Abstract
Background
Atrial fibrillation (AF) frequently occurs in patients with chronic kidney disease (CKD); however, the long‐term impact of development of AF on the risk of death among patients with CKD is unknown.
Methods and Results
We studied adults with CKD (glomerular filtration rate <60 mL/min per 1.73 m2 by the Chronic Kidney Disease Epidemiology Collaboration equation) identified between 2002 and 2010 who were enrolled in Kaiser Permanente Northern California and had no previously documented AF. Incident AF was identified using primary hospital discharge diagnoses or ≥2 outpatient visits for AF. Death was comprehensively ascertained from health plan administrative databases, Social Security Administration vital status files, and the California death certificate registry. Covariates included demographics, comorbidity, ambulatory blood pressure, laboratory values (hemoglobin, proteinuria), and longitudinal medication use. Among 81 088 adults with CKD, 6269 (7.7%) developed clinically recognized incident AF during a mean follow‐up of 4.8±2.7 years. There were 2388 cases of death that occurred after incident AF (145 per 1000 person‐years) compared with 18 865 cases of death during periods without AF (51 per 1000 person‐years, P<0.001). After adjustment for potential confounders, incident AF was associated with a 66% increase in relative rate of death (adjusted hazard ratio 1.66, 95% CI 1.57 to 1.77).
Conclusion
Incident AF is independently associated with an increased risk of death in adults with CKD. Further study is needed to understand the mechanisms by which CKD is associated with AF and to identify potentially modifiable risk factors to decrease the burden of AF and subsequent risk of death in this high‐risk population.
Keywords: atrial fibrillation, kidney disease, mortality
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in the general population.1 The prevalence of AF is even higher among patients with kidney disease, occurring in 7% to 20% among patients with end‐stage renal disease (ESRD) on dialysis,2 a rate that is 2‐ to 3‐fold higher than reported among the general population.3 Recent data from the US Renal Data System reported that the prevalence of AF continues to increase among patients with ESRD.3 Moreover, AF is independently associated with increased risk for ischemic stroke and death among patients with ESRD on dialysis.4–5
Recently, several studies have also found high incidence and prevalence of AF among the larger population of patients with chronic kidney disease (CKD) not yet requiring dialysis.6–10 A recent study estimated the prevalence of AF to be 18% in a multicenter cohort with a wide range of kidney function.10 Although it is well established that AF is associated with poor clinical outcomes in the general population and in ESRD patients, much less is known about the long‐term impact of AF in patients with CKD.
To address this knowledge gap, we examined the association of incident AF on the risk of death among a large, diverse, community‐based cohort of adults with known CKD.
Methods
Source Population
The source population included members of Kaiser Permanente Northern California, a large integrated healthcare delivery system providing comprehensive care to >3.4 million patients in the San Francisco and greater Bay area.
Study Population
The study sample included all adult members (aged ≥21 years) who had an estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2, as calculated by the Chronic Kidney Disease Epidemiology Collaboration equation11 between January 1, 2002, and December 31, 2010. Subjects had to have ≥2 outpatient eGFR measures of <60 mL/min per 1.73 m2 separated by at least 90 days, and all subsequent eGFR measures <60 mL/min per 1.73 m2 were included in the analyses. Based on guidelines from Kidney Disease Improving Global Outcomes, we categorized kidney function as follows: 45 to 59, 30 to 44, 15 to 29 and <15 mL/min per 1.73 m2.12 The date of the first eGFR that qualified as CKD was considered the index date. Among 113 851 participants who met initial inclusion criteria, we excluded 3078 patients with prior ESRD and 29 685 with previously documented AF. Prior AF was defined as having ≥1 primary or secondary hospital discharge diagnosis, ambulatory visit, and/or emergency department visit with an International Classification of Diseases, ninth revision, code of 427.31 or 427.32.13–15 The final analytic sample included 81 088 subjects.
The study was approved by the institutional review board of the Kaiser Foundation Research Institute. Waiver of informed consent was obtained, given the nature of the study.
