been fully elucidated.
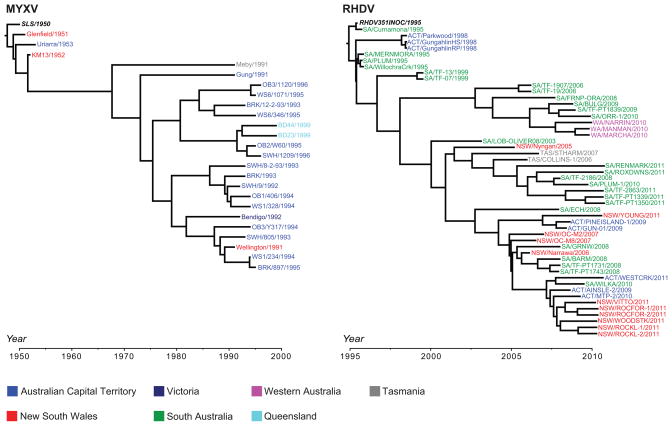
Finally, despite the fact that RHDV has been released thousands of times in Australia, it is noteworthy that all those field isolates sampled to date derive from the initial variant that escaped quarantine in 1995 (Figure 3). Hence, in the entirely RHDV-naïve environment (although with RCV-A1 present in some localities), those early viral strains that achieved rapid adaptation to the rabbit host may have been able to outcompete re-released strains that retained the genotype of the original inoculum [45], co-evolving with rabbits to maintain high virulence. A similar outcome was observed in the case of MYXV. Here, the MYXV strain (SLS) initially released in Australia in 1950 has been shown to be the ancestor of all later sampled viruses, despite multiple later releases of this virus and the LU strain, while the initially released LU strain dominated in Europe despite multiple re-releases [18, 53].
Figure 3. Geographic structure of MYXV and RHDV in Australia.
Time-scaled phylogenetic trees were estimated using the Bayesian Markov chain Monte Carlo method available in the BEAST package [81]. Both MYXV and RHDV analyses utilized the GTR+Γ model of nucleotide substitution, a relaxed molecular clock, and a constant population size coalescent prior. The MYXV data set comprised 25 complete genome sequences (alignment of 163,555 nt), while the RHDV data set comprised 51 capsid (VP60) sequences of 1,737 nt. The sequence names are colored according to the Australian state/territory from where they were isolated. The sequences in bold italics are the strains originally released in 1950 (MYXV) and 1995 (RHDV).
The evolution of resistance to viral biocontrol agents
As well as improving our knowledge of virus evolution, the release of MYXV and RHDV provide valuable information about the rapidity with which host resistance evolves, although the underlying mechanisms are still opaque. The impact of MYXV in Australia was severely dampened because rabbits quickly became resistant to the virus [54]. For example, uninfected rabbits captured in the region of Lake Urana in New South Wales, experienced a drop in mortality rate from 54% to 26% within a year [55]. More work is required to fully understand the mechanisms of host resistance, but it seems likely that resistant rabbits have an enhanced type 1 cytokine and natural killer cell response, and so can better control viral infection [56, 57]. Rabbits in Europe also gained resistance, with the seemingly longer time-scale likely reflecting a lack of intensive surveillance: studies in the UK showed that in 1969 the mortality rate was still over 90%, but decreased to 59% in 1970 and 13% in 1974 [58].
Resistance is even less well understood in the case of RHDV. A role for histo-blood antigens (HBGA) as attachment co-factors has been proposed [59, 60], such that there is an association between HBGA-type and RHDV susceptibility [61], although whether other cell receptors play a role, including any in the liver, is uncertain. In addition, young rabbits exhibit innate resistance to fatal RHDV compared to adults, but this protection is lost by 10 weeks [62]. While the genetic basis to resistance is unknown, it can be overcome by experimental immunosuppression of young rabbits using corticosteroids [63]. In addition, maternal antibodies are also able to prevent RHDV infection [30, 62]. Importantly, resistance in the case of RHDV appears to predominantly involve prevention of infection [64], while in MYXV resistance is associated with enhanced control of virus replication and dissemination. Finally, non-pathogenic RCV-A1 clearly offers at least partial protection to RHDV [25–27]. Indeed, RCV-A1 antibody prevalence was highest in wet areas, and this may be a major reason why RHDV has been less effective in these localities [65], although the underlying mechanisms are again unclear [29].
