Abstract
While neutrophil elastase (NE), released by activated neutrophils, is a key mediator of secondary pathogenesis in adult models of brain ischemia and spinal cord injury, no studies to date have examined this protease in the context of the injured immature brain, where there is notable vulnerability resulting from inadequate antioxidant reserves and prolonged exposure to infiltrating neutrophils. We thus reasoned that NE may be a key determinant of secondary pathogenesis, and as such, adversely influence long-term neurological recovery. To address this hypothesis, wild-type (WT) and NE knockout (KO) mice were subjected to a controlled cortical impact at post-natal day 21, approximating a toddler-aged child. To determine if NE is required for neutrophil infiltration into the injured brain, and whether this protease contributes to vasogenic edema, we quantified neutrophil numbers and measured water content in the brains of each of these genotypes. While leukocyte trafficking was indistinguishable between genotypes, vasogenic edema was markedly attenuated in the NE KO. To determine if early pathogenesis is dependent on NE, indices of cell death (TUNEL and activated caspase-3) were quantified across genotypes. NE KO mice showed a reduction in these markers of cell death in the injured hippocampus, which corresponded to greater preservation of neuronal integrity as well as reduced expression of heme oxygenase-1, a marker of oxidative stress. WT mice, treated with a competitive inhibitor of NE at 2, 6 and 12 h post-injury, likewise showed a reduction in cell death and oxidative stress compared to vehicle-treated controls. We next examined the long-term behavioral and structural consequences of NE deficiency. NE KO mice showed an improvement in long-term spatial memory retention and amelioration of injury-induced hyperactivity. However, volumetric and stereological analyses found comparable tissue loss in the injured cortex and hippocampus independent of genotype. Further, WT mice treated acutely with the NE inhibitor showed no long-term behavioral or structural improvements. Together, these findings validate the central role of NE in both acute pathogenesis and chronic functional recovery, and support future exploration of the therapeutic window, taking into account the prolonged period of neutrophil trafficking into the injured immature brain.
Keywords: Traumatic brain injury, neutrophil elastase, cell death, behavior, pathology, pediatric
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of childhood morbidity worldwide (Langlois, 2000; Faul et al., 2010). The developing brain is particularly vulnerable to injury, with long-term cognitive deficits becoming apparent over time alongside brain maturation (Anderson et al., 2005; Anderson et al., 2012). These impairments include persistent cognitive deficits and hyperactivity which emerge alongside progressive atrophy of key neuroanatomical structures, features which are paralleled in our model of TBI to the immature mouse brain (Tong et al., 2002; Pullela et al., 2006).
Behavioral consequences of injury are preceded by early pathogenic events including acute neuronal cell death, which coincides with the influx of polymorphonuclear neutrophils from the peripheral circulation into the injured brain (Clark et al., 1994). Previously, we have found that neutrophil infiltration into the immature injured mouse brain is more prominent compared to the adult and protracted over an extended period of time, indicating an exacerbated inflammatory response in the young brain which may in part underlie age-dependent vulnerability to injury (Claus et al., 2010).
Lining the blood vessels and subarachnoid spaces within hours post-injury, neutrophils are typically the first innate immune cells to transmigrate across the vasculature and infiltrate the parenchyma of the injured brain, peaking in numbers by 12–48 hours (Clark et al., 1994; Holmin et al., 1995; Hausmann et al., 1999). These leukocytes then assume an activated, neurotoxic phenotype (Allen et al., 2012) and are capable of releasing a plethora of agents including myeloperoxidase, matrix metalloproteinases (MMPs), pro-inflammatory cytokines and reactive oxygen species, all of which contribute to pathogenesis secondary to the initial insult (Dinkel et al., 2004; Nguyen et al., 2007). Neutrophil depletion (Kenne et al., 2012), as well as strategies to reduce neutrophil adhesion and infiltration (Knoblach and Faden, 2002; Semple et al., 2010a; Bao et al., 2012), affords varying degrees of neuroprotection in adult models of TBI, providing evidence of a biologically significant role for neutrophils in acute brain injury.
Neutrophil elastase (NE), perhaps most studied in acute and chronic lung disease, is recognized as one of the most destructive proteolytic enzymes. At the vascular interface it mediates cellular damage and dysfunction, and when released by infiltrating neutrophils into the adjacent parenchyma, NE promotes degradation of the extracellular matrix and triggers pathways leading to cell death (Kawabata et al., 2002; Zhou et al., 2012). A chymotrypsin-like serine protease with potent catalytic activity, biologically active NE is stored in neutrophil azurophilic granules at high concentrations and released into the extracellular space upon activation (Shapiro, 2002; Korkmaz et al., 2008). In addition to elastin, NE is known to degrade almost all components of the extracellular matrix (including collagen types I–IV, proteoglycan, fibronectin and cadherins), as well as key plasma proteins such as immunoglobulin, coagulation and complement factors, and other proteases including MMP-9 (Weiss, 1989; Delclaux et al., 1996; Kawabata et al., 2002; Korkmaz et al., 2008). By cleavage to enhance or abolish the function of the cytokines interleukin-6 and -8, NE is also thought to play a role in exacerbating ongoing inflammation (Bank and Ansorge, 2001; Meyer-Hoffert and Wiedow, 2011). Further, by facilitating the conversion of xanthine dehydrogenase to xanthine oxidase, NE activity likely promotes the generation of oxidative stress (Phan et al., 1992; Aoshiba et al., 2001).
Accumulating evidence implicates neutrophil-mediated NE in pathogenesis and neurovascular dysfunction in adult models of brain ischemia and spinal cord injury. That is, specific targeting of NE by genetic deletion or pharmacological inhibition is acutely neuroprotective in rodent and rabbit models of transient focal ischemia (Shimakura et al., 2000; Matayoshi et al., 2009; Stowe et al., 2009; Ikegame et al., 2010), as well as traumatic and ischemic spinal cord injury (Tonai et al., 2001; Iwamoto et al., 2009). This is the first study to define the role of NE in the unique setting of the developing brain, where injury produces an age-specific pro-inflammatory state superimposed on an environment that is rapidly undergoing both structural and behavioral maturation. The immature brain may be particularly sensitive to NE exposure, due to inherently lower anti-oxidant levels, which renders a reduced capacity to deal with the consequences of NE proteolytic activity (Fan et al., 2003; Potts et al., 2006). We therefore hypothesized that NE, released by activated neutrophils, defines an environment that is toxic to early neuronal survival and unfavorable to long-term neurological recovery. Here, we aimed to define the contribution of NE to acute secondary pathogenesis in a murine model of TBI at postnatal day 21, using complementary genetic and pharmacological approaches, and then determine whether modulation of NE activity influences long-term brain development in terms of structure and function after injury.
MATERIALS AND METHODS
Animals
B6.129X1-Elanetm1Sds/J mice (henceforth referred to as ‘NE KO’) are a targeted mutant strain with the selective knockout of exon 2 of the neutrophil elastase Ela2 gene (Belaaouaj et al., 1998). Homozygote breeders were kindly provided by Dr. M. Looney (Department of Medicine, University of California San Francisco; UCSF) and backcrossed with C57Bl/6J mice for multiple generations (The Jackson Laboratory, Bar Harbor, ME) to obtain heterozygotes. These heterozygotes were bred to obtain NE KO and wild-type (WT) littermate pups for experiments. Genotyping was performed on tail snips using FINNZYMES Phire® Animal Tissue Direct PCR Kit (Thermo Fisher Scientific, Rockford, IL) and primers as specified by The Jackson Laboratory (strain #006112). NE KO mice are viable and fertile, do not present as immuno-compromised, and produce litters at normal Mendelian ratios (Belaaouaj et al., 1998; Shapiro, 2002). They have normal numbers of circulating neutrophils (Grenda et al., 2002), and express normal levels of adhesion molecules including alpha and beta integrins, L-selectin and PECAM (Young et al 2004).
In addition, for studies involving the pharmacological inhibition of NE, male C57Bl/6J pups at p17 with an accompanying lactating mother were purchased from The Jackson Laboratory and housed in the Laboratory Animal Resource Center at UCSF Parnassus. ZN200,355 (AstraZeneca, London, UK; henceforth referred to as the ‘NE inhibitor’), a selective trifluoromethyl ketone-based competitive peptide inhibitor of human and mouse NE, was dissolved in sterile isotonic saline to a concentration of 0.5 mg/ml. 10 mg/kg body weight was injected intra-peritoneal at 2, 6 and 12 h post-surgery, with sterile saline only used as the vehicle-control. Dosage and timing were based upon published pharmacokinetics (Williams et al., 1991; Mehta et al., 1994; Tiefenbacher et al., 1997), demonstrated efficiency in a murine model of ischemic stroke (Stowe et al., 2009), and the time course of NE activity in the injured immature brain (figure 1a). ZN200,355 has been established as a potent inhibitor of human and mouse neutrophil elastase without affecting other proteolytic enzymes such as chymotrypsin, thrombin or cathepsin G (Sommerhoff et al., 1991; Starcher et al., 1996).
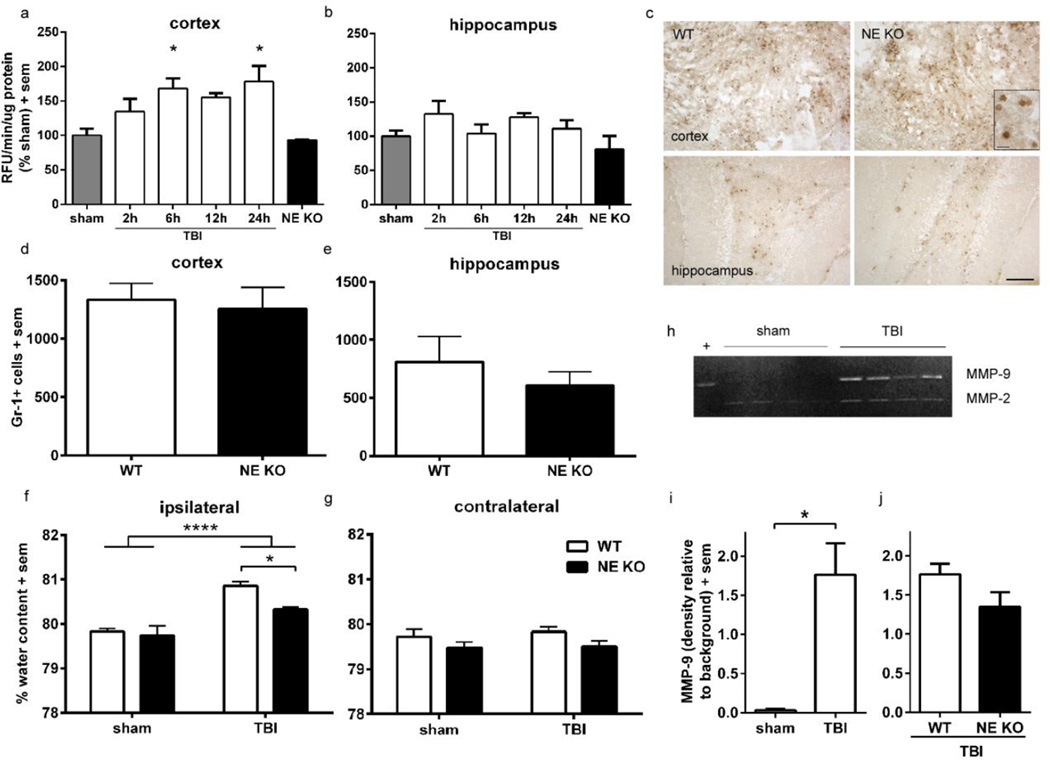
Figure 1. Deficiency of NE protects against vasogenic edema but does not alter neutrophil infiltration or MMP-9 activity at 24 h post-injury.
Neutrophil elastase activity was measured by a fluorogenic kinetic assay, in the cortex (a) and hippocampus (b) of WT mice across an acute time course following TBI at p21 (n=4/time point; 1-way ANOVA and Dunnett’s post-hoc, *p<0.05 at 6 h and 24 h post-injury compared to sham-operated controls). Panel c illustrates representative images of Gr-1+ cells in the cortex and hippocampus of WT and NE KO mice at 24 h post-injury (scale bar =200 µm), with an insert depicting positively-stained cells at higher magnification (scale bar =20 µm). Quantification of Gr-1+ cells found similar numbers in the injured cortex (d) and hippocampus (e) of NE KO mice compared to WT controls (n=6/group). Vasogenic edema was next measured by the wet weight-dry weight method, at 24 h after sham or TBI at p21 (f, g). Although edema was evident in TBI mice compared to sham controls (2-way ANOVA effect of injury, ****p<0.0001) water content was significantly lower in the ipsilateral hemisphere of brain-injured NE KO mice compared to brain-injured WT mice (n=7–8/group; *p<0.05 from planned comparison analysis). No changes in water content were observed in the contralateral hemisphere. Lastly, MMP-9 activity was measured by gelatin zymography at 24 h post-injury or sham (h). MMP-9 was up-regulated at 24 h after injury (i; t-test *p<0.05), but to a similar level in both brain-injured WT and NE KO mice (j; t-test, n.s.). Abbreviations: MMP-9 = matrix metalloproteinase-9; RFU = relative fluorescent units.
Standard rodent chow and water were available ad libitum, and the housing room was maintained on a 12 hour light/dark cycle. All surgical and behavioral procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the UCSF Institutional Animal Care and Use Committee. 175 mice were used in total for this study, and all experiments including histology and behavioral analyses were conducted blinding to injury and genotype or treatment group. One mouse (vehicle-treated TBI) was excluded at day 4 post-injury due to critical weight loss (>15%), and one mouse (WT TBI) died acutely post-surgery.
Controlled cortical impact model
The controlled cortical impact model of TBI was induced as previously described (Tong et al., 2002; Pullela et al., 2006) at postnatal day (p) 21, an age that approximates a toddler-aged child based upon structural, biochemical and behavioral characteristics (Semple et al., 2013). At p21, pups were weaned and anesthetized with 1.25 % 2, 2, 2-tribromoethanol (‘Avertin;’ St. Louis, MO) in isotonic saline i.p. at 0.02 ml/g body weight. In brief, the skull was exposed by a midline skin incision and a 5.0 mm circular craniotomy was made mid-way between bregma and lambda, with the medial edge 0.5 mm lateral to the midline. Mice were randomly allocated for brain injury or sham-operation. The injury was generated at 4.5 m/s velocity to 1.73 mm depth of penetration, for a sustained depression of 150 ms using a 3.0 mm convex impactor tip. Sham-operated mice underwent identical surgical procedures, including craniotomy, without receiving the cortical impact. Anesthesia for tissue collection was by overdose with Avertin (1.25% in saline for juveniles and 2.5% for adults).
NE activity assay
Neutrophil elastase activity was determined by a kinetic assay, using a fluorogenic substrate to quantify the proteolytic activity of NE over an acute time course. Brains were collected at 2, 6, 12 and 24 h post-injury and 2 h post-sham operation, for rapid dissection of the injured cortex and hippocampus, which were immediately weighed and frozen on dry ice. Tissue samples were pulverized in liquid nitrogen, followed by homogenization of the powdered tissue in lysis buffer (0.25 M sucrose in 5 mM Tris, pH 7.5). The supernatant was collected and stored at −80 °C for subsequent gelatin zymography (see below). The pellet was washed then re-suspended in extraction buffer (10 mM Bis-Tris pH 6.1, 2 M NaCl), and the resulting supernatant stored at −80 °C for the NE activity assay. Protein concentrations were determined using a BCA Protein Assay (Pierce, Rockford, IL).
