Abstract
Background
A large animal model with a continuous expanding infrarenal aortic aneurysm gives access to a more realistic AAA model with anatomy and physiology similar to humans, and thus allows for new experimental research in the natural history and treatment options of the disease.
Methods
10 pigs (group A) underwent infrarenal aortic dissection, balloon dilatation, infusion of elastase into the lumen and placement of a stenosing cuff around the aorta. 10 control pigs (group B) underwent a sham procedure. The subsequent 28 days the AP-diameters of the aneurysms were measured using ultrasound, hereafter the pigs were euthanized for inspection and AAA wall sampling for histological analysis.
Results
In group A, all pigs developed continuous expanding AAA's with a mean increase in AP-diameter to 16.26 ± 0.93 mm equivalent to a 57% increase. In group B the AP-diameters increased to 11.33 ± 0.13 mm equivalent to 9.3% which was significantly less than in group A (p < 0.001). In group A, a significant negative association between the preoperative weight and the resulting AP-diameters was found. Histology shoved more or less complete resolution of the elastic tissue in the tunica media in group A. The most frequent complication was a neurological deficit in the lower limbs.
Conclusion
In pigs it's possible to induce continuous expanding AAA's based upon proteolytic degradation and pathological flow, resembling the real life dynamics of human aneurysms. Because the lumbars are preserved, it's also a potential model for further studies of novel endovascular devices and their complications.
Keywords: Abdominal aortic aneurysm, Balloon dilatation, Elastase, Porcine model, Endoleaks
Highlights
-
•
A large porcine animal model of AAA disease that mimics human aneurysm pathology.
-
•
The first large AAA animal model to demonstrate a continuous AAA expansion over time.
-
•
A potential model for further research into the natural history and prognosis of AAA's.
-
•
Due to preserved lumbars a potential model for further EVAR/Endoleak research.
1. Introduction
Abdominal aortic aneurysms (AAA) in human adults defined present when the infrarenal aortic diameter exceeds 3.0 cm, are most often asymptomatic but highly lethal with an overall mortality around 80–90% when rupture occurs [1]. The disease is a major health problem, as it affects 5–9% of the male population over the age of 65 years [2].
Today surgical or endovascular repair are the only treatments. Consequently, many animal models have been developed to study the natural history and treatment options of AAA's in vivo.
When Ponseti IV et al., in 1952 studied the disease Epidemic lathyrism by putting white rats on a diet containing 50% of sweet peas (Lathyrus odoratus), whose seeds contain β-aminopropionitrile that prevents the cross-linking of collagen, he accidentally in 6 of 8 rats induced thoracic aortic medial necrosis with aneurysm formation and dissection, and thus came across the first animal model with aneurysm disease [3]. Since then a broad spectrum of techniques has been used in attempts to develop arterial/aortic aneurysm disease in various animal models. These models can generally be divided into three main categories [4], genetically predisposed animals like the blotchy mouse [5], early used physical models characterized by physical destruction of the vessel integrity for example by surgical resection of the aortic media and adventitia [6], or by mechanically inducing a crushing injury to the aortic wall [7] and finally chemically induced models with extra- and/or intraluminal application of calcium chloride (CaCl2) [8] and/or elastase [9] or by continuous subcutaneous infusion of angiotensin-II in Apo −/− mice [10]. Since it is known that increased elastolytic activity in the media plays a key role in the initial pathophysiology in human aneurysms [11], the use of elastase based models seems most proper when studying the natural history of the disease. However, small animal models don't allow experiments to sophisticate endovascular treatment or develop novel surgical treatment possibilities. Attempts in larger animals have been performed, but so far none that mimics the natural history including initial elastin fragmentation and proteolytic degradation, have been able to demonstrate a continuous progressive AAA expansion over time [12–14], which is a key characteristic of the disease, since it's a dynamic condition with changes in properties over time. Consequently, the aim of this study was to develop a reliable, large AAA animal model with a satisfactory and consistent AAA formation, that shows no sign of halting in the progressive expansion over time, mimicking the real life dynamics of human aneurysm disease, which makes it a potential model for further research into the natural history, prognosis and treatment options of the disease.