Predictor Variable
The primary predictor was diagnosed incident AF from cohort entry through December 2010. Incident AF was defined using previously described, validated approaches13–15 based on the first occurrence of hospitalization with a primary discharge diagnosis of AF or ≥2 ambulatory visits for AF based on International Classification of Diseases, ninth revision, codes 427.31 or 427.32 found in health plan inpatient and ambulatory visit databases.
Follow‐Up and Outcomes
Follow‐up occurred until the outcome of all‐cause mortality was reached, the patient disenrolled from the health plan, or the end of the study period on December 31, 2010. Deaths were identified from health plan administrative databases, Social Security Administration vital status files, and the California state death certificate registry during the follow‐up period.16 Patients were censored at ESRD, which was defined as receipt of chronic dialysis or renal transplant and was identified from a comprehensive health plan ESRD treatment registry.17
Covariates
Data were collected on demographic characteristics (age, sex, and self‐reported race or ethnicity) from health plan administrative databases and selected socioeconomic features (educational attainment, annual household income) from 2010 US census block data.16,18 Comorbid conditions (diabetes mellitus, hypertension, coronary heart disease, stroke, heart failure, peripheral artery disease, dyslipidemia, lung disease, liver disease, and hyperthyroidism) were determined using validated algorithms based on relevant diagnoses and procedures, dispensed prescription medications, and/or laboratory results from health plan databases.19 The most recent outpatient systolic and diastolic blood pressure values before the index date were obtained from ambulatory visit databases, which have been shown to reliably reflect chronic blood pressure levels in our population.20 Hemoglobin level at entry was obtained from ambulatory health plan laboratory databases and categorized (in g/L) as <9, 9 to 9.9, 10 to 10.9, 11 to 11.9, 12 to 12.9, 13 to 13.9, and >14. Proteinuria at entry (in the absence of possible concomitant urinary tract infection) was defined based on urine dipstick results obtained from ambulatory health plan laboratory databases and quantified as none/trace, 1+, 2+, 3+, or 4+.16 Baseline use of antihypertensive medications (angiotensin converting enzyme inhibitors or angiotensin receptor blockers, calcium channel blockers, diuretics, beta blockers), statins, other lipid‐lowering agents, warfarin, and antiplatelet medications in the 120 days before or on the index date were obtained from health plan ambulatory pharmacy databases.
Statistical Methods
All analyses were performed using SAS statistical software version 9.1. Differences between subjects with and without incident AF were compared using the Student t test for continuous variables and the chi‐square test for categorical variables. We performed multivariable Cox proportional hazards regression to examine the association between development of incident AF during follow‐up and risk of death. Follow‐up for each subject started on the index date and continued until disenrollment from the health plan, ESRD, end of the study period, or occurrence of the outcome event (ie, death). AF was a time‐updated exposure. If a patient developed AF during follow‐up, they contributed time to the no AF exposure group before being diagnosed with incident AF. After being diagnosed with AF, they would contribute person‐time to the incident AF exposure group. Variables included in models were based on variables that were significantly different between the study population and controls on bivariate analyses or have been shown previously to be associated either with kidney function or AF.6,21–22 We identified a priori potential confounder covariates that were time updated throughout the duration of follow‐up or after AF diagnosis as appropriate: age, sex, race, household income status, educational attainment, diabetes mellitus, dyslipidemia, chronic lung disease, chronic liver disease, and thyroid disease. We also identified potential mediators of the association between AF and death that were fixed at the time of AF diagnosis among patients who developed incident AF: eGFR category, proteinuria, hemoglobin category, hypertension status, systolic blood pressure, history of stroke or transient ischemic attack, history of heart failure, history of coronary heart disease, history of peripheral artery disease, and baseline use of relevant medications (beta blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, calcium channel blockers, diuretics, statins, other lipid‐lowering agents, warfarin, and antiplatelet agents).
