Abstract
Background
We investigated the hypothesis that the favorable effects of gastrointestinal (GI) intervention on hypertension (HTN) and cardiovascular (CV) disturbances are mediated by antagonizing overdrive of the sympathetic nervous system (SNS).
Methods and Results
Hypertensive patients with metabolic disturbances underwent laparoscopic Roux‐en‐Y gastric bypass surgery, and spontaneously hypertensive rats (SHRs) underwent RYGB or sham surgery. Blood pressure (BP), heart rate (HR), endothelium‐dependent flow‐mediated dilation, and anthropometric as well as laboratory parameters were measured at baseline and during follow‐up. Changes of BP and HR in response to cold stress, renal sympathetic nervous activity (RSNA), vasoconstriction induced by electrical field stimulation, microinjection of nucleus of the solitary tract (NTS), and CV function and structure were examined in SHRs with or without surgery. Compared with baseline, BP and HR were significantly reduced in both hypertensive patients with type 2 diabetes and rats. Impaired endothelial‐dependent vasodilatation and metabolic disturbances in hypertensive patients were also ameliorated after surgery. CV disturbances were reversed by surgery in SHRs. Under acute cold exposure, the variations in BP and HR were smaller in surgically treated SHRs, compared to sham SHRs. RSNA and vasoconstriction induced by perivascular nerve stimulation as well as NTS‐mediated changes of BP were decreased in surgically treated SHRs, compared to sham SHR. Weight loss did not affect BP and RSNA in sham SHRs.
Conclusions
GI intervention ameliorates HTN in both hypertensive patients and rats by inhibiting overdrive of the SNS. Therefore, targeting gastrointestine could be a novel strategy to treat HTN with metabolic disturbances.
Keywords: gastric bypass surgery, gastrointestinal intervention, hypertension, sympathetic nervous system
Introduction
The autonomic nervous system (ANS), including the sympathetic nervous system (SNS) and parasympathetic nervous system, is a collection of afferent and efferent neurons that link the central nervous system (CNS) with visceral effectors. The neural control of the circulation operates through parasympathetic neurons that innervate the heart and sympathetic efferent neurons that innervate blood vessels, heart, kidneys, and the adrenal medulla.1–2 The sympathetic nerves dominantly regulate cardiovascular (CV) structure and function by promoting the release of adrenal catecholamine and activation of the renin‐angiotensin‐aldosterone system.3 It is well known that activation of the SNS contributes to the development of hypertension (HTN) as well as CV remodeling and dysfunction.4–8 Currently, adrenergic receptor blockers, renal sympathetic nerve denervation (RSND), and electric stimulation of the carotid sinus are used to treat HTN and its complications.9–11
However, little attention is given to the effect of gastrointestinal (GI) intervention to autonomic nerves, especially GI nerve innervation, on CV regulation. Bariatric surgery, also called metabolic surgery, can induce substantial and sustained weight loss and is a highly effective treatment for severe obesity and diabetes.12–14 A substantial number of clinical and experimental studies have shown that GI intervention also resolved HTN and improved the prognosis of CV events (CVEs).15–20 Interestingly, the improvement in high blood pressure (BP) occurs before any appreciable weight loss after metabolic surgery.17 The precise mechanisms underlying the resolution of HTN after metabolic surgery have not been determined, although several mechanisms, such as metabolic surgery‐induced weight loss, changes in gut hormones, and increased urinary sodium excretion, have been postulated.20–22 It has recently been reported that metabolic surgery resulted in a significant reduction in heart rate (HR) and an enhancement in HR recovery.15,23 Subjects who had undergone gastric bypass had significantly lower muscle sympathetic nerve activity, compared to obese subjects.24 These studies suggest that metabolic surgery had an impact on visceral SNS. Therefore, we hypothesized that the beneficial effect of metabolic surgery on HTN may stem from inhibition of sympathetic nerve activity. In this study, we showed that GI intervention resolved HTN and improved CV disturbances by antagonizing the activation of the SNS, independent of weight loss, in both diabetic hypertensive patients and genetic hypertensive rats.
Methods
Patient Study
All studies were performed according to the principles of the Declaration of Helsinki. The institute's ethics committee of Daping Hospital approved the study. Ten male and 11 female hypertensive patients with type 2 diabetes (T2DM) were recruited (mean age, 45.2±10.4 years). Subjects were classified by the following criteria: (1) HTN, BP ≥140/90 mm Hg, or treated HTN; (2) diabetes, fasting plasma glucose (FPG) ≥7.0 mmol/L, and/or oral glucose tolerance test (OGTT; 2 hours ≥11.1 mmol/L), or treated diabetes; and (3) obesity, body mass index (BMI) ≥28 kg/m2 or waist circumference (WC) higher than 90 cm in men or 85 cm in women.25 The bariatric surgery indication for Chinese T2DM is: (1) BMI ≥35 kg/m2, T2DM with or without complications, should be considered for GI metabolic surgery; (2) BMI 30 to 35 kg/m2, T2DM, if difficult to control blood glucose or complications, especially CV risk, by lifestyle changes and drug therapy, GI metabolic surgery should be one of the treatment choices; (3) BMI 28.0 to 29.9 kg/m2, T2DM with central obesity (WC >85 cm for female and >90 cm for male) and at least another 2 criteria of metabolic syndrome (high triglycerides [TGs], low high‐density lipoprotein cholesterol [HDL‐C] levels, or high BP). For these patients, GI metabolic surgery may be considered for the treatment of choice; and (4) BMI 25.0 to 27.9 kg/m2, patients with T2DM should be informed consent and in strict accord with research programs. However, these procedures should be considered only as a pure clinical research preceding approval of the ethics committee, and not a wide recommendation.26 All patients were recruited in our hospital, and the time frame for patient recruitment was from November 2011 to July 2013 and they received laparoscopic Roux‐en‐Y gastric bypass (RYGB).27 All patients were informed of the risks and benefits of each procedure and provided written, informed consent. All patients underwent complete evaluation before and after the metabolic operation and then were followed up at 1, 3, 6, and 12 months. Some measurements were duplicated, including 24‐hour ambulatory BP, flow‐mediated dilation (FMD), anthropometric and clinical parameters, as well as blood glucose and lipids.
Anthropometry Assessments
A general physical examination was conducted at each visit at the same time of day, with the participant wearing light clothing and no shoes. All procedures were performed by the same examiner using the same calibrated scale. WC was measured using a nonelastic tape at the midpoint between the bottom of the rib cage and the top of the iliac crest at minimal respiration to the nearest 0.1 cm. Height (m) was recorded to the nearest 0.1 cm on a portable standiometer, and weight was measured to the nearest 0.1 kg with the patient standing motionless in the center of the scale after adjustment for the estimated weight of clothing. BMI was calculated as weight/height2.
Human BP Monitoring
Ambulatory blood pressure monitoring (ABPM) was performed with a Spacelabs 90207 (Spacelabs Medical, Issaqua, WV) at baseline and at 6 months. The cuff size was adjusted to the upper‐arm circumference. ABPM measurements were performed every 15 minutes during the daytime and every 20 to 30 minutes at night. ABPM profiles were divided into daytime (8:00 am to 8:00 pm) and nighttime periods (12:00 am to 6:00 am).28 Office BP was measured by a physician using a mercury sphygmomanometer after the participant had rested at least 5 minutes in the sitting position. First and fifth phases of Korotkoff sounds were taken as systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively. Three measurements were taken at 2‐minute intervals and recorded, and the average was used to define the SBP and DBP.
Endothelium‐Dependent Endothelial Function
Endothelium‐dependent dilation of the brachial artery was measured by ultrasound (7.5‐MHz ultrasound Doppler probe, HY6000PRO; HAIYING Group Co, Ltd, Wuxi, China). The guidelines for determining and analyzing FMD, as previouslydescribed,29were strictly followed. Measurements were performed on the brachial artery 4.5 cm above the antecubital fossa before inflation of a pneumatic cuff on the upper arm to 250 mm Hg for 5 minutes and at 1 minute after cuff release. FMD was expressed as percentage dilation from the baseline diameter to that observed 1 minute after cuff release.
