Abstract
Background
Periesophageal vagal nerve injury is recognized as a rare complication in atrial fibrillation ablation procedures. We investigated the factors associated with the occurrence of symptomatic periesophageal vagal nerve injury after pulmonary vein antrum isolation.
Methods and Results
Overall, 535 consecutive patients who underwent sole pulmonary vein antrum isolation were included. Point‐by‐point radiofrequency applications were applied using irrigated‐tip catheters under minimal sedation without esophageal temperature monitoring. In the initial 165 patients, the ablation settings for the posterior left atrium were a maximum energy of 25 to 30 W and a duration of 30 seconds. In the subsequent 370 patients, the power was additionally limited to 20 to 25 W at specific parts of the posterior left atrium where the ablation line transversed the esophagus. Symptomatic gastric hypomotility was found in 13 patients, and all were observed during the initial period (7.9%). No other collateral damage was observed. Logistic regression analysis revealed that the body mass index was the only independent predictor for identifying patients with gastric hypomotility (odds ratio 0.770; 95% confidence interval 0.643 to 0.922; P=0.0045) during the initial period. The prevalence of gastric hypomotility was significantly higher in the initial study period than subsequently (0 of 370, 0%; P<0.0001). All except for 1 patient recovered completely with conservative treatment within 4 months after the procedure.
Conclusions
Periesophageal vagal nerve injury is more common collateral damage than direct esophageal injury in pulmonary vein antrum isolation procedures. Titrating the radiofrequency energy at specific areas where the ablation line transverses the esophagus and taking account of the body mass index might reduce occurrences not only of direct esophageal damage but also of periesophageal vagal nerve injury in pulmonary vein antrum isolation procedures.
Keywords: atrial fibrillation, catheter ablation, collateral damage, complication, periesophageal vagal nerve injury, pulmonary vein isolation
Introduction
Since it was reported that paroxysmal atrial fibrillation (AF) is most often triggered by sources inside the pulmonary veins (PVs),1 radiofrequency (RF) catheter ablation of AF has been increasingly performed. PV electrical isolation subsequently evolved to include the whole PV antrum to avoid PV stenosis, and several studies confirmed that PV antrum isolation (PVAI) results in a better clinical outcome than segmental PV isolation.2–5 Despite PVAI having become the standard strategy for AF ablation, several procedure‐related complications have been recognized over the past 15 years.6–7 Collateral damage to the esophagus, such as direct esophageal injury8 and periesophageal vagal nerve injury (PNI),9–11 is an important complication of AF ablation procedures. Several attempts have been made to protect patients from direct esophageal injury because the esophagus could be visualized by various modalities. At present, however, no definitive method of preventing PNI has been established. The objectives of this study were to identify the factors associated with the occurrence of PNI in PVAI procedures using RF energy.
Methods
Study Population
The study was comprised of 535 consecutive patients undergoing their first PVAI for paroxysmal AF or persistent AF at our institution between October 2010 and November 2013 after irrigated‐tip ablation catheters were approved in Japan. Patients undergoing additional substrate modification in the left atrium (LA) after PVAI were excluded from this study to avoid the impact of additional substrate modification on the periesophageal vagal nerve. AF was classified according to the Heart Rhythm Society, European Heart Rhythm Association, and European Cardiac Arrhythmia Society 2012 consensus statement on catheter and surgical ablation of AF.7 The study was approved by an institutional review committee, and the participants gave informed consent.
Electrophysiological Study
All antiarrhythmic medications were discontinued at least 5 half‐lives prior to the ablation. All patients were anticoagulated with warfarin for at least 1 month before the procedure, and therapeutic anticoagulation was maintained with intravenous heparin following warfarin discontinuation 3 days prior to the intervention. Transesophageal echocardiography was performed within 24 hours preprocedurally to exclude any LA thrombi. Warfarin was restarted on the day of the procedure. An enhanced cardiac computed tomography scan was performed for the evaluation of any relevant cardiac anatomy before the procedure. The surface electrocardiogram (ECG) and bipolar intracardiac electrograms were continuously monitored and stored on a computer‐based digital recording system (LabSystem PRO; Bard Electrophysiology). Bipolar electrograms were filtered from 30 to 500 Hz. A 7‐Fr, 20‐pole or 14‐pole 2‐site mapping catheter (Irvine Biomedical Inc.) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording, and internal cardioversion. The electrophysiological study was performed under minimal sedation with pentazocine and hydroxyzine pamoate.