Fifty micrograms of tissue lysate was loaded into a black 96-well microplate in triplicate, followed by addition of assay buffer (50 mM NaOAc pH 7.8, 200 mM NaCl). Human NE (10 nM) and α-1 antitrypsin from human plasma (1uM) were loaded as positive and negative controls, respectively (Calbiochem/Millipore, Billerica, MA). A selective fluorogenic probe MEOSuc-AAPV-AMC (Calbiochem) was added to each well (240 uM/well) immediately prior to loading the plate into a Spectramax Gemini XS microplate spectrofluorometer (Molecular Devices, Sunnyvale, CA). The fluorescent arbitrary units were read every 2 min over a 3 h period at 460 nm after excitation at 380 nm. Data were reduced to Vmax (milli-units/min) and analysis was restricted to the time interval which displayed linear dependence (5000 to 10,000 sec) using Softmax Pro v5.0 (Molecular Devices). Data were acquired as relative fluorescent units (RFU) per min relative to the protein concentration, and expressed as % sham values.
Gelatin Zymography
Ten micrograms of lysate was analyzed by gel zymography, on a 10% SDS-polyacrylamide gel copolymerized with 1 mg/ml gelatin substrate, as described previously (Noble et al., 2002). Following electrophoresis, proteins were incubated with renaturing buffer (Biorad Life Science, Hercules, CA) followed by incubation with a developing buffer (Biorad) at 37 °C. After 72 h, gels were stained with Coomassie blue (0.5% in 40% methanol and 10% acetic acid) and de-stained (40% methanol and 10% acetic acid). Negative staining is indicative of the location of active gelatinases which have cleaved the gelatin substrate. Purified human MMP-9 protein was loaded as a positive control (Abcam, Cambridge, MA). The identity of MMP’s was based upon molecular weights. Samples were run on duplicate gels (4 samples per group). To avoid potential variability arising from comparison across different gels, comparisons were only made between sham versus WT TBI, or TBI samples from different genotypes/treatments, whereby all samples included in the analysis were run simultaneously side-by-side on the same gel. Gels were scanned for digitalization, and band intensity was quantified using ImageJ Analysis Software (NIH).
Western blotting
Cortical and hippocampal tissue samples were dissected fresh, weighed and snap frozen on dry ice for storage at −80°C. Samples were homogenized in Glo Lysis buffer (Promega, Madison, WI) containing a Complete protease inhibitor cocktail tablet (Roche Applied Sciences, Indianapolis, IN), and protein concentrations were determined by a BCA protein assay (Pierce). Samples (50 ug protein) were boiled in Laemmli buffer containing 5% β-mercaptoethanol, then separated in a 10% SDS-polyacrylamide gel and transferred onto an activated PVDF Immobilon-FL membrane (Millipore). Membranes were incubated with Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE) for 1 hour, followed by overnight incubation at 4 °C with antibodies against NeuN (monoclonal anti-mouse, 1:5000, clone A60; Chemicon/Millipore) and β-actin (polyclonal rabbit, 1:20,000; Sigma Aldrich, St Louis, MO). Secondary antibodies were next applied for 1 h (IRDye800-conjugated goat anti-mouse IgG at 1:20,000, and IRDye680-conjugated goat anti-rabbit IgG at 1:10,000; Li-Cor Biosciences). Signal was detected with an Odyssey Infrared Imager at wavelengths of 700 and 800 nm, and band analysis was performed using Odyssey v1.2 software (Li-Cor Biosciences). The intensity of NeuN bands are expressed relative to each samples’ level of β-actin, as a loading control.
Edema measurements
Vasogenic edema as a result of TBI was determined using the wet weight-dry weight method (Elliott et al., 2008; Kenne et al., 2012). After terminal anesthesia, mice were decapitated and brains rapidly collected. The olfactory bulbs, brain stem and cerebellum were removed and discarded, and each hemisphere was immediately weighed on a small square of aluminum foil (‘wet weight’). Samples were dehydrated in an oven at 70 °C for 72 h, and then re-weighed (‘dry weight’). Percentage water content in each hemisphere sample was calculated as [(wet weight − dry weight)/wet weight] × 100.
Immunohistochemistry
Anesthetized mice were perfused transcardially with ice-cold saline followed by 4% paraformaldehyde (PFA) in 0.1M phosphate-buffered saline. Brains were removed and post-fixed overnight in 4% PFA, then transferred into a 30% sucrose solution for 72 h. Brains were then embedded in Neg50™ (Richard-Allan Scientific, Thermo Fisher Scientific, MI) for storage at −80 °C. Coronal sections spanning the entire cortex were cut at 20 µm or 40 µm (for 24 h and 2 month time points, respectively) and collected serially onto Fisherbrand Superfrost* Plus slides (Thermo Fisher Scientific).
Immunohistochemistry was performed on 20 µm coronal sections to detect infiltrated neutrophils (Gr-1+), apoptosis (active caspase-3+) or the oxidative stress response (heme-oxygenase; HO-1+). Briefly, sections were thawed and immersed in hydrogen peroxide (either 3% in methanol or 0.3% in phosphate buffered saline), followed by 1 h incubation in blocking solution containing 10% species-appropriate serum, 0.2% Triton-X100 and 0.1% bovine serum albumin. Sections were then incubated overnight at 4 °C in serum-containing blocking solution with the following antibodies: monoclonal rat anti-mouse Gr-1 (Ly6G and Ly6C, 1:50; clone RB6-8C5, BD Biosciences, San Jose, CA)(Stirling et al., 2009); rabbit polyclonal anti-cleaved (active) caspase-3 (1:500, Millipore); or rabbit polyclonal HO-1 (1:5000; Enzo Life Sciences, Farmingdale, NY). Negative controls were adjacent sections on which the same procedure was performed in the absence of primary antibody.
A subsequent 1 h incubation with the biotinylated secondary antibodies rabbit anti-rat IgG (mouse pre-absorbed; 1:200) or goat anti-rabbit IgG (1:500, Vector Laboratories Inc., Burlingame, CA), was followed by detection with the Vectastain ABC kit (Vector Laboratories Inc.) and nickel-enhanced 3, 3’-diaminobenzidine tetrahydrochloride, localized Gr-1 and active caspase-3, respectively. Although the Gr-1 antibody may label monocytes in addition to infiltrated neutrophils, adjacent sections stained with cresyl violet revealed poly-lobulated nuclei, confirming the identity of these cells as neutrophils (Claus et al., 2010). In addition, very few monocytes are present in the brain at the time point assessed (24 h postinjury), which are typically recruited to the injured brain from 72 h onwards (Clark et al., 1994; Semple et al., 2010b).
Staining was quantified by a blinded investigator on 5 equally-spaced coronal sections per brain, spanning 2000 µm to encapsulate the entire lesioned area, with the first section taken from the same anatomical plane for all brains (~Bregma −1.0 mm). A contour of the ipsilateral cortex and ipsilateral hippocampus was outlined using StereoInvestigator software (MicroBrightField Inc, Williston, VT), and all positively stained cells were counted within these boundaries (summed per animal, averaged per group). There was minimal positive staining on the contralateral side (data not shown). A cell was considered positively stained when it had a clearly defined membrane or nuclear staining, for Gr-1 and caspase-3, respectively (Claus et al., 2010).
Binding of the HO-1 primary antibody was detected by application of a FITC-conjugated goat anti-rabbit IgG (1:200, Jackson Immuno Research) and nuclei were counter-stained with 4',6-diamidino-2-phenylindole (DAPI). HO-1/DAPI staining was visualized using a Nikon Optiphot light microscope and Spot™ Imaging Solutions software to overlay images captured under green and blue filters. Three non-overlapping images were captured at 20 × of both the injured cortex and hippocampus of each section (3 sections per brain; total of 18 images per brain). Immuno-fluorescent cells with a glial-like morphology and DAPI-positive nucleus were counted as HO-1 positive cells, using Metamorph analysis software (Molecular Devices, Sunnyvale, CA) (Weinzierl et al., 2004).
Detection of cell death by TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP nick 3’-end labeling (TUNEL) was performed on sections collected 24 h post-injury to quantify dying cells, using the in situ Cell Death Detection kit according to the manufacturer’s instructions (Roche Diagnostics). TUNEL-positive cells were counted within the contoured ipsilateral cortex and hippocampus as described for Gr-1 and caspase-3 immunohistochemistry, on five sections per brain.
Cortical and hippocampal volume measurements
An estimation of intact cortical and hippocampal volumes was performed on 40 µm cresyl violet-stained coronal sections collected at 2 months post-injury. The unbiased Cavalieri method (Schmitz and Hof, 2005) was performed using Stereo Investigator (MicroBrightField v10.21.1, Williston, VT), with a Nikon E600W microscope configured with a motorized stage, MAC 5000 controller and Retiga 2000R color digital camera (QImaging, Surrey, BC, Canada). An average of 15 sections were measured per brain, between Bregma 1.5 to −3.8 mm, using a sampling interval of 8 and a grid size of 200 µm or 75 µm, for the cortex and hippocampus respectively. Measurements were confined to the dorsal hemispheres which contained the impacted region. The inferior boundary for cortical measurements was defined by a horizontal line underlining the most ventromedial point of the corpus callosum. For hippocampal measurements, the inferior boundary was defined anteriorly by a horizontal line from the most ventral point of the 3rd ventricle at the midline, and posteriorly by the most dorsal point of the posterior commissure. The Gundersen mean coefficient of error (m=1) for individual estimates maintained ≤0.10, and group means are expressed as estimated volume (mm3).
Hippocampal cell counts
Neuronal numbers in the ipsilateral hilus and dentate gyrus (DG) were estimated using the optical fractionator method on StereoInvestigator (Gundersen et al., 1988, West et al., 1991). This design-based stereological method is widely used to obtain unbiased estimations of hippocampal neuronal numbers (Schmitz and Hof, 2005). Briefly, the regions of interest (ipsilateral upper DG blade, lower DG blade and hilus) were contoured on 40 µm cresyl violet-stained sections using a 4× objective. The sectioning interval was 4, with an average of 14 sections spanning the hippocampus measured per brain. A 60× oil immersion objective was used for cell counting. Systematic random sampling was achieved by counting cells at a regular pre-determined interval (grid size of 80 × 80 µm) within a dissector counting frame (20 × 20 µm) superimposed upon the contoured region of interest. A dissector height of 10 µm with a top guard zone of 1 µm allowed for quantification of cells in a three-dimensional manner, and only cells that fell within the inclusion borders of the dissector were counted. The Gundersen mean coefficient of error (m=1) for individual estimates was maintained at ≤0.10 for the DG blades and ≤0.20 for the hilus. The total number of cells per contoured region was estimated by the following equation: N= ∑Q · (t/h)(1/asf)(1/ssf); where Q is the number of cells counted, t is the measured section thickness, h is the dissector height, asf is the area sampling fraction, and ssf is the section sampling fraction (Schmitz and Hof, 2005).
Behavioral assessment
All behavioral testing and housing during this period was conducted within the Neurobehavioral Core for Rehabilitation Research at UCSF. An extensive battery of behavioral assessments was performed in the following order: open field, rotarod, elevated plus maze, elevated zero maze, three-chamber social task and Morris water maze (n=10–13 per group). Mice were separated into individual clean cages 24 h prior to commencement of testing for overnight isolation and habituation. All testing was conducted between 9:00 am and 6:00 pm daily.
Rotarod
The accelerating rotarod (Ugo Basile 7650, Comerio VA) was used to assess general motor function, coordination and motor learning. The latency to fall was recorded in sec, and mice were tested across three consecutive days, three times per session with an inter-session interval of approximately 1 h (Pullela et al., 2006).
Open field
Exploratory behaviors were assessed over a 10 min session in an automated open field arena (40.64 cm × 60.64 cm; Kinder Scientific, Poway, CA). Interfaced Motor Monitor software allowed for calculation of parameters including total distance travelled, and relative time spent in the center versus the periphery (Pullela et al., 2006).
Elevated plus maze and zero mazes
The elevated plus maze and elevated zero mazes (Kinder Scientific) assess anxiety based upon the natural tendency of rodents to avoid the open arms in preference for enclosed areas (Gould et al., 2009). For both tasks, mice were placed individually on the apparatus and allowed free access for a 10 min period. Times spent in the open versus closed arms/areas, and total distance moved, were assessed as previously described (Pullela et al., 2006).
Three-chamber social approach task
The three-chamber paradigm allows for the evaluation of social affiliation and social recognition in mice (Yang et al., 2011). The task was performed as described in detail previously (Semple et al., 2012). In brief, three consecutive stages of 10 min each allow for (1) habituation in an empty 3-chambered box; (2) a choice between an empty cup and a cup containing a novel stimulus mouse; and (3) choice between a second novel stimulus mouse and the first, now familiar mouse. Stages 2 and 3 are measures of the test mouse’s preference for sociability and social recognition, respectively. ‘Stimulus’ mice used for the three-chamber task were age-matched naïve male C57Bl/6J mice. Data was expressed as time spent in each chamber (% of total time). This task was implemented in our laboratory after the behavioral assessments were completed for the NE inhibitor study, thus data was only obtained for the NE KO versus WT experiments.
Morris water maze
The Morris water maze was used to assess spatial learning and memory as previously described (Pullela et al., 2006; Davis et al., 2012), using a 140 cm-diameter circular pool filled with opaque water. Mice underwent two daily sessions for 5 consecutive days, and each session consisted of three 60 sec trials with a 10–15 min inter-trial interval. During days 1 and 2, the platform was raised above the water surface and clearly labeled with a flag (‘visible platform’). The platform location was rotated to different quadrants during this training stage, and mice that failed to reach the platform within 60 sec were led there by the investigator. During days 3, 4 and 5 (‘hidden platform’), the platform was hidden just below the water surface and maintained in a constant location, such that mice were required to use spatial cues from the room to locate it. Movements were tracked with an overhead mounted video camera interfaced with Noldus EthoVision software, for quantification of the latency to locate the platform, as well as swim velocity (cm/s). At the conclusion of days 3, 4 and 5, the platform was removed from the pool and a 60 sec ‘probe trial’ was conducted for each mouse. A final probe trial was performed one week later, to further assess spatial memory retention. Only probe trials conducted at the end of the hidden trials (day 5) and one week later are presented, quantified as the time spent in the target quadrant (where the platform was previously located) compared to the other quadrants.
Statistical analysis
Statistical analyses were performed using Prism v.5.0 (GraphPad Software, Inc, La Jolla, CA), with a significance level of p<0.05. An a priori analysis strategy was applied, to address specific questions regarding (a) the effect of injury on outcome measures, and (b) the effect of NE deficiency or drug treatment in brain-injured mice. To determine whether NE deficiency or drug treatment differentially affected brain-injured mice, planned unpaired two-tailed t-tests were used for direct comparisons of the two TBI-injured groups (WT versus NE KO, or vehicle versus inhibitor-treated), e.g. for regional cell counts. Mann-Whitney tests were applied for non-parametric data (e.g. MMP-9 activity). One-way analysis of variance (ANOVA) was used to examine NE activity, followed by Dunnett’s post-hoc analyses to directly compare the time course of NE activity to sham values (baseline). 2-way ANOVA’s were used to compare 2 or more groups or factors (injury × genotype, drug treatment and/or time). If a significant interaction was detected, the main effects were disregarded and multiple comparison analyses were considered in isolation. If the interaction was not statistically significant but both main effects did reject the null hypothesis, we next conducted planned comparisons between our specific groups of interest (TBI WT versus TBI NE KO; or TBI saline versus TBI NE inhibitor-treated). Repeated measures (RM) were used for the rotarod and three-chamber tasks, with a between-subjects factor of group (injury/drug or genotype) and a within-subjects factor of session or chamber, respectively. Analysis of the MWM was conducted using the nlme package in the statistical program R (Pinheiro et al., 2012; Team, 2012). A linear mixed model was fitted to the visible and hidden sessions separately, with predictors of session, genotype (or drug) and injury. This mixed model approach provides greater sensitivity and statistical power to identifying real main effects compared to the traditionally-used analyses for this task (Young et al., 2009). A 2-way ANOVA was also conducted specifically at session 4 (final visible platform session), to determine whether acquisition of this task was equivalent between groups. Relative time spent in each quadrant during probe trials were compared by 1-way RM ANOVAs within each group, with Dunnett’s multiple-comparisons analyses to detect a potential preference for the target quadrant. P-values are reported to 4 decimal points, and results are expressed as mean ± standard error of the mean (sem).