Laboratory Measurements
After an overnight fast, laboratory tests were obtained for measurement of FPG, OGTT (2 hour), plasma insulin, glycated hemoglobin (HbA1C), high‐sensitivity C‐reactive protein (hs‐CRP), total cholesterol (TC), TGs, HDL‐C, and low‐density lipoprotein cholesterol (LDL‐C). Oral glucose tolerance (2‐hour) was tested during an OGTT (75‐g glucose load). Fasting blood samples were collected in EDTA and assayed for hs‐CRP by a validated high‐sensitivity assay using turbidimetric immunoassay (Orion Diagnostica, Espoo, Finland). Samples were stored frozen at −70°C until analysis. The homeostasis model assessment of insulin resistance (HOMA‐IR) was calculated on the basis of FPG and serum insulin levels.27
Animal Treatments
Eight‐week‐old male spontaneously hypertensive rats (SHRs) were obtained from Charles River Laboratories (Bar Harbor, ME). Rats were housed under a 12‐hour day/night cycle with free access to food and water. The institute's animal care and use committee approved all animal protocols. Rats were randomly assigned to either the RYGB (surgery) or sham surgery group (sham). A subgroup of sham SHRs was restricted and consumed 30% less chow than other sham SHRs. There were 12 animals in each group. The animal study protocol is shown in Figure 1A. The 24‐hour urine sample was collected in metabolic cages to measure urinary sodium excretion.
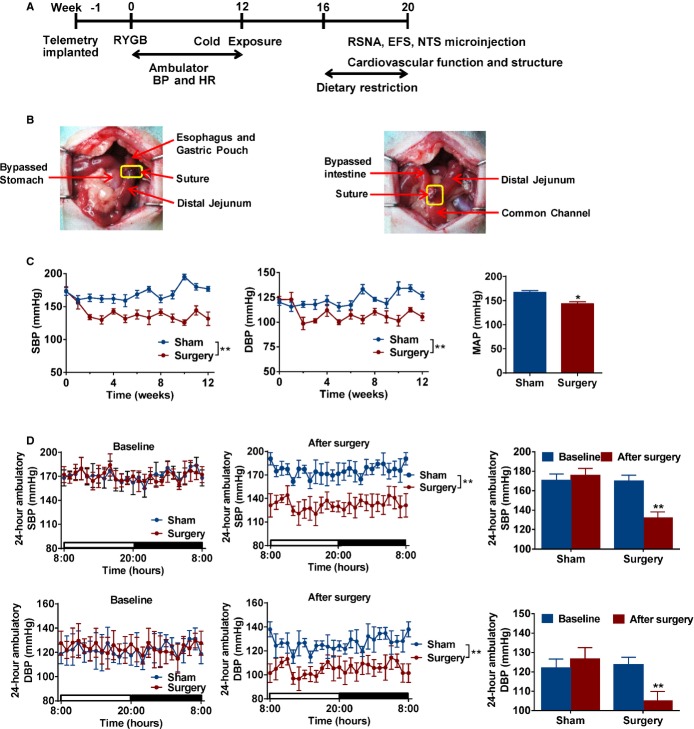
Figure 1.
Effect of metabolic surgery on blood pressure in spontaneous hypertensive rats. A, Experimental flow chart. Telemetric transmitters were implanted before RYGB or sham surgery. SHRs were subjected to cold exposure at week 12. Dietary restriction was performed in a subgroup of sham SHRs at 16 to 20 weeks. RSNA, EFS, NTS microinjection, and cardiovascular function and structure were analyzed at the end of 20 weeks. B, Representative diagram of RYGB surgery on SHRs. Left panel showed that the gastric pouch of the stomach was anastomosed to the distal cut end of the jejunum. Right panel shows that the proximal cut end of the jejunum was anastomosed to the side of the lower jejunum. C, Time‐related changes of blood pressure in SHR. Blood pressure was determined using radiotelemetry. Mean arterial pressure (MAP) from sham and surgery SHRs was directly recorded by a cannula under anesthesia at 20 weeks. *P<0.05; **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=7. D, RYGB reduced SBP and DBP in SHRs. Twenty‐four‐hour blood pressure was determined using radiotelemetry at baseline (week 0) and after surgery (week 12) in SHRs. **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=7. BP indicates blood pressure; DBP, diastolic blood pressure; EFS, electrical field stimulation; HR, heart rate; NTS, nucleus of the solitary tract; RSNA, renal sympathetic nervous activity; RYGB, Roux‐en‐Y gastric bypass; SHRs, spontaneously hypertensive rats; SBP, systolic blood pressure.
Animal BP Measurements
SHRs were surgically implanted with telemetric transmitters (TA11PA‐C40; Data Sciences International, Saint Paul, MN) before metabolic surgery. The catheter portion of the implant was placed into the distal portion of the descending aorta. Rats were allowed to recover from surgery for 7 days, and then 24‐hour SBP, DBP, and HR were measured by telemetry in conscious and unrestrained rats at week 0 (baseline) and week 12 after surgery. BP and HR were recorded weekly for 1 hour at 10:00 am. We collected data for 10 seconds every minute and used the mean values for statistical analysis.30
Metabolic Surgery and Postoperative Care of SHRs
RYGB is the most common type of metabolic surgery. After surgical implantation of the telemetric transmitters, SHR rats were allowed to recover for 1 week and then RYGB was performed, as previously reported.31 After overnight food deprivation, rats were deeply anesthetized with isoflurane (4% induction, 1.5% maintenance; VIP‐3000 Vaporizer; Matrix, Orchard Park, NY)32 and abdominal organs exposed by laparotomy. First, a gastric pouch of ≈10% of the total stomach volume was formed using scissors, and the distal cut end of the stomach was sutured. Second, the jejunum was transected ≈10 cm from the ligament of Treitz, and the distal cut end was anastomosed to the gastric pouch. Third, the proximal cut end of the jejunum was anastomosed to the side of the lower jejunum (≈20 cm from the ligament of Treitz). For sham surgery, transections of the GI tract were performed at all sites where enterotomies were performed for the RYGB, but were reanastomosed to maintain the physiological circuit of food through the bowel. On the day after surgery, rats were given access to a limited amount of Ensure. Three days after surgery, rats were fed ad libitum with normal chow. Two rats (16.7%) in the RYGB group and 1 (8.3%) in the sham surgery group died of complications during the operation or within the first 24 hours after surgery.
Cold Exposure‐Induced BP and HR Variability of SHRs
SHRs were subjected to a periodic change of ambient temperature at week 12. The rhythm of the temperature fluctuation was as follows. A starting temperature of 26°C was ramped downward over 10 minutes to reach 4°C and was held there for 5 minutes. The temperature was then ramped upward over 10 minutes to reach 26°C and was held there for 5 minutes. Another 30‐minute cycle was then started. The temperature settings were in accord with previous studies.33–34 All rats underwent 3 cycles, changes of SBP, DBP, and HR were measured with the telemetry system, and variations in those parameters were calculated as the difference between highest and lowest values in every cycle.
Echocardiography of SHRs
Echocardiography was performed with the VisualSonics Vevo 770 imaging system (VisualSonics Inc., Toronto, Ontario, Canada) using a 710 scan head in animals anesthetized with isoflurane, as mentioned above, after 20 weeks, as previously reported.35 The left ventricle internal diameter (LVID), interventricular septum thickness (IVST), and left ventricular posterior wall (LVPW) were measured in the short‐axis view from M‐mode recordings in end diastole and end systole. The peak early diastolic filling velocity (E wave) and peak late diastolic filling velocity (A wave) were taken using the apical 4‐chamber view.
Microinjection of the Nucleus of the Solitary Tract
Animals were placed in a stereotaxic apparatus, and a partial craniotomy of the occipital bone was performed. The dorsal surface of the brainstem was exposed. The coordinates for the nucleus of the solitary tract (NTS) were determined from the atlas, which were 0.5 mm rostral to the caudal tip of the area postrema, 0.5 mm lateral to the midline, and 0.5 mm below the dorsal surface of the brain stem. After baseline mean arterial blood pressure (MAP) and HR recording, microinjections of gamma‐aminobutyric acid (GABA; 0.1, 1, and 10 nmol/L) into the NTS were made with glass pipettes (tip size, 20 to 40 μm) coupled to a pressure injection apparatus (PicoPump; World Precision Instruments, Sarasota, FL). The volume of injection (60 nL) was estimated from displacement of the fluid meniscus in the pipette using a calibrated reticule. Microinjection sites were marked with 2% pontamine sky blue.
Measurement of Plasma Glucagon‐Like Peptide 1
Plasma was kept from RYGB and sham SHRs. Plasma glucagon‐like peptide 1 (GLP‐1) levels were assayed by the Glucagon‐Like Peptide‐1 (Active) ELISA kit (Linco Research, Inc., Saint Charles, MO), according to the manufacturer's instructions.