2. Materials and methods
20 female Danish Landrace pigs were divided into either intervention group A (n = 10, mean weight 34 kg (range 31–38 kg)) or control group B (n = 10, mean weight 34 kg (range 30–38 kg)).
2.1. Anesthesia and surgical procedure
Anesthesia was induced by intramuscular injection (mg/kg BW) of 1.25 mg tiletaminchdrochlorid, 1.25 mg zolazepamhydrochlorid, 0.25 mg butorphanoltartrat, 1.25 mg ketaminhydroclorid and 1.25 mg xylazin. After tracheal intubation, the pigs were placed in the supine position and ventilated with oxygen 4 L/min and atm. air (1:1, v/v) and anesthesia was extended by continuous intravenous infusion of 10 mg propofol and 25 μg fentanyl per kg BW/h.
A transabdominal ultrasound scan of the infrarenal aorta in the systolic state was performed in both the transverse and longitudinal plane to measure the preoperative external anterior-posterior diameter (AP0). After 1500 mg of intravenous cefuroxime a midline longitudinal laparotomy was made and a retrocolic prerenal transperitoneal approach to the infrarenal aorta was performed. The aorta was dissected from the lowest renal artery to the trifurcation (Fig. 1A). After intravenous administration of 5000 IU of unfractionated heparin sulfate, the lumbars and the inferior mesenteric artery and the aorta itself were temporarily clamped. The sham group B had the clamps removed after 30 min. In group A a 2.5 mm arteriotomy in the aorta was made at the level of the inferior mesenteric artery through which a 10 mm × 4 cm high-pressure balloon catheter (Johnson & Johnson – Cordis PowerFlex P3) was placed and inflated for 5 min (Fig. 1B). Hereafter a curved beaded knop needle was introduced through which 10 ml of porcine pancreatic elastase (Sigma–Aldrich Denmark A/S, E1250-100MG, Type-I ≥4.0 units/mg protein) was gradually manually infused into the aortic lumen over 30 min. Then a 12 mm × 4 cm high-pressure balloon catheter (Johnson & Johnson – Cordis PowerFlex P3) was introduced and inflated for 5 min where after the arteriotomy was sutured and the aorta declamped. To establish turbulent flow in the infrarenal aortic segment a stenosing nylon cable-tie strap (120 mm × 5 mm) was placed as a cuff around the aorta just below the renal artery and narrowed until a thrill indicating turbulent flow in the infrarenal aortic segment could be palpated and postoperatively be visualized by Doppler sonography (Fig. 1C and D). Finally the retroperitoneum and laparotomy were closed.
Fig. 1.
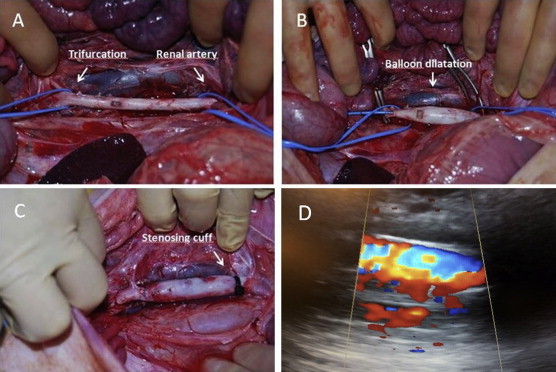
Induction of infrarenal AAA. A Surgical isolation of the infrarenal aorta. B Proximal and distal clamping and temporary clamping of the side branches. Endovascular balloon dilatation to 10 mm with 10 atm. for 5 min. Infusion of 10 ml of porcine elastase for 30 min. Endovascular balloon dilatation to 12 mm with 6 atm. for 5 min. C Placement of an infrarenal stenosing plastic cuff to stimulate turbulent flow. (Notice the early intraoperative AAA formation after the procedure). D Postoperative Doppler sonography showing turbulent flow in the infrarenal dilated segment.