Parallel evolution in Australia and Europe
One of the characteristics of MYXV and RHDV that makes their study particularly informative is that the emergence of these two viruses occurred in parallel on two continents – Australia and Europe (Figure 4). Although both MYXV and RHDV were introduced into New Zealand, the latter illegally, MYXV failed to establish, while RHDV spread rapidly [66].
Figure 4. Parallel evolution of MYXV and RHDV on two continents.
Schematic view of the evolutionary outcomes that followed the release of MYXV and RHDV in Australia (left) and Europe (right). Both viruses spread rapidly in both continents and are now endemic in the rabbit population. MYXV attenuated in Australia and Europe, whereas RHDV retained its high virulence in both locations. An increase in host resistance was observed for both viruses, although jumping to hosts other than lagomorphs has not been documented in Australia and Europe for either virus, although new RHDV strains are emerging in Europe. Rabbits facing right represent MYXV infection, whereas rabbits facing left denote RHDV infection.
Perhaps the most obvious manifestation of this parallel evolution is that MYXV virulence declined markedly in both Australia and Europe; less virulent strains than those initially released came to dominate the field within a few years [15]. While mosquitoes were initially the main vectors of MYXV in Australia, the virus was largely transmitted via fleas in Europe (Spilopsyllus cuniculi and Xenopsylla cunicularis) [67]. Both fleas were later introduced into Australia to boost MYXV transmission; however, S. cuniculi failed to survive in arid regions and the release of X. cunicularis co-occurred with the emergence of RHDV, so that its impact was difficult to assess. Notably, there is also evidence of selection for viral attenuation as transmission via fleas increased when rabbits survived longer but viral loads remained high [16]. Despite this cross-continent similarity, it is striking how few MYXV mutations have occurred in parallel on both continents; hence, although there has been a clear convergence in phenotype – with similar virulence grades observed in the field in both continents – there has been no equivalent convergence at the genotypic scale [18]. As noted above, this is compatible with the idea that in a large double-strand DNA virus such as MYXV there is sufficient genomic flexibility to allow multiple solutions to the same adaptive problem [3].
A rather different, but still partly parallel, evolutionary trajectory was seen with RHDV. This virus was deliberately introduced by humans into Australia, but not so in Europe. The impact on the rabbit population was similar to MYXV, but with large-scale geographic variation, again highlighting the importance of local differences in host and environment. However, in marked contrast to MYXV, to date there is no evidence for RHDV attenuation in either Europe or Australia. Although more data are required, it is possible that highly virulent RHDV might have a near optimal transmission rate which explains its maintenance since emergence. Such consistently high virulence supports the frequent transmission of RHDV following blow fly feeding on rabbit carcasses, in contrast to MYXV in which live but diseased animals are the main vehicle for transmission (although aerosol transmission has also been proposed; [15]). In addition, it is possible that the trajectory of virulence evolution differs between MYXV and RHDV because of the far smaller (~50%) and less dense rabbit population in Australia at the time of RHDV release, which resulted in locally varying selection pressures [4].
Viral biocontrol and the evolution of host range
In Australia, the European rabbit is regarded as an invasive pest. Other than hares (another lagomorph) that are also considered pests, no closely related mammalian species are found in Australia. Hence, while little is known about the factors that shape host range in poxviruses and caliciviruses, it is likely that the unique mammalian fauna of Australia limits the opportunities for inadvertent cross-species transmission to other mammals. Indeed, any cross-species transmission to marsupials would be expected to represent a major adaptive challenge requiring multiple host-gain mutations, although transmission to non-native eutherian species (such as rats or feral pigs) would be predicted to be more likely. To date there is no evidence of transmission of either MYXV or RHDV to animal species other than lagomorphs in Australia, supporting the initial safety studies undertaken prior to their release [68]. Hence, when used with care, viral biocontrol can be safely undertaken. More importantly, there is similarly no evidence of host jumping to non-lagomorphs in Europe, where placental mammals are obviously abundant and where the vector-mediated mode of transmission will enhance exposure to different species. For example, a recent study found RHDV RNA in the liver of mice that were living close to rabbits, although with no evidence for viral replication or illness [69]. At face value this suggests that cross-species viral transmission may sometimes be more difficult than is generally envisaged, even among relatively closely related host species, and for caliciviruses such as RHDV in which phylogenetic studies suggest frequent host jumping over evolutionary time [70]. In contrast, the parvoviruses used as an anti-cat biocontrol on Marion Island now appear to be remarkable host generalists, with mutations at a limited number of amino acid residues in the virus capsid responsible for the successful transfer of this virus to a diverse array of carnivore species [71]. Revealing the factors that mediate successful cross-species transmission, including possible host barriers, will do much to inform us about the factors that control successful emergence (Box 3).
