Abstract
Background
A prolonged PR interval is known to be a poor prognostic factor in cardiovascular disease. The aim of this study was to investigate the association between PR interval and clinical outcome in patients undergoing radiofrequency catheter ablation (RFCA) of atrial fibrillation (AF).
Methods and Results
We prospectively included 576 patients with AF (75.5% male, 57.8±11.6 years old, 68.8% paroxysmal AF) who underwent RFCA. We analyzed preprocedural sinus rhythm ECGs obtained in the absence of antiarrhythmic drug, and all enrolled patients were categorized into 4 groups based on the quartile values of the PR interval (166, 182, and 202 ms), and were analyzed according to the left atrium (LA) volume (CT; Computed tomography), LA voltage (NavX), and clinical outcome of AF ablation. Based on quartile value of PR interval, the highest quartile of PR interval (Q4; PR ≥202 ms) was oldest (P<0.001), and most likely to have persistent AF (P<0.001) and hypertension (P=0.013) compared with the other groups. However, there was no significant difference in LA conduction velocity and atrial effective refractory period. Q4 had the greatest LA dimension (P<0.001) and volume index (P<0.001), and lowest LA appendage‐emptying velocity (P<0.032) and LA voltage (P<0.001) compared with the others. For 13.1±7.5 months, the classification based on the PR interval was a significant predictor of AF recurrence after RFCA of AF (HR=1.969, 95% CI 1.343 to 2.886, P=0.001).
Conclusions
The PR interval was closely associated with advanced LA remodeling due to AF, and had a noninvasive significant predictive value of clinical recurrence of AF after RFCA.
Keywords: atrial fibrillation, catheter ablation, PR interval, recurrence, remodeling
Introduction
Attention to the P wave indices, such as the maximum duration or dispersion, and PR intervals has been increasing for various cardiovascular diseases including atrial fibrillation (AF). The P wave indices are significantly prolonged in hypertension,1 diabetes,2 and cardiovascular disease.3 A prolonged PR interval has been reported to be associated with an increased risk of coronary artery disease, heart failure, and pacemaker implantations.4 A large community‐based cohort study revealed a higher incidence of AF among patients with a prolonged PR interval.5 The maintenance mechanism of AF represents a heterogeneous focal scarring of atrial tissue and atrial structural remodeling, which results in an inhomogeneous recovery and slow conduction.6 Atrial substrate remodeling is initiated by hemodynamic, oxidative, or ischemic stress and induces atrial inflammation, matrix remodeling, and atrial fibrosis. Remodeling associated with abnormal propagation of atrial impulses might be reflected in the electrocardiography findings, such as the PR interval or abnormal P wave indices. A previous study has shown a higher recurrence rate of AF after cardioversion in patients who had longer P wave indices.7 However, most AF studies on P waves were cross‐sectional designed and had a short follow‐up duration or small sample size without any invasive mapping validation.8 Therefore, we analyzed and compared the P wave indices and PR intervals with clinical or prognostic factors and detailed intracardiac electrophysiologic parameters in patients who underwent radiofrequency catheter ablation (RFCA) of AF. Our hypothesis was that the PR interval or P wave indices are associated with the electroanatomical remodeling of the left atrium (LA) and predict the clinical outcome of the RFCA of AF during the long‐term follow‐up.
Methods
Study Population
The study protocol adhered to the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University Health System. All patients provided written informed consent. Among 1253 consecutive patients in the Yonsei AF Ablation Cohort (June 2009 to September 2013), 576 patients were included who had a pre‐procedural ECG with sinus rhythm without any anti‐arrhythmic drug effect. The majority of the patient population (68.8%) had paroxysmal AF (PAF) and 31.2% had persistent AF. The study's exclusion criteria were as follows: (1) permanent AF refractory to electrical cardioversion; (2) AF with moderate to severe mitral stenosis or regurgitation, any mechanical or bioprosthetic heart valve, or mitral valve repair; (3) associated structural heart disease other than left ventricular hypertrophy; (4) clinically significant atrioventricular (AV) block ≥2nd degree, and (5) prior AF ablation. We also excluded (6) patients who had taken amiodarone due to its long half‐life, and (7) those did not have sinus rhythm ECG taken within a day before the procedure. Three‐dimensional (3D) spiral computed tomography (CT) scans (64 Channel, Light Speed Volume CT; Philips, Brilliance 63, Netherlands) were performed to visually define the pulmonary vein (PV) anatomy. All antiarrhythmic drugs were discontinued for a period corresponding to at least 5 half‐lives. Anticoagulation therapy was maintained before the catheter ablation.
Measurement of the Electrocardiographic Parameters
We analyzed the standard 12‐lead ECG in all patients (GE Healthcare, Marquette, MAC5500, Waukesha, WI). The paper speed was 25 mm/s with a calibration of 10 mm/mV. The heart rate, PR interval, QRS, QTc, and P‐axis were automatically measured by the ECG system. The P wave analyses were conducted in leads I, II, III, aVR, aVL, aVF, and V1 by a technician who was blinded to the clinical characteristics. The duration and amplitude of the P waves were manually measured using a customized software (Cardio Calipers, version 3.3). In lead V1, both the positive and negative P‐terminal forces were measured. The technician was asked to repeat the measurement of same ECG parameters, and the correlation coefficient for the intraclass correlation was 0.96 (P<0.001).