2.2. Postoperative care
5000 IU of unfractionated heparin sulfate was given subcutaneously each day to reduce the risk of thromboembolic events. On the 3rd 7th 14th 21st and 28th postoperative days the pigs were anaesthetized and the AP-diameter of the infrarenal aorta was again measured using transabdominal ultrasound. Hereafter the pigs were euthanized with a lethal overdose of intravenous phenobarbital and the infrarenal aortic segment was removed and fixed in 10% buffered formalin.
2.3. Histology
The specimens were sliced and stained with routine protocols for human tissue for ×100 and ×400 microscopy with hematoxylin and eosin stain, Verhoeff's stain for elastin and immunohistochemical staining for human smooth muscle desmin (that was found to react satisfactory with tissue from pigs). Desmin staining was done in a Ventana autostainer using antibody clone D33 from DAKO.
2.4. Statistics
A sample size calculation with 80% power and 5% significance revealed that at least 7 pigs were required in each group to detect a difference in means of at least 15% with a standard deviation of 10% after 28 days.
To compare the mean aortic AP-diameter, at the start of the experiment and the fixed time points, the independent samples t-test and two-way repeated measurement ANOVA was used as appropriate for comparison between groups. Results were presented as means ± SD and with 95% confidence intervals P-values <0.05 were considered significant. To evaluate the association between the preoperative weight and the resulting relative aortic expansions in group A the Spearman's rank correlation coefficient/Spearman's rho was calculated and P-values <0.05 were considered significant. The software used was IBM SPSS Statistics 21.0 (SPSS Inc. Chicago, IL, USA).
3. Results
3.1. Macroscopic
After 28 days all 10 pigs in group A developed macroscopically AAA's with a mean increase in AP-diameter to 16.26 ± 0.93 mm equivalent to an increase of 57% ± 10.17 SD (Range 31%). In control group B there were no signs of developing aneurysms as mean AP-diameter increased to 11.33 ± 0.13 mm equivalent to an increase of 9.3% ± 1.37 SD (Range 3.5%) in accordance with normal physiological growth. The Two-way repeated measurement ANOVA revealed a significant difference between group A and B. (p < 0.001) (Fig. 2).
Fig. 2.
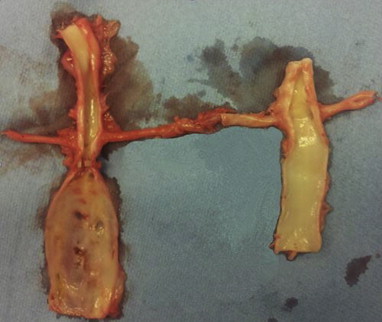
Notice the significantly lager infrarenal AP-diameter (AP28) in group A (left side) 28 days after surgery compared to the control group B (right side).
Already intraoperatively after the traumatizing balloon dilatation and elastase treatment the aortic segments seemed significantly enlarged. During the subsequent 28 days the infrarenal AP-diameters in this group kept expanding at a higher velocity compared to control group B with no sign of halting in the progressive expansion (Fig. 3). Already on the third postoperative day the difference in mean AP-diameters between the two groups was highly significant with a P-value <0.001 and this could be observed throughout the experiment (Table 1.).
Fig. 3.
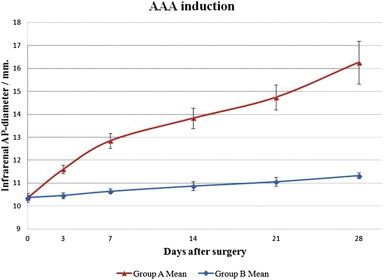
In intervention group A a progressive aneurysmatic expansion of the infrarenal aortas could be seen during the 28 postoperative days. In this observation period the infrarenal aortas in control group B showed only physiological expansion/growth.
Table 1.