Box 3. Outstanding questions.
What mutations and genes act as virulence determinants in MYXV and RHDV? Do they support the hypothesis that there are more possible virulence determinants in MYXV?
What host genes confer resistance to MYXV and RHDV?
Will RHDV virulence continue to increase?
What aspects of virulence evolution following a species jump are predictable? How is this mediated by local differences in ecology?
What is the host range of mammalian caliciviruses and were hosts other than rabbits involved in the emergence of RHDV?
What host and virus factors restrict host range in MYXV and RHDV?
It will also be important to reveal the viral genetic basis to these emergence events. As noted above, a simple prediction is that a limited number of similar mutations will facilitate host adaptation, including virulence evolution, in the case of RNA viruses such as RHDV because highly constrained genomes mean that there are few viable adaptive pathways. In contrast, the large genome sizes of double-stranded DNA (dsDNA) viruses such as MYXV may provide opportunities for multiple mutations to control host range, although what these mutations are remains uncertain in both cases.
Concluding remarks
Emerging viruses are the subject of intensive research activity. Indeed, the deliberate release of both MYXV and RHDV shows how rapidly a virus can spread and establish itself across an entire continent, and with remarkable host mortality. From an evolutionary perspective these biocontrol agents provide fascinating and informative insights into the trajectory and determinants of virulence evolution, the dynamics of viral evolution in a naïve host population, and patterns and determinants of cross-species transmission. General conclusions that can be drawn from these grand natural experiments are that (i) there is a strong selection for increased transmissibility, but this does not always include viral attenuation (or high virulence) and evidence for an evolutionary trade-off between virulence and transmissibility is only seen in MYXV, (ii) there has been a parallel evolution of virulence in Australia and Europe despite very different ecological settings, and (iii) host-jumps to non-target species are not an inevitable consequence of viral evolution. Although the use of viruses as biocontrol agents will always be controversial, both MYXV and RHDV have undoubtedly been of enormous benefit to Australia’s ecosystem and agricultural industries, reducing the rabbit population to approximately 10% of its initial size. It is also likely that a better understanding of the evolution of these viruses will help predict the course and outcomes of future emergence events.
Highlights.
Virus biocontrol agents provide unique insights into disease emergence and evolution.
MYXV and RHDV have experienced different trajectories of virulence evolution.
Host resistance appeared to both MYXV and RHDV, reducing the impact of biocontrol.
There has been no inadvertent jumping of MYXV and RHDV to non-target host species.