Echocardiographic and CT Evaluation of the Heart
All patients underwent trans‐thoracic echocardiography (Sonos 5500, Philips Medical System, Andover, MA or Vivid 7; GE Vingmed Ultrasound, Horten, Norway) prior to the RFCA. The chamber size, transmitral Doppler flow velocity, and ratio of the early diastolic mitral peak mitral inflow velocity and early diastolic mitral annular velocity (E/Em) were acquired following the American Society of Echocardiography guidelines.9–10 Transesophageal echocardiography was performed to exclude any intracardiac thrombi. The emptying velocity of the LA appendage was measured in all patients.
Electroanatomical Mapping and LA CT Measurements
A 3D electroanatomical map (NavX; St. Jude Medical Inc, Minnetonka, MN) was generated using a circular PV mapping catheter (Lasso; Biosense‐Webster Inc, Diamond Bar, CA). The NavX system‐generated 3D geometry of the LA and PVs was merged with the corresponding 3D spiral CT images. LA voltage maps were generated after circumferential PV isolation by obtaining contact bipolar electrograms from 350 to 500 points on the LA endocardium during atrial pacing at 500 ms, and the mean LA voltage was calculated as previously described.11–12 A blinded technician analyzed the color‐coded CT‐merged NavX voltage maps with customized software (Image Pro) as previously described.12–14 To calculate the LA conduction velocity, the conduction distance from the earliest activation site at the septum to the latest activation point at the LA appendage was measured on the anterior–posterior view of the isochronal map and was divided by the time difference as previously described (Figure 1).12,14 The reference distance was measured by the interelectrode distances of the coronary sinus catheter (duodecapolar catheter; St. Jude Medical Inc, Minnetonka, MN). The 3D spiral CT images of the LA were analyzed on an imaging processing workstation (Aquarius, Terarecon Inc, USA). Each LA image was divided into portions according to the embryological origin: the venous LA, anterior LA, and LA appendage.14 The effective refractory periods were measured at 4 regions in both atria (high right atrium, low right atrium, proximal and distal coronary sinus).
Figure 1.
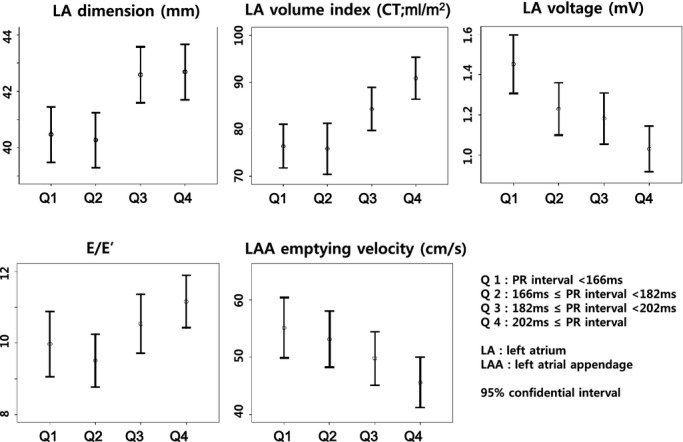
The association between a prolonged PR interval and left atrial remodeling. CT indicates computed tomography; LAA, left atrium appendage.
Radiofrequency Catheter Ablation
An open‐irrigation 3.5‐mm‐tip deflectable catheter (Celsius, Johnson & Johnson Inc, Diamond Bar, CA; Coolflex, St. Jude Medical Inc, Minnetonka, MN; 30 to 35 W; 47°C) was used for the RFCA (Stockert generator; Biosense Webster Inc, Diamond Bar, CA). All patients initially underwent circumferential PV isolation and the creation of cavo‐tricuspid isthmus block. For the patients with persistent AF, we added a roof line, posterior inferior line, and anterior line15 as a standard lesion set. Depending on the operator's decision, additional ablation of the superior vena cava, non‐PV foci, or complex fractionated electrograms was conducted. The procedure ended when there was no immediate recurrence of AF after cardioversion with an isoproterenol infusion (5 to 10 μg/min). If there were non‐PV foci under the isoproterenol infusion, we ablated them all.