Urinary Sodium Excretion Measurement
SHRs were acclimatized to the metabolic cages (Techniplast 3701M001; Tecniplast Gazzada S.a r.l., Buguggiate, Italy) for 48 hours, then 24‐hour urine was collected through metabolic cages to measure urinary sodium excretion.
Renal Sympathetic Nervous Activity and Baroreflex Analysis of SHRs
Renal sympathetic nervous activity (RSNA) and baroreflex were analyzed after 20 weeks, as previously reported.36 Briefly, rats were anesthetized with pentobarbital sodium (45 mg/kg, IP), and the trachea was cannulated to facilitate mechanical respiration. The femoral artery was catheterized for BP measurement. HR was derived from the BP pulse by a PowerLab Chart system (ADInstruments, Dunedin, New Zealand). The femoral vein was cannulated for intravenous injections. Body temperature was kept at 37°C by a temperature controller. The left renal sympathetic nerves were exposed, identified, and dissected free of the surrounding connective tissue and then a pair of recording electrodes were attached to the nerves. Pressor doses of phenylephrine (10 μg/kg, IV; Sigma‐Aldrich, St. Louis, MO) were acutely administered to induce reflex bradycardia. Both the nerve and the electrodes were covered with a fast‐setting silicone (Wacker Sil‐Gel, Wacker Chemie AG, Munich, Germany). The signal was amplified (bandpass, 100 to 3000 Hz) with a preamplifier (FZG‐81; Shanghai Institute of Physiology, Shanghai, China). The distal terminal of the renal nerve was cut to avoid afferent activity. The baseline RSNA represented 100% (after subtraction of the background noise level from the absolute value). Arterial baroreflex was evaluated by the mean index relating changes in RSNA to changes in MAP and expressed as mVs per mm Hg.
Vascular Constriction Measurement of SHRs
Endothelium‐dependent vascular constriction of freshly isolated arteries was studied using a myograph system (Danish Myo Technology A/S, Aarhus, Denmark), as previously described.30,37After rats were anesthetized with pentobarbital sodium, the mesenteric and femoral vascular bed was removed and placed in a cold Krebs solution containing 118 mmol/L of NaCl, 25 mmol/L of NaHCO3, 11 mmol/L of d‐glucose, 4.7 mmol/L of KCl, 1.2 mmol/L of KH2PO4, 1.17 mmol/L of MgSO4, and 2.5 mmol/L of CaCl2. Arterial segments (2 to 2.5 mm in length) were mounted in the myograph apparatus. Each ring was bathed in Krebs solutionaerated with 95% O2 and 5% CO2 at 37°C (pH 7.4). After measurement of the passive‐tension internal circumference characteristics, the tension was set to the estimated in vivo internal circumference. After a 60‐minute stabilization period, the functional integrity of the rings was confirmed by contraction in response to KCl (60 mmol/L). The presence of endothelium was confirmed by a relaxant response to acetylcholine (Ach; 1 mmol/L) in rings that had been contracted by exposure to phenylephrine (PE; 1 mmol/L). Segments relaxing >80% were considered being endothelium intact, whereas those relaxing <5% were defined as being endothelium denuded. PE and Ach were purchased from Sigma‐Aldrich. Vasoconstriction induced by perivascular nerve stimulation was measured by electrical field stimulation (EFS) using a stimulator (SD9 Grass Stimulator; Natus Neurology Incorporated, Grass Products, Warwick, RI) connected to 2 platinum electrodes placed on each side of the vessel parallel to its longitudinal axis. Frequency‐response curves to EFS (2, 4, 8, 16, 32, and 64 Hz) were performed. The parameters used for EFS were 200 mA, 0.3 ms, and 2 to 64 Hz for 30 seconds with an interval of 1 minute between each stimulus, which was the time required to recover basal tone. In every experiment, the first frequency‐response curve served as the control and the time between the first and second frequency‐response curve was 60 minutes. EFS‐induced contractions and concentration‐response curves in response to noradrenaline treatment were obtained from separate preparations.
Histopathological Examination
After 20 weeks, samples were processed as previously described.38 Frozen sections of heart ventricles and mesenteric arteries (10 μm) were obtained and stained with hematoxylin‐eosin (H&E) or with Masson's trichrome reagent for measurement of collagen deposition. Microscopic visualization and photographs were obtained, and the measurements were performed by a blinded investigator using NIS‐Elements 3.2 software (Nikon, Tokyo, Japan).
Statistical Analysis
All results in figures are presented as mean±SEM. Kolmogorov‐Smirnov's test or Shapiro‐Wilk's test was used to determine whether each variable had a normal distribution. Comparisons between groups were made with the Student t test or 1‐way ANOVA with Bonferroni's multiple‐comparison post‐hoc test. The paired t test was used for the comparisons of characteristics of patients at baseline and after surgery. Repeated‐measures analysis was used for the comparisons of repeated measured quantitative variables. A linear regression analysis was performed to assess the relationship between BP and HR of all patients at 5 time points, that is, before surgery and 1, 3, 6, and 12 months after surgery. For each time point, mean values of the patients' BP and HR were used in the analysis. P<0.05 was regarded as a significant effect or between‐group difference. All tests were 2‐tailed, and analyses were performed using either GraphPad Prism (GraphPad Software Inc., La Jolla, CA) or SPSS (version 12.0; SPSS, Inc., Chicago, IL).
Results
Metabolic Surgery Lowered BP in Genetic Hypertensive Rats
In this study, we used SHRs, an established animal model of genetic HTN. After surgical implantation of the telemetric transmitters, SHR rats were allowed to recover for 1 week and then RYGB was performed (Figure 1A and 1B). Radiotelemetry showed that metabolic surgery significantly reduced BP, and these hypotensive effects were maintained for an extended period of time (Figure 1C). Furthermore, 24‐hour BP was significantly lowered in SHRs with surgery, compared to sham SHRs and SHRs before surgery (Figure 1D). These data indicate that metabolic surgery reduced BP in genetic hypertensive rats in the absence of metabolic disturbances.
Metabolic Surgery Improved Vascular Remodeling and Dysfunction
After surgical treatment for 20 weeks, mesenteric and femoral arteries were isolated from SHRs. In SHRs with surgery, H&E staining showed larger lumen diameters and thinner walls, as determined by the lower wall‐to‐lumen ratio, compared to sham SHRs (Figure 2A). Endothelial‐dependent relaxations were also improved in mesenteric and femoral arteries from SHRs with surgery, compared to sham SHRs (Figure 2B). These results suggest that the reduction in BP was related to the improvement of vascular relaxation.
Figure 2.
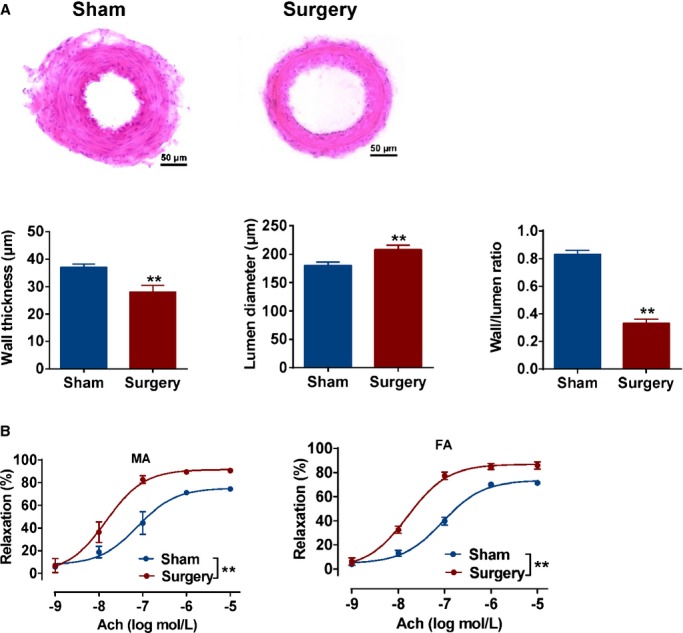
Effects of metabolic surgery on vascular remodeling and relaxation in spontaneously hypertensive rats (SHRs). A, Representative images of hematoxylin‐eosin staining cross‐sections of mesenteric arteries isolated from sham and surgery SHRs. Statistical analysis of mesenteric artery wall thickness, lumen diameter, and the ratio of artery wall to the lumen. Scale bar denotes 50 μm. **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=3. B, Acetylcholine (Ach)‐induced relaxation in the mesenteric arteries (MA) and femoral arteries (FA) from sham and surgery SHRs. **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=3.