Acknowledgments
FDG is support by the Swiss National Science Foundation grant number P2ZHP3_151594. ECH is supported by an NHMRC Australia Fellowship, ARC grant DP140103362, and NIH grant R01 AI093804-01A1. We thank Peter Kerr and Tanja Strive for valuable comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morens DM, et al. Emerging infections: a perpetual challenge. Lancet Infect Dis. 2008;8:710–719. doi: 10.1016/S1473-3099(08)70256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parrish CR, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev. 2008;72:457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes EC. What can we predict about viral evolution and emergence? Curr Opin Virol. 2013;3:180–184. doi: 10.1016/j.coviro.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox T, et al. PestSmart Toolkit publication. Invasive Animals Cooperative Research Centre; Canberra, Australia: 2013. Benefits of Rabbit Biocontrol in Australia. [Google Scholar]
- 5.van Rensburg PJJ, et al. Effects of feline panleucopaenia on the population characteristics of feral cats on marion island. J Appl Ecol. 1987;24:63–73. [Google Scholar]
- 6.Bester MN, et al. A review of the successful eradication of feral cats from sub-Antarctic Marion Island, Southern Indian Ocean. S Afr J Wildl Res. 2002;32:65–73. [Google Scholar]
- 7.Nettles VF, et al. A survey of wild swine in the United States for evidence of hog cholera. J Wildl Dis. 1989;25:61–65. doi: 10.7589/0090-3558-25.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Kaden V, et al. Classical swine fever (CSF) in wild boar: the role of the transplacental infection in the perpetuation of CSF. J Vet Med B Infect Dis Vet Public Health. 2005;52:161–164. doi: 10.1111/j.1439-0450.2005.00838.x. [DOI] [PubMed] [Google Scholar]
- 9.Panizzi AR. History and contemporary perspectives of the integrated pest management of soybean in Brazil. Neotrop Entomol. 2013;42:119–127. doi: 10.1007/s13744-013-0111-y. [DOI] [PubMed] [Google Scholar]
- 10.Inceoglu AB, et al. Recombinant baculoviruses for insect control. Pest Manag Sci. 2001;57:981–987. doi: 10.1002/ps.393. [DOI] [PubMed] [Google Scholar]
- 11.Moscardi F. Assessment of the application of baculoviruses for control of Lepidoptera. Annu Rev Entomol. 1999;44:257–289. doi: 10.1146/annurev.ento.44.1.257. [DOI] [PubMed] [Google Scholar]
- 12.Elsworth P, et al. Increased virulence of rabbit haemorrhagic disease virus associated with genetic resistance in wild Australian rabbits (Oryctolagus cuniculus) Virology. 2014;464–465:415–423. doi: 10.1016/j.virol.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beutler B, et al. Genetic analysis of resistance to viral infection. Nat Rev Immunol. 2007;7:753–766. doi: 10.1038/nri2174. [DOI] [PubMed] [Google Scholar]
- 14.Fenner F, Woodroofe GM. Changes in the virulence and antigenic structure of strains of myxoma virus recovered from australian wild rabbits between 1950 and 1964. Aust J Exp Bidl nicd Sei. 1965;43:359–370. doi: 10.1038/icb.1965.69. [DOI] [PubMed] [Google Scholar]
- 15.Kerr PJ. Myxomatosis in Australia and Europe: a model for emerging infectious diseases. Antiviral Res. 2012;93:387–415. doi: 10.1016/j.antiviral.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Mead-Briggs AR, Vaughan JA. The differential transmissibility of myxoma virus strains of differing virulence grades by the rabbit flea Spilopsyllus cuniculi (Dale) J Hyg (Lond) 1975;75:237–247. doi: 10.1017/s0022172400047276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alizon S, van Baalen M. Emergence of a convex trade-off between transmission and virulence. Am Nat. 2005;165:E155–167. doi: 10.1086/430053. [DOI] [PubMed] [Google Scholar]
- 18.Kerr PJ, et al. Evolutionary history and attenuation of myxoma virus on two continents. PLoS Pathog. 2012;8:e1002950. doi: 10.1371/journal.ppat.1002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerr PJ, et al. Genome scale evolution of myxoma virus reveals host-pathogen adaptation and rapid geographic spread. J Virol. 2013;87:12900–12915. doi: 10.1128/JVI.02060-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrester NL, et al. Unravelling the paradox of rabbit haemorrhagic disease virus emergence, using phylogenetic analysis; possible implications for rabbit conservation strategies. Biol Conserv. 2006;131:296–306. [Google Scholar]