Postablation Management and Follow‐Up
All patients were followed with all anti‐arrhythmic drugs discontinued after the RFCA. The patients visited the outpatient clinic regularly at 1, 3, 6, and 12 months and then every 6 months thereafter or whenever symptoms occurred after the RFCA. All patients underwent electrocardiography during every visit and 24‐ or 48‐hour Holter recording and/or event recording at 3, 6, and every 6 months, according to the 2012 HRS/EHRA/ECAS Expert Consensus Statement guidelines.16 However, whenever patients reported symptoms of palpitations, Holter monitor or event monitor recordings were obtained and evaluated for possible arrhythmia recurrences. We defined a recurrence of AF as any episode of AF or atrial tachycardia lasting for at least 30 s in duration.17 Any ECG documentation of an AF recurrence within a 3‐month blanking period was diagnosed as an early recurrence, and 3 months after a procedure as a clinical recurrence.17
Data Analysis
Normally distributed continuous variables were expressed as the mean± SD. We compared the PR interval during sinus rhythm prior to the ablation to the degree of electroanatomical remodeling of the LA, echocardiographic parameters, and clinical outcome. The cutoff values of the parametric variables were determined by median and quartile values. Statistical significance of the comparisons depending on the quartile of PR interval was assessed using the ANOVA test. We also used ANOVA and correlation analysis to assess the association between LA conduction velocity or effective refractory periods and PR interval. A Kaplan–Meier and Cox regression analysis was used to analyze the recurrent AF‐free survival after the catheter ablation, and log rank test P<0.05 was considered statically significant. Uni‐ and multivariable linear regression analyses were used to analyze the association with the PR interval. Log minus log graph for analyzing the proportionality assumption of PR interval shows parallel pattern according to the quartile of PR interval. This means that the effects of PR interval are constant regardless of time (the proportional assumption of Cox regression analysis). Variance inflation factors were used to measure the colinearity in the multivariable regression analysis. A P‐value <0.05 was considered statistically significant.
Results
A Long PR Interval Is Related to the Clinical Risk Factors of AF
All patients were classified into 4 groups based on the quartile value of the PR interval as depicted in Table 1. Patients who had a long PR interval were older (P<0.001), likely to have less paroxysmal AF (P<0.001), and more hypertension (P=0.013) compared to those with a short PR interval, but the CHADS2 scores did not significantly differ between the groups (P=0.098). The procedure time (P=0.042) and ablation time (P=0.019) were also prolonged in the long PR interval group.
Table 1.
Baseline Characteristics Depending on the PR Interval
| Q1 (<166 ms) (n=139) | Q2 (166 to 182 ms) (n=128) | Q3 (182 to 202 ms) (n=161) | Q4 (≥202 ms) (n=148) | P Value | |
|---|---|---|---|---|---|
| Male, n (%) | 104 (74.8) | 102 (79.7) | 118 (73.3) | 111 (75.0) | 0.640 |
| Age, years | 55.1±12.4 | 56.9±11.0 | 58.5±12.0 | 60.5±10.3 | 0.001* |
| Paroxysmal AF, n (%) | 107 (77.0) | 104 (81.3) | 106 (65.8) | 79 (53.4) | <0.001* |
| BMI, kg/m2 | 24.6±2.7 | 24.6±2.9 | 25.0±2.7 | 24.9±3.1 | 0.593 |
| CHADS2 score | 0.9±1.1 | 0.9±1.0 | 1.2±1.2 | 1.0±1.0 | 0.098 |
| Heart failure, n (%) | 15 (10.8) | 4 (3.1) | 18 (11.2) | 6 (4.1) | 0.048* |
| Hypertension, n (%) | 51 (36.7) | 63 (49.2) | 88 (54.7) | 74 (50.0) | 0.013* |
| Age >75 years, n (%) | 5 (3.6) | 6 (4.7) | 10 (6.2) | 7 (4.7) | 0.766 |
| Diabetes, n (%) | 15 (10.8) | 16 (12.5) | 28 (17.4) | 27 (18.2) | 0.204 |
| Stroke/TIA, n (%) | 22 (15.8) | 13 (10.2) | 24 (14.9) | 15 (10.1) | 0.330 |
| β‐blocker, n (%) | 48 (34.5) | 46 (35.9) | 50 (31.1) | 41 (27.7) | 0.458 |
| Procedure & follow‐up | |||||
| Ablation time, s | 4657.0±1596.0 | 4730.9±1473.1 | 4993.2±1477.1 | 5186.6±1766.6 | 0.019* |
| Procedure time, min | 182.9±53.9 | 181.4±44.7 | 186.5±39.9 | 196.2±51.3 | 0.042* |
| Early recurrence, n (%) | 33 (23.7) | 40 (31.2) | 37 (23.0) | 50 (33.8) | 0.107 |
| Clinical recurrence, n (%) | 20 (14.4) | 6 (4.7) | 21 (13.0) | 33 (22.3) | 0.003* |
| Follow‐up duration, month | 13.6±7.6 | 13.4±7.4 | 13.2±7.6 | 12.4±7.6 | 0.574 |
AF indicates atrial fibrillation; BMI, body mass index; TIA, transient ischemic attack.
P < 0.05.
A Long PR Interval Is Associated With a Long P Wave Duration and Advanced Electroanatomical Remodeling of the LA
Table 2 summarizes the electrophysiological and anatomical characteristics of AF depending on the quartile values of the PR interval during sinus rhythm. As the PR interval prolonged, the heart rate was lower (P=0.010) and the P wave in lead II (P<0.001) and negative P terminal force in V1 (P<0.001) increased in duration. Likewise, the QTc interval was noted to increase with the PR interval (P<0.001). Figure 1 shows that as the PR interval prolonged, the LA dimension (P<0.001) and volume (P<0.001) increased and the LA voltage decreased (P<0.001), and the emptying velocity of the LA appendage decreased linearly (P=0.032). These findings suggest that the advanced electroanatomical remodeling of the LA was associated with the prolonged PR interval (Figure 2). The LA conduction velocity and effective refractory periods did not show any linear relationship to the PR interval.