RESULTS
Neutrophil infiltration, NE and MMP-9 activity in the immature injured brain
NE activity in the injured immature brain was first determined by cleavage of a fluorogenic substrate. In the injured cortex, NE activity was elevated compared to levels in sham-operated mice, peaking at 6 and 24 h post-injury (figure 1a; 1-way ANOVA F5, 15=4.51, p=0.0104; Dunnett’s multiple comparisons, p<0.05; n=4/group). NE levels in the injured hippocampus (figure 1b) tended to be slightly elevated in samples from injured mice; however, this was not significantly different from sham (1-way ANOVA F5, 15=1.72, p=0.1902). In both cortex and hippocampus, NE activity in samples from NE KO mice at 24 h post-injury was equivalent to levels in WT sham control mice, at or below the minimal detection level of the assay, confirming a lack of NE activity in this strain.
As the contribution of NE activity to neutrophil recruitment remains controversial (Woodman et al., 1993; Belaaouaj et al., 1998; Hirche et al., 2004), we quantified Gr-1-labeled neutrophils in the injured brain during the peak of infiltration (24 h post-injury). A pronounced accumulation of Gr-1 positive cells was evident in the injured hemisphere of both WT and NE KO mice, distributed throughout the core lesion site and peri-contusional parenchyma (figures 1c). Absence of NE did not impair neutrophil influx, as a similar number of Gr-1 positive cells was seen in WT and NE KO mice in both the injured cortex (unpaired t-test, t10=0.34, p=0.7443) and underlying hippocampus (t10=0.81, p=0.4360; n=6/group; figure 1d–e).
The activity of MMP-9, a potent metalloproteinase released by infiltrating neutrophils whose activity may be dependent upon NE cleavage, was next examined by gelatin zymography. Pro-MMP-9 was elevated in the injured cortex of WT mice at 24 h post-injury compared to minimally detectable levels in sham controls (figure 1h and i; Mann-Whitney test p=0.0286), as previously described by our group and others (Hadass et al., 2013). However, direct comparison between pro-MMP-9 levels in WT and NE KO mice revealed a similar level regardless of the presence of NE (figure 1j, Mann-Whitney test p=0.2000; n=4/group).
Vasogenic edema is attenuated in brain-injured NE KO mice
Neutrophils, and neutrophil-derived NE in particular, have been implicated in edema formation in the ischemic brain (Stowe et al., 2009; Ikegame et al., 2010). We therefore examined the effect of genetic deficiency of NE on vasogenic edema in the immature injured brain. Although the interaction between injury and genotype was not signficant (F1, 25=2.64, p=0.1170), edema was evident in the ipsilateral hemisphere at 24 h post-injury compared to sham controls as expected (figure 1f; 2-way ANOVA effect of injury, F1, 25=34.62, p<0.0001). In addition, there was a significant effect of genotype (F1, 24=5.13, p=0.0324). Planned comparisons analysis between TBI WT and TBI NE KO mice was performed to better understand these effects; here, we found that NE KO mice had lower ipsilateral water content after injury compared to WT injured mice (Bonferroni’s test p<0.05), similar to sham controls, indicative of reduced injury-mediated vasogenic edema post-injury. In the contralateral hemisphere (figure 1g), although there was a non-significant trend towards an overall effect of genotype (F1, 25=3.99, p=0.0568), TBI did not induce a change in water content (effect of injury F1, 25=0.26, p=0.6137) nor was there an injury × genotype interaction (F1, 25=0.08, p=0.7799).
Hippocampal cell death is reduced in NE KO mice
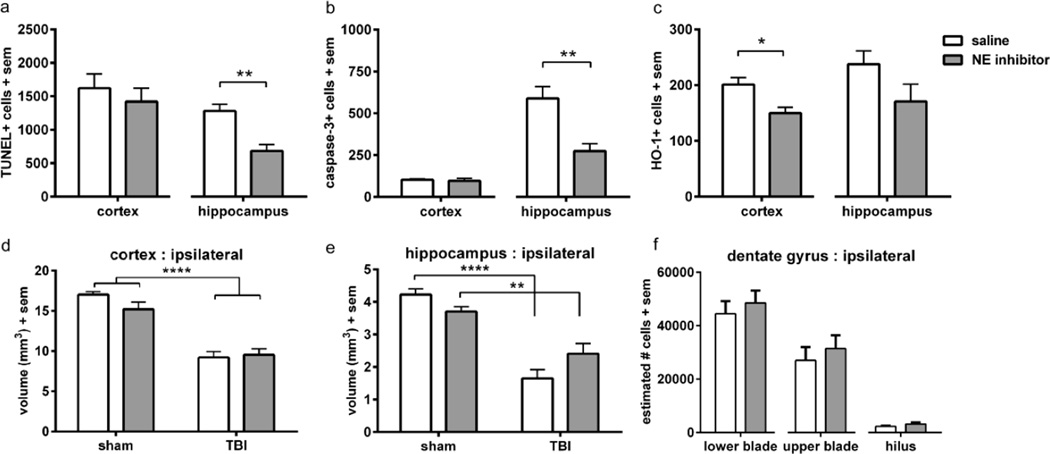
Cell death and neuronal integrity were assessed at 24 h post-injury, to determine the contribution of NE to acute pathogenesis in the injured immature brain. TUNEL staining, as a measure of DNA fragmentation, was abundant throughout the injured cortex and hippocampus at this time, particularly evident within the granule cell layer (GCL) of the hippocampus dentate gyrus (figure 2a). Quantification revealed similar numbers in the injured cortex in both genotypes (unpaired t-test t10=1.50, p=0.1647; n=6/group). In contrast, fewer TUNEL-positive cells were found in the injured hippocampus of NE KO mice, at ~ 60% of WT levels (unpaired t-test t10=2.24, p=0.0492).
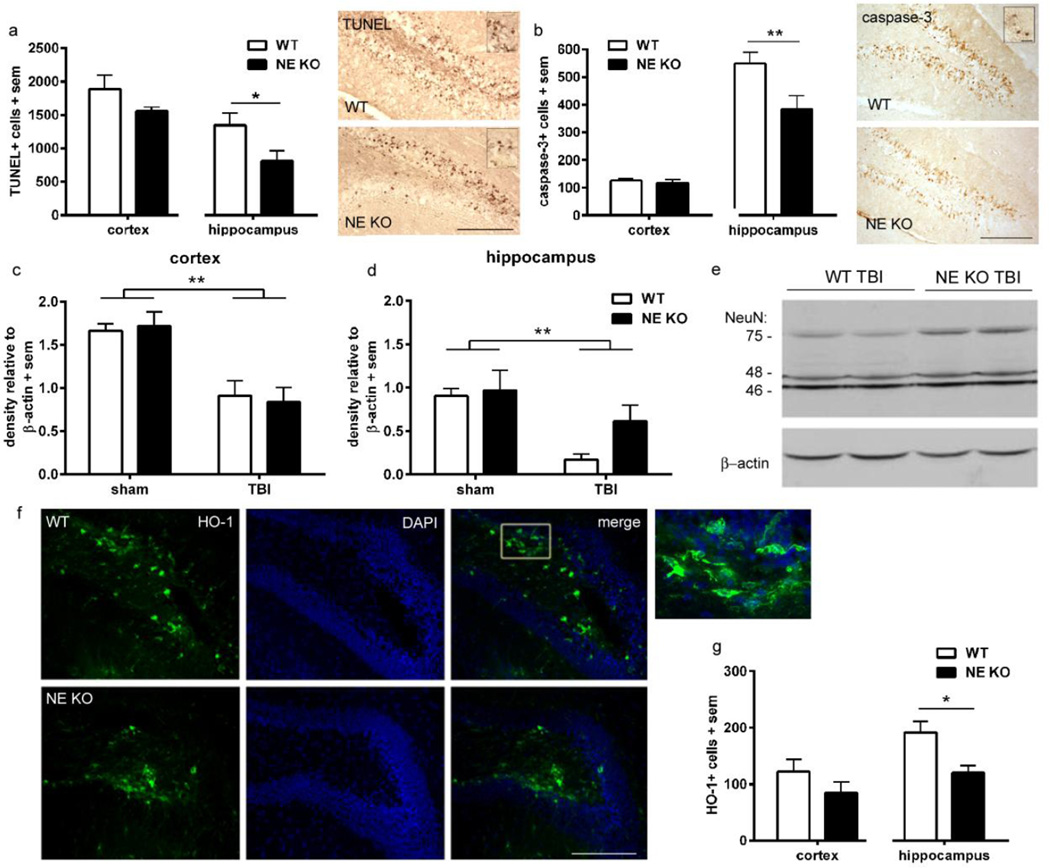
Figure 2. NE deficiency is acutely neuroprotective in the immature injured brain.
TUNEL staining was quantified at 24 h post-injury (a), revealing fewer dying cells in the injured hippocampus of NE KO mice compared to WT mice (n=6/group; t-test *p<0.05; scale bar=100 µm; insert =20 µm). The contribution of apoptosis to cell death was confirmed by immuno-staining for activated (cleaved) caspase-3 (b), which was also reduced in the hippocampus of NE KO compared to WT mice (t-test **p<0.01; scale bar =100 µm; insert =20 µm). The consequence of acute cell death on neuronal viability was measured by western blot for the neuronal marker NeuN, and the 75 kDa band was quantified. At 24 h post-injury, NeuN was notably reduced in the cortex (c) of both WT and NE KO mice after TBI compared to sham controls (2-way ANOVA effect of injury ***p<0.0002). In the hippocampus (d), NeuN loss was also evident after injury (2-way ANOVA effect of injury p=0.0047; n=4/group). Representative NeuN bands from hippocampal TBI samples (WT and NE KO) are presented in panel e. Heme-oxygenase (HO-1) levels were also quantified at 24 h post-injury, as an indication of the pathological and oxidative state. Representative staining in injured hippocampus is illustrated in panel f (scale bar =200 µm; insert depicts higher magnification of HO-1+ cells from the box annotated in the WT merged image). Fewer HO-1 positive cells were found in the hippocampus of NE KO mice compared to WT (n=6/group; t-test *p<0.05). Abbreviations: DAPI = 4',6-diamidino-2-phenylindole; HO-1 = heme-oxygenase 1; TUNEL = terminal deoxynucleotidyl transferase-mediated dUTP nick 3’-end labeling.
TUNEL staining does not allow for the differentiation of necrotic versus apoptotic cell death (Kraupp et al., 1995). Thus, the presence of activated (cleaved) caspase-3, a key protease involved in apoptotic cell death pathways, was also assessed on adjacent sections (figure 2b; n=6/group). Immuno-reactivity for caspase-3 appeared to be most abundant in the hippocampus with fewer cells scattered throughout the injured cortex, suggesting that necrosis rather than apoptosis primarily contributes to cell death in the cortex. Caspase-3+ cell numbers in the injured cortex were similar in WT and NE KO mice (unpaired t-test, t10=0.62, p=0.5475). In the injured hippocampus, in parallel with TUNEL staining, we noted a large number of caspase-3-positive cells within the dentate gyrus, confirming the vulnerability of this region to apoptotic cell death. Quantification revealed a 30% reduction in caspase-3-positive cells within the injured hippocampus of NE KO mice compared to WT mice (unpaired t-test, t10=2.56, p=0.0284).
Thirdly, we sought to confirm whether reduced cell death within the NE KO hippocampus corresponded with changes in acute neuronal loss, by Western blotting for the mature neuronal marker NeuN (figure 2c–e) (Bao et al., 2012). In homogenates of the cortex and hippocampus from sham and injured mouse brains, we found three distinct bands at 46, 48 and 75 kDa for NeuN, as previously reported (Dredge and Jensen, 2011). For unknown reasons, the 75 kDa band was the only band whose intensity was found to be injury-dependent and thus was quantified (n=4/group). Densitometry demonstrated that 75 kDa NeuN decreased in cortical TBI samples by ~50 % compared to sham controls (2-way ANOVA effect of injury, F1, 12=28.82, p=0.0002), which was consistent in both WT and NE KO mice (effect of genotype F1, 12=0.003, p=0.9551; interaction F1, 12=0.18, p=0.6824). In the hippocampus, we again saw a reduction in NeuN in response to injury (F1, 12=11.96, p=0.0047), in the absence of either an overall effect of genotype (F1, 12=2.61, p=0.1325) or an injury × genotype interaction (F1,12=1.44, p=0.2530). Although not statistically significant, brain-injured NE KO mice appeared to show greater preservation of NeuN in the hippocampus (mean 37 % reduction versus to their sham controls), compared to an 81 % reduction of NeuN in WT TBI mice compared to WT controls.
Induction of heme-oxygenase, a marker of oxidative stress, is reduced in NE KO mice
Based upon evidence that NE activity generates reactive oxygen species (Aoshiba et al., 2001; Fischer and Voynow, 2002) - for example, by facilitating xanthine dehydrogenase production of superoxide anions and hydrogen peroxide (Phan et al., 1992) - we hypothesized that NE may also contribute to the initiation and propagation of oxidative stress in the injured brain. The inducible form of heme-oxygenase (HO-1), the rate-limiting enzyme required for heme catabolism, is elevated in the rodent and human brain after injury in response to oxidative stress (Fukuda et al., 1996; Cousar et al., 2006). We thus quantified HO-1 by immunofluorescence at 24 h post-injury as an indirect marker of the oxidative stress response. HO-1 was primarily localized to glial-like cells with processes, distributed throughout the injured cortex and hippocampus. In the dentate gyrus, a region that showed pronounced vulnerability to cell death, HO-1-positive cells were most abundant within the hilus (figure 2f). Quantification found a significant difference between WT and NE KO in the injured hippocampus (unpaired t-test t10=3.06, p=0.0121), but similar numbers in the injured cortex of both genotypes (unpaired t-test t10=1.33, p=0.2148). These data indicate a reduced oxidative stress response in the NE KO hippocampus after injury (figure 2g; n=6/group).
Long-term loss of cortical and hippocampal tissue in both WT and NE KO mice
To determine whether NE contributes to progressive tissue loss after injury, histopathology was examined in the brains of NE KO and WT mice at 2 months post-injury (~3 months of age). Injury induced a large cavity in the dorsal parietal lobe cortex, and deformation of the underlying dorsal hippocampus (figure 3a). Volumetric analyses confirmed this loss (figure 3b), with a 30 % reduction of the ipsilateral dorsal cortex compared to the contralateral side (2-way ANOVA effect of injury F1, 42=65.58, p<0.0001). However, cortical volumes were similar in brain-injured WT and NE KO mice (effect of genotype, F1, 42=0.58, p=0.4493; injury × genotype interaction F1, 42=0.13, p=0.7188). The volume of the contralateral dorsal cortex (figure 3c) was unchanged by either injury (F1, 42=1.61, p=0.2121) or genotype (F1, 42=1.64, p=0.2077; injury × genotype interaction F1, 42=3.12, p=0.0848).