Schematic representation of the comparison between group A and B.
| Group A / Group B | Day 0 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | |
|---|---|---|---|---|---|---|---|
| Mean weight, Kg | |||||||
| Group A | 33.8 ± 2.44 | 34.3 ± 2.66 | 36.2 ± 1.99 | 40.7 ± 2.41 | 45.9 ± 2.69 | 50.4 ± 3.37 | |
| Group B | 34.0 ± 2.58 | 34.4 ± 3.31 | 37.8 ± 2.10 | 42.2 ± 1.76 | 46.1 ± 3.05 | 51.1 ± 2.46 | |
| P value | 0.86 | 0.95 | 0.10 | 0.13 | 0.90 | 0.59 | |
| Mean AP diameter ± SD, mm | |||||||
| Group A | 10.36 ± 0.11 | 11.6 ± 0.18 | 12.85 ± 0.32 | 13.83 ± 0.44 | 14.74 ± 0.54 | 16.26 ± 0.93 | |
| Range A | 0.3 | 0.6 | 0.9 | 1.2 | 1.6 | 2.9 | |
| Group B | 10.37 ± 0.19 | 10.46 ± 0.13 | 10.64 ± 0.11 | 10.87 ± 0.19 | 11.06 ± 0.2 | 11.33 ± 0.13 | |
| Range B | 0.6 | 0.3 | 0.3 | 0.5 | 0.6 | 0.4 | |
| P value | 0.89 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Mean increase ± SD, %a | |||||||
| Group A | 11.98 ± 2.62 | 24.04 ± 3.67 | 3.52 ± 5.19 | 42.32 ± 6.31 | 57.03 ± 10.17 | ||
| Range A | 8.1 | 10.1 | 14.6 | 18.2 | 31.0 | ||
| Group B | 0.89 ± 1.59 | 2.63 ± 1.91 | 4.85 ± 1.85 | 6.69 ± 2.14 | 9.26 ± 1.37 | ||
| Range B | 5.8 | 6.9 | 4.9 | 5.9 | 3.5 | ||
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
Mean increase ± SD, % with respect to the preoperative/Day 0 aortic AP-diameter.
Preoperative weight influenced the final outcome in intervention group A, as lower preoperative weights resulted in larger AP-diameters after 28 days. The Spearman's rho for the association between preoperative weight and the resulting relative aortic expansion was found to be ρ = −0.951 with a P-value <0.001 suggesting a highly significant negative association (Fig. 4).
Fig. 4.
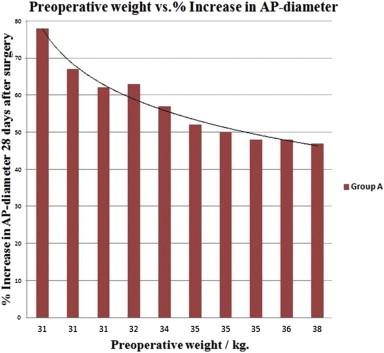
Lower preoperative weights resulted in significantly greater aortic expansions in the intervention group A.
3.2. Microscopic
Using Verhoeff's Stain for elastic tissue (Fig. 5A–D) microscopy of the removed aortic segments in group A showed a preserved endothelium and as expected, more or less complete resolution of the elastic tissue in the tunica media, suggesting elastolytic breakdown initiated by the infused elastase. In areas with partially preserved elastic lamellae (all localized in the anti-luminal side of the tunica media) the fibers seemed disorganized, thinned and fragmented, suggesting early fracture-disruption of the lamellar architecture caused by the wall stress during balloon dilatation.
Fig. 5.