Table 2.
The Electrophysiologic Characteristics Depending on the PR Interval
| Q1 (<166 ms) (n=139) | Q2 (166 to 182 ms) (n=128) | Q3 (182 to 202 ms) (n=161) | Q4 (≥202 ms) (n=148) | P Value | |
|---|---|---|---|---|---|
| Electrocardiogram | |||||
| Heart rate/min | 63.8±15.3 | 61.0±10.0 | 60.7±8.3 | 59.5±10.2 | 0.010* |
| P wave duration in lead II, ms | 116.0±15.5 | 122.7±14.3 | 124.0±16.4 | 131.2±18.9 | <0.001* |
| P wave amplitude in lead II, mV | 15.1±5.1 | 15.5±4.9 | 15.1±5.1 | 14.6±4.6 | 0.512 |
| Negative P duration in lead V1, ms | 50.8±25.0 | 52.6±22.9 | 59.6±25.8 | 62.6±25.2 | <0.001* |
| Negative P amplitude in lead V1, mV | 6.1±3.4 | 5.9±3.7 | 6.6±3.7 | 6.6±3.0 | 0.221 |
| QRS duration, ms | 101.0±32.9 | 99.8±12.7 | 99.6±14.9 | 105.2±16.3 | 0.077 |
| QTc, ms | 429.2±46.0 | 431.9±24.8 | 439.3±31.6 | 445.3±30.0 | <0.001* |
| Echocardiogram | |||||
| LA dimension, mm | 40.5±5.9 | 40.3±5.5 | 42.6±6.4 | 42.7±6.1 | <0.001* |
| LA volume index, mL/m2 | 33.9±12.4 | 31.5±11.1 | 36.8±13.2 | 38.2±11.4 | <0.001* |
| LV ejection fraction, % | 62.6±7.7 | 64.0±6.7 | 60.9±9.9 | 63.8±8.3 | 0.004* |
| E/Em | 10.0±5.3 | 9.5±4.1 | 10.5±5.1 | 11.2±4.4 | 0.031* |
| LV mass index, g/m2 | 94.1±22.7 | 92.3±21.1 | 95.5±21.7 | 94.7±22.7 | 0.786 |
| LVEDD, mm | 50.1±4.1 | 49.7±4.2 | 50.1±4.3 | 49.5±5.0 | 0.624 |
| LVESD, mm | 33.9±3.9 | 32.8±4.4 | 34.4±4.9 | 33.2±4.7 | 0.025 |
| LAA velocity (n=342), cm/s | 55.1±23.3 | 53.1±22.3 | 49.8±22.8 | 45.6±21.2 | 0.032* |
| 3D‐CT | |||||
| LA volume/BSA, mL/m2 | 76.4±23.4 | 75.9±26.2 | 84.3±25.7 | 90.9±23.1 | <0.001* |
| Anterior LA/BSA, mL/m2 | 42.4±14.6 | 43.9±15.1 | 48.4±17.4 | 52.9±16.9 | <0.001* |
| LAA/BSA, mL/m2 | 7.5±8.7 | 6.5±3.2 | 7.4±4.8 | 8.1±5.2 | 0.288 |
| NavX electroanatomical map | |||||
| LA voltage (n=−425), mV | 1.5±0.7 | 1.2±0.6 | 1.2±0.7 | 1.0±0.6 | <0.001* |
| LA conduction velocity (n=345), m/s | 0.4±0.3 | 0.5±0.3 | 0.4±0.3 | 0.4±0.3 | 0.404 |
| Effective refractory period (n=443) | |||||
| High right atrium, ms | 227.9±26.9 | 233.2±27.3 | 229.8±27.8 | 230.7±24.2 | 0.549 |
| Low right atrium, ms | 232.0±33.2 | 232.5±28.5 | 226.8±35.3 | 228.6±34.0 | 0.551 |
| Proximal coronary sinus, ms | 241.5±32.9 | 244.9±40.0 | 256.4±218.0 | 250.0±154.3 | 0.833 |
| Distal coronary sinus, ms | 254.3±41.6 | 255.7±41.2 | 249.7±40.9 | 245.5±44.3 | 0.516 |
3D‐CT indicates 3‐dimensional computed tomography; E/Em, the ratio of the transmitral and myocardial early diastolic velocity; LA, left atrium; LAA, LA appendage; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter.
P < 0.05.
Figure 2.