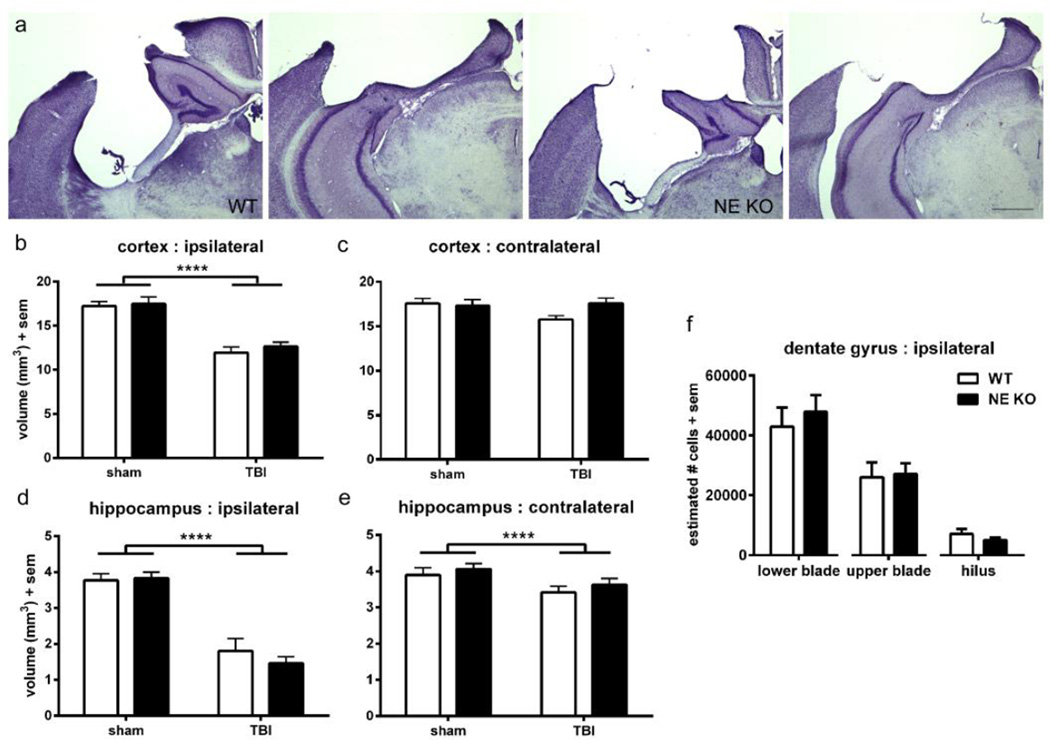
Figure 3. NE gene deficiency does not preserve injury-induced tissue damage long-term.
Stereological analysis was performed on cresyl violet stained sections to assess atrophy in the dorsal cortex and hippocampus at 3 months of age (~ 2 months post-injury). Representative images are presented from WT and NE KO brains both anteriorly and posteriorly (a; scale bar =500 µm). Quantification revealed a reduction of cortical volume in TBI mice compared to sham controls (2-way ANOVA effect of injury, ****p<0.0001), which was similar in WT and NE KO mice (b). Contralateral cortex volume was not affected by either injury or genotype (c). Hippocampal volumes were affected by injury both ipsilateral and contralateral to the impact site (d; 2-way ANOVA effect of injury ****p<0.0001), to a similar extent in both WT and NE KO mice. The surviving neuronal population in specific regions of the ipsilateral hippocampus DG (e) was calculated using the optical fractionator method. Consistent with volumetric analyses, no differences were observed between WT and NE KO mice (t-tests n.s). n=10–12/group.
The dorsal hippocampus, ipsilateral to the injury site, was also considerably affected by TBI (figure 3d), showing an injury-related volume reduction of 57 % compared to the contralateral hippocampus (2-way ANOVA effect of injury F1, 42=97.78, p<0.0001). However, volumetric loss after injury was similar in both WT and NE KO mice (effect of genotype F1, 42=0.43, p=0.5149; injury × genotype interaction F1, 42=0.86, p=0.3586). Interestingly, the unilateral traumatic impact also influenced the contralateral dorsal hippocampus (figure 3e), which showed a volumetric reduction of ~10 % compared to sham-operated controls (figure 3c, 2-way ANOVA effect of injury F1, 42=6.74, p=0.0130). This atrophy was not affected by genotype (F1, 42=1.15, p=0.2907; interaction F1, 42=0.02, p=0.8902). Lastly, volumetric changes in the dorsal dentate gyrus (DG) specifically were measured. Injury significantly reduced the DG ipsilateral to the impact site by 53 % compared to the contralateral DG (2-way ANOVA effect of injury F1, 42=81.91, p=0<0.0001), to a similar extent in both WT and NE KO mice (effect of genotype F1, 42=0.49, p=0.4881; injury × genotype interaction F1, 42=0.58, p=0.4513). The volume of the contralateral DG was unaffected by either injury or genotype (data not shown).
Complementary to volumetric analysis, we conducted stereological quantification using the optical fractionator method on cresyl violet-stained coronal sections to estimate the number of surviving GCL cells in the hippocampal DG (figure 3f). Comparing brain-injured WT and NE KO mice, no differences were found in the number of surviving cells in the lower DG blade (unpaired t-test t18=0.59, p=0.5618), upper DG blade (t18=0.18, p=0.8554) or the hilus (t18=1.12, p=0.2784).
Injury-induced hyperactivity is attenuated in NE KO mice
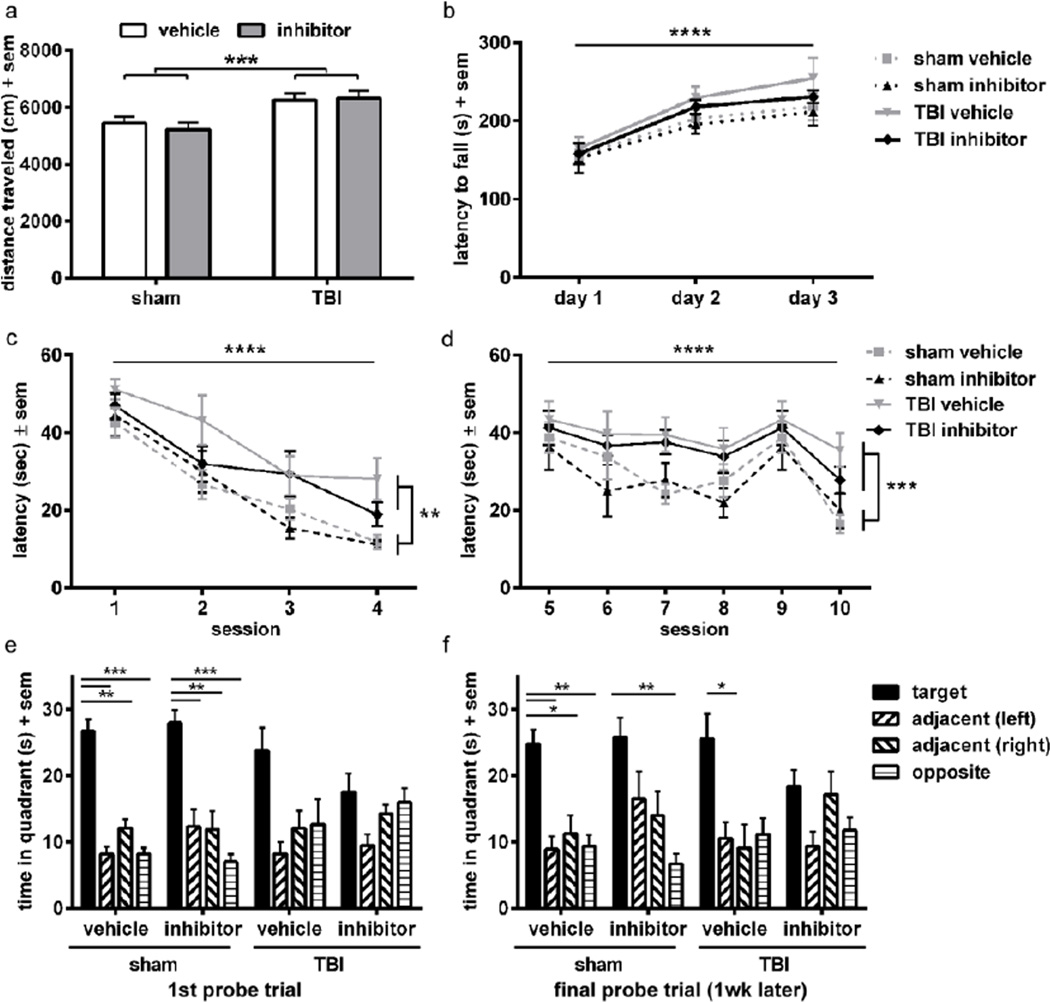
An extensive battery of behavioral assays was conducted at 2 months post-injury to determine whether NE deficiency afforded an improvement in long-term outcomes after TBI. Using the open field paradigm, we detected an overall increase in the distance moved after injury (2-way ANOVA effect of injury, F1, 43=6.23, p=0.0165), indicative of a robust hyperactivity phenotype in this model which is consistent with previous studies (figure 4a) (Pullela et al., 2006). Analysis also revealed an effect of genotype (F1, 43=15.44, p=0.0003), although the interaction between injury and genotype did not quite reach statistical significance (F1,43=3.33, p=0.0749). We proceeded with planned comparison analyses between our key groups of interest, and found that the distance moved was lower for brain-injured NE KO mice (at a level similar to sham-operated controls) compared to WT TBI mice (Bonferroni’s test, p<0.001), suggesting a rescue of this hyperactive phenotype. In accordance with this finding, the time spent at rest was altered by both injury and genotype (2-way ANOVA injury × genotype interaction F1, 43=7.75, p=0.0080). Compared to sham-operated controls of both genotypes (mean WT sham = 10.8 %; NE KO sham = 10.2 % time at rest), WT TBI mice spent half as much time immobile (5.1 %). In comparison, time at rest by brain-injured NE KO mice was significantly higher than WT TBI mice (Bonferroni’s test, p<0.01; 10.4 % time at rest), at levels comparable to sham-operated controls (data not shown).
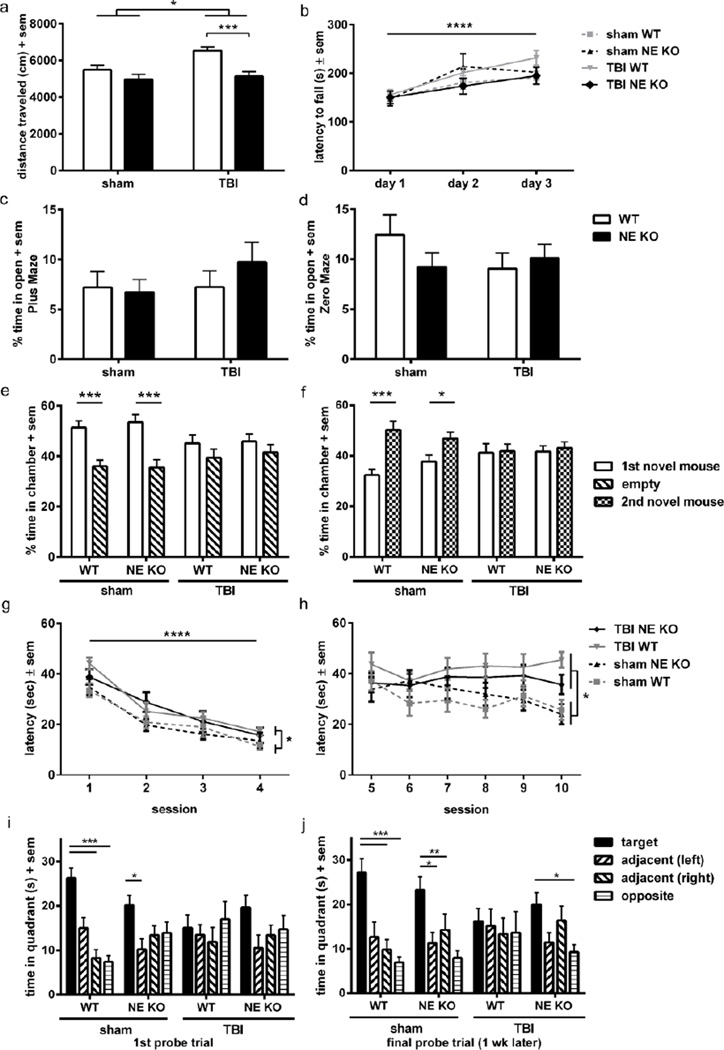
Figure 4. NE deficiency ameliorates injury-induced hyperactivity and improves spatial memory retention, but does not influence measures of anxiety, motor performance or social preference.
Behavioral assessments were performed at 3 months of age (~ 2 months post-injury) to compare functional outcomes in the presence or absence of NE. Although brain-injured mice overall showed an injury-dependent increase in distance traveled in the open field compared to sham controls (a; 2-way ANOVA effect of injury *p<0.05), brain-injured NE KO mice moved less distance compared to brain-injured WT mice (***p<0.001 from planned Bonferroni’s analysis; n=11–13/group). General motor function as tested on the rotarod across 3 consecutive days (b) was not affected by either TBI or NE deficiency, although all groups showed improvement (increased latency to fall) over time (2-way ANOVA effect of time, ****p<0.0001). Anxiety levels, measured as the percentage time spent in the open in the Plus and Zero mazes (c, d), were no different between any of the groups. The three-chamber task was used to test for sociability and social novelty. Sham mice showed normal sociability and social novelty, with a preference for the first stimulus mouse over the empty chamber (e; 2-way ANOVA effect of stimulus p=0.0006; ***p<0.001 from multiple comparisons analysis), and a preference for the second novel mouse over the first (f; 2-way ANOVA effect of stimulus p=0.0091; *p<0.05; ***p<0.001 from multiple comparisons analyses). Brain-injured mice failed to show this preference; however, this was comparable across both genotypes. The Morris water maze (MWM) was also performed to assess spatial learning and memory. In this task, all mice showed improvement over time during the visible platform sessions (g; linear mixed effects ANOVA effect of time ****p<0.0001), although brain-injured mice showed a longer latency to reach the platform overall compared to sham controls, indicating impaired task learning after injury (effect of injury *p<0.05). When the platform was hidden in subsequent sessions (h), brain-injured mice showed again exhibited a longer latency to reach the platform compared to sham controls (effect of injury *p<0.05), indicating poorer spatial memory. Performance in both the visible and hidden platforms of the MWM was not affected by genotype. At the end of the MWM testing period, both WT and NE KO sham-operated mice showed memory retention in the probe trial (i), spending more time in the target quadrant compared to other quadrants, whereas brain-injured mice did not (***p<0.001 and *p<0.05 from Dunnett’s analyses after 1-way RM ANOVAs within each group). When tested again one week later (j), TBI NE KO mice showed a preference for the target quadrant, indicating memory retention similar to sham-operated mice, whereas TBI WT mice spent equivalent time in each quadrant (***p<0.001, **p<0.01 and *p<0.05). n = 11–13/group.
Sensorimotor function and anxiety are unchanged by either TBI or NE deficiency
Rotarod performance across three consecutive days was quantified as a measure of sensorimotor function (figure 4b). All mice showed improvement over time (2-way RM ANOVA effect of time, F2, 88=41.70, p<0.0001), however, neither injury nor genotype affected rotarod performance (effect of group F3, 44=0.68, p=0.5700; time × group interaction F6, 88=2.11, p=0.0597).
Anxiety was quantified as percentage of time spent in the open arms of the elevated plus and zero mazes (figure 4c–d). Consistent with previous studies (Pullela et al., 2006), TBI did not alter the percentage time spent in the open areas in either the plus or zero maze when mice were tested at adulthood after injury at p21 (2-way ANOVA effect of injury, F1, 44=0.88, p=0.3543 and F1, 44=0.62, p=0.4354, respectively). In addition, genetic absence of NE did not alter anxiety in either task (effect of genotype F1, 44=0.39, p=0.5334 for plus maze; F1, 44=0.46, p=0.4998 for zero maze; injury × genotype interaction F1, 44=0.84, p=0.3644 for plus maze; F1, 44=1.85, p=0.1806).