Based on a priori hypotheses, we conducted stratified multivariable analyses for age (<60, 60 to 70, and ≥70 years), sex (men versus women), race (white, black, and Asian/Pacific Islander), and entry eGFR level (45 to 59, 30 to <45, and <30 mL/min per 1.73 m2).
In a sensitivity analysis, we adjusted for interim stroke and transient ischemic attack to test whether these interim events mediated the association between incident AF and risk of death. We also performed a second sensitivity analysis to determine whether the development of AF was a proxy for progression of kidney disease by conducting a parallel matched cohort analysis using a highly stratified extended Cox regression model. In this parallel matched cohort analysis, t1 was the time of incident AF diagnosis for patients who developed incident AF. We matched each incident AF patient (n=6269) with patients who did not have AF at t1 based on sex, age (±5 years), eGFR category (<30, 30 to 44, or 45 to 59 mL/min per 1.73 m2), and being alive at the time of t1 (n=49 140), with an average matching ratio of 1:7. We followed both the AF and non‐AF controls until the end of follow‐up, ESRD, disenrollment, or death. We adjusted for covariates in the models based on the specifications outlined above for the primary analysis.
Results
Baseline Characteristics
The total study population included 81 088 adults with CKD. At cohort entry, mean age was 72.9±11.3 years, 51.1% were women, and 67.1% were white. Overall, 27.9% of subjects had diabetes mellitus, 80.5% had hypertension, 6.4% had coronary heart disease, 8.5% had heart failure, and 4.2% had hyperthyroidism. At entry, 62.8% had eGFR 45 to 59 mL/min per 1.73 m2, 28.2% had eGFR 30 to 44 mL/min per 1.73 m2, 7.8% had eGFR 15 to 29 mL/min per 1.73 m2, and 1.2% had eGFR<15 mL/min per 1.73 m2. Furthermore, 39.1% of subjects were receiving beta blockers, 46.1% were receiving angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and 35.7% were receiving statins at entry.
Through 2010, a total of 6269 subjects (7.7%) developed incident AF. Compared with subjects who did not develop AF, those who developed incident AF were more likely to be older, male, and white; have a history of hypertension or cardiovascular conditions; have higher systolic blood pressure; and have lower eGFR at baseline (Table 1).
Table 1.
Baseline Characteristics of 81 088 Adults With Chronic Kidney Disease*
| Characteristic | No Incident Atrial Fibrillation (n=74 819) | Incident Atrial Fibrillation (n=6269) | P Value |
|---|---|---|---|
| Mean (SD) age, y | 72.5 (11.4) | 77.5 (8.4) | <0.0001 |
| Women, % | 51.4 | 48.5 | <0.0001 |
| Race, % | <0.0001 | ||
| White | 66.3 | 77.1 | |
| Black | 7.3 | 4.1 | |
| Hispanic | 0.2 | 0.2 | |
| Asian/Pacific Islander | 9.9 | 6.7 | |
| Socioeconomic status | |||
| Annual household income <$35 000 (%) | 14.8 | 13.8 | 0.03 |
| Less than 9th grade education, % | 4.4 | 3.6 | <0.005 |
| Medical history, % | |||
| Diabetes mellitus | 28.2 | 23.7 | <0.0001 |
| Hypertension | 80.2 | 84.0 | <0.0001 |