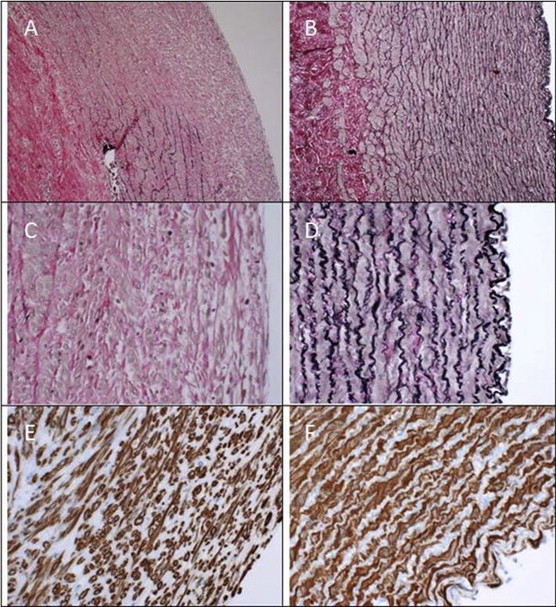
Histological findings in group A and B listed in the left and right column respectively with the luminal side to the right. A–D: Verhoeff's Stain for elastic tissue enlarged ×100 and ×400. E–F: Immunoperoxidase staining for smooth muscle actin enlarged ×400. Notice the widespread resolution of the medial elastic lamellae and that the fibers in areas with residual elastic tissue are stretched and thinned. Smooth muscle cells are disorganized and appear atrophic especially towards the luminal side of the media.
Immunoperoxidase staining for smooth muscle actin (Fig. 5E and F) revealed light to moderate muscle atrophy in the tunica media.
The tunica adventitia appeared largely unaffected.
3.3. Complications and adverse outcomes
The overall success rate regarding group A in our series was 58.8% (10/17) as various complications were initially encountered and 7 pigs had to be euthanized prematurely to attain the 10 pigs in group A. The most frequent complication was a neurological deficit in the lower limbs without ischemia ranging from a mild palsy to severe paralysis in four pigs. Three postoperative aortic ruptures were seen after respectively 4 h, 3 days and 7 days. On post mortem inspection all pigs showed moderate to severe fibrosis surrounding the infrarenal aorta.
4. Discussion
This study has shown that it's possible to induce AAA's in pigs weighing 31–38 kg, with a mean increase in AP-diameter of 57% ± 10,17 SD after 28 days. The model seems relatively reliable and consistent in its aneurysm development. Besides being a model which, due to the elastase induced elastolytic degradation, mimics the initial events in human aneurysm pathophysiology, it's also unique compared to other models because it's in fact the first large animal model of AAA disease to demonstrate a continuous progressive AAA expansion over time, resembling the dynamic properties of real life aneurysm disease. This makes it a potential model for further research into the natural history, prognosis and treatment options of the AAA disease. Because earlier published papers describing similar models all have failed in producing continuous expanding AAA's [12–14], we believe that the stenosing cuff producing turbulence may be key, as it continuously generates wall stress to the damaged endothelium surpassing its regenerative repair potential. Whether this process continues beyond the period of observation remains to be examined, but we saw a tendency towards increasing expansion rates towards the end of the observation period.
Because of the fact that the lumbars are preserved also makes it a potential model to study novel endovascular devices and especially type-I and -II endoleaks.
However, to make this model directly applicable to clinical trials investigating endovascular devices and their complications even larger animals may be required. One approach to this would be to prolong the follow-up time until the animals reach adult size around 70 kg, although this may imply a risk of preterm rupture. Another and perhaps more feasible approach would be to induce aneurysms in even larger pigs around 50–70 kg. Unfortunately this study showed, that when using the described procedure, there is a significant negative association between the preoperative weight and the resulting relative aortic expansion after 28 days. This could be explained by the fact that the aortic walls in the larger animals are thicker and therefore less permeable to the elastase solution and that the preoperative aortic diameters (AP0) are wider and therefore less traumatized by the balloon dilatations.
In our initial studies during the evolution of the model, we tried to induce AAA in pigs weighing around 50–55 kg by infusing elastase and placing a stenosing cuff around aorta as described by Moláček J et al. [15], but even in combination with balloon dilatation to mechanically induce a crushing injury to the aortic wall resulting in fracture-disruption and ablation of the medial lamellar architecture as described by Zatina MA et al. [7] this approach yielded no continuous expanding AAA's. First by gradually downgrading the pigs' weight we began to see results.