- 21.Kerr PJ, et al. Origin and phylodynamics of rabbit hemorrhagic disease virus. J Virol. 2009;83:12129–12138. doi: 10.1128/JVI.01523-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gall-Recule G, et al. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet Rec. 2011;168:137–138. doi: 10.1136/vr.d697. [DOI] [PubMed] [Google Scholar]
- 23.Bergin IL, et al. Novel calicivirus identified in rabbits, Michigan, USA. Emerg Infect Dis. 2009;15:1955–1962. doi: 10.3201/eid1512.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutze G, et al. The initial impact of rabbit hemorrhagic disease on European rabbit populations in South Australia. J Wildl Dis. 1998;34:221–227. doi: 10.7589/0090-3558-34.2.221. [DOI] [PubMed] [Google Scholar]
- 25.Cooke BD, Fenner F. Rabbit haemorrhagic disease and the biological control of wild rabbits, Oryctolagus cuniculus, in Australia and New Zealand. Wildl Res. 2002;29:689–706. [Google Scholar]
- 26.Cooke BD. Australia’s War Against Rabbits: The Story of Rabbit Haemorrhagic Disease. CSIRO Publishing; 2014. [Google Scholar]
- 27.Henzell RP, et al. Factors affecting the survival of Australian wild rabbits exposed to rabbit haemorrhagic disease. Wildl Res. 2002;29:523–542. [Google Scholar]
- 28.Jahnke M, et al. Evolution and phylogeography of the nonpathogenic calicivirus RCV-A1 in wild rabbits in Australia. J Virol. 2010;84:12397–12404. doi: 10.1128/JVI.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strive T, et al. The non-pathogenic Australian rabbit calicivirus RCV-A1 provides temporal and partial cross protection to lethal rabbit haemorrhagic disease virus infection which is not dependent on antibody titres. Vet Res. 2013;44:51. doi: 10.1186/1297-9716-44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrantes J, et al. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Vet Res. 2012;43:12. doi: 10.1186/1297-9716-43-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mutze GJ, et al. Is increased juvenile infection the key to recovery of wild rabbit populations from the impact of rabbit haemorrhagic disease? Eur J Wildlife Res. 2014;60:489–499. [Google Scholar]
- 32.Kerr PJ, et al. Molecular and serological analysis of the epidemiology of myxoma virus in rabbits. Vet Microbiol. 2010;143:167–178. doi: 10.1016/j.vetmic.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Morales M, et al. Genome comparison of a nonpathogenic myxoma virus field strain with its ancestor, the virulent Lausanne strain. J Virol. 2009;83:2397–2403. doi: 10.1128/JVI.02189-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saint KM, et al. Genetic variation in Australian isolates of myxoma virus: an evolutionary and epidemiological study. Arch Virol. 2001;146:1105–1123. doi: 10.1007/s007050170109. [DOI] [PubMed] [Google Scholar]
- 35.Muller A, et al. Partial sequencing of recent Portuguese myxoma virus field isolates exhibits a high degree of genetic stability. Vet Microbiol. 2010;140:161–166. doi: 10.1016/j.vetmic.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Cameron C, et al. The complete DNA sequence of myxoma virus. Virology. 1999;264:298–318. doi: 10.1006/viro.1999.0001. [DOI] [PubMed] [Google Scholar]
- 37.Holmes EC. Error thresholds and the constraints to RNA virus evolution. Trends Microbiol. 2003;11:543–546. doi: 10.1016/j.tim.2003.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmes EC. The evolutionary genetics of emerging viruses. Annu Rev Ecol Evol Syst. 2009;40:353–372. [Google Scholar]
- 39.Weaver SC, et al. Venezuelan equine encephalitis. Annu Rev Entomol. 2004;49:141–174. doi: 10.1146/annurev.ento.49.061802.123422. [DOI] [PubMed] [Google Scholar]
- 40.Weaver SC, et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group Lancet. 1996;348:436–440. doi: 10.1016/s0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]
- 41.Anishchenko M, et al. Venezuelan encephalitis emergence mediated by a phylogenetically predicted viral mutation. Proc Natl Acad Sci USA. 2006;103:4994–4999. doi: 10.1073/pnas.0509961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brault AC, et al. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat Genet. 2007;39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinnear M, Linde CC. Capsid gene divergence in rabbit hemorrhagic disease virus. J Gen Virol. 2010;91:174–181. doi: 10.1099/vir.0.014076-0. [DOI] [PubMed] [Google Scholar]