Catheter Ablation Protocol
The details of the ablation protocol were reported previously.12 After 1 transseptal puncture, 2 long sheaths (SL0; AF Division, St Jude Medical) were introduced into both superior PVs. Pulmonary venography during ventricular pacing and contrast esophagography (Figure 1) was performed to obtain the relative locations of the PV ostia and the esophagus. Heparin was administered 100 IU/kg of body weight following the transseptal puncture, and heparinized saline was additionally infused to maintain the activated clotting time at 300 to 350 seconds. Two decapolar circular mapping catheters (Lasso, Biosense Webster) were placed in the superior and inferior PVs, and the left‐ and right‐sided ipsilateral PVs were circumferentially ablated under the guidance of a 3‐dimensional mapping system (CARTO3; Biosense Webster). Posteriorly, ablation was performed anatomically in the LA, ≈1 to 3 cm from the PV ostia. Anteriorly, ablation was performed on the edge of the left PVs guided by early PV potentials. The electrophysiological end point of the PVAI was the achievement of a bidirectional conduction block between the LA and PVs, and the anatomic end point was the creation of complete continuous circumferential lesions around the ipsilateral veins.13
Figure 1.
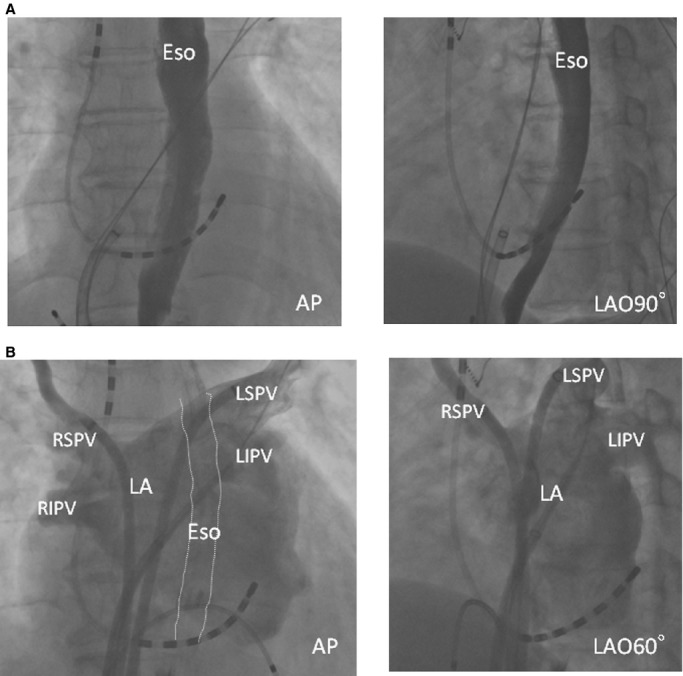
Representative esophagraphy and pulmonary venography. A, Esophagrams reveal the esophageal courses. B, Pulmonary venography shows the anatomy of the PV antrum. AP indicates anteroposterior view; Eso, esophagus; LA, left atrium; LAO, left oblique view; LIPV, left inferior PV; LSPV, left superior PV; PV, pulmonary vein; RIPV, right inferior PV; RSPV, right superior PV.
After completing the PVAI, a 30‐mg bolus of ATP was injected to unmask any dormant PV conduction, and any gap responsible for dormant conduction was eliminated by additional RF applications.14 The cavotricuspid isthmus was also ablated to create a bidirectional conduction block if common atrial flutter was detected on the 12‐lead electrogram or if cavotricuspid isthmus‐dependent flutter was identified during the procedure.15 When superior vena cava potentials were identified, electrical superior vena cava isolation was added.