| Coronary heart disease | 6.3 | 8.5 | <0.0001 |
| Ischemic stroke | 2.0 | 2.0 | 1.0 |
| Transient ischemic attack | 0.7 | 1.0 | 0.03 |
| Chronic heart failure | 7.9 | 14.9 | <0.0001 |
| Peripheral arterial disease | 2.6 | 3.4 | 0.0007 |
| Dyslipidemia | 51.5 | 51.0 | 0.40 |
| Chronic lung disease | 27.1 | 32.1 | <0.0001 |
| Chronic liver disease | 1.5 | 0.9 | 0.0001 |
| Hyperthyroidism | 4.2 | 4.5 | 0.2 |
| Estimated GFR category, mL/min per 1.73 m2 (%) | <0.0001 | ||
| 45 to 59 | 63.2 | 57.7 | |
| 30 to 44 | 27.7 | 34.5 | |
| 15 to 29 | 7.8 | 7.5 | |
| <15 | 1.3 | 0.3 | |
| Mean (SD) systolic blood pressure category, mm Hg (%) | <0.0001 | ||
| ≤120 | 19.3 | 19.0 | |
| 121 to 129 | 13.8 | 11.2 | |
| 130 to 139 | 24.2 | 23.1 | |
| 140 to 159 | 26.1 | 28.3 | |
| 160 to 179 | 11.8 | 13.6 | |
| ≥180 | 4.8 | 4.8 | |
| Mean (SD) diastolic blood pressure category, mm Hg (%) | <0.0001 | ||
| ≤80 | 70.8 | 73.8 | |
| 81 to 84 | 9.9 | 8.9 | |
| 85 to 89 | 7.6 | 7.2 | |
| 90 to 99 | 8.4 | 8.0 | |
| 100 to 109 | 2.5 | 1.7 | |
| ≥110 | 0.8 | 0.4 | |
| Mean (SD) hemoglobin category, g/dL (%) | <0.0001 | ||
| <9.0 | 1.3 | 0.6 | |
| 9.0 to 9.9 | 2.3 | 1.7 | |
| 10.0 to 10.9 | 5.9 | 4.8 | |
| 11.0 to 11.9 | 12.2 | 11.9 | |
| 12.0 to 12.9 | 20.1 | 20.8 | |
| 13.0 to 13.9 | 23.6 | 25.6 | |
| ≥14.0 | 34.6 | 34.6 | |
| Albuminuria by urine dipstick, % | <0.0001 | ||
| Negative/trace | 91.2 | 92.6 | |
| 1+ | 3.8 | 3.8 | |
| 2+ | 2.9 | 2.5 | |
| 3+ | 0.6 | 0.3 | |
| 4+ | 1.6 | 0.9 | |
| Medication use, % | |||
| Beta blocker | 38.6 | 45.0 | <0.0001 |
| Angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker | 46.0 | 48.1 | 0.002 |
| Calcium channel blockers | 21.7 | 26.1 | <0.0001 |
| Diuretics | 54.5 | 59.0 | <0.0001 |
| Statin | 35.6 | 35.8 | 0.80 |
| Other lipid‐lowering therapy | 3.3 | 2.8 | 0.07 |
| Warfarin | 1.9 | 4.1 | <0.0001 |
| Antiplatelet agents | 3.3 | 4.2 | 0.0002 |
GFR indicates glomerular filtration rate.
Continuous variables are reported as means with SDs. Categorical variables are reported as frequencies and percentages.
Incident AF and Risk of Death
Mean follow‐up among all subjects was 4.8±2.7 years. During follow‐up, 5623 (6.9%) developed ESRD, and 13 703 (16.9%) disenrolled before the end of the study period. There were 2388 cases of death that occurred after development of incident AF (145 per 1000 person‐years) compared with 18 865 cases of death during periods without AF (51 per 1000 person‐years, P<0.001). The median time to death for participants with AF was 1.7 years (interquartile range 0.6 to 3.2 years) compared with 3.3 years (interquartile range 1.8 to 5.2 years) for those without AF.
In unadjusted analyses, incident AF was associated with a >2‐fold increase in rate of death (Table 2). The greatest attenuation in the rate of death was after adjustment for age (adjusted hazard ratio [HR] 1.64, 95 % CI 1.57 to 1.71). After adjustment for demographics, comorbidity, and medication use, incident AF was associated with a 66% higher relative rate of death among patients with CKD (Table 2).
Table 2.