Metabolic Surgery Reserved Cardiac Hypertrophy and Dysfunction
To further examine the effect of metabolic surgery on cardiac structure and function, hearts were isolated from SHRs and stained as described above. Heart weight, the ratio of heart weight to body weight, and myocardial collagen volume fraction were significantly reduced in SHRs with surgery, compared to sham SHR (Figure 3A and 3B). M‐mode echocardiography images analysis showed that SHRs with surgery had larger LVID, lower IVST, and LVPW thickness, as well as decreased mitral valve E/A ratio, compared to sham SHRs (Figure 3C and 3D). These results suggest that metabolic surgery also improves cardiac remodeling and dysfunction.
Figure 3.
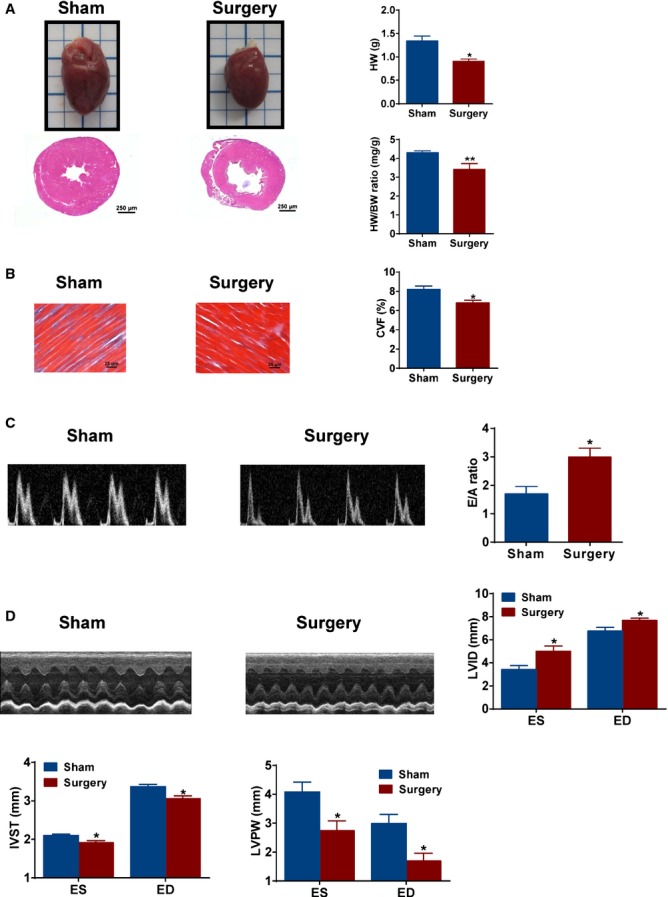
Effects of metabolic surgery on cardiac hypertrophy and dysfunction of SHRs. A, Representative images of cardiac morphology and hematoxylin‐eosin staining of transverse section in sham and surgery SHRs. The ratio of heart weight (HW) and heart weight/body weight (HW/BW) in sham and surgery SHRs (right panels). Scale bar denotes 250 μm. *P<0.05; **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=4. B, Representative photomicrographs of Masson trichrome staining for myocardium in red and cardiac fibrosis in blue. Scale bar denotes 25 μm. *P<0.05 vs. sham SHRs. Data are means±SEM. Each n=4. C, Representative E and A waves of mitral valve in sham and surgery SHRs. E/A ratio of mitral valve was analyzed. *P<0.05 vs. sham SHRs. Data are means±SEM. Each n=4. D, Representative images of M‐mode echocardiography in sham and surgery SHRs. Left ventricular internal dimension (LVID), interventricular septum thickness (IVST), left ventricular posterior wall (LVPW) at end of systole (ES), and end of diastole (ED) in M‐mode echocardiography. *P<0.05 vs. sham SHRs. Data are means±SEM. Each n=4. SHRs indicates spontaneously hypertensive rats.
Metabolic Surgery Antagonized Overdrive of SNS
The question remained as to what mechanism involved in metabolic surgery could mediate the improvement of CV disturbances. It is well known that SNS overdrive plays an important role in the development of HTN and CV disorders. Compared to sham SHR, we found a time‐related reduction in HR in SHRs with surgery, which was validated by the 24‐hour ambulatory BP monitoring (Figure 4A). To further confirm the role of SNS, SHRs were exposed to cold stress in a temperature‐controlled chamber. A larger variability of BP and HR was observed in sham SHRs, but not in SHRs with surgery (Figure 4B). Our results indicated that SNS overdrive in SHRs can be antagonized by metabolic surgery through inhibition of both peripheral and central sympathetic nerve activity.
Figure 4.
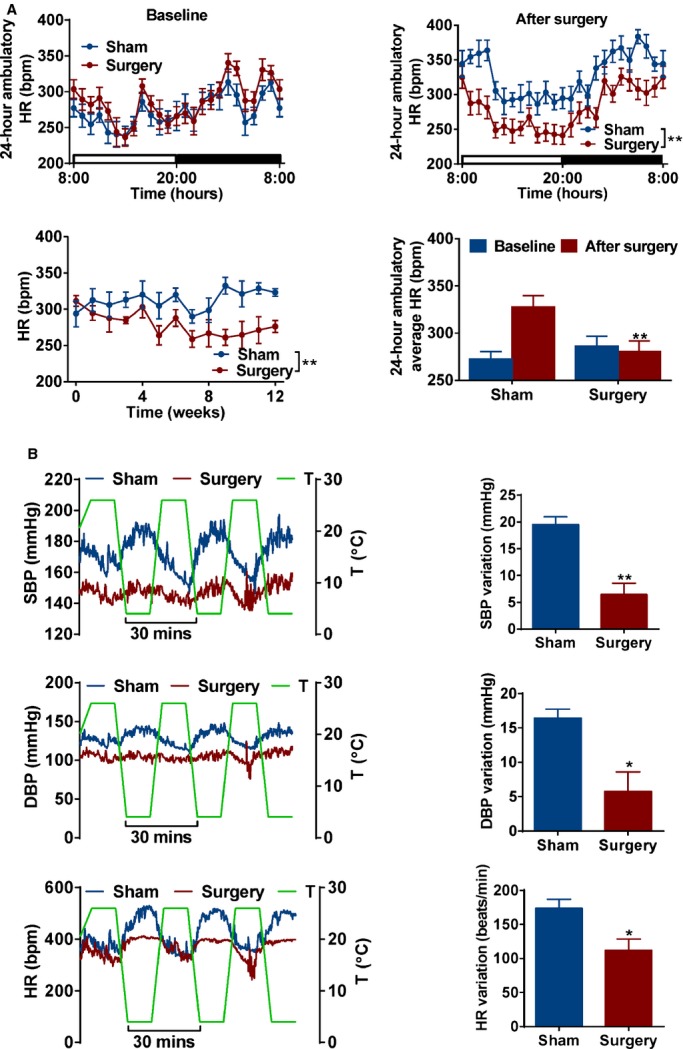
Effects of metabolic surgery on the sympathetic activity of SHRs. A, 24‐hour ambulatory heart rate (HR) in baseline (upper‐left panel) or after surgery at 12 weeks (upper‐right panel) in sham and surgery SHRs. The time courses of HR (lower‐left panel) and average of 24‐hour ambulatory (lower‐right panel) HR in sham and surgery SHR. *P<0.05; **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=7. B, Effect of cold stress on blood pressure and HR variability. *P<0.05; **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=3. bpm indicates beats per minute; DBP, diastolic blood pressure; SBP, systolic blood pressure; SHRs, spontaneously hypertensive rats.