Power Setting During Pulmonary Vein Antrum Isolation
During the PVAI, RF current was delivered point by point for 30 seconds with a 3.5‐mm externally irrigated‐tip quadripolar ablation catheter (Thermocool, Biosense‐Webster) with power up to 35 W, a target temperature of ≤38°C, and an irrigation rate of 30 mL/min. Between October 2010 and July 2011, the power of the RF energy applied to the LA posterior wall was 25 to 30 W, uniformly. From August 2011, the power was limited to 20 to 25 W on the specific part of the posterior wall where the ablation line transversed the esophagus (Figure 2, yellow points). The power delivered at the area was basically 20 W; however, if the decrease in amplitude of the local bipolar signal was small, the power was increased to 25 W, based on the operator's judgment. The location of the esophagus was identified by contrast esophagography just before the start of RF delivery. Throughout the entire study period, RF energy delivery was discontinued when the patient complained of any strong pain, and prophylactic treatment with proton‐pump inhibitors for more than 2 weeks and antibiotics for 3 days were started to prevent any reflux esophagitis from the day of the ablation procedure in all patients.
Figure 2.
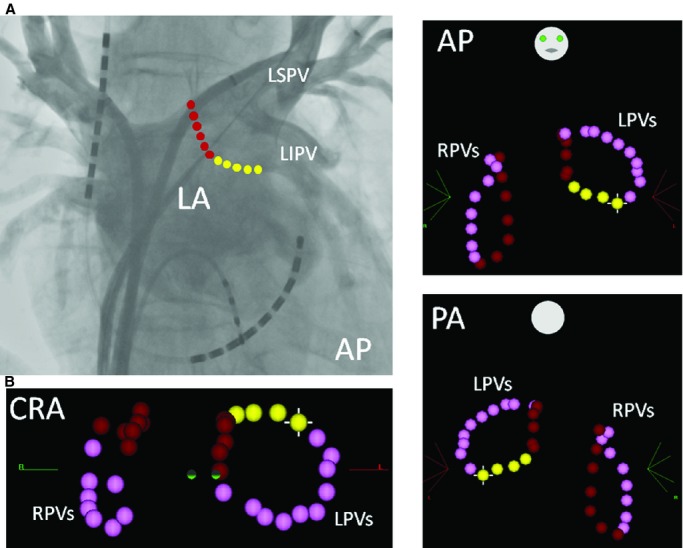
A, A point‐by‐point RF ablation line on the posterior LA is shown on the pulmonary venography image. B, A point‐by‐point circumferential ablation line is shown on a 3‐dimensional mapping system (CARTO3). Each dot indicates 1 RF application with a duration of 30 seconds. The pink dots show the ablation points on the anterior part of the PVs, and the red (longitudinal line) and yellow dots (transverse line) show the ablation points on the posterior LA. After August 2011, the energy power was limited to 20 to 25 W at the sites where the ablation line transversed the esophagus (yellow dots). AP indicates anteroposterior view; CRA, cranial view; LA, left atrium; LIPV, left inferior PV; LPVs, left pulmonary veins; LSPV, left superior PV; PA, posteroanterior view; PV, pulmonary vein; RF, radiofrequency; RPVs, right pulmonary veins.
Diagnostic Definition of Periesophageal Vagal Nerve Injury
Patients were diagnosed as having PNI if they exhibited the following symptoms and findings after AF ablation: acute onset of characteristic prolonged symptoms of gastric delayed emptying, such as nausea, vomiting, postprandial fullness, bloating, constipation, or epigastric pain and the finding of gastric hypomotility assessed by abdominal x‐ray. If necessary, gastric endoscopy was also performed.
Statistical Analysis
Continuous data are expressed as mean±SD for normally distributed variables or as the median (25th, 75th percentiles) for non‐normally distributed variables and were compared using a Student's t test or Mann–Whitney U test, respectively. Categorical variables were compared using the chi‐square test. All parameters with significance of <0.05 in univariate analysis were entered into multiple logistic regression analysis. A probability value of P<0.05 indicated statistical significance.
Results
Prevalence of Periesophageal Vagal Nerve Injury
Between October 2010 and July 2011, 165 patients (30.8%) underwent PVAI, and another 370 patients (69.2%) underwent PVAI between August 2011 and November 2013. PVAI was successfully performed in all 535 patients, and no additional LA ablation was performed in this population. Reduction of the power delivered to a specific area did not lead to any difficulty in performing the PVAI. A total of 13 patients (2.4%) showed the above symptoms after the PVAI and were diagnosed by abdominal x‐ray as having gastric hypomotility (Figure 3A). All patients with gastric hypomotility underwent AF ablation during the former period. No esophageal fistulae, pyloric spasms, or any other vagal nerve symptoms were observed in any of the patients.