Association Between Incident Atrial Fibrillation and Subsequent Risk of Death Among Adults With Chronic Kidney Disease (N=81 088)
| Hazard Ratio (95% CI) | |
|---|---|
| Unadjusted | 2.10 (2.01 to 2.20) |
| Adjusted for age | 1.64 (1.57 to 1.71) |
| Adjusted for age, sex, and race | 1.61 (1.54 to 1.68) |
| Adjusted for age, sex, race, socioeconomic characteristics, comorbid conditions, systolic blood pressure, eGFR, proteinuria, and hemoglobin level* | 1.59 (1.51 to 1.68) |
| Adjusted for age, sex, race, socioeconomic characteristics, comorbid conditions, systolic blood pressure, eGFR, proteinuria, hemoglobin level, and medication use* | 1.66 (1.57 to 1.77) |
| Adjusted for interim stroke and TIA events | 1.57 (1.48 to 1.67) |
ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; TIA, transient ischemic attack.
Included age, sex, race, education, income level, diabetes mellitus, hypertension, coronary heart disease, baseline ischemic stroke, baseline transient ischemic attack, heart failure, peripheral arterial disease, dyslipidemia, chronic lung disease, chronic liver disease, systolic blood pressure, eGFR, proteinuria, and hemoglobin.
Included age, sex, race, education, income level, diabetes mellitus, hypertension, coronary heart disease, baseline ischemic stroke, baseline transient ischemic attack, heart failure, peripheral arterial disease, dyslipidemia, chronic lung disease, chronic liver disease, systolic blood pressure, eGFR, proteinuria, hemoglobin, and baseline medication use (beta blockers, ACE inhibitors or ARBs, calcium channel blockers, diuretics, statins, other lipid‐lowering agents, warfarin, antiplatelet agents).
In adjusted models stratified by age, sex, race, and baseline eGFR level, we found that incident AF was consistently associated with a higher adjusted rate of death in all of the targeted patient subgroups except patients aged <60 years (Figure).
Figure 1.
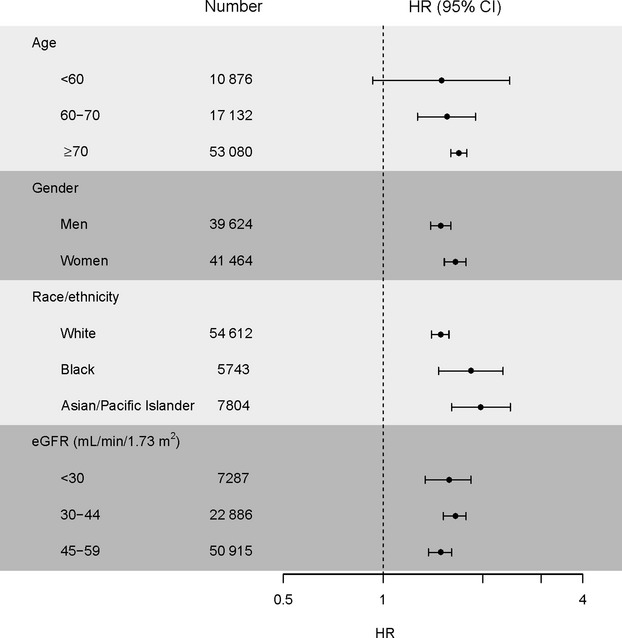
Multivariable association between atrial fibrillation and risk of death among chronic kidney disease subgroups. Models included age, sex, race, education, income level, eGFR level, albuminuria, hemoglobin, diabetes mellitus, hypertension, coronary heart disease, ischemic stroke, transient ischemic attack, heart failure, peripheral arterial disease, dyslipidemia, chronic lung disease, chronic liver disease, hyperthyroidism, and baseline medication use (beta blockers, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, calcium channel blockers, diuretics, statins, other lipid lowering agents, warfarin, antiplatelet agents). eGFR indicates estimated glomerular filtration rate; HR, hazard ratio.