Sociability deficits after TBI are not altered in NE KO mice
Preferences for sociability and social novelty were evaluated by the three-chamber social approach task. When offered a choice between an empty outer chamber or one containing a novel stimulus mouse, both sham WT and NE KO mice showed a preference for social proximity to the novel stimulus mouse (2-way RM ANOVA effect of stimulus, F3, 44=13.63, p=0.0006; Bonferroni’s test, p<0.001; figure 4e). In contrast, brain-injured mice, regardless of genotype, did not show a preference for either chamber (effect of group, F3, 44=1.66, p=0.1902; interaction F3, 44=1.38, p=0.2602). The next stage of the test offers mice a choice between the first (now familiar) novel mouse compared to a second novel mouse, as a measure of social novelty and social recognition (figure 4f). Both WT and NE KO sham-operated mice again showed a strong preference for the chamber containing the second novel mouse (2-way RM ANOVA effect of stimulus F1, 44=7.45, p=0.0091; Bonferroni’s test, p<0.001 and p<0.05, for WT sham and NE KO sham, respectively). In contrast, brain-injured mice of both genotypes spent equivalent time in each chamber, indicating a lack of preference for social novelty. No differences were noted between groups (F3, 44=0.52, p=0.6679) nor was there an interaction between stimulus preference and group (F3, 44=2.15, p=0.1074), thus NE deficiency did not rescue TBI-induced social deficits.
Long-term spatial memory retention is improved in NE KO mice
Spatial learning and memory were assessed in the MWM at 2 months post-injury, comparing sham and brain-injured WT and NE KO mice. Swim velocity (m/s) was firstly quantified across the visible platform trials; however, this measure was not affected by either injury or genotype (data not shown). Latency to find the platform during the visible sessions is a test of task learning based on spatial orientation (figure 4g). All mice showed improvement over time, with a reduction in latency to the visible platform over the four visible sessions (linear mixed effects ANOVA effect of time, F1, 528=164.97, p<0.0001). Braininjured mice were slower to reach the platform compared to sham-operated mice (effect of injury F1, 528=5.44, p=0.0201), indicating poorer learning of the task. By session 4, however, TBI mice were able to locate the visible platform within a comparable latency to sham-operated controls (2-way ANOVA of session 4 only; no effect of injury, F1, 140=2.96, p=0.0877). No differences were observed between WT and NE KO mice overall (effect of genotype F1, 528=0.11, p=0.7444; injury × genotype interaction F1, 528=0.14, p=0.7109).
The platform was hidden from view for the subsequent MWM sessions (sessions 5–10), providing a test of short-term spatial memory (figure 4h). Brain-injured mice showed an increased latency in the hidden sessions compared to sham-operated mice overall (linear mixed effects ANOVA effect of injury F1, 813=5.21, p=0.0228). Surprisingly, there was little improvement across time (effect of session F1, 813=2.98, p=0.0846), and performance by WT and NE KO mice was indistinguishable overall (effect of genotype, F1, 528=0.36, p=0.5488; injury × genotype interaction F1, 813=1.73, p=0.1887).
At the end of the MWM testing period we removed the platform completely for a probe trial, and examined time spent in the target quadrant (where the platform was previously located) compared to the other quadrants (figure 4i). WT sham-operated mice spent more time in the target quadrant (1-way RM ANOVA F10, 30=14.60, p=0.0004; Dunnett’s multiple comparisons test p<0.001 vs. target quadrant). Sham-operated NE KO mice also showed memory retention at this time (F12, 36=3.28, p=0.0289; Dunnett’s test p<0.05). In contrast, brain-injured mice of both genotypes failed to show a preference for the target quadrant (1-way RM ANOVA F10, 30=0.35, p=0.6365 and F12, 36=1.66, p=0.1989 for WT and NE KO, respectively).
When tested again one week later (figure 4j), both WT and NE KO sham-operated mice maintained a strong preference for the target quadrant (1-way RM ANOVA, F10, 30=9.02, p=0.0035 for WT; F12, 36=4.40, p=0.0248 for NE KO; Dunnett’s tests p<0.05, p<0.001 and p<0.0001 vs. target quadrant, as shown graphically). Brain-injured WT mice showed a persistent deficit in memory retention, with no preference between quadrants at this time (F10, 30=0.17, p=0.8548). In comparison, brain-injured NE KO mice showed memory retention, with a preference for the target quadrant which was similar to shamoperated controls (F12, 36=3.75, p=0.0169; Dunnett’s test p<0.05).
Treatment with an NE inhibitor protects the acutely injured hippocampus
A separate cohort of WT mice were subjected to TBI or sham-operation at p21, followed by acute treatment at 2, 6 and 12 h post-surgery with either vehicle-control or the competitive NE inhibitor ZN200,355. In line with the NE KO study described above, neutrophil infiltration into the acutely injured brain (24 h) was not altered by inhibition of NE, with similar numbers of Gr-1+ neutrophils identified in NE inhibitor-treated and vehicle-control mice in the injured cortex (unpaired t-test t8=1.42, p=0.1979; 1206 ± 124 and 976 ± 106 cells, respectively) and hippocampus (unpaired t-test t8=0.88, p=0.4076; 825 ± 238 and 584 ± 155 cells, respectively; n=5/group). The ability of these neutrophils to release the potent gelatinase pro-MMP-9 at this time was also unchanged by treatment with the NE inhibitor (Mann-Whitney test p=0.2000), with a median band density of 1.69 and 1.52 for vehicle and NE inhibitor-treated samples, respectively (n=4/group).
Cell death was quantified by TUNEL (figure 5a) and activated caspase-3 immuno-labeling (figure 5b) at 24 h post-injury. TUNEL-positive cells in the injured cortex was similar in vehicle and NE inhibitor-treated mice (unpaired t-test t8=0.68, p=0.5205). In contrast, the number of TUNEL-positive cells in the injured hippocampus was reduced by 47% after treatment with the NE inhibitor compared to vehicle controls (unpaired t-test t8=4.36, p=0.0033; n=5/group). Consistent with these observations, the number of cells stained positive for activated caspase-3 was comparable in the injured cortex (unpaired t-test t8=0.37, p=0.7207), but reduced by 53% in the injured hippocampus of NE inhibitor-treated mice compared to saline controls (unpaired t-test t8=3.93, p=0.0057; n=5/group). Acute treatment with the NE inhibitor also reduced the numbers of glial-like cells expressing HO-1 in the cortex compared to saline controls (figure 5c; unpaired t-test t8=3.09, p=0.0214). In the hippocampus, however, the number of HO-1+ cells was not significantly affected by NE inhibition (t8=1.70, p=0.1397).
Figure 5. Treatment with the NE inhibitor is acutely neuroprotective, but does not alter long-term neuroanatomical outcomes.
NE inhibitor or saline-vehicle was administered i.p. at 2, 6 and 12 h post-injury, for analysis at 24 h (n=5/group). Cell death assessed by TUNEL (a) was unchanged in the injured cortex but reduced in the injured hippocampus of NE inhibitor-treated mice compared to vehicle-treated controls (t-test *p<0.01). Caspase-3 immuno-staining (b) confirmed this region-specific effect, with a reduction in apoptotic cells in the hippocampus of NE inhibitor-treated mice compared to saline controls (t-test **p<0.01). In contrast, HO-1+ cells (c) were significantly reduced in the cortex of NE inhibitor-treated mice compared to vehicle, but not different in the hippocampus (t-test *p<0.05). Two months post-injury, the volume of intact ipsilateral dorsal cortex (d) was reduced in brain-injured mice compared to sham controls (2-way ANOVA effect of injury ****p<0.0001), but comparable in mice treated acutely with either saline-vehicle or the NE inhibitor. Likewise, the ipsilateral hippocampal volume differed between sham and injured mice but not saline and NE inhibitor-treated (2-way ANOVA interaction p=0.0135; ****p<0.0001 and **p<0.01 from multiple comparison analyses). Likewise, surviving neurons in the ipsilateral DG were similar regardless of drug treatment (f; t-tests n.s.). n=9–10/group. Abbreviations: HO-1 = heme-oxygenase 1; TUNEL = terminal deoxynucleotidyl transferase-mediated dUTP nick 3’-end labeling.
In contrast to the NE KO study described above, quantification of NeuN density by Western Blot at 24 h post-injury did not reflect the observed treatment effect on cell death, as NeuN intensity in vehicle and NE inhibitor-treated homogenates from either injured cortex or hippocampus were comparable (data not shown; n=4/group). Further, vasogenic edema was unaffected by NE inhibition, with both vehicle-control and NE inhibitor-treated mice showing equivalent water content in the ipsilateral hemisphere at 24 h post-injury (unpaired t-test, t10=0.61, p=0.5535; n=7–8/group).
Early treatment with an NE inhibitor does not alter long-term loss of cortical and hippocampal structures
Long-term tissue integrity was assessed by volumetric and stereological analyses at 2 months post-injury (figure 5d–f). A significant loss of dorsal cortex volume was evident at this time relative to sham-operated mice (2-way ANOVA effect of injury F1, 33=85.11, p<0.0001), which was comparable in both vehicle-control and NE inhibitor-treated mice (effect of drug F1, 33=1.01, p=0.3226; injury × drug interaction F1, 33=2.11, p=0.1556). Volumetric loss of dorsal hippocampal tissue was also considerable after injury (2-way ANOVA interaction F1, 33=6.79, p=0.0135), with Bonferroni’s multiple comparison analyses revealing a loss of hippocampal volume in TBI animals compared to sham controls in both vehicle and NE inhibitor-treated mice (p<0.0001 and p<0.01 sham versus TBI, for vehicle and NE inhibitor-treated mice, respectively). Contralateral to the injury site, both cortex and hippocampus also showed modest evidence of injury-induced volumetric loss (2-way ANOVA effect of injury, F1, 33=5.17, p=0.0296 for cortex; F1, 33=6.49, p=0.0155 for hippocampus; data not shown). However, acute NE inhibition did not alleviate contralateral volume loss in either region (n.s. effect of drug; n.s. injury × drug interaction). Lastly, surviving cells within the ipsilateral DG (granule cell layer) were quantified in brain-injured mice (figure 5f). Acute treatment with the NE inhibitor or vehicle-control resulted in similar cell numbers in the upper blade (unpaired t-test t17=0.62, p=0.5456), lower blade (t17=0.61, p=0.5484) and hilus (t17=1.27, p=0.2226).
Treatment with an NE inhibitor does not ameliorate hyperactivity or long-term spatial memory retention
Hyperactivity, as measured by an increase in distance traveled in the open field arena (figure 6a), was detected at 2 months after TBI at p21 in this cohort (2-way ANOVA effect of injury, F1, 34=15.12, p=0.0004), consistent with previous reports (Pullela et al., 2006). However, contrary to TBI NE KO mice which showed an amelioration of this phenotype compared to WT brain-injured controls (figure 4a), treatment with the NE inhibitor did not alter distance traveled compared to vehicle-treated mice (effect of drug, F1,34=0.10, p=0.7586; injury × drug interaction F1, 34=0.35, p=0.5604).
Figure 6. Treatment with the NE inhibitor ZN200,355 does not alter long-term functional outcomes after TBI at p21.
TBI mice show hyperactivity in the open field as compared to sham controls (a; 2-way ANOVA effect of injury ***p<0.001), however, this was not affected by treatment with the NE inhibitor. Although all mice showed improvement over time on the accelerating rotarod (b; 2-way RM ANOVA effect of time ****p<0.0001), none of the groups differed from each other. Spatial task learning was assessed in the MWM visible sessions (c), revealing an impairment in TBI mice compared to sham (linear mixed effects ANOVA effect of injury **p<0.01), although all groups showed improvement (reduced latency to the platform) over sequential sessions (effect of time ****p<0.0001). Similarly in the MWM hidden platform sessions (d), brain-injured mice exhibited a higher latency to reach the platform compared to sham controls overall (effect of injury ***p<0.001), although all groups again showed improvement across sessions (effect of time ****p<0.0001). No differences were observed between vehicle and NE inhibitor-treated mice in either the visible or hidden platform sessions of the MWM. A probe trial conducted at the end of the testing week (e) revealed a preference for the target quadrant in both vehicle and NE inhibitor-treated sham control mice (***p<0.001 and **p<0.01 from Dunnett’s analyses after 1-way RM ANOVAs within each group), whereas TBI mice did not show such a preference, indicating memory retention deficits in brain-injured mice. When tested again one week later (f), vehicle and NE inhibitor-treated sham groups as well as the vehicle TBI mice showed memory retention, whereas NE inhibitor-treated TBI mice did not (**p<0.01 and *p<0.05). n=9–10/group.
Sensorimotor behavior was assessed by the rotarod (figure 6b). All groups showed improvement across repeated testing (2-way RM ANOVA effect of time F2, 68=49.02, p<0.0001), but neither injury nor treatment with the NE inhibitor influenced rotarod performance (effect of group F3, 34=1.14, p=0.3463; injury × drug interaction F6, 68=0.48, p=0.8205). Also consistent with the NE KO and WT cohorts, neither injury nor NE inhibitor treatment had an impact on anxiety behaviors in the elevated plus and zero mazes (data not shown).
Next, we tested mice for spatial learning and memory in the MWM task. Neither injury nor drug administration altered swimming velocity in the visible platform sessions (data not shown), indicating a lack of visual or motor deficits in this task. All groups showed improvement over time in both the visible (effect of time F1, 417=220.97, p<0.0001) and hidden sessions (effect of time, F1, 645=38.50, p<0.0001; figure 6c–d). However, brain-injured mice showed significant impairments in the MWM task, with TBI mice taking a longer latency to reach the platform during both visible and hidden sessions (linear mixed effects ANOVA effect of injury F1, 34=10.57, p=0.0026, and F1, 34=15.50, p=0.0004; drug × injury interaction F1, 34=1.04, p=0.3148, and F1, 34=0.07, p=0.7883; for visible and hidden, respectively). In contrast, no differences were found between vehicle and inhibitor-treated mice in either the visible (effect of drug F1, 34=1.20, p=0.2810) or hidden sessions (F1, 34=0.91, p=0.3460), indicating that administration of the NE inhibitor acutely after injury did not alter spatial learning and memory.
Probe trials were conducted following hidden platform testing. At the end of the testing week (figure 6e), sham-operated mice treated with either NE inhibitor or vehicle showed a strong preference for the target quadrant over all other quadrants (1-way RM ANOVA F8, 24=3.23, p<0.0001 and F8, 24=1.01, p=0.0003, respectively; Dunnett’s multiple comparison analyses for comparisons with target quadrant, as annotated graphically). TBI impaired memory retention at this time, with a lack of preference for the target quadrant displayed by brain-injured mice treated with either vehicle (1-way RM ANOVA, F8, 24=1.16, p=0.0524) or the NE inhibitor (F8, 24=4.05, p=0.1416). An additional probe trial was conducted one week after MWM testing (figure 6f). At this time, sham-operated mice treated with either the NE inhibitor or vehicle retained a strong preference for the target quadrant over other quadrants (1-way RM ANOVA F8, 24=9.67, p=0.0005 and F8, 24=4.81, p=0.0384, respectively). Surprisingly, in contrast to WT TBI mice in the previous experiment (figure 4), vehicle-treated TBI mice also showed memory retention (1-way RM ANOVA F8, 24=4.66, p=0.0177). However, brain injured mice that received acute treatment with the NE inhibitor failed to show preference for the target quadrant (1-way RM ANOVA F8, 24=2.10, p=0.1495).