- 44.Esteves PJ, et al. Detection of positive selection in the major capsid protein VP60 of the rabbit haemorrhagic disease virus (RHDV) Virus Res. 2008;137:253–256. doi: 10.1016/j.virusres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Kovaliski J, et al. Molecular epidemiology of rabbit haemorrhagic disease virus in Australia: when one became many. Mol Ecol. 2014;23:408–420. doi: 10.1111/mec.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forrester NL, et al. Recombination in rabbit haemorrhagic disease virus: possible impact on evolution and epidemiology. Virology. 2008;376:390–396. doi: 10.1016/j.virol.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Capucci L, et al. A further step in the evolution of rabbit hemorrhagic disease virus: the appearance of the first consistent antigenic variant. Virus Res. 1998;58:115–126. doi: 10.1016/s0168-1702(98)00106-3. [DOI] [PubMed] [Google Scholar]
- 48.Abrantes J, et al. Detection of RHDVa on the Iberian Peninsula: isolation of an RHDVa strain from a Spanish rabbitry. Arch Virol. 2014;159:321–326. doi: 10.1007/s00705-013-1808-2. [DOI] [PubMed] [Google Scholar]
- 49.Grazioli S, et al. Rabbit haemorrhagic disease virus (RHDV) subtype “A” (RHDVa) is replacing the original strain in some Italian regions. In: Brocchi E, Lavazza A, editors. Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna.Veterinary virology in the new Millennium: Fifth International Congress of Veterinary Virology; Brescia, Italy. 2000. pp. 27–30. [Google Scholar]
- 50.McIntosh MT, et al. A pandemic strain of calicivirus threatens rabbit industries in the Americas. Virol J. 2007;4:96. doi: 10.1186/1743-422X-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalton KP, et al. Spread of new variant RHDV in domestic rabbits on the Iberian Peninsula. Vet Microbiol. 2014;169:67–73. doi: 10.1016/j.vetmic.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 52.Dalton KP, et al. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg Infect Dis. 2012;18:2009–2012. doi: 10.3201/eid1812.120341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berman D, et al. Should the 40-year-old practice of releasing virulent myxoma virus to control rabbits (Oryctolagus cuniculus) be continued? Wildl Res. 2006;33:549. [Google Scholar]
- 54.Marshall ID, Fenner F. Studies in the epidemiology of infectious myxomatosis of rabbits. V. Changes in the innate resistance of Australian wild rabbits exposed to myxomatosis. J Hyg (Lond) 1958;56:288–302. doi: 10.1017/s0022172400037773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall ID, Douglas GW. Studies in the epidemiology of infectious myxomatosis of rabbits. VIII. Further observations on changes in the innate resistance of Australian wild rabbits exposed to myxomatosis. J Hyg (Lond) 1961;59:117–122. doi: 10.1017/s0022172400038766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Best SM, Kerr PJ. Coevolution of host and virus: the pathogenesis of virulent and attenuated strains of myxoma virus in resistant and susceptible European rabbits. Virology. 2000;267:36–48. doi: 10.1006/viro.1999.0104. [DOI] [PubMed] [Google Scholar]
- 57.Best SM, et al. Coevolution of host and virus: cellular localization of virus in myxoma virus infection of resistant and susceptible European rabbits. Virology. 2000;277:76–91. doi: 10.1006/viro.2000.0505. [DOI] [PubMed] [Google Scholar]
- 58.Ross J, Sanders MF. Innate resistance to myxomatosis in wild rabbits in England. J Hyg (Lond) 1977;79:411–415. doi: 10.1017/s0022172400053262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Pendu J, et al. Mendelian resistance to human norovirus infections. Semin Immunol. 2006;18:375–386. doi: 10.1016/j.smim.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruvoen-Clouet N, et al. Binding of rabbit hemorrhagic disease virus to antigens of the ABH histo-blood group family. J Virol. 2000;74:11950–11954. doi: 10.1128/jvi.74.24.11950-11954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nystrom K, et al. Histo-blood group antigens act as attachment factors of rabbit hemorrhagic disease virus infection in a virus strain-dependent manner. PLoS Pathog. 2011;7:e1002188. doi: 10.1371/journal.ppat.1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson AJ, et al. Statistical models for the effect of age and maternal antibodies on the development of rabbit haemorrhagic disease in Australian wild rabbits. Wildl Res. 2002;29:663–671. [Google Scholar]