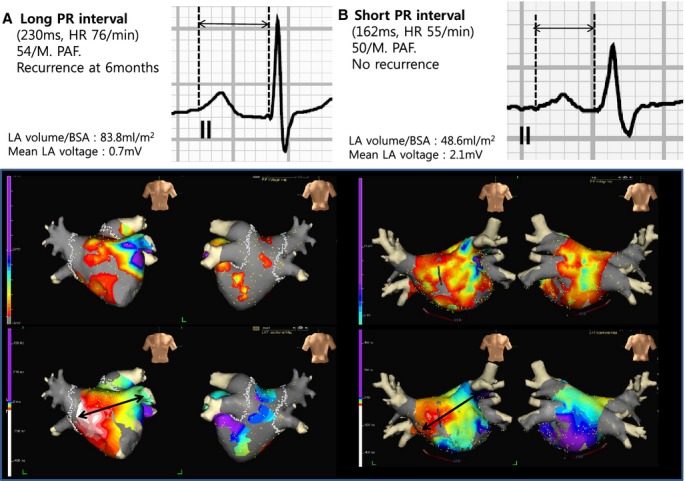
A, Patients with a prolonged PR interval had an enlarged LA size and decreased LA voltage. B, Patients with a short PR interval had a relatively small LA size and preserved LA voltage compared to those with a prolonged PR interval. PR interval prolongation led to the clinical recurrence of AF after the RFCA. AF indicates atrial fibrillation; BSA, body surface area; LA, left atrium; PAF, paroxysmal AF; RFCA, radiofrequency catheter ablation.
A Long PR Interval Is a Predictor of an AF Recurrence After Catheter Ablation
The overall clinical recurrence rate was 13.9% during 13.1±7.5 (3 to 37) months of follow‐up, and the recurrence rate significantly increased as the PR interval increased (P=0.003, Table 1). A multivariable Cox analysis showed that a long PR interval was a predictor of an AF recurrence after the RFCA of AF (HR=1.969, 95% CI 1.343 to 2.886, P=0.001; Table 3). A Kaplan–Meier curve showed a significant difference in the recurrent AF‐free survival according to the PR interval (log rank P<0.001; Figure 3A). The results by continuous version of PR interval were also consistent with analysis by PR interval quartile in multivariable analysis (HR 1.014, 95% CI 1.006 to 1.023, P<0.001). These results were also consistent with the analysis of the 283 patients who were followed up for longer than 1 year (19.1±5.9 months, log rank P=0.003; Figure 3B).
Table 3.
Multivariable Analysis of the Clinical Recurrence of AF
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Male | 1.011 | 0.574 to 1.780 | 0.970 | 1.033 | 0.458 to 2.327 | 0.938 |
| Age | 1.014 | 0.992 to 1.036 | 0.209 | 0.970 | 0.942 to 0.999 | 0.046* |
| Persistent AF | 2.042 | 1.248 to 3.344 | 0.005 | 2.251 | 0.914 to 5.546 | 0.078 |
| BMI | 0.959 | 0.877 to 1.048 | 0.353 | |||
| Congestive heart failure | 0.241 | 0.033 to 1.740 | 0.158 | |||
| Hypertension | 0.880 | 0.538 to 1.441 | 0.612 | |||
| Age >75 years | 1.624 | 0.651 to 4.049 | 0.299 | |||
| DM | 1.371 | 0.745 to 2.522 | 0.310 | |||
| Stroke/TIA | 1.207 | 0.615 to 2.372 | 0.584 | |||
| LA dimension | 1.027 | 0.987 to 1.069 | 0.186 | 1.001 | 0.939 to 1.066 | 0.981 |
| LA volume index (echo) | 1.014 | 0.995 to 1.033 | 0.145 | |||
| LV ejection fraction | 1.011 | 0.980 to 1.043 | 0.489 | |||
| E/Em | 1.009 | 0.964 to 1.057 | 0.701 | |||
| LAA emptying velocity | 0.989 | 0.973 to 1.005 | 0.173 | |||
| LA volume index (3D‐CT) | 1.010 | 1.000 to 1.021 | 0.059 | 0.996 | 0.979 to 1.013 | 0.613 |
| Mean LA voltage | 0.464 | 0.269 to 0.802 | 0.006 | 0.326 | 0.149 to 0.714 | 0.005* |
| Ablation time | 1.000 | 1.000 to 1.000 | 0.148 | |||
| Sinus node dysfunction or 1 AVB | 0.735 | 0.495 to 1.092 | 0.127 | 0.843 | 0.510 to 1.396 | 0.507 |
| Early recurrence | 3.698 | 2.254 to 6.068 | <0.001 | 5.725 | 2.784 to 11.771 | <0.001* |
| PR interval (quartile) | 1.429 | 1.129 to 1.809 | 0.003 | 1.969 | 1.343 to 2.886 | 0.001* |
Sample size was 265 (all parameters of which were measured), recurrence events was 36. Reference of PR interval (quartile) is Q1. AVB, atrioventricular block; 3D‐CT indicates 3‐dimensional‐computed tomography; AF, atrial fibrillation; BMI, body mass index; DM, diabetes mellitus; E/Em, the ratio of the transmitral and myocardial early diastolic velocity; LAA, left atrial appendage; LV, left ventricle; TIA, transient ischemic attack.
P < 0.05.
Figure 3.