If future studies will try to induce AAA's in even larger/heavier animals, one might expect that the AAA inducing procedure has to be modified according to the weight range of interest, e.g. a larger volume and more concentrated elastase solution given over a prolonged period of time. Possibly equally important is the possibility of adding further proteolytic substances that exerts an additional pathophysiologic reactive breakdown of the aortic wall. A. Czerski et al. [14] recently demonstrated that by supplementing the elastase with intraluminal collagenase infusion and an external swab soaked in calcium chloride radically improves the immediate aneurysm formation, even though this regime alone was not enough to create continuous expanding AAA's in pigs in the 20–30 kg weight range.
To minimize the risk of complications the procedure has to be carefully implemented. The overall success rate of 58.8% (10/17) regarding group A in our study could desirably be higher. The 7 pigs that had to be euthanized prematurely due to complications were all operated upon during the initial phase of the study. However, as we gained experience in the procedure, we managed to reduce the risks of complications considerably and in the last 6 pigs in group A we saw no major complications. We decided to include the seven pigs in this paper, as we wish to point out the importance that the procedure has to be carried out accurately and safely and that a learning curve may be required to achieve this.
An ischemic spinal cord lesion due to the aortic clamping may explain the neurological deficit in the lower limbs being the most frequent complication. Experiments on dogs investigating the spinal cords critical ischemia time, found that neurological deficits caused by spinal ischemia for up to 3 h are largely reversible [16]. As the average aortic clamping time for group A in our study was around 50 min, the lower limb paralysis may be reversible, and this survey may be the objective of future studies.
Another disadvantage of the described procedure is the necessity of a laparotomy and the resulting retroperitoneal fibrosis. Recently Lederman et al. [17] introduced a totally endovascular approach of chemically induced AAA creation avoiding these issues. Unfortunately, in this model the extension of the aneurysm is limited by the distance of adjacent lumbars, making it challenging when trying to create aneurysms with short or wide neck anatomy in order to study e.g. endoleaks type-1, as a lumbar is often seen about 0.5–1 cm distal to the renal arteries. A totally endovascular approach also makes it impossible to place an external stenosing cuff, which we believe is essential.
A possible solution to this problem could be a compromise with a combined procedure where the aneurysm is induced endovascularly with an assisting laparoscopic or maybe even a SILS (Single Incision Laparoscopic Surgery) procedure. The latter in order to dissect the aortic neck and place the cuff and temporarily clamp the proximal lumbar.
The perfect large AAA animal model has yet to be seen, but we believe that a combination of different techniques may be the next step in this quest.
Conflict of interest statement
The authors declare no conflicts of interest.
Fundings
Health Research Fund of Central Denmark Region. The Danish Heart Foundation, grant number 10-04-R78-A2817-22602. The funding sources had no involvement in the in study design, collection, analysis, interpretation of data or in the writing or the decision to submit this article for publication.
Ethical approval
The procedure was conducted under local project license J.nr.2010/561-1844 in conformity with the Danish legislations regarding animal welfare and experimental surgery. During the operative procedure and the 28 days observation period, the pigs were housed at the DCA – Danish Centre for Food and Agriculture, Aarhus University Foulum.
Consent
Because this study is preformed on animals there is no need/possibility for a written and signed consent.
Author contribution
Brian O. Kloster: Study design, data collection, data analysis, interpretation and writing. Jes S. Lindholt: Study design, data analysis, interpretation and writing. Lars Lund: Study design. Gorm Soendergaard: Histological analysis.
Guarantor
Brian O. Kloster, Corresponding author, Vascular Research Unit, Department of Vascular Surgery, Viborg Hospital, Heibergs Alle 4, 8800 Viborg, Denmark, E-mail: Brian.Kloster@ki.au.dk, Phone: +45 26793032.
Contributor Information
Brian O. Kloster, Email: Brian.Kloster@ki.au.dk.
Lars Lund, Email: lars.lund@rsyd.dk.
Jes S. Lindholt, Email: Jes.Sanddal.Lindholt@rsyd.dk.