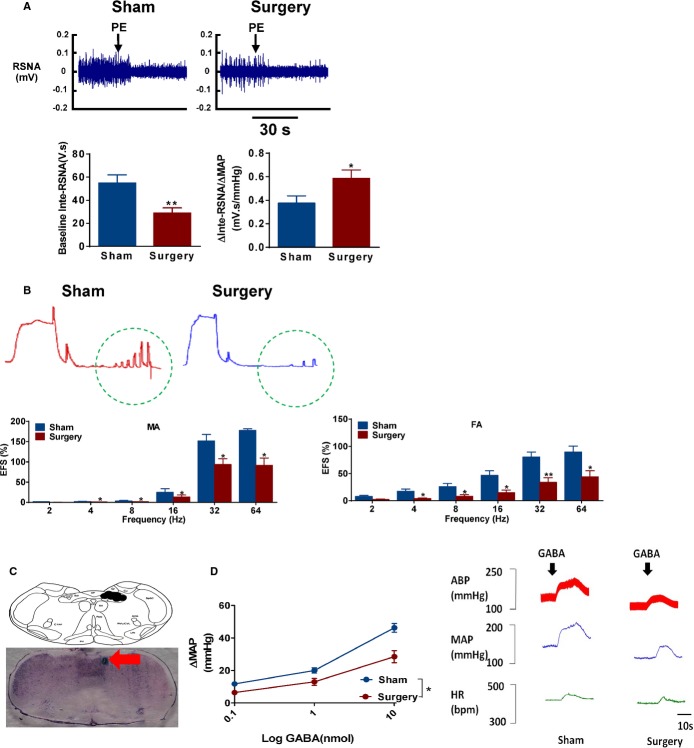
Effect of Metabolic Surgery on Peripheral and Central Sympathetic Nerve Activity
To examine the effects of metabolic surgery on sympathetic outflow in SHRs, RSNA and baroreflex sensitivity were measured after intravenous injection of PE in anesthetized rats. As shown in Figure 5A, the basal RSNA was lower in SHRs with surgery, compared to sham SHR. After bolus injections of PE, the increasing BP resulted in a profound bradycardia and sympathetic inhibition in SHRs with surgery (Figure 5A). Stimulating the perivascular nerve by EFS can induce a vasoconstriction. We found that EFS induced a frequency‐dependent contractile response, which was significantly lower in isolated vascular segments from SHRs with surgery, compared to sham SHR (Figure 5B). Furthermore, microinjection of GABA into the NTS significantly reduced changes of artery BP in SHRs with surgery, compared to sham SHR. These indicated that GI intervention may be an effective treatment of HTN through inhibition both peripheral and central sympathetic nerve activity (Figure 5C and 5D).
Figure 5.
Effect of metabolic surgery on the peripheral and central sympathetic nerve activity. A, Segments of original recordings of PE‐induced responses of renal sympathetic nerve activity (RSNA) in sham and surgery SHRs (upper panels). Histograms showed baseline of inte‐RSNA and the ratio of changes of inte‐RSNA to changes of MAP in sham and surgery SHRs (lower panels). **P<0.01 vs. sham SHR. Data are means±SEM. Each n=7. B, Effect of RYGB on vasoconstriction response to electrical field stimulation (EFS; 2 to 64 Hz) induced in the mesenteric arteries (MA) and femoral arteries (FA) from sham and surgery SHRs. The results are expressed as a percentage of the initial contraction elicited by KCl (60 mmol/L). *P<0.05; **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=4. C, Site of NTS microinjections in the transverse section of the brain stem in rats. Red arrow indicates the site of NTS microinjection. D, Immediate blood pressure effects of GABA microinjection into the NTS of sham and surgery SHRs. *P < 0.05 vs. sham SHR. Data are means±SEM. Each n=4. ABP indicates arterial blood pressure; GABA, gamma‐aminobutyric acid; HR, heart rate; MAP, mean arterial blood pressure; NTS, nucleus of the solitary tract; PE, phenylephrine; RYGB, Roux‐en‐Y gastric bypass; SHRs, spontaneously hypertensive rats.
In addition, SHRs with surgery reduced food intake and body weight (Figure 6A and 6B), but had a similar sodium intake and 24‐hour urinary sodium level, compared to sham SHR (Figure 6C). However, plasma GLP‐1 concentration was significantly higher in SHRs with surgery, compared to sham SHR (Figure 6D). In order to rule out the effect of weight loss on BP and SNS, 1 subgroup of sham SHRs was restricted with food intake for 4 weeks to approach the same body weight as SHRs with surgery. Interestingly, there were no significantly differences in BP, HR, and RSNA between sham SHRs with and without dietary restriction (Figure 6E). These results strongly support that the effect of GI intervention on BP is independent of weight loss.
Figure 6.
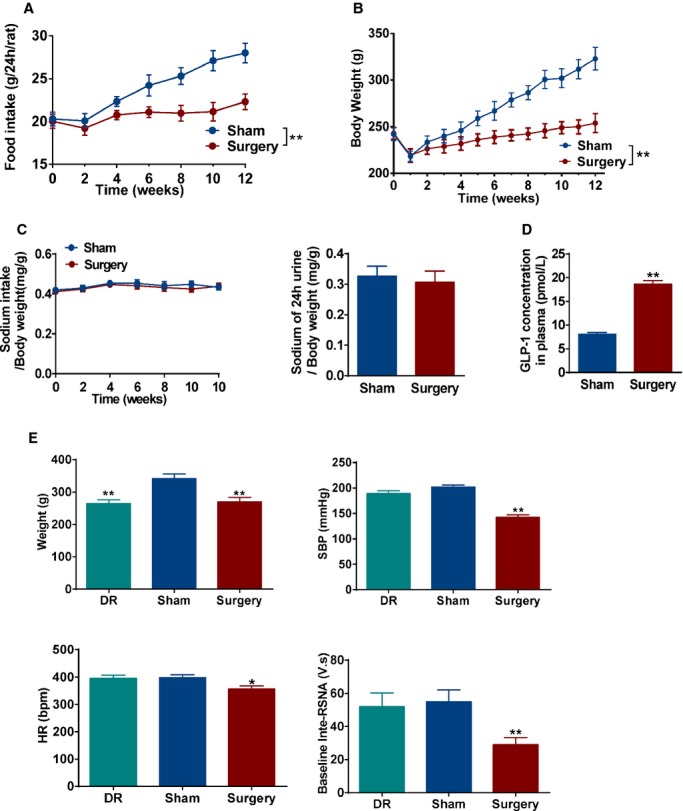
Effect of metabolic surgery on blood pressure is independent on weight loss. A and B, The food intake (A) and time courses of body weight (B) in sham and surgery SHRs. *P<0.05; **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=7. C, The ratio of sodium intake/body weight (left panel) and 24‐hour urinary sodium excretion of body weight (right panel) from sham and surgery SHRs. Data are means±SEM. Each n=7. D, Plasma GLP‐1 concentration in sham and surgery SHRs. **P<0.01 vs. sham SHR. Data are means±SEM. Each n=7. E, Effect of dietary restriction on body weight, SBP, HR, and baseline‐inte‐RSNA. *P<0.05; **P<0.01 vs. sham SHRs. Data are means±SEM. Each n=7. GLP indicates glucagon‐like peptide‐1; HR, heart rate; RSNA, renal sympathetic nervous activity; SBP, systolic blood pressure; SHRs, spontaneously hypertensive rats.
Effect of Metabolic Surgery on BP in Diabetic Hypertensive Patients
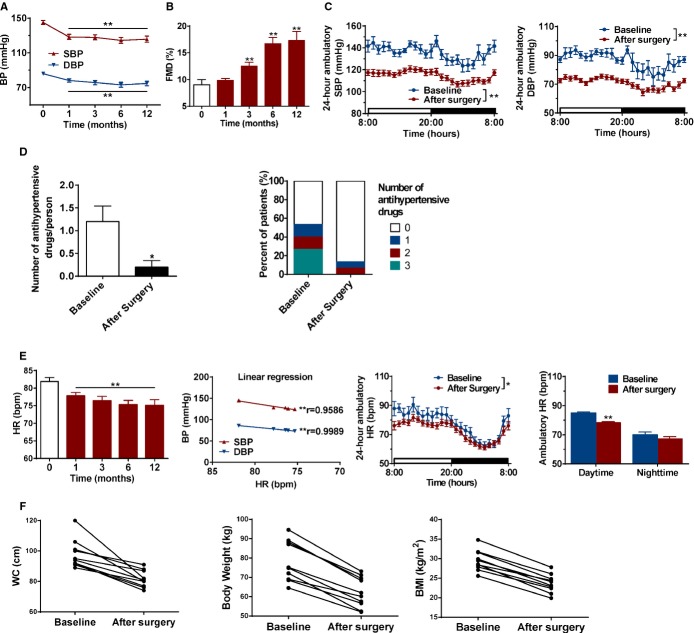
As a proof of principle, we further evaluated whether metabolic surgery lowers BP and improves vascular function through inhibition of SNS in human HTN with T2DM. Compared with presurgery levels, there was a reduced BP in hypertensive patients underwent metabolic surgery (Figure 7A). Additionally, the endothelium‐dependent vasodilatation was significantly improved in hypertensive patients underwent metabolic surgery after the third month (Figure 7B). ABPM further confirmed a reduction in BP after metabolic surgery (Figure 7C). Furthermore, metabolic surgery significantly reduced the kinds and dosage of antihypertensive drugs taken by hypertensive patients (Figure 7D). Consistent with the findings in SHRs, hypertensive patients also showed a time‐dependent reduction in HR after surgery, especially in the daytime, which was significantly correlated with reduction in BP (Figure 7E). In addition, waist circumstance, body weight, BMI blood lipid and glucose levels were significantly improved in hypertensive patients with T2DM (Figure 7F; Table). These results suggest that metabolic surgery can lower BP and ameliorate vascular dysfunction in hypertensive patients with comorbid metabolic disorders that might antagonize the overdrive of SNS (Figure 8).