Long-term, selective neurological improvement is replicated in NE KO mice, treated acutely with an NE inhibitor
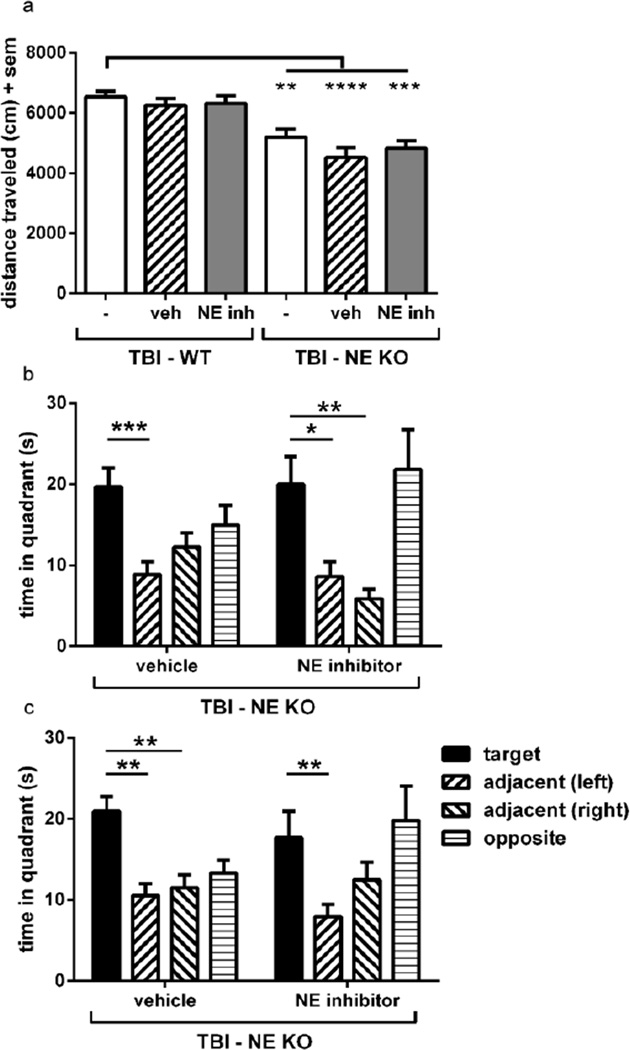
This last finding, of poorer memory retention in brain-injured NE inhibitor-treated mice compared to vehicle-treated controls, raised the question of whether acute treatment with the NE inhibitor potentially had off-target effects which might, inadvertently, be detrimental to spatial memory retention. To address this, we induced TBI in a cohort of NE KO mice and treated with either saline-vehicle or ZN200,355 (n=12–14/group) using the same dosing protocol as previously described (10 mg/kg administered i.p. at 2, 6 and 12 h post-injury), and tested their performance in the MWM and open field at 2 months post-injury. In the open field task (figure 7a), the distance traveled was compared between groups by 1-way ANOVA (F5, 63=10.85, p<0.0001). All brain-injured NE KO groups [NE KO with no treatment (as shown previously – figure 5a), NE KO treated with vehicle, and NE KO treated with the NE inhibitor] showed a reduction in activity compared to the hyperactivity exhibited by WT TBI mice (Dunnett’s multiple comparison analyses annotated graphically), confirming rescue of the hyperactive phenotype which typically results after TBI in WT mice. No confounding effects were observed in NE KO mice treated with or without the NE inhibitor.
Figure 7. Administration of the NE inhibitor ZN200,355 to brain-injured NE KO mice confirms the functional benefits afforded by NE deficiency, without any confounding effects.
Injury-induced hyperactivity in the open field (a) was ameliorated to a similar extent in brain-injured untreated NE KO mice, brain-injured NE KO mice which received vehicle treatment, and brain-injured NE KO which received the NE inhibitor (1-way ANOVA p<0.0001; Dunnett’s analyses ****p<0.0001, ***p<0.001 and **p<0.01). Treatment with the NE inhibitor did not alter the response seen in untreated or vehicle-treated NE KO mice alone. WT data in (a) is reproduced from figures 4a and 6a, for comparison. NE inhibitor treatment also had no effect on brain-injured NE KO mice in terms of spatial memory retention in the MWM probe trials at the end of the testing period (b) and one week later (c), with both vehicle and NE inhibitor-treated NE KO mice showing preference for the target quadrant (1-way RM ANOVAs with Dunnett’s analyses). n=12–14/group.
Brain-injured NE KO mice showed a preference for the target quadrant, indicating memory retention, in the MWM probe trials at the end of the MWM testing week (figure 7b). This was somewhat in contrast to the equivalently-timed probe trial in earlier experiments, whereby preference for the target quadrant failed to reach statistical significance for TBI NE KO mice at this time (figure 4i). In the probe trial conducted one week later (figure 7c), brain-injured NE KO mice treated with either vehicle or the NE inhibitor both showed a preference of the target quadrant, consistent with the preservation of memory retention in brain-injured NE KO mice as shown previously (figure 4j).Further, this effect was evident independent of treatment with the NE inhibitor (1-way RM ANOVA’s with Dunnett’s analyses annotated graphically). Of note, despite showing a preference for the target quadrant, these mice also appeared to spend a considerable amount of time in the opposite quadrant (where they first entered the pool), such that it is unclear whether quadrant preferences are strictly dependent upon spatial memory retention or confounded by quadrant biases due to unknown external cues. Regardless, together these results from the open field and MWM confirm the long-term functional benefits afforded by NE deficiency, and the lack of effect of the NE inhibitor on these outcome measures.
DISCUSSION
Here we have investigated the role of neutrophil-mediated NE in both acute and chronic outcomes after TBI, in the context of the developing brain, using a two-armed approach of genetic deletion and pharmacological inhibition. Activated neutrophils are known contributors to oxidative stress, releasing proteases that collectively create an environment that is hostile to cell survival (Meyer-Hoffert and Wiedow, 2011; Allen et al., 2012). However, strategies to systemically deplete these leukocytes or minimize their trafficking into the parenchyma of the injured brain have had limited success in terms of neuroprotection (Rhodes, 2011). It has been hypothesized that the blunt inhibition of the inflammatory response may be too simplistic of an approach, and that potential neutrophil subtypes, duration of inhibition, and specific mechanisms underlying the consequences of inflammatory cell accumulation (e.g. roles in tissue destruction and would healing) are likely determinants of treatment efficacy. Therefore, we here focused on NE as a specific potent mediator released by activated neutrophils, which is reputed to be a critical determinant of vascular tissue injury in peripheral inflammatory conditions (Zeiher et al., 2002; Meyer-Hoffert and Wiedow, 2011). For the first time, we have demonstrated that deleting the NE gene or inhibiting NE activity results in robust alleviation of acute cell death, strongly implicating NE in early post-injury pathogenesis. While the long-term loss of cortical and hippocampal volumes were not rescued in brain-injured NE KO mice, deficits in cognitive memory and hyperactivity were markedly attenuated. A similar long-term neurobehavioral benefit was not seen in WT mice treated acutely with an NE inhibitor, an outcome that may reflect a suboptimal dosing regime and/or therapeutic window.
NE mediates acute pathogenesis in the injured immature brain
To assess the pathogenicity of NE in the acutely injured brain, we evaluated cell death, neuronal integrity, vasogenic edema, and induction of HO-1, a sensitive indicator of oxidative stress. Acute cell death was clearly reduced by NE gene deficiency in our model, which led to a non-significant trend towards the preservation of neuronal integrity at 24 h post-injury. Although each of these methods in isolation have their limitations (immuno-labeling of TUNEL and caspase-3, and NeuN measured by Western Blot), it is noteworthy that all three measures indicate a lesser degree of cell death in the brains of NE KO mice. Of note, the benefits afforded by NE deficiency were greatest in the hippocampus, contrary to evidence of relatively higher neutrophil numbers accumulating into the injured cortex. Regional specificity of neuroprotection has also been reported after neutrophil depletion in ischemic-reperfusion injury (Beray-Berthat et al., 2003), however, the underlying mechanisms are unclear. This spatial susceptibility may relate to distance from the impact site; the cortex contains the irreversibly damaged lesion core, which is likely beyond possible rescue by therapeutic interventions. In contrast, the more distally located hippocampus may represent the peri-contusional penumbra, a region of microvascular disruption where cells are stressed but potentially salvageable (Liu et al., 2010; Newcombe et al., 2013). In support of this theory, we detected greater numbers of cells positive for activated caspase-3 in the injured hippocampus compared to the cortex, suggesting that hippocampal cell death at this time is largely apoptotic secondary to the initial impact.
Acute post-injury treatment with a specific NE inhibitor also afforded a degree of neuroprotection, with a reduction in cell death and oxidative stress. Consistent with our results, treatment with the same NE inhibitor at 0 and 4 h during reperfusion after transient middle cerebral artery occlusion in mice reduced infarct volume, BBB disruption and edema by 24 h (Stowe et al., 2009). Other inhibitors of NE (ONO-5046 or sivelestat) have also shown efficacy at reducing neuronal loss, edema and vascular permeability after focal ischemia in rats or rabbits (Shimakura et al., 2000; Matayoshi et al., 2009; Ikegame et al., 2010).
It has been proposed that NE activity may promote the ongoing extravasation of neutrophils, by regulating leukocyte adhesion and transmigration (Woodman et al., 1993), ‘clearing the path’ via the degradation of matrix proteins, and jeopardizing BBB integrity (Nagy et al., 1998). While there is some evidence to support this function under tissue-specific and stimulus-specific conditions (Young et al., 2004; Young et al., 2007), NE gene-deficient neutrophils show a normal capacity for transmigration both in vivo and in vitro (Belaaouaj et al., 1998; Tkalcevic et al., 2000; Allport et al., 2002; Hirche et al., 2004), suggesting a largely redundant role for this protease in leukocyte infiltration into tissues. This hypothesis is supported by our current data, as we saw no effect on neutrophil infiltration after TBI in either NE KO or NE inhibitor-treated mice.
Amelioration of brain water content in NE KO mice, despite normal neutrophil infiltration, provides evidence that NE rather than neutrophils per se directly mediates edema formation. Data from ischemic stroke models support this hypothesis, as treatment with an NE inhibitor was shown to reduce brain edema, postulated to result from the preservation of endothelial cell integrity and survival (Ikegame et al., 2010). In contrast, in the current study, pharmacological inhibition of NE did not protect against vasogenic edema in brain-injured mice. This raises a question about the degree to which NE activity was restricted by pharmacological inhibition in our paradigm, and the possibility that incomplete inhibition may account for less robust findings in drug-treated mice compared to NE deficient animals. Unfortunately, it was not technically feasible to directly measure the degree of NE inhibition in this study, as binding between the inhibitor and substrate molecules is voided during assay sample preparation (unpublished observations). Regardless, given that genetic NE deficiency resulted in selective sparing of long-term functional deficits but acute NE inhibition did not, we postulate that the presence or absence of NE-mediated edema may be a critical determinant of long-term outcomes.
Neither NE deficiency nor acute NE inhibition altered the level of MMP-9 activity at 24 h post-injury. One limitation here is that only pro-MMP-9 could be detected by gelatin zymography, suggesting that active MMP-9 levels may be below the detection limits of this assay (Frankowski et al., 2012). In the context of robust neutrophil infiltration which was independent of NE manipulation in this study, the finding that pro-MMP-9 levels are also unaffected is consistent with previous evidence identifying neutrophils as a key contributor to MMP-9 production (Nguyen et al., 2007). However, NE is known to activate MMP-9 and can cleave its natural inhibitor, suggesting that NE may enhance MMP-9 activity (Delclaux et al., 1996; Jackson et al., 2010). That the absence of NE did not alter pro-MMP-9 levels is consistent with results from Stowe and colleagues (2009), who showed additional neuroprotective effects of pharmacological NE inhibition when administered to MMP-9 KO mice after ischemic brain injury (Stowe et al., 2009); together, these findings suggest that NE contributes to pathogenesis in the injured brain by mechanisms that are independent of interactions with MMP-9.
Lastly, we examined the intracellular expression of HO-1 in the acutely injured brain. The robust activation of HO-1 is a ubiquitous cellular response to oxidative stressors including free heme released from the hydrolysis of heme-proteins, as well as signaling by NFκB and heat shock proteins (Ryter and Tyrrell, 2000; Chang et al., 2005). After TBI, HO-1 expression is primarily localized to astrocytes, macrophages and microglia (Fukuda et al., 1995; Fukuda et al., 1996). An increase in HO-1 is a reflection of the pathological state of the tissue, thus the reduction in HO-1 levels seen in NE KO and NE inhibitortreated mice compared to their respective controls indicates a shift towards reduced pathogenesis. Together, these findings implicate NE as an important mediator of cellular stress in the injured brain.
NE deficiency provides long-term functional benefits in the absence of tissue sparing
Given that injury to the developing brain has long-term functional consequences, we investigated whether manipulation of neutrophil-mediated pathogenesis early post-injury influenced long-term outcomes when animals had matured to adulthood. Consistent with previous studies (Pullela et al., 2006), this model of experimental TBI to the p21 mouse did not produce any motor deficits or an anxiety phenotype, and these characteristics were not altered by NE manipulation.
In accordance with acute neuroprotection, NE gene deficiency resulted in long-term benefit on behavioral outcomes, with NE KO mice showing a restoration of TBI-induced hyperactivity and time spent at rest to sham levels, as well as preserved spatial memory retention at adulthood. This may be a reflection of NE KO mice showing functional improvement over time post-injury to a ‘normal’ acquisition of age-appropriate skills; or alternatively, that they never exhibited TBI-induced deficits afforded by acute neuroprotection. Further experiments involving repeated behavioral assessments across the time course of development after injury may address this question.
Of note, however, this functional benefit did not correspond to long-term sparing of tissue in either the injured cortex or hippocampus. Interestingly, other investigators have reported similar discrepancies between functional and structural outcomes in experimental TBI models. For example, Whalen and colleagues reported a reduction in motor and memory function in mice deficient in poly(ADP-ribose) despite comparable cortical contusion volumes (Whalen et al., 1999), while therapeutic hypothermia improved functional outcomes without altering lesion volumes in brain-injured rats (Dixon et al., 1998). Yet others have reported histological sparing in the absence of functional benefit (Clark et al., 2000). While there is no clear explanation for such disparity, it is likely that the pathological processes and substrates which lead to irreversible cell loss over time are incompletely understood. While there is considerable evidence that performance in the MWM relates to hippocampal integrity (Broadbent et al., 2004), we found no differences in the number of surviving hippocampal DG cells between genotypes, suggesting that the preservation in spatial memory retention displayed by NE KO mice is not associated with varying degrees of DG cell loss. Further, we cannot exclude the possibility that NE deficiency or inhibition afforded sparing of neurons in brain regions not examined in the current study, such as the CA1–3 regions of the hippocampus, or neuroanatomical structures which may mediate TBI-induced hyperactivity.
It is also worth considering the mismatch between tissue integrity acutely and chronically post-injury. We originally hypothesized that acute neuroprotection afforded by NE gene deficiency would translate into greater tissue sparing long-term, however, this was not the case. It is becoming evident that the injured immature brain shows an extended period of secondary pathogenesis resulting in progressive expansion of the lesion and continuing loss of hippocampal neurons over time (Pullela et al., 2006; Tsuru-Aoyagi et al., 2009). In the young brain, this pathogenesis is superimposed upon a dynamic system which is rapidly developing, with ongoing neurogenesis, synaptic pruning and myelination occurring well into adolescence (Semple et al., 2013). In line with an emerging role of proteases in synaptic modulation and subsequent cognitive function (Lee et al., 2008), it is plausible that NE activity in the immature brain may influence synaptic reorganization after injury, such that the absence of NE in gene-deficient mice results in improved behavioral outcomes despite the absence of overt tissue sparing. Evidently, the extent to which neutrophil-dependent and independent mechanisms participate in acute cell loss, and the relationship between post-injury tissue disruption and ultimate lesion volumes, requires further elucidation. Nonetheless, as improving functional outcomes is of primary importance to enhance quality of life for brain-injured patients, the long-term reduction in hyperactivity and modest preservation of spatial memory retention in NE KO mice are encouraging.