References
- 1.Pearce W.H., Zarins C.K., Bacharach J.M. Atherosclerotic peripheral vascular disease symposium II: controversies in abdominal aortic aneurysm repair. Circulation. 2008;118:2860–2863. doi: 10.1161/CIRCULATIONAHA.108.191176. [DOI] [PubMed] [Google Scholar]
- 2.Thompson Robert W., Curci John A., Ennis Terri L., Mao Dongli, Pagano Monica B., Pham Christine T.N. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann NY Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- 3.Ponseti Ignacio V., Baird William A. Scoliosis and dissecting aneurysm of the aorta in rate fed with Lathyrus odoratus seeds. Am J Pathol. 1952;28:1059–1077. [PMC free article] [PubMed] [Google Scholar]
- 4.Tsui Janice C. Experimental models of abdominal aortic aneurysms. Open Cardiovasc Med J. 2010;4:221–230. doi: 10.2174/1874192401004010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews E.J., White W.J., Bullock L.P. Spontaneous aortic aneurysms in blotchy mice. Am J Pathol. 1975;78:199–210. [PMC free article] [PubMed] [Google Scholar]
- 6.White J.C., Sayre G.P., Whisnant J.P. Experimental destruction of the media for the production of intracranial arterial aneurysms. J Neurosurg. 1961;18:741–745. doi: 10.3171/jns.1961.18.6.0741. [DOI] [PubMed] [Google Scholar]
- 7.Zatina Michael A., Zarins Christopher K., Gewertz Bruce L., Glagov Seymour. Role of medial lamellar architecture in the pathogenesis of aortic aneurysms. J Vasc Surg. 1984;1:442–448. [PubMed] [Google Scholar]
- 8.Gertz S.D., Kurgan A., Eisenberg D. Aneurysm of the rabbit common carotid artery induced by periarterial application of calcium chloride in vivo. J Clin Invest. 1988;81:649–656. doi: 10.1172/JCI113368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anidjar S., L Salzmann J., Gentric D., Lagneau P., Camilleri J.P., Michel J.B. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82:973–981. doi: 10.1161/01.cir.82.3.973. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty A., Manning M.W., Cassis L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curci John A., Thompson Robert W. Adaptive cellular immunity in aortic aneurysms: cause, consequence, or context? J Clin Invest. 2004;114:168–171. doi: 10.1172/JCI22309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynecek Robert L., DeRubertis Brian G., Trocciola Susan M., Zhang Honglei, Prince Martin R., Ennis Terri L. The creation of an infrarenal aneurysm within the native abdominal aorta of swine. Surgery. 2007;142:143–149. doi: 10.1016/j.surg.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Marinov G.R.1, Marois Y., Pâris E., Roby P., Formichi M., Douville Y. Can the infusion of elastase in the abdominal aorta of the Yucatán miniature swine consistently produce experimental aneurysms? J Invest Surg. 1997;10:129–150. doi: 10.3109/08941939709032144. [DOI] [PubMed] [Google Scholar]
- 14.Czerski A., Bujok J., Gnus J., Hauzer W., Ratajczak K., Nowak M. Experimental methods of abdominal aortic aneurysm creation in swine as a large animal model. J Physiol Pharmacol. 2013 Apr;64(2):185–192. [PubMed] [Google Scholar]
- 15.Moláček Jiří, Třeška Vladislav, Kobr Jiří, Čertík Bohuslav, Skalický Tomáš, Kuntscher Vilém. Optimization of the model of abdominal aortic aneurysm – experiment in an animal model. J Vasc Res. 2009;46:1–5. doi: 10.1159/000135659. [DOI] [PubMed] [Google Scholar]
- 16.Bitar Alatorre W.E., Garcia Martinez D., Rosales Corral S.A., Flores Soto M.E., Velarde Silva G., Portilla de Buen E. Critical ischemia time in a model of spinal cord section. A study performed on dogs. Eur Spine J. 2007 Apr;16(4):563–572. doi: 10.1007/s00586-006-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederman A., Fernando T.S.N., Rimarcs F., Luis F., Otoch Jose P., Aun Ricardo. Endovascular model of abdominal aortic aneurysm induction in swine. Vasc Med. 2014 May 30;19(3):167–174. doi: 10.1177/1358863X14534006. [DOI] [PubMed] [Google Scholar]