In a sensitivity analysis, we adjusted for interim stroke and transient ischemic attack events. With this final adjustment, the association between incident AF and risk of death was only mildly attenuated (HR 1.57, 95% CI 1.48 to 1.67]) (Table 2). In sensitivity analyses among a subgroup of 55 409 CKD patients within our cohort, each incident AF patient was matched with patients who did not develop AF, based on age, sex, eGFR category, and vital status at the time of the incident AF diagnosis. In this parallel matched cohort analysis, the multivariable association of incident AF with death was similar to the main analysis using the entire cohort (HR 1.41, 95% CI 1.32 to 1.49) (Table 3).
Table 3.
Association Between Incident Atrial Fibrillation and Subsequent Risk of Death Among Adults With Chronic Kidney Disease in a Parallel Matched Cohort Study Design (n=55 409)
| Hazard Ratio (95% CI) | |
|---|---|
| Unadjusted | 1.48 (1.41 to 1.54) |
| Adjusted for age, sex, and race | 1.34 (1.28 to 1.40) |
| Adjusted for age, sex, race, socioeconomic characteristics, comorbid conditions, systolic blood pressure, eGFR, proteinuria, and hemoglobin level* | 1.37 (1.28 to 1.40) |
| Adjusted for age, sex, race, socioeconomic characteristics, comorbid conditions, systolic blood pressure, eGFR, proteinuria, hemoglobin level, and medication use* | 1.41(1.32 to 1.49) |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate.
Included age, sex, race, education, income level, diabetes mellitus, hypertension, coronary heart disease, baseline ischemic stroke, baseline transient ischemic attack, heart failure, peripheral arterial disease, dyslipidemia, chronic lung disease, chronic liver disease, systolic blood pressure, eGFR, proteinuria, and hemoglobin.
Included age, sex, race, education, income level, diabetes mellitus, hypertension, coronary heart disease, baseline ischemic stroke, baseline transient ischemic attack, heart failure, peripheral arterial disease, dyslipidemia, chronic lung disease, chronic liver disease, systolic blood pressure, eGFR, proteinuria, hemoglobin, and baseline medication use (beta blockers, ACE inhibitors or ARBs, calcium channel blockers, diuretics, statins, other lipid‐lowering agents, warfarin, antiplatelet agents).
Discussion
Among a large, diverse cohort of adults with CKD, we found that incident AF was independently associated with a 66% (95% CI 57% to 77%) higher relative rate of death, even after adjustment for a broad set of potential confounders. This association was consistent among all age, sex, racial, and baseline eGFR subgroups (except adults aged <60 years). Interim stroke and transient ischemic attack did not fully explain this association. Although previous literature has shown that AF is associated with higher rates of death among patients with ESRD receiving dialysis, our results extend these findings to the much larger CKD population.
A limited number of studies have reported on the incidence of AF among patients with CKD.6 A study of the Medicare 5% CKD population found that the 2‐year incidence of AF was 14.4% among patients with stage 3 to 5 CKD.23 In the Atherosclerosis Risk in Community (ARIC) study, there was a graded, increased risk of incident AF with lower baseline eGFR or higher level of albuminuria, even after adjustment for other risk factors.6 Our study confirmed high incidence of AF among patients with CKD. Similar to other studies, patients in our study who developed AF were older in age, were more likely to be white, and had a higher burden of comorbid diseases. Several possible mechanisms may explain the high rate of incident AF among patients with CKD, including older age and a high burden of risk factors such as hypertension and cardiovascular disease;7 high rates of inflammation, which has been linked to both CKD and AF24–31; increase in left atrial size among CKD patients;7 and activation of the renin–angiotensin–aldosterone system.32–33
Among patients with ESRD on dialysis, AF is independently associated with higher rates of death.4 Among >17 000 international dialysis patients, for example, patients with AF at study enrollment had a 16% (95% CI 8% to 25%) higher rate of death.5 Within the nationally comprehensive US Renal Data System between 1989 and 2006, the adjusted 1‐year risk of death was 45% higher for dialysis patients with AF compared with those who did not have documented AF.3 Very few studies have examined the risk of mortality with incident AF among the much larger population of CKD patients. A large study of the 2006 5% Medicare population found that incident AF was associated with a 27% increased rate of death among patients with CKD23; however, 55.7% of this population of CKD patients had unknown stage of CKD, all analyses were based on administrative data, and only a limited number of baseline covariates were available for this study. A single‐center study of 387 Japanese patients with AF reported higher mortality rates among patients with lower eGFR and high CHADS2 score compared with patients with preserved eGFR and low CHADS2 score.34 In our study, based on a large, well‐characterized, diverse, community‐based cohort of US adults with CKD, we found a 66% higher relative rate of death among those who developed incident AF, even after adjustment for a wide variety of possible confounders, including blood pressure, laboratory values, and medication use. The association between incident AF and death was similar in various patient subgroups by age, sex, race, and eGFR level (except for patients aged <60 years).