Acute pharmacological inhibition of NE did not alter long-term outcomes
In contrast to absence of the NE gene, acute NE inhibition failed to alleviate either behavioral or structural outcomes at adulthood after injury, despite evidence of neuroprotection acutely post-injury. The NE inhibitor ZN200,355 did not appear to have any off-target effects, as administration to NE KO mice neither negated nor amplified the functional benefits afforded by NE deficiency. One potential confound here is that vehicle-treated C57Bl/6J mice used in the inhibitor-treated experiments performed somewhat differently in the MWM compared to C57Bl/6 WT littermates used in the NE KO study. Despite our best efforts to generate a consistent genetic background by extensive backcrossing, it is conceivable that phenotypic differences may arise due to residual genetic material in loci flanking manipulated genes (Eisener-Dorman et al., 2009).
Another possible explanation for discrepancies between the gene deficiency and pharmacological inhibition studies is the unique, prolonged nature of the inflammatory response after injury in the developing brain. Neutrophil infiltration into the immature brain is elevated compared to that seen in the adult brain after injury, and extends up to two weeks after injury (Claus et al., 2010). This observation is consistent with others who have identified age-related differences in how the brain responds to inflammatory signals, for example, adult mice are more resistant to inflammation, with less leukocyte recruitment and barrier disruption in response to pro-inflammatory cytokine stimulation compared to 1–3 week old mice (Anthony et al., 1997; Anthony et al., 1998). Despite this, in the current study we adopted a conservative approach for the drug treatment regime, limiting treatment to the first 12 h post-injury, with the goal to ‘first do no harm’ and avoid potential interference with brain development after injury at this age. We hypothesized that acute reduction of NE activity would be sufficient to prevent or diminish the propagation of secondary damage after TBI, potentially with long-term benefit on brain structure and function. In light of our results, however, the lack of long-term effects after acute NE inhibition in our current study may relate to drug administration over an insufficient duration to suppress this prolonged period of neutrophil activity, and subsequent secondary brain damage which occurs. Future preclinical studies are required to address treatment timing and duration, as well as dissect signaling pathways responsible for both neutrophil activation and effector functions in the injured brain.
It is also worth noting here that all of the previous studies examining NE inhibition in the ischemic injured brain have focused solely on acute outcome measures (Shimakura et al., 2000; Matayoshi et al., 2009; Ikegame et al., 2010). Whether observed neuroprotection in these studies translates to long-term functional and neuroanatomical benefit was not determined, and remains unclear. Thus our complex findings in the current study highlight the necessity of examining chronic outcomes after acute drug treatment, particularly in the developing brain in which ongoing maturation may interact with lesion progression.
Contribution of different neutrophil phenotypes in CNS injury
Despite abundant preclinical research, the precise contribution of neutrophils to CNS injury remains controversial (Emerich et al., 2002; Easton, 2013). For example, in the context of stroke, reducing neutrophil infiltration via CD18 or ICAM-1 inhibition generally improves outcomes in animal models (Prestigiacomo et al., 1999); on the contrary, stimulation of neutrophils prior to stroke reduces the burden of disease (Ahmed et al., 2000). Further, clinical trials do not show benefit in stroke patients by neutrophil blockage (Becker, 2002; Krams et al., 2003). Conflicting findings such as these suggest that the contribution of neutrophils to brain injury is likely complex, dependent on cell numbers, timing, stimulus and brain region, as well as effector functions. There is increasing evidence that neutrophils show functional heterogeneity in vivo and in vitro, enabling them to respond to different stimuli in a context-dependent manner (Fridlender et al., 2009). For example, neutrophil phenotypes are adopted depending upon environmental cues in the context of tumor metastasis, with ‘N1’ designated as the pro-inflammatory subtype and ‘N2’ being anti-inflammatory and pro-angiogenic (Piccard et al., 2012). Given its role in promoting oxidative stress and inflammation, we hypothesize that NE is likely to be produced by N1 neutrophils, however this has not yet been confirmed. Of note, a recent study in ischemic stroke suggests that polarization of neutrophils from an N1 to N2 phenotype is associated with neutrophil clearance and resolution of inflammation, and that this transformation can be modulated by activation of the peroxisome proliferator-activated receptor-γ nuclear receptor (Cuartero et al., 2013). Future studies are needed to elucidate the contribution of neutrophil subsets to pathogenesis after TBI, across the time course of parenchyma accumulation post-injury, and particularly in the context of prolonged and exacerbated neutrophil infiltration as seen after injury to the immature brain (Claus et al., 2010).
Clinical relevance and translation
Although limited in scope compared to preclinical studies, there is evidence of a robust neutrophilic response after TBI in humans. In addition to post-mortem evidence of inflammatory infiltrates in injured brain parenchyma (Holmin et al., 1995; Hausmann et al., 1999), increased neutrophil activation has been reported in both adults (Rhind et al., 2010; Junger et al., 2013) and children (Fitrolaki et al., 2013) after severe TBI relative to healthy controls. Further, neutrophil-derived proteases including NE and MMP-9 are elevated in patient serum after CNS injury and stroke (Cojocaru et al., 1006; Iwatsuki et al., 1998; Roberts et al., 2013), and patients with severe head trauma (Glasgow Coma Score < 8) also present with elevated serum NE compared to controls (Mussack et al., 2001). Additional preclinical studies to fully elucidate the role of neutrophil-mediated NE would thus have strong clinical relevance.
However, new therapeutic options in pediatric populations must be approached with great caution, as treatment would be superimposed upon a brain which is still undergoing considerable maturational changes. Interventions which may prove efficacious in the adult brain can not necessarily be extrapolated to function similarly or safely in the developing brain (Natale et al., 2006). Of note, inhibition of human NE using the compound sivelestat (ONO 5046, Ono Pharmaceuticals Co., Ltd.) has demonstrated safety in a pediatric population for acute respiratory distress syndrome (Kikuchi et al., 2006; Matsumoto et al., 2009), as well as efficacy attenuating the post-operative inflammatory response and improving clinical outcomes after cardiopulmonary surgery in children (Inoue et al., 2013; Kohira et al., 2013). Although acute NE inhibition failed to improve long-term outcomes in the current study, these clinical findings suggest that administration of optimized NE inhibitors to pediatric patients may be a safe and feasible option after pediatric TBI. Particularly given the uniquely exacerbated neutrophil response to injury which is observed in the immature brain, targeting this cell population remains an attractive option.
CONCLUSIONS
In summary, reduction of NE by both gene deficiency and drug inhibition mediates robust, acute neuroprotection after injury to the immature brain, indicating a contribution of NE to neuropathology. While pharmacological inhibition of NE did not alter the long-term functional or histological consequences of TBI, NE gene deficiency resulted in improved behavioral outcomes, validating NE as an important therapeutic target in pediatric TBI. These findings demonstrate the central role of NE to acute pathogenesis, and support further exploration of a therapeutic window during the prolonged period of neutrophil trafficking into the injured immature brain. The lack of tissue sparing chronically, in contrast to acute neuroprotection, highlights the importance of examining long-term outcomes to thoroughly evaluate the consequences of experimental manipulations to the developing brain.
Table 1.
Summary of acute and long-term outcomes after pediatric TBI in NE KO and NE inhibitor-treated mice
| Measure | Marker | Gene deficiency |
Pharmacological inhibition |
|
|---|---|---|---|---|
| Acute outcomes | Neutrophil infiltration | Gr-1 IHC | no difference | no difference |
| Cell death | TUNEL, caspase-3 | reduced in hpc | reduced in hpc | |
| Oxidative stress | HO-1 | reduced in hpc | reduced in ctx | |
| Brain edema | water content | reduced | no difference | |
| Neuronal integrity | stereology | no difference | no difference | |
| Long-term outcomes | Hyperactivity | open field | reduced | no difference |
| Spatial memory | MWM probe trials | memory retention | no retention |
Change indicated relative to control (WT or saline-vehicle groups, respectively). Ctx = injured cortex only; hpc = injured hippocampus only; HO-1 = heme-oxygenase-1; IHC = immunohistochemistry; MWM = Morris Water Maze; NE = neutrophil elastase.
Highlights.
Neutrophils release neutrophil elastase (NE) in the injured, immature brain.
NE is a key mediator of cell injury in the acutely injured, immature brain.
Pharmacologic inhibition or genetic deficiency of NE confers acute neuroprotection.
Acute pharmacologic blockade of NE does not alleviate behavioral deficits.
Genetic deficiency of NE supports selective, long-term behavioral recovery.
ACKNOWLEDGEMENTS
The authors would like to thank S.A. Canchola (Neurobehavioral Core for Rehabilitation Research, UCSF) for assistance with behavioral assays; L.M. Coussens (Oregon Health and Science University) for guidance with the NE activity assay; S. LaHue and A. Ekeledo for technical assistance; K. Eilertson (Gladstone Institutes Bioinformatics Core, UCSF) for assistance with MWM statistical analyses; and L. Martensson (AstraZeneca) for kindly providing the NE inhibitor ZN200,355.
FUNDING: This work was supported by NIH/NINDS R01 NS050159 and NS077767 (L.J. Noble-Haeusslein), a CJ Martin Biomedical Postdoctoral Fellowship from the National Health and Medical Research Council of Australia (B.D. Semple), and a Sir Keith Murdoch Postdoctoral Fellowship from the American Australian Association (B.D. Semple).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
Contributor Information
Alpa Trivedi, Email: Alpa.Mahuvakar@ucsf.edu.
Kayleen Gimlin, Email: Kayleen.Gimlin@ucsf.edu.
Linda J Noble-Haeusslein, Email: Linda.Noble@ucsf.edu.
REFERENCES
- Ahmed SH, He YY, Nassief A, Xu J, Xu XM, Hsu CYea. Effects of lipopolysaccharide priming on acute ischemic brain injury. Stroke. 2000;31:193–199. doi: 10.1161/01.str.31.1.193. [DOI] [PubMed] [Google Scholar]
- Allen C, Thornton P, Denes A, McColl BW, Pierozynski A, Monestier M, Pinteaux E, Rothwell N, Allan SM. Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J Immunol. 2012;189:381–392. doi: 10.4049/jimmunol.1200409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport JR, Lim YC, Shipley JM, Senior RM, Shapiro SD, Matsuyoshi N, Vestweber D, Luscinskas FW. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J Leukoc Biol. 2002;71:821–828. [PubMed] [Google Scholar]
- Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. 10 years outcome from childhood traumatic brain injury. Int J Dev Neurosci. 2012;30:217–224. doi: 10.1016/j.ijdevneu.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8:923–926. doi: 10.1016/s0960-9822(07)00373-9. [DOI] [PubMed] [Google Scholar]
- Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120:435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Yasuda K, Yasui S, Tamaoki J, Nagai A. Serine proteases increase oxidative stress in lung cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L556–L564. doi: 10.1152/ajplung.2001.281.3.L556. [DOI] [PubMed] [Google Scholar]
- Bank U, Ansorge S. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity control. J Leukoc Biol. 2001;69:197–206. [PubMed] [Google Scholar]
- Bao F, Shultz SR, Hepburn JD, Omana V, Weaver LC, Cain DP, Brown A. A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats. J Neurotrauma. 2012;29:2375–2392. doi: 10.1089/neu.2012.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;19:s18–s22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4:615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- Beray-Berthat V, Croci N, Plotkine M, Margaill I. Polymorphonuclear neutrophils contribute to infarction and oxidative stress in the cortex but not in the striatum after ischemia–reperfusion in rats. Brain Res. 2003;987:32–38. doi: 10.1016/s0006-8993(03)03224-4. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Claus CP, Vreman HJ, Wong RJ, Noble-Haeusslein LJ. Heme regulation in traumatic brain injury: relevance to the adult and developing brain. J Cereb Blood Flow Metab. 2005;25:1401–1417. doi: 10.1038/sj.jcbfm.9600147. [DOI] [PubMed] [Google Scholar]
- Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma. 1994;11:499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- Clark RS, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, Jin KL, Graham SH. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem. 2000;74:740–753. doi: 10.1046/j.1471-4159.2000.740740.x. [DOI] [PubMed] [Google Scholar]
- Claus CP, Tsuru-Aoyagi K, Adwanikar H, Walker B, Whetstone W, Noble-Haeusslein LJ. Age is a determinant of the inflammatory response and loss of cortical volume after traumatic brain injury. Dev Neurosci. 2010;32:454–465. doi: 10.1159/000316805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojocaru IM, Cojocaru M, Burcin C. Evaluation of granulocyte elastase as a sensitive diagnostic parameter of inflammation in first ischemic stroke. Rom J Intern Med. 1006;44:317–321. [PubMed] [Google Scholar]
- Cousar JL, Lai Y, Marco CD, Bayir H, Adelson PD, Janesko-Feldman KL, Kochanek PM, Clark RS. Heme oxygenase 1 in cerebrospinal fluid from infants and children after severe traumatic brain injury. Dev Neurosci. 2006;28:342–347. doi: 10.1159/000094160. [DOI] [PubMed] [Google Scholar]
- Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbí AL, Lizasoain I, Moro MA. N2 neutrophils, novel players in brain inflammation after stroke. Stroke. 2013;44:3498–3508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Haley T, Duvoisin RM, Raber J. Measures of anxiety, sensorimotor function, and memory in male and female mGluR4−/− mice. Behav Brain Res. 2012;229:21–28. doi: 10.1016/j.bbr.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclaux C, Delacourt C, D'Ortho MP, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol. 1996;14:288–295. doi: 10.1165/ajrcmb.14.3.8845180. [DOI] [PubMed] [Google Scholar]
- Dinkel K, Dhabhar FS, Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci USA. 2004;101:331–336. doi: 10.1073/pnas.0303510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Markgraf CG, Angileri F, Pike BR, Wolfson B, Newcomb JK, Bismar MM, Blanco AJ, Clifton GL, Hayes RL. Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat. J Neurotrauma. 1998;15:95–103. doi: 10.1089/neu.1998.15.95. [DOI] [PubMed] [Google Scholar]
- Dredge BK, Jensen KB. NeuN/Rbfox3 Nuclear and Cytoplasmic Isoforms Differentially Regulate Alternative Splicing and Nonsense-Mediated Decay of Rbfox2. PLoS ONE. 2011;6:e21585. doi: 10.1371/journal.pone.0021585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton AS. Neutrophils and stroke - can neutrophils mitigate disease in the central nervous system? Int Immunopharmacol. 2013;17:1218–1225. doi: 10.1016/j.intimp.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Eisener-Dorman AF, Lawrence DA, Bolivar VJ. Cautionary insights on knockout mouse studies: the gene or not the gene? Brain Behav Immun. 2009;23:318–324. doi: 10.1016/j.bbi.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MB, Jallo JJ, Tuma RF. An investigation of cerebral edema and injury volume assessments for controlled cortical impact injury. J Neurosci Methods. 2008;168:320–324. doi: 10.1016/j.jneumeth.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Dean RLr, Bartus RT. The role of leukocytes following cerebral ischemia: pathogenic variable or bystander reaction to emerging infarct? Exp Neurol. 2002;173:168–181. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- Fan P, Yamauchi T, Noble L, Ferriero D. Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. J Neurotrauma. 2003;20:437–445. doi: 10.1089/089771503765355513. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26:447–452. doi: 10.1165/ajrcmb.26.4.4473. [DOI] [PubMed] [Google Scholar]