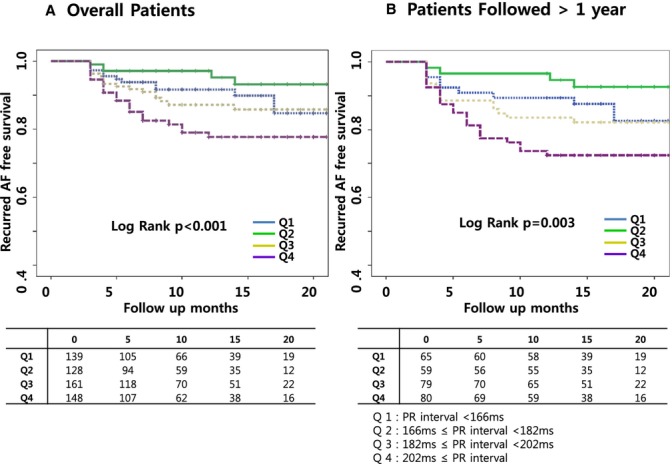
The Kaplan–Meier curve for the recurrent AF‐free survival after the RFCA of AF. A, The patients with a prolonged PR interval had a poor recurrent AF‐free survival after the RFCA of AF as compared to those with a short PR interval. B, These results were also consistent with the analysis of the patients with a follow‐up of >1 year. AF indicates atrial fibrillation; RFCA, radiofrequency catheter ablation.
Discussion
The current study showed that a prolonged PR interval reflected atrial remodeling due to AF, and was associated with a greater LA size, reduced LA voltage, and higher prevalence of persistent AF and hypertension compared to that for a short PR interval. In addition, the prolonged PR interval, a noninvasive parameter, was a significant predictor of a clinical recurrence of AF after the RFCA.
PR Interval as Prognostic Parameter for Cardiac Disease and AF
The PR interval reflects an electrical impulse that propagates from the peri‐sinus nodal tissue to the Purkinje‐ventricular muscle junction. Hence, a prolonged PR interval is affected by the atrial conduction properties, AV nodal conduction delays, and infranodal conduction or myocardial disease. The major portion of the PR interval includes the P wave duration, and there have been many studies regarding the P wave duration or dispersion related to aging,18 uncontrolled hypertension,1 diabetes,2 or obesity.19 Furthermore, a prolonged P wave duration and dispersion have been known to be associated with aortic stenosis,20 mitral stenosis,21 and coronary artery disease,3 as well as hyperthyroidism,22 obstructive sleep apnea,23 or a higher incidence of AF.24 In the current study, we found a close association between the PR interval and P wave duration, and a prolonged PR interval predicted the clinical recurrence of AF after catheter ablation. The prolonged PR interval was associated with an increased risk of AF, pacemaker implantations, and all‐cause mortality in the Framingham Heart Study,5 but was not associated with mortality or hospitalization in another population study.25
Potential Mechanisms of PR Prolongation in AF
Increased atrial pressure, ischemia, and hemodynamic changes result in atrial remodeling including atrial fibrosis and inflammation.26 This remodeling leads to an inhomogeneous recovery and slow conduction throughout the atrium. As AF leads to atrial remodeling and sinus node dysfunction,27–28 the PR interval becomes prolonged. In the current study, an enlarged atrial size or decreased atrial endocardial voltage was associated with an increased PR interval and P wave duration. In addition, the patients with a prolonged PR interval had a significantly decreased heart rate compared to those with a short PR interval in accordance with a study in male athletes, where the predictors of AF were a prolonged PR interval and resting bradycardia.29 Moreover, a low heart rate with moderate exercise is known to be a long‐term predictor of incident AF30 related to an increased vagal tone and autonomic remodeling. A recent meta‐analysis on genomewide association studies identified the genetic loci that are associated with the PR interval, and this suggests that genetic variations also play important roles in the atrial and AV conduction.31–32 Ultimately, the PR interval may be a significant predictor of the clinical recurrence after RFCA of AF, which would represent the remodeling of the sinus node, atrial substrate, AV node, or cardiac autonomic nervous system, as well as a genetic propensity. However, it is notable that patients in Q1 (PR interval <166 ms) had a poorer clinical outcome than those in Q2 (PR interval 166 to 182 ms) in the current study, and for this reason further study is warranted.
Limitations
This study was a retrospective observational study from a Cohort registry that included a highly selective group of patients referred for AF catheter ablation. The enrolled population in this study had a relatively low number of comorbidities. Therefore, these results cannot be generalized to a wider population of AF. Although there was no statistical difference between the groups, 32.1% of the patients included were on β‐blockers. We did not measure the AH or HV interval to determine conduction in the AV node; thus, we could not explain how the conduction delay in the AV node might have affected the PR interval prolongation. Although we tried to keep the consistent standard ablation protocols for PAF and persistent AF as much as we could, there were some additional ablations depending on the operator's decision. These individually variable ablation lesions can affect the outcome after RFCA. The clinical recurrence rate after a single procedure was relatively low in this study compared with the previous reports.33 It might be due to a small‐size heart and thin‐walled atria in Asian patients. Because of frequently recurring AF at baseline, we measured conduction time after circumferential PV isolation and cardioversion. If there was frequently recurring AF after cardioversion, we could not measure conduction time. This selection bias can contribute to the lack of relationship between PR interval and conduction velocity. Rate‐related conduction slowing was reported to be predictive for the AF mechanism,34 so that measuring PR interval with different pacing cycle length might be valuable for further study.
Conclusions
A prolonged PR interval reflects atrial remodeling in patients with AF, and is a significant predictor of the clinical recurrence of AF after RFCA.