- 63.Marques RM, et al. Immunosuppression abrogates resistance of young rabbits to Rabbit Haemorrhagic Disease (RHD) Vet Res. 2014;45:14. doi: 10.1186/1297-9716-45-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elsworth PG, et al. Rabbit haemorrhagic disease: are Australian rabbits (Oryctolagus cuniculus) evolving resistance to infection with Czech CAPM 351 RHDV? Epidemiol Infect. 2012;140:1972–1981. doi: 10.1017/S0950268811002743. [DOI] [PubMed] [Google Scholar]
- 65.Cooke BD, et al. Rabbit haemorrhagic disease: does a pre-existing RHDV-like virus reduce the effectiveness of RHD as a biological control in Australia? Wildl Res. 2002;29:673–682. [Google Scholar]
- 66.Parkes JP, et al. Epidemiology of rabbit haemorrhagic disease (RHD) in the South Island, New Zealand, 1997–2001. Wildl Res. 2002;29:543–555. [Google Scholar]
- 67.Cooke BD. Notes on the comparative reproductive biology and the laboratory breeding of the rabbit flea Xenopsylla cunicularis smit (Siphonaptera, Pulicidae) Aust J Zool. 1990;38:527–534. [Google Scholar]
- 68.Ratcliffe FN, et al. Myxomatosis in Australia; a step towards the biological control of the rabbit. Nature. 1952;170:7–11. doi: 10.1038/170007a0. [DOI] [PubMed] [Google Scholar]
- 69.Merchan T, et al. Detection of rabbit haemorrhagic disease virus (RHDV) in nonspecific vertebrate hosts sympatric to the European wild rabbit (Oryctolagus cuniculus) Infect Genet Evol. 2011;11:1469–1474. doi: 10.1016/j.meegid.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Kitchen A, et al. Family level phylogenies reveal modes of macroevolution in RNA viruses. Proc Natl Acad Sci USA. 2011;108:238–243. doi: 10.1073/pnas.1011090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allison AB, et al. Frequent cross-species transmission of parvoviruses among diverse carnivore hosts. J Virol. 2013;87:2342–2347. doi: 10.1128/JVI.02428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kerr PJ, et al. Comparative analysis of the complete genome sequence of the California MSW strain of myxoma virus reveals potential host adaptations. J Virol. 2013;87:12080–12089. doi: 10.1128/JVI.01923-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fenner F, Fantini B. Biological Control of Vertebrate Pests: The History of Myxomatosis, An Experiment in Evolution. CABI Publishing; 1999. [Google Scholar]
- 74.Lombardi L, et al. Habitat-related differences in rabbit (Oryctolagus cuniculus) abundance, distribution, and activity. J Mammal. 2003;84:26–36. [Google Scholar]
- 75.Kritas SK, et al. A pathogenic myxoma virus in vaccinated and non-vaccinated commercial rabbits. Res Vet Sci. 2008;85:622–624. doi: 10.1016/j.rvsc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Meyers G, et al. Rabbit hemorrhagic disease virus-molecular cloning and nucleotide sequencing of a calicivirus genome. Virology. 1991;184:664–676. doi: 10.1016/0042-6822(91)90436-f. [DOI] [PubMed] [Google Scholar]
- 77.Liu SJ, et al. A new viral disease in rabbit. Anim Husb Vet Met. 1984;16:253–255. [Google Scholar]
- 78.Cancellotti FM, Renzi M. Epidemiology and current situation of viral haemorrhagic disease of rabbits and the European brown hare syndrome in Italy. Rev Sci Tech. 1991;10:409–422. doi: 10.20506/rst.10.2.558. [DOI] [PubMed] [Google Scholar]
- 79.Villafuerte R, et al. Incidence of viral hemorrhagic disease in wild rabbit populations in Spain. Mammalia. 1995;59:651–659. [Google Scholar]
- 80.Asgari S, et al. Field evidence for mechanical transmission of rabbit haemorrhagic disease virus (RHDV) by flies (Diptera:Calliphoridae) among wild rabbits in Australia. Virus Res. 1998;54:123–132. doi: 10.1016/s0168-1702(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 81.Drummond AJ, et al. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]