- Fitrolaki DM, Dimitriou H, Kalmanti M, Briassoulis G. CD64-neutrophil expression and stress metabolic patterns in early sepsis and severe traumatic brain injury in children. BMC Pediatrics. 2013;13:31. doi: 10.1186/1471-2431-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankowski H, Gu YH, Heo JH, Milner R, del Zoppo GJ. Use of gel zymography to examine matrix metalloproteinases (gelatinase) expression in brain tissue or in primary glial cultures. Methods Mol Biol. 2012;814:221–233. doi: 10.1007/978-1-61779-452-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling Lea. Polarization of tumor-associated neutrophil phenotype by TGF-beta: 'N1' versus 'N2' TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Panter SS, Sharp FR, Noble LJ. Induction of heme oxygenase-1 (HO-1) after traumatic brain injury in the rat. Neurosci Lett. 1995;199:127–130. doi: 10.1016/0304-3940(95)12042-3. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Richmon JD, Sato M, Sharp FR, Panter SS, Noble LJ. Induction of heme oxygenase-1 (HO-1) in glia after traumatic brain injury. Brain Res. 1996;736:68–75. doi: 10.1016/0006-8993(96)00680-4. [DOI] [PubMed] [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE. The Open Field Test. Neuromethods. 2009;42:1–20. [Google Scholar]
- Grenda DS, Johnson SE, Mayer JR, McLemore ML, Benson KF, Horwitz M, Link DC. Mice expressing a neutrophil elastase mutation derived from patients with severe congenital neutropenia have normal granulopoiesis. Blood. 2002;100:3221–3228. doi: 10.1182/blood-2002-05-1372. [DOI] [PubMed] [Google Scholar]
- Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM, Zhang C, Giritharan AB, Purnell W, Robinson CRn, Shin D, Schroeder VA, Suckow MA, Simonyi AY, Sun G, Mobashery S, Cui J, Chang M, Gu Z. Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One. 2013;8:e76904. doi: 10.1371/journal.pone.0076904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R, Kaiser A, Lang C, Bohnert M, Betz P. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int J Legal Med. 1999;112:227–232. doi: 10.1007/s004140050241. [DOI] [PubMed] [Google Scholar]
- Hirche TO, Atkinson JJ, Bahr S, Belaaouaj A. Deficiency in neutrophil elastase does not impair neutrophil recruitment to inflamed sites. Am J Respir Cell Mol Biol. 2004;30:576–584. doi: 10.1165/rcmb.2003-0253OC. [DOI] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T, Shetye J, Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta Neurochir (Wien) 1995;132:110–119. doi: 10.1007/BF01404857. [DOI] [PubMed] [Google Scholar]
- Ikegame Y, Yamashita K, Hayashi S, Yoshimura S, Nakashima S, Iwama T. Neutrophil elastase inhibitor prevents ischemic brain damage via reduction of vasogenic edema. Hypertension Res. 2010;33:704–707. doi: 10.1038/hr.2010.58. [DOI] [PubMed] [Google Scholar]
- Inoue N, Oka N, Kitamura T, Shibata K, Itatani K, Tomoyasu T, Miyaji K. Neutrophil elastase inhibitor sivelestat attenuates perioperative inflammatory response in pediatric heart surgery with cardiopulmonary bypass. Int Heart J. 2013;54:149–153. doi: 10.1536/ihj.54.149. [DOI] [PubMed] [Google Scholar]
- Iwamoto S, Higashi A, Ueno T, Goto M, Iguro Y, Sakata R. Protective effect of sivelestat sodium hydrate (ONO-5046) on ischemic spinal cord injury. Interact Cardiovasc Thorac Surg. 2009;8:606–609. doi: 10.1510/icvts.2008.197244. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Kumura E, Yoshimine T, Yamamoto K, Sato M, Hayakawa T. Increase in jugular levels of polymorphonuclear neutrophil elastase in patients with acute cerebral infarction. Neurol Res. 1998;20:397–402. doi: 10.1080/01616412.1998.11740537. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Xu X, Wilson L, Weathington NM, Clancy JP, Blalock JE, Gaggar A. Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction. Mol Med. 2010;16:159–166. doi: 10.2119/molmed.2009.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger WG, Rhind SG, Rizoli SB, Cuschieri J, Baker AJ, Shek PN, Hoyt DB, Bulger EM. Prehospital hypertonic saline resuscitation attenuates the activation and promotes apoptosis of neutrophils in patients with severe traumatic brain injury. Shock. 2013;40:366–374. doi: 10.1097/SHK.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol. 2002;451:1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- Kenne E, Erlandsson A, Lindbom L, Hillered L, Clausen F. Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J Neuroinflammation. 2012;9 doi: 10.1186/1742-2094-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Kugimiya T, Katashima S. Usefulness of sivelestat sodium administration in patients with aspiration pneumonia. J Anesth. 2006;20 doi: 10.1007/s00540-006-0435-4. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Administration of either anti-intercellular adhesion molecule-1 or a nonspecific control antibody improves recovery after traumatic brain injury in the rat. J Neurotrauma. 2002;19:1039–1050. doi: 10.1089/089771502760341956. [DOI] [PubMed] [Google Scholar]
- Kohira S, Oka N, Inoue N, Itatani K, Hanayama N, Kitamura T, Fujii M, Takeda A, Oshima H, Tojo K, Yoshitake S, Miyaji K. Effect of the Neutrophil Elastase Inhibitor Sivelestat on Perioperative Inflammatory Response After Pediatric Heart Surgery with Cardiopulmonary Bypass: A Prospective Randomized Study. Artif Organs. 2013;37:1027–1033. doi: 10.1111/aor.12103. [DOI] [PubMed] [Google Scholar]
- Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: Physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA Investigators AS. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- Kraupp BG, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (tunel assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: A cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Langlois JA. Atlanta, GA: Division of Acute Care, Rehabilitation Research and Disability Prevention, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Department of Health and Human Services; 2000. Traumatic brain injury in the United States: Assessing outcomes in children: Summary and recommendations from the Expert Working Group, October 26–27. [Google Scholar]
- Lee TW, Tsang VW, Birch NP. Synaptic plasticity-associated proteases and protease inhibitors in the brain linked to the processing of extracellular matrix and cell adhesion molecules. Neuron Glia Biol. 2008;4:223–234. doi: 10.1017/S1740925X09990172. [DOI] [PubMed] [Google Scholar]
- Liu S, Levine SR, Winn HR. Targeting ischemic penumbra Part 1: from physiology to therapeutic strategy. J Exp Stroke Transl Med. 2010;3:47–55. doi: 10.6030/1939-067x-3.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matayoshi H, Hirata T, Yamashita S, Ishida K, Mizukami Y, Gondo T, Matsumoto M, Sakabe T. Neutrophil elastase inhibitor attenuates hippocampal neuronal damage after transient forebrain ischemia in rats. Brain Res. 2009;1259:98–106. doi: 10.1016/j.brainres.2008.12.070. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Hidaka S, Goto K, Hagiwara S, Shingu C, Iwasaka H, Noguchi T. Sivelestat treatment for acute respiratory distress syndrome in an infant. J Anesth. 2009;23:288–291. doi: 10.1007/s00540-008-0735-y. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Nichols WW, Nicolini FA, Hendricks J, Donnelly WH, Saldeen TG. Neutrophil elastase inhibitor ICI 200,880 protects against attenuation of coronary flow reserve and myocardial dysfunction following temporary coronary artery occlusion in the dog. Cardiovasc Res. 1994;28:947–956. doi: 10.1093/cvr/28.7.947. [DOI] [PubMed] [Google Scholar]
- Meyer-Hoffert U, Wiedow O. Neutrophil serine proteases: mediators of innate immune responses. Curr Opin Hematol. 2011;18:19–24. doi: 10.1097/MOH.0b013e32834115d1. [DOI] [PubMed] [Google Scholar]
- Mussack T, Biberthaler P, Gippner-Steppert C, Kanz KG, Wiedemann E, Mutschler W, Jochum M. Early cellular brain damage and systemic inflammatory response after cardiopulmonary resuscitation or isolated severe head trauma: a comparative pilot study on common pathomechanisms. Resuscitation. 2001;49:193–199. doi: 10.1016/s0300-9572(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Kolev K, Csonka E, Vastag M, Machovich R. Perturbation of the integrity of the bloodbrain barrier by fibrinolytic enzymes. Blood Coagul Fibrinolysis. 1998;9:471–478. doi: 10.1097/00001721-199809000-00003. [DOI] [PubMed] [Google Scholar]
- Natale JE, Joseph JG, Pretzlaff RK, Silber TJ, Guerguerian AM. Clinical trials in pediatric traumatic brain injury: unique challenges and potential responses. Dev Neurosci. 2006;28:276–290. doi: 10.1159/000094154. [DOI] [PubMed] [Google Scholar]
- Newcombe VF, Williams GB, Outtrim JG, Chatfield D, Gulia Abate M, Geeraerts T, Manktelow A, Room H, Mariappen L, Hutchinson PJ, Coles JP, Menon DK. Microstructural basis of contusion expansion in traumatic brain injury: insights from diffusion tensor imaging. J Cereb Blood Flow Metab. 2013;33:855–862. doi: 10.1038/jcbfm.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HX, O’Barr TJ, Anderson AJ. Polymorphonuclear leukocytes promote neurotoxicity through release of matrix metalloproteinases, reactive oxygen species, and TNF-α. J Neurochem. 2007;102:900–912. doi: 10.1111/j.1471-4159.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan SH, Gannon DE, Ward PA, Karmiol S. Mechanism of neutrophil-induced xanthine dehydrogenase to xanthine oxidase conversion in endothelial cells: evidence of a role for elastase. Am J Respir Cell Mol Biol. 1992;6:270–278. doi: 10.1165/ajrcmb/6.3.270. [DOI] [PubMed] [Google Scholar]
- Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RDC. nlme: Linear and Nonlinear Mixed Effects Models. R package version. 2012;3:1–104. [Google Scholar]
- Potts M, Koh S-E, Whetstone W, Walker B, Yoneyama T, Claus C, Manvelyan H, Noble-Haeusslein L. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. Neuroreport. 2006;3:143–153. doi: 10.1016/j.nurx.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestigiacomo CJ, Kim SC, Connolly JES, Liao H, Yan SF, Pinsky DJ. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused by not nonreperfused stroke. Stroke. 1999;30:1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- Pullela R, Raber J, Pfankuch T, Ferriero DM, Claus CP, Koh S-E, Yamauchi T, Rola R, Fike JR, Noble-Haeusslein LJ. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Dev Neurosci. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- Rhind SG, Crnko NT, Baker AJ, Morrison LJ, Shek PN, Scarpelini S, Rizoli SB. Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. J Neuroinflammation. 2010;18:5. doi: 10.1186/1742-2094-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. Peripheral immune cells in the pathology of traumatic brain injury? Curr Opin Crit Care. 2011;17:122–130. doi: 10.1097/MCC.0b013e3283447948. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Jenne CN, Léger C, Kramer AH, Gallagher CN, Todd S, Parney IF, Doig CJ, Yong VW, Kubes P, Zygun DA. A prospective evaluation of the temporal matrix metalloproteinase response after severe traumatic brain injury in humans. J Neurotrauma. 2013;30:1717–1726. doi: 10.1089/neu.2012.2841. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;15:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Semple BD, Canchola SA, Noble-Haeusslein L. Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J Neurotrauma. 2012;29:2672–2683. doi: 10.1089/neu.2012.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Bye N, Ziebell JM, Morganti-Kossmann MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol Dis. 2010a;40:394–403. doi: 10.1016/j.nbd.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010b;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD. Neutrophil Elastase: Path Clearer, Pathogen Killer, or Just Pathologic? Am J Respir Cell Mol Biol. 2002;26:266–268. doi: 10.1165/ajrcmb.26.3.f233. [DOI] [PubMed] [Google Scholar]
- Shimakura A, Kamanaka Y, Ikeda Y, Kondo K, Suzuki Y, Umenura K. Neutrophil elastase inhibition reduces cerebral ischemic damage in the middle cerebral artery occlusion. Brain Res. 2000;858:55–60. doi: 10.1016/s0006-8993(99)02431-2. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 Leukocytes after Spinal Cord Injury in Mice Alters Wound Healing and Worsens Neurological Outcome. The Journal of Neuroscience. 2009;29:753–764. doi: 10.1523/JNEUROSCI.4918-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe AM, Adair-Kirk TL, Gonzales ER, Perez RS, Shah AR, Park TS, Gidday JM. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol Dis. 2009;35:82–90. doi: 10.1016/j.nbd.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2012. http://www.R-project.org/. [Google Scholar]
- Tiefenbacher CP, Ebert M, Niroomand F, Batkai S, Tillmanns H, Zimmermann R, Kübler W. Inhibition of elastase improves myocardial function after repetitive ischaemia and myocardial infarction in the rat heart. Pflugers Arch. 1997;433:563–570. doi: 10.1007/s004240050315. [DOI] [PubMed] [Google Scholar]
- Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E, Nishisho T. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem. 2001;78:1064–1072. doi: 10.1046/j.1471-4159.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- Tong W, Igarashi T, Ferriero DM, Noble LJ. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp Neurol. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- Tsuru-Aoyagi K, Potts M, Trivedi A, Pfankuch T, Raber J, Wendland M, Claus C, Koh S-E, Ferriero D, Noble-Haeusslein L. Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann Neurol. 2009;65:540–549. doi: 10.1002/ana.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinzierl M, Mautes AE, Whetstone W, Lin Y, Noble-Haeusslein LJ. Endothelin-mediated induction of heme oxygenase-1 in the spinal cord is attenuated in transgenic mice overexpressing superoxide dismutase. Brain Res. 2004;1030:125–132. doi: 10.1016/j.brainres.2004.09.060. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;329:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Clark RS, Dixon CE, Robichaud P, Marion DW, Vagni V, Graham SH, Virag L, Hasko G, Stachlewitz R, Szabo C, Kochanek PM. Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) J Cereb Blood Flow Metab. 1999;19:835–842. doi: 10.1097/00004647-199908000-00002. [DOI] [PubMed] [Google Scholar]
- Williams JC, Falcone RC, Knee C, Stein RL, Strimpler AM, Reaves B, Giles RE, Krell RD. Biologic characterization of ICI 200,880 and ICI 200,355, novel inhibitors of human neutrophil elastase. Am Rev Respir Dis. 1991;144:875–883. doi: 10.1164/ajrccm/144.4.875. [DOI] [PubMed] [Google Scholar]
- Woodman RC, Reinhardt PH, Kanwar S, Johnston FL, Kubes P. Effects of human neutrophil elastase (HNE) on neutrophil function in vitro and in inflamed microvessels. Blood. 1993;82:2188–2195. [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011 doi: 10.1002/0471142301.ns0826s56. Chapter 8, Unit 8.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Clark MH, Goffus A, Hoane MR. Mixed effects modeling of Morris water maze data: Advantages and cautionary notes. Learning and Motivation. 2009;40:160–177. [Google Scholar]
- Young RE, Voisin MB, Wang S, Dangerfield J, Nourshargh S. Role of neutrophil elastase in LTB4-induced neutrophil transmigration in vivo assessed with a specific inhibitor and neutrophil elastase deficient mice. Br J Pharmacol. 2007;151:628–637. doi: 10.1038/sj.bjp.0707267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RE, Thompson RD, Larbi KY, La M, Roberts CE, Shapiro SD, Perritti M, Nourshargh S. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J Immunol. 2004;172:4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]
- Zeiher BG, Matsuoka S, Kawabata K, Repine JE. Neutrophil elastase and acute lung injury: Prospects for sivelestat and other neutrophil elastase inhibitors as therapeutics. Crit Care Med. 2002;30:S281–S287. doi: 10.1097/00003246-200205001-00018. [DOI] [PubMed] [Google Scholar]
- Zhou X, Dai Q, Huang X. Neutrophils in acute lung injury. Front Biosci (Landmark Ed) 2012;17:2278–2283. doi: 10.2741/4051. [DOI] [PubMed] [Google Scholar]