Several possible mechanisms may contribute to how AF could increase the risk of death. AF promotes systemic inflammation,27–31 which has been strongly associated with death in patients with CKD35. AF is associated with adverse outcomes such as heart failure and stroke,36–38 which may also increase risk of death. In fact, one study noted that patients with CKD and AF have double the rates of stroke compared with AF patients without CKD.39 In addition, AF may reflect a generalized increase in cardiovascular burden that placed patients with CKD at higher risk for death.
Our study had several strengths. We examined a very large and diverse sample of well‐characterized, community‐based adults across the spectrum of CKD, with outcomes through 2010. We were able to capture documented incident AF in both the inpatient and outpatient settings through validated diagnosis codes in health plan automated databases. We also had serial calibrated outpatient serum creatinine measurements available on entry into the study cohort and used rigorous criteria to confirm the presence and severity of CKD. We had detailed data on comorbid diseases, laboratory values, and pertinent medication use, which has been a limitation of prior studies. Our primary end point was death, which was comprehensively captured from a combination of health plan, statewide, and national data sources. Our study also had a few limitations. We were not able to quantify the severity of proteinuria because only urine dipstick results were available. We were not able to determine specific cause of death. We were also unable to determine the exact mechanisms explaining the association between AF and death. The study population was selected based on clinical outpatient eGFR measures. Consequently, those included in the study may over‐represent patients at greater risk for CKD and cardiovascular disease, which may bias our estimates of the association of incident AF with risk of death. We cannot completely rule out residual confounding, although we were able to statistically adjust for a wide variety of potential explanatory factors. We conducted our study among health plan members within a large integrated healthcare delivery system in northern California, so our findings may not be completely generalizable to all healthcare settings or to uninsured patients.
In conclusion, incident AF is associated with a 66% (95% CI 57% to 77%) higher relative rate of death among patients with CKD, independent of known clinical risk factors and medical therapies. Further study is needed to understand the mechanisms by which CKD is associated with AF and to identify potentially modifiable risk factors to reduce the burden of AF and subsequent risk of death among this high‐risk population.