Figure 7.
Effect of metabolic surgery on blood pressure (BP) in hypertensive patients with type 2 diabetes. A, Time courses of systolic blood pressure (SBP) and diastolic blood pressure (DBP) in hypertensive patients. BP was measured by mercury sphygmomanometer. **P<0.01 vs. baseline. Data are means±SEM. Each n=9 to 21. B, Flow‐mediated dilation (FMD) in hypertensive patients who underwent metabolic surgery was measured at 0 (baseline), 1, 3, 6, and 12 months. **P<0.01 vs. baseline. Data are means±SEM. Each n=9 to 21. C, The 24‐hour ambulatory SBP and DBP was monitored from hypertensive patients at baseline or 6 months after metabolic surgery. **P<0.01 vs. baseline. Data are means±SEM. Each n=17 to 21. D, Usage of antihypertensive drugs from hypertensive patients at baseline or after metabolic surgery. *P<0.05 vs. baseline. Data are means±SEM. Baseline (n=21) and after surgery (n=17). E, The time courses of RYGB reduced HR from hypertensive patients and a linear correlation between HR and BP. The 24‐hour ambulatory HR change after surgery, daytime, and nighttime HR were measured at month 6. *P<0.05; **P<0.01 vs. baseline. Data are means±SEM. Each n=9 to 21. F, Scatter plot graphic for WC, body weight, and BMI of hypertensive patients at baseline or 12 months after metabolic surgery. Baseline and after surgery (n=10). BMI indicates body mass index; HR, heart rate; RYGB, Roux‐en‐Y gastric bypass; WC, waist circumference.
Table 1.
Clinical and Biochemical Characteristics of Patients at Baseline and After Surgery
| Characteristics | Baseline | After Surgery |
|---|---|---|
| Weight, kg | 75.9±12.2 | 59.1±8.1** |
| BMI, kg/m2 | 29.1±3.7 | 23±2.3** |
| WC, cm | 95.4±8.9 | 79.2±6.3** |
| OGTT 0 minutes, mmol/L | 8.2±3.3 | 6.9±2.9 |
| OGTT 120 minutes, mmol/L | 15.7±4.8 | 8.4±3.7** |
| HOMA‐IR | 7.1±9.5 | 3.1±1.4 |
| HbA1c, % | 7.8±1.8 | 6.6±1.2* |
| hs‐CRP, g/L | 4.3±4.3 | 1±1.3** |
| TC, mmol/L | 5.2±1.5 | 4.2±1.3* |
| TG, mmol/L | 3.1±2.4 | 1.9±1.6 |
| HDL‐C, mmol/L | 1.3±0.7 | 1.4±0.3 |
| LDL‐C, mmol/L | 2.6±0.6 | 2.6±0.9 |
Four males and 6 females are included in Table, with mean age 46±12 years old. Data are presented as mean±SD. BMI indicates body mass index; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; OGTT, oral glucose tolerance test; TC, total cholesterol; TG, triglyceride; WC, waist circumference.
*P<0.05; **P<0.01 after surgery (at 6 months) vs. baseline.
Figure 8.
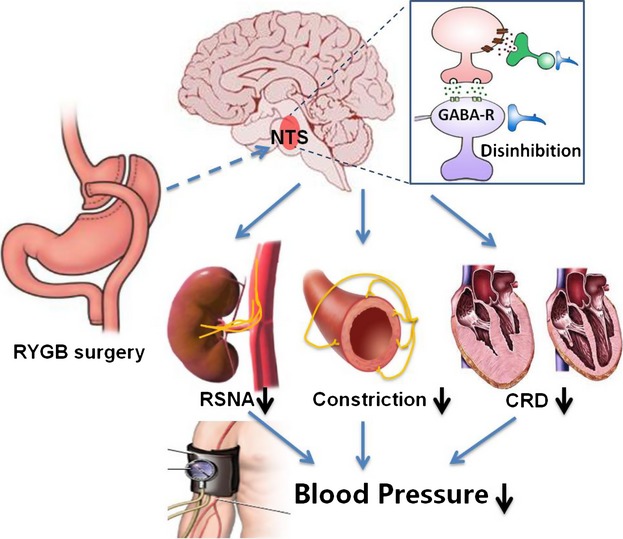
The possible role of gastrointestinal intervention in reducing blood pressure through inhibition of sympathetic nerve activity. Roux‐en‐Y gastric bypass (RYGB) surgery transmits a signal to the nucleus of solitary tract (NTS). Disinhibition of NTS neurons reduces renal sympathetic activity (RSNA), baroreflex, cold exposure‐induced changes in blood pressure and heart rate, and vasoconstriction by perivascular nerve stimulation as well as improves cardiac remodeling and dysfunction (CRD), which lowers blood pressure in the genetic hypertensive model. GABA indicates gamma‐aminobutyric acid.
Discussion
The present study provides evidences for the beneficial effect of metabolic surgery on HTN and cardiac‐metabolic risks. Our studies confirmed that GI surgical intervention can significantly inhibit the overdrive of SNS24 in genetic hypertensive rats, which results in reducing BP and improvement of CV remodeling and dysfunction. In addition, metabolic surgery inhibited RSNA and the peripheral vascular nerve induced vasoconstriction as well as central baroreflex in these hypertensive rats. Importantly, this beneficial effect of metabolic surgery was also shown in hypertensive patients with T2DM. Furthermore, metabolic surgery reduced HR in a time‐dependent manner in hypertensive patients. Taken together, we propose a mechanism by which metabolic surgery ameliorates HTN and cardiac/metabolic disturbances by antagonizing SNS activation.
Metabolic surgery is currently the most effective treatment option for obesity and diabetes.12–14 In addition, metabolic surgery can effectively ameliorate HTN over the long term and reduce future CVEs15–20; however, the underlying mechanisms remain elusive. Several studies suggest that the reduction in BP could be related to metabolic surgery‐induced weight loss.39–40 However, clinical trials and experimental studies indicated that a quick reduction in BP occurred before the metabolic surgery‐mediated weight loss.17 A meta‐analysis reported that there was resolution of HTN in 61.7% of patients and resolution or improvement of HTN in 78.5% of patients after metabolic surgery.14 Antihypertensive therapy was reduced or discontinued in 70% of patients receiving metabolic surgery.41 We also showed that the beneficial effect of metabolic surgery on BP was independent of weight loss in surgically treated hypertensive rats and was independent of surgical trauma and food intake.15,18 However, the mechanism underlying the metabolic surgery‐mediated antihypertensive effect remains unknown.
Although the precise mechanisms mediating HTN remission after metabolic surgery remains poorly understood, it is apparent that rearrangements of GI anatomy can exert several discrete antihypertensive effects beyond those related to reduce sodium intake and body weight. Proposed multiple factors include the changes in gut hormones, insulin sensitivity, GI nerve innervation, and intestinal microbial flora.42–44,37,45–47 Antagonizing the SNS by metabolic surgery could be one of the factors responsible for hypotension. It is well known that overdrive of SNS contributes to the pathogenesis of HTN and cardiometabolic disease.4–8 Administration of α‐ or β‐adrenergic blockers or clonidine, a drug that stimulates central α2‐receptors and reduces SNS activity, can prevent high‐fat‐diet–induced HTN in obese dogs. Combined α‐ and β‐adrenergic blocker treatment reduced ambulatory BP more in obese hypertensive patients than in lean hypertensive patients.48 In this study, metabolic surgery reduced HR in both hypertensive patients and rats in a time‐dependent manner. Furthermore, variations in BP and HR under cold exposure were strikingly smaller in the surgically treated hypertensive rats than that in sham hypertensive rats. These findings suggest that the metabolic surgery‐mediated hypotension was closely associated with the inhibition of overdrive of the SNS.