Sources of Funding
This work was supported by a Korea Health 21 R&D Project (A085136), Ministry of Health and Welfare, National Research Foundation of Korea, the Ministry of Science (7‐2013‐0362), Ministry of Science, ICT & Future Planning (MSIP).
Disclosures
None.
References
- 1.Dilaveris PE, Gialafos EJ, Chrissos D, Andrikopoulos GK, Richter DJ, Lazaki E, Gialafos JE. Detection of hypertensive patients at risk for paroxysmal atrial fibrillation during sinus rhythm by computer‐assisted P wave analysis. J Hypertens. 1999; 17:1463-1470. [DOI] [PubMed] [Google Scholar]
- 2.Yazici M, Ozdemir K, Altunkeser BB, Kayrak M, Duzenli MA, Vatankulu MA, Soylu A, Ulgen MS. The effect of diabetes mellitus on the P‐wave dispersion. Circ J. 2007; 71:880-883. [DOI] [PubMed] [Google Scholar]
- 3.Celik T, Iyisoy A, Kursaklioglu H, Kilic S, Kose S, Amasyali B, Isik E. Effects of primary percutaneous coronary intervention on P wave dispersion. Ann Noninvasive Electrocardiol. 2005; 10:342-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crisel RK, Farzaneh‐Far R, Na B, Whooley MA. First‐degree atrioventricular block is associated with heart failure and death in persons with stable coronary artery disease: data from the Heart and Soul Study. Eur Heart J. 2011; 32:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton‐Cheh C, Levy D, Benjamin EJ, Vasan RS, Wang TJ. Long‐term outcomes in individuals with prolonged PR interval or first‐degree atrioventricular block. JAMA. 2009; 301:2571-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spach MS. Mounting evidence that fibrosis generates a major mechanism for atrial fibrillation. Circ Res. 2007; 101:743-745. [DOI] [PubMed] [Google Scholar]
- 7.Dogan A, Avsar A, Ozturk M. P‐wave dispersion for predicting maintenance of sinus rhythm after cardioversion of atrial fibrillation. Am J Cardiol. 2004; 93:368-371. [DOI] [PubMed] [Google Scholar]
- 8.Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009; 2:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009; 22:107-133. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJChamber Quantification Writing G, American Society of Echocardiography's G, Standards C, European Association of E. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440-1463. [DOI] [PubMed] [Google Scholar]
- 11.Lin YJ, Tai CT, Kao T, Chang SL, Wongcharoen W, Lo LW, Tuan TC, Udyavar AR, Chen YJ, Higa S, Ueng KC, Chen SA. Consistency of complex fractionated atrial electrograms during atrial fibrillation. Heart Rhythm. 2008; 5:406-412. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Pak HN, Kim SK, Jang JK, Choi JI, Lim HE, Hwang C, Kim YH. Electrophysiologic characteristics of complex fractionated atrial electrograms in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009; 20:266-272. [DOI] [PubMed] [Google Scholar]
- 13.Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, Dimitri H, Roberts‐Thomson KC, Wilson L, De Sciscio P, Young GD, Sanders P. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor”. J Am Coll Cardiol. 2009; 53:1182-1191. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, Choi JI, Hwang C, Kim YH. The relationship between endocardial voltage and regional volume in electroanatomical remodeled left atria in patients with atrial fibrillation: comparison of three‐dimensional computed tomographic images and voltage mapping. J Cardiovasc Electrophysiol. 2009; 20:1349-1356. [DOI] [PubMed] [Google Scholar]
- 15.Pak HN, Oh YS, Lim HE, Kim YH, Hwang C. Comparison of voltage map‐guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 2011; 8:199-206. [DOI] [PubMed] [Google Scholar]
- 16.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber DHeart Rhythm Society Task Force on C, Surgical Ablation of Atrial F. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012; 9:632-696.e621. [DOI] [PubMed] [Google Scholar]
- 17.European Heart Rhythm A, European Cardiac Arrhythmia S, American College of C, American Heart A, Society of Thoracic S. Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007; 4:816-861. [DOI] [PubMed] [Google Scholar]
- 18.Asad N, Spodick DH. Prevalence of interatrial block in a general hospital population. Am J Cardiol. 2003; 91:609-610. [DOI] [PubMed] [Google Scholar]
- 19.Kosar F, Aksoy Y, Ari F, Keskin L, Sahin I. P‐wave duration and dispersion in obese subjects. Ann Noninvasive Electrocardiol. 2008; 13:3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turhan H, Yetkin E, Atak R, Altinok T, Senen K, Ileri M, Sasmaz H, Cehreli S, Kutuk E. Increased P‐wave duration and P‐wave dispersion in patients with aortic stenosis. Ann Noninvasive Electrocardiol. 2003; 8:18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turhan H, Yetkin E, Senen K, Yilmaz MB, Ileri M, Atak R, Cehreli S, Kutuk E. Effects of percutaneous mitral balloon valvuloplasty on P‐wave dispersion in patients with mitral stenosis. Am J Cardiol. 2002; 89:607-609. [DOI] [PubMed] [Google Scholar]