Sources of Funding
Disclosures
Go has received a research grant from Astra Zeneca.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014; 129:e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, Hou Q, Shireman TI. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid‐eligible dialysis patients. Kidney Int. 2012; 81:469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011; 22:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovesi S, Vincenti A, Rossi E, Pogliani D, Acquistapace I, Stella A, Valsecchi MG. Atrial fibrillation and morbidity and mortality in a cohort of long‐term hemodialysis patients. Am J Kidney Dis. 2008; 51:255-262. [DOI] [PubMed] [Google Scholar]
- 5.Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, Kerr PG, Young EW, Robinson BM. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010; 77:1098-1106. [DOI] [PubMed] [Google Scholar]
- 6.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011; 123:2946-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, Lerma EV. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010; 5:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, Warnock DG, Muntner P. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011; 4:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horio T, Iwashima Y, Kamide K, Tokudome T, Yoshihara F, Nakamura S, Kawano Y. Chronic kidney disease as an independent risk factor for new‐onset atrial fibrillation in hypertensive patients. J Hypertens. 2010; 28:1738-1744. [DOI] [PubMed] [Google Scholar]
- 10.Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL, Dries DL, Bazzano L, Mohler ER, Wright JT, Feldman HI. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 2010; 159:1102-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, de Jong PE, Coresh J, Nahas ME, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011; 80:17-28. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370-2375. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003; 290:2685-2692. [DOI] [PubMed] [Google Scholar]
- 15.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end‐stage renal disease in adults with chronic kidney disease. Circulation. 2013; 127:569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351:1296-1305. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end‐stage renal disease: 25‐year follow‐up. Arch Intern Med. 2009; 169:342-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006; 296:2105-2111. [DOI] [PubMed] [Google Scholar]
- 19.Karter AJ, Moffet HH, Liu J, Parker MM, Ahmed AT, Go AS, Selby JV. Glycemic response to newly initiated diabetes therapies. Am J Manag Care. 2007; 13:598-606. [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander M, Tekawa I, Hunkeler E, Fireman B, Rowell R, Selby JV, Massie BM, Cooper W. Evaluating hypertension control in a managed care setting. Arch Intern Med. 1999; 159:2673-2677. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994; 271:840-844. [PubMed] [Google Scholar]
- 22.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 158:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson SE, Shroff GR, Li S, Herzog CA. Impact of chronic kidney disease on risk of incident atrial fibrillation and subsequent survival in medicare patients. J Am Heart Assoc. 2012; 1:e00209710.1161/JAHA.112.002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Mitra N, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DSfor the CSI. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012; 7:1938-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upadhyay A, Larson MG, Guo CY, Vasan RS, Lipinska I, O'Donnell CJ, Kathiresan S, Meigs JB, Keaney JF, Jr, Rong J, Benjamin EJ, Fox CS. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant. 2011; 26:920-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003; 107:87-92. [DOI] [PubMed] [Google Scholar]
- 27.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001; 104:2886-2891. [DOI] [PubMed] [Google Scholar]
- 28.Hatzinikolaou‐Kotsakou E, Tziakas D, Hotidis A, Stakos D, Floros D, Papanas N, Chalikias G, Maltezos E, Hatseras DI. Relation of C‐reactive protein to the first onset and the recurrence rate in lone atrial fibrillation. Am J Cardiol. 2006; 97:659-661. [DOI] [PubMed] [Google Scholar]
- 29.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005; 112:1266-1273. [DOI] [PubMed] [Google Scholar]
- 30.Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends Mol Med. 2011; 17:556-563. [DOI] [PubMed] [Google Scholar]
- 31.Negi S, Sovari AA, Dudley SC., Jr Atrial fibrillation: the emerging role of inflammation and oxidative stress. Cardiovasc Hematol Disord Drug Targets. 2010; 10:262-268. [DOI] [PubMed] [Google Scholar]
- 32.Iravanian S, Dudley SC., Jr The renin‐angiotensin‐aldosterone system (RAAS) and cardiac arrhythmias. Heart rhythm. 2008; 5:S12-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khatib R, Joseph P, Briel M, Yusuf S, Healey J. 2012. Blockade of the renin‐angiotensin‐aldosterone system (RAAS) for primary prevention of non‐valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol; 165:17-24. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa K, Hirai T, Takashima S, Fukuda N, Ohara K, Sasahara E, Taguchi Y, Dougu N, Nozawa T, Tanaka K, Inoue H. Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011; 107:912-916. [DOI] [PubMed] [Google Scholar]
- 35.Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. C‐reactive protein and albumin as predictors of all‐cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005; 68:766-772. [DOI] [PubMed] [Google Scholar]
- 36.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998; 32:695-703. [DOI] [PubMed] [Google Scholar]
- 37.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987; 147:1561-1564. [PubMed] [Google Scholar]
- 38.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983-988. [DOI] [PubMed] [Google Scholar]
- 39.Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6:2599-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]