It is well documented that renal sympathetic nerves mediate most of the chronic effects of SNS activation on BP.49 In addition, the impact of GI autonomic nerves on BP is well recognized. One early study reported that an appropriate amount of electrical stimulation of the central cut end of the abdominal vagus raised BP by 30 mm Hg.50 HTN is referred to as neurogenic if it is the result of abnormal stimulation of the ANS, rather than the result of a primary vascular or renal defect. This abnormality can originate in the afferent arm of the system or in the central circuitry. The level of nucleus activity at rest is presumed to be the most crucial parameter for long‐term BP control.10 This neural activity is set by a core network of neurons that reside in the rostral ventrolateral medulla, the spinal cord, the hypothalamus, and the NTS.10
Recent studies show that dietary factors, gut flora, and hormones can affect the nervous system through a gut‐brain cross‐talk.42,51–52 Endogenous GI noradrenalin is being released, especially in the duodenum, where the concentration of noradrenalin is the highest.53 Sensory stimulation in the GI tract is involved in the regulation of the SNS through the CNS.52 A recent study reported that there was a vagal remodeling and denervation of the stomach and part of intestine following metabolic surgery.54 In addition to hormones and circulating factors, the gut communicates with the brain through primary visceral afferent nerve fibers, such as GI or renal nerves, comprised in the vagus and the dorsal root/spinal cord pathway.55 Thus, metabolic surgery alters existing gut‐brain communication and results in an alteration of the neural control of the target organs, such as the heart, blood vessels, and kidney. In this study, we showed that RSNA and vasoconstriction by perivascular nerve stimulation were significantly inhibited in surgically treated hypertensive rats, compared to sham hypertensive rats. To further identify which specific circuit or reflex pathway or cerebral nucleus is affected, the effect of metabolic surgery on the central baroreflex pathway was examined. The NTS is a termination site for primary afferent fibers from baroreceptors, and other peripheral CV receptors, that contain BP‐sensitive neurons.10 GABA is a well‐known neurotransmitter that exerts inhibitory actions in the brain, mediated through its receptors.56–58 We showed that microinjection of GABA into the NTS caused smaller changes of BP in SHRs with surgery, compared to sham SHRs. These striking results indicate that GI intervention may be an effective method for treatment of HTN through inhibition of both peripheral and central sympathetic nerve activity.
Although the reduction of renal sympathetic afferent and efferent activity by percutaneous, catheter‐based endovascular radiofrequency ablation effectively lowers BP, a recent clinical trial did not observe a significant effect on SBP in patients with resistant HTN.59 Furthermore, there was controversial or inconsistent evidence concerning the improvement of hyperglycemia, obesity, and dyslipidemia through RSND.60–63 In contrast, most clinical studies, including ours, have shown that metabolic surgery had a satisfying outcome in regard to weight loss, remission of hyperglycemia, and improvement of dyslipidemia.64 Obesity, T2DM, and obstructive sleep apnea syndrome are common causes of resistant HTN. Thus, metabolic surgery might be a novel option for the management of resistant HTN, especially for hypertensive patients with the complications of obesity and T2DM.65
In summary, we demonstrate that metabolic surgery treatment lowers BP in hypertensive patients and genetic hypertensive rats. Our mechanistic evidence suggests that this CV benefit is likely to be the result of decreasing excessive sympathetic activation. Our findings provide insights into the mechanism of metabolic surgery in the regulation of BP. GI intervention may represent a promising intervention in resistant hypertension with metabolic disturbances.
Sources of Funding
This work was supported by grants from the National Basic Research Program of China (2012CB517805 and 2012CB517806), the National Natural Science Foundation of China (91339000, 91339112, and 81130006), Program for Changjiang Scholars and Innovative Research Team in University (IRT1216), the International Science and Technology Cooperation/Base and Platform Construction of Chongqing Science and Technology Commission (cstc2011gjhz0006), and the Clinical Fund of Third Military Medical University (2010XLC38).
Disclosures
None.
References
- 1.Blessing WW. The Lower Brainstem and Bodily Homeostasis. 1997New York, NY: Oxford University Press [Google Scholar]
- 2.Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. 1990New York, NY: Oxford University Press [Google Scholar]
- 3.Mancia G, Daffonchio A, Di Rienzo M, Ferrari A, Grassi G. Methods to quantify sympathetic cardiovascular influences. Eur Heart J. 1998; 19:F7. [PubMed] [Google Scholar]
- 4.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012; 33:1058-1066. [DOI] [PubMed] [Google Scholar]
- 5.Esler M, Lambert E, Schlaich M. Point: chronic activation of the sympathetic nervous system is the dominant contributor to systemic hypertension. J Appl Physiol. 2010; 109:1996-1998. [DOI] [PubMed] [Google Scholar]
- 6.Levick SP, Murray DB, Janicki JS, Brower GL. Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension. 2010; 55:270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffrin EL. Vascular remodeling in hypertension mechanisms and treatment. Hypertension. 2012; 59:367-374. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Wang H, Luo W, Guo C, Wang J, Chen Y, Chang L, Eitzman DT. Leptin‐induced endothelial dysfunction is mediated by sympathetic nervous system activity. J Am Heart Assoc. 2013; 2:e00029910.1161/JAHA.113.000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation mechanisms and potential for hypertension therapy. Hypertension. 2011; 57:880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006; 7:335-346. [DOI] [PubMed] [Google Scholar]
- 11.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S. Catheter‐based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof‐of‐principle cohort study. Lancet. 2009; 373:1275-1281. [DOI] [PubMed] [Google Scholar]
- 12.Cummings DE. Metabolic surgery for type 2 diabetes. Nat Med. 2012; 18:656. [DOI] [PubMed] [Google Scholar]
- 13.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012; 366:1567-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery. JAMA. 2004; 292:1724-1737. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez A, Becerril S, Valentí V, Moncada R, Méndez‐Giménez L, Ramírez B, Lancha A, Martín M, Burrell MA, Catalán V. Short‐term effects of sleeve gastrectomy and caloric restriction on blood pressure in diet‐induced obese rats. Obes Surg. 2012; 22:1481-1490. [DOI] [PubMed] [Google Scholar]
- 16.Arterburn D, Schauer DP, Wise RE, Gersin KS, Fischer DR, Selwyn CA, Jr, Erisman A, Tsevat J. Change in predicted 10‐year cardiovascular risk following laparoscopic roux‐en‐y gastric bypass surgery. Obes Surg. 2009; 19:184-189. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed AR, Rickards G, Coniglio D, Xia Y, Johnson J, Boss T, O'Malley W. Laparoscopic roux‐en‐y gastric bypass and its early effect on blood pressure. Obes Surg. 2009; 19:845-849. [DOI] [PubMed] [Google Scholar]
- 18.Cowan GS, Buffington CK. Significant changes in blood pressure, glucose, and lipids with gastric bypass surgery. World J Surg. 1998; 22:987-992. [DOI] [PubMed] [Google Scholar]
- 19.Hallersund P, Sjöström L, Olbers T, Lönroth H, Jacobson P, Wallenius V, Näslund I, Carlsson LM, Fändriks L. Gastric bypass surgery is followed by lowered blood pressure and increased diuresis‐long term results from the Swedish Obese Subjects (SOS) study. PLoS One. 2012; 7:e49696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjöström L, Lindroos A‐K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004; 351:2683-2693. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen NT, Perez RV, Fleming N, Rivers R, Wolfe BM. Effect of prolonged pneumoperitoneum on intraoperative urine output during laparoscopic gastric bypass. J Am Coll Surg. 2002; 195:476-483. [DOI] [PubMed] [Google Scholar]
- 22.Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux‐en‐Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012; 26:2231-2239. [DOI] [PubMed] [Google Scholar]
- 23.Wasmund SL, Owan T, Yanowitz FG, Adams TD, Hunt SC, Hamdan MH, Litwin SE. Improved heart rate recovery after marked weight loss induced by gastric bypass surgery: two‐year follow up in the Utah Obesity Study. Heart Rhythm. 2011; 8:84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curry TB, Somaraju M, Hines CN, Groenewald CB, Miles JM, Joyner MJ, Charkoudian N. Sympathetic support of energy expenditure and sympathetic nervous system activity after gastric bypass surgery. Obesity. 2013; 21:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti G, Zimmet P, Shaw J, Grundy SM. The IDF consensus worldwide definition of the metabolic syndrome. Brussels: Int Diabetes Fed. 2005; 366:1059-1602. [Google Scholar]