- 22.Katircibasi MT, Deniz F, Pamukcu B, Binici S, Atar I. Effects of short‐term propylthiouracil treatment on P wave duration and P wave dispersion in patients with overt hyperthyroidism. Exp Clin Endocrinol Diabetes. 2007; 115:376-379. [DOI] [PubMed] [Google Scholar]
- 23.Can I, Aytemir K, Demir AU, Deniz A, Ciftci O, Tokgozoglu L, Oto A, Sahin A. P‐wave duration and dispersion in patients with obstructive sleep apnea. Int J Cardiol. 2009; 133:e85-e89. [DOI] [PubMed] [Google Scholar]
- 24.De Bacquer D, Willekens J, De Backer G. Long‐term prognostic value of P‐wave characteristics for the development of atrial fibrillation in subjects aged 55 to 74 years at baseline. Am J Cardiol. 2007; 100:850-854. [DOI] [PubMed] [Google Scholar]
- 25.Aro AL, Anttonen O, Kerola T, Junttila MJ, Tikkanen JT, Rissanen HA, Reunanen A, Huikuri HV. Prognostic significance of prolonged PR interval in the general population. Eur Heart J. 2014; 35:123-129. [DOI] [PubMed] [Google Scholar]
- 26.Everett TH, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007; 4:S24-S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hocini M, Sanders P, Deisenhofer I, Jais P, Hsu LF, Scavee C, Weerasoriya R, Raybaud F, Macle L, Shah DC, Garrigue S, Le Metayer P, Clementy J, Haissaguerre M. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003; 108:1172-1175. [DOI] [PubMed] [Google Scholar]
- 28.Tse HF, Lau CP, Ayers GM. Heterogeneous changes in electrophysiologic properties in the paroxysmal and chronically fibrillating human atrium. J Cardiovasc Electrophysiol. 1999; 10:125-135. [DOI] [PubMed] [Google Scholar]
- 29.Grimsmo J, Grundvold I, Maehlum S, Arnesen H. High prevalence of atrial fibrillation in long‐term endurance cross‐country skiers: echocardiographic findings and possible predictors—a 28‐30 years follow‐up study. Eur J Cardiovasc Prev Rehabil. 2010; 17:100-105. [DOI] [PubMed] [Google Scholar]
- 30.Grundvold I, Skretteberg PT, Liestol K, Erikssen G, Engeseth K, Gjesdal K, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Low heart rates predict incident atrial fibrillation in healthy middle‐aged men. Circ Arrhythm Electrophysiol. 2013; 6:726-731. [DOI] [PubMed] [Google Scholar]
- 31.Pfeufer A, van Noord C, Marciante KD, Arking DE, Larson MG, Smith AV, Tarasov KV, Muller M, Sotoodehnia N, Sinner MF, Verwoert GC, Li M, Kao WH, Kottgen A, Coresh J, Bis JC, Psaty BM, Rice K, Rotter JI, Rivadeneira F, Hofman A, Kors JA, Stricker BH, Uitterlinden AG, van Duijn CM, Beckmann BM, Sauter W, Gieger C, Lubitz SA, Newton‐Cheh C, Wang TJ, Magnani JW, Schnabel RB, Chung MK, Barnard J, Smith JD, Van Wagoner DR, Vasan RS, Aspelund T, Eiriksdottir G, Harris TB, Launer LJ, Najjar SS, Lakatta E, Schlessinger D, Uda M, Abecasis GR, Muller‐Myhsok B, Ehret GB, Boerwinkle E, Chakravarti A, Soliman EZ, Lunetta KL, Perz S, Wichmann HE, Meitinger T, Levy D, Gudnason V, Ellinor PT, Sanna S, Kaab S, Witteman JC, Alonso A, Benjamin EJ, Heckbert SR. Genome‐wide association study of PR interval. Nat Genet. 2010; 42:153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler AM, Yin X, Evans DS, Nalls MA, Smith EN, Tanaka T, Li G, Buxbaum SG, Whitsel EA, Alonso A, Arking DE, Benjamin EJ, Berenson GS, Bis JC, Chen W, Deo R, Ellinor PT, Heckbert SR, Heiss G, Hsueh WC, Keating BJ, Kerr KF, Li Y, Limacher MC, Liu Y, Lubitz SA, Marciante KD, Mehra R, Meng YA, Newman AB, Newton‐Cheh C, North KE, Palmer CD, Psaty BM, Quibrera PM, Redline S, Reiner AP, Rotter JI, Schnabel RB, Schork NJ, Singleton AB, Smith JG, Soliman EZ, Srinivasan SR, Zhang ZM, Zonderman AB, Ferrucci L, Murray SS, Evans MK, Sotoodehnia N, Magnani JW, Avery CL. Novel loci associated with PR interval in a genome‐wide association study of 10 African American cohorts. Circ Cardiovasc Genet. 2012; 5:639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale AInvestigators R‐. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of paroxysmal atrial fibrillation (RAAFT‐2): a randomized trial. JAMA. 2014; 311:692-700. [DOI] [PubMed] [Google Scholar]
- 34.Lalani GG, Schricker A, Gibson M, Rostamian A, Krummen DE, Narayan SM. Atrial conduction slows immediately before the onset of human atrial fibrillation: a bi‐atrial contact mapping study of transitions to atrial fibrillation. J Am Coll Cardiol. 2012; 59:595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]