- 26. Bariatric Surgical in the Treatment of Patients with Type 2 Diabetes. A position statement from the Chinese Diabetes Society. China J Pract Surg. 2011; 31:367-370. [Google Scholar]
- 27.Huang C‐K, Shabbir A, Lo C‐H, Tai C‐M, Chen Y‐S, Houng J‐Y. Laparoscopic Roux‐en‐Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25–35. Obes Surg. 2011; 21:1344-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He H, Zhao Z, Chen J, Ni Y, Zhong J, Yan Z, Li Y, Liu D, Pletcher MJ, Zhu Z. Metformin‐based treatment for obesity‐related hypertension: a randomized, double‐blind, placebo‐controlled trial. J Hypertens. 2012; 30:1430-1439. [DOI] [PubMed] [Google Scholar]
- 29.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard‐Herman M, Herrington D. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial arterya report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002; 39:257-265. [DOI] [PubMed] [Google Scholar]
- 30.Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z. Activation of TRPV1 by dietary capsaicin improves endothelium‐dependent vasorelaxation and prevents hypertension. Cell Metab. 2010; 12:130-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud H‐R. Meal‐induced hormone responses in a rat model of Roux‐en‐Y gastric bypass surgery. Endocrinology. 2010; 151:1588-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown AR, Antle MC, Hu B, Teskey GC. High frequency stimulation of the subthalamic nucleus acutely rescues motor deficits and neocortical movement representations following 6‐hydroxydopamine administration in rats. Exp Neurol. 2011; 231:82-90. [DOI] [PubMed] [Google Scholar]
- 33.Shechtman O, Fregly MJ, van Bergen P, Papanek PE. Prevention of cold‐induced increase in blood pressure of rats by captopril. Hypertension. 1991; 17:763-770. [DOI] [PubMed] [Google Scholar]
- 34.Sun Z, Fregly MJ, Cade JR. Effect of renal denervation on elevation of blood pressure in cold‐exposed rats. Can J Physiol Pharmacol. 1995; 73:72-78. [DOI] [PubMed] [Google Scholar]
- 35.Pleger ST, Remppis A, Heidt B, Völkers M, Chuprun JK, Kuhn M, Zhou R‐H, Gao E, Szabo G, Weichenhan D. S100a1 gene therapy preserves in vivo cardiac function after myocardial infarction. Mol Ther. 2005; 12:1120-1129. [DOI] [PubMed] [Google Scholar]
- 36.Wang W‐Z, Wang L‐G, Gao L, Wang W. Contribution of AMPA/kainate receptors in the rostral ventrolateral medulla to the hypotensive and sympathoinhibitory effects of clonidine. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R1232-R1238. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, Ma L, Zhao Z, Liu D, Zhu Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon‐like peptide‐1 secretion and improves glucose homeostasis. Diabetes. 2012; 61:2155-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aceros H, Farah G, Cobos‐Puc L, Stabile A, Noiseux N, Mukaddam‐Daher S. Moxonidine improves cardiac structure and performance in SHR through inhibition of cytokines, p38 MAPK and Akt. Br J Pharmacol. 2011; 164:946-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernstrom JD, Courcoulas AP, Houck PR, Fernstrom MH. Long‐term changes in blood pressure in extremely obese patients who have undergone bariatric surgery. Arch Surg. 2006; 141:276. [DOI] [PubMed] [Google Scholar]
- 40.Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass‐induced weight loss. Ann Surg. 2003; 237:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012; 366:1577-1585. [DOI] [PubMed] [Google Scholar]
- 42.Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011; 12:453-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010; 61:393-411. [DOI] [PubMed] [Google Scholar]
- 44.Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR. Metabolic surgery profoundly influences gut microbial–host metabolic cross‐talk. Gut. 2011; 60:1214-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon‐like peptide 1‐dependent mechanism. Hypertension. 2012; 60:833-841. [DOI] [PubMed] [Google Scholar]
- 46.Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, Ni Y, Liu D, Zhu Z. Blood pressure‐lowering effects of GLP‐1 receptor agonists exenatide and liraglutide: a meta‐analysis of clinical trials. Diabetes Obes Metab. 2013; 15:737-749. [DOI] [PubMed] [Google Scholar]
- 47.Linz D, Wirth K, Linz W, Heuer HO, Frick W, Hofmeister A, Heinelt U, Arndt P, Schwahn U, Böhm M. Antihypertensive and laxative effects by pharmacological inhibition of sodium‐proton‐exchanger subtype 3–mediated sodium absorption in the gut. Hypertension. 2012; 60:1560-1567. [DOI] [PubMed] [Google Scholar]
- 48.Wofford MR, Anderson DC, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of α‐and β‐adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001; 14:694-698. [DOI] [PubMed] [Google Scholar]
- 49.Wofford MR, Hall JE. Pathophysiology and treatment of obesity hypertension. Curr Pharm Des. 2004; 10:3621-3637. [DOI] [PubMed] [Google Scholar]
- 50.Cragg BG, Evans D. Some reflexes mediated by the afferent fibers of the abdominal vagus in the rabbit and cat. Exp Neurol. 1960; 2:1-12. [DOI] [PubMed] [Google Scholar]
- 51.Cryan JF, Dinan TG. Mind‐altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012; 13:701-712. [DOI] [PubMed] [Google Scholar]
- 52.Mul JD, Begg DP, Barrera JG, Li B, Matter EK, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. High‐fat diet changes the temporal profile of GLP‐1 receptor‐mediatedhypophagia in rats. Am J Physiol Regul Integr Comp Physiol. 2013; 305:R68-R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taubin H, Djahanguiri B, Landsberg L. Noradrenaline concentration and turnover in different regions of the gastrointestinal tract of the rat: an approach to the evaluation of sympathetic activity in the gut. Gut. 1972; 13:790-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gautron L, Zechner J, Aguirre V. Vagal innervation patterns following Roux‐en‐Y gastric bypass in the mouse. Int J Obes. 2013; 37:1603-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berthoud H‐R, Shin AC, Zheng H. Obes Surg and gut–brain communication. Physiol Behav. 2011; 105:106-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dietrich WD, Lowry OH, Loewy AD. The distribution of glutamate, GABA and aspartate in the nucleus tractus solitarius of the cat. Brain Res. 1982; 237:254-260. [DOI] [PubMed] [Google Scholar]
- 57.Izzo PN, Sykes RM, Spyer KM. Gamma‐aminobutyric acid immunoreactive structures in the nucleus tractus solitarius: a light and electron microscopic study. Brain Res. 1992; 591:69-78. [DOI] [PubMed] [Google Scholar]
- 58.Potts JT, Paton JF, Mitchell JH, Garry MG, Kline G, Anguelov PT, Lee SM. Contraction‐sensitive skeletal muscle afferents inhibit arterial baroreceptor signalling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience. 2003; 119:201-214. [DOI] [PubMed] [Google Scholar]
- 59.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha‐Singh K, Townsend RR, Bakris GLSYMPLICITY HTN‐3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014; 370:1393-1401. [DOI] [PubMed] [Google Scholar]
- 60.D'Agord Schaan B, Lacchini S, Bertoluci MC, Irigoyen MC, Machado UF, Schmid H. Impact of renal denervation on renal content of glut1, albuminuria and urinary tgf‐ß1 in streptozotocin‐induced diabetic rats. Auton Neurosci. 2003; 104:88-94. [DOI] [PubMed] [Google Scholar]
- 61.Witkowski A, Prejbisz A, Florczak E, Kądziela J, Śliwiński P, Bieleń P, Michałowska I, Kabat M, Warchoł E, Januszewicz M. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011; 58:559-565. [DOI] [PubMed] [Google Scholar]
- 62.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertensionclinical perspective a pilot study. Circulation. 2011; 123:1940-1946. [DOI] [PubMed] [Google Scholar]
- 63.Katayama T, Sueta D, Kataoka K, Hasegawa Y, Koibuchi N, Toyama K, Uekawa K, MingJie M, Nakagawa T, Maeda M, Ogawa H, Kim‐Mitsuyama S. Long‐term renal denervation normalizes disrupted blood pressure circadian rhythm and ameliorates cardiovascular Injury in a rat model of metabolic syndrome. J Am Heart Assoc. 2013; 2:e00019710.1161/JAHA.113.000197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seravalle G, Colombo M, Perego P, Giardini V, Volpe M, Dell'Oro R, Mancia G, Grassi G. Long‐term sympathoinhibitory effects of surgically induced weight loss in severe obese patients. Hypertension. 2014; 64:431-437. [DOI] [PubMed] [Google Scholar]
- 65.Mark AL, Norris AW, Rahmouni K. Sympathetic inhibition after bariatric surgery. Hypertension. 2014; 64:235-236. [DOI] [PMC free article] [PubMed] [Google Scholar]