Figure 3.
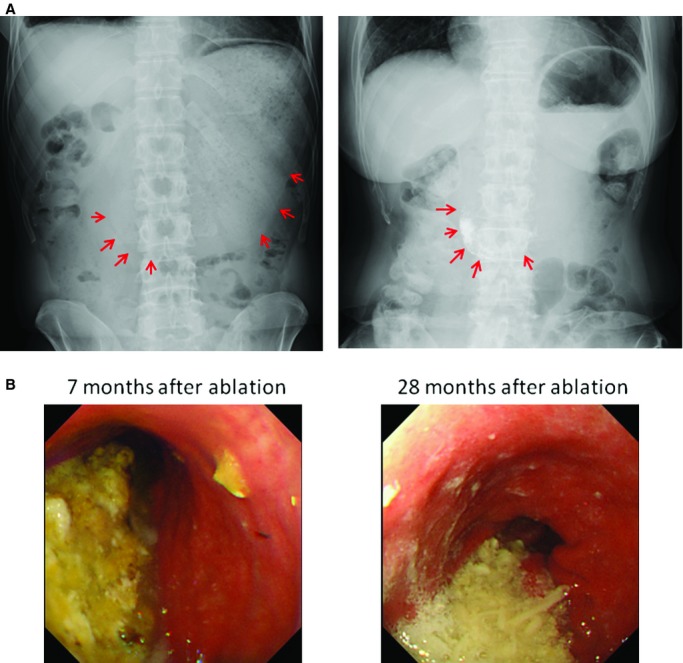
Representative images of gastric hypomotility resulting from AF ablation. A, Abdominal x‐rays taken on the day after catheter ablation show dilatation of the stomach and massive amounts of retained food (left image: case 13; right image: case 11). The red arrows show the contour of the dilated stomach. Note that the contrast medium used in the esophagraphy during the procedure is still retained in the stomach on the next day (right image). B, Massive amounts of retained food were found by gastric endoscopy despite an overnight period of fasting even 28 months after the ablation procedure (case 5).
Factors Associated With Periesophageal Vagal Nerve Injury During the Initial Period
The baseline characteristics of patients with and without PNI are shown in Table 1. During the initial period, there were no significant differences between patients with and without gastric hypomotility in terms of baseline clinical characteristics, echocardiographic parameters, inflammatory markers, or neurohumoral factors except for body mass index (BMI). The distance between the esophagus and LA at the level of the inferior PV measured on cardiac computed tomography was also similar between the 2 groups (Table 1 and Figure 4). Gastric hypomotility was prevalent in 12 of 104 healthy‐weight patients (11.5%), 1 of 51 overweight patients (2.0%), and 0 of 10 obese patients (0%). Logistic regression analysis revealed that the baseline BMI (per 1 kg/m2) was the only independent predictor for identifying patients with gastric hypomotility during the initial period (odds ratio 0.770; 95% confidence interval 0.643 to 0.922; P=0.0045).
Table 1.
Characteristics in Patients With and Without Periesophageal Vagal Nerve Injury
| Period | October 2010–July 2011 | August 2011–November 2013 | P Value | |||
|---|---|---|---|---|---|---|
| Eso Injury (+) | Eso Injury (−) | P Value | Total | |||
| N | 13 | 152 | 165 | 370 | ||
| Age, y | 64.5±7.3 | 64.0±10.5 | 0.874 | 64.0±10.3 | 62.9±11.4 | 0.245 |
| Female, n (%) | 5 (38.5%) | 37 (24.3%) | 0.282 | 42 (25.5%) | 105 (28.3%) | 0.533 |
| Structural heart disease, n (%) | 2 (15.4%) | 17 (11.2%) | 0.662 | 19 (11.5%) | 35 (9.5%) | 0.470 |
| Non‐paroxysmal AF, n (%) | 0 (0%) | 10 (6.6%) | 0.193 | 10 (6.1%) | 20 (5.4%) | 0.763 |
| Body mass index, kg/m2 | 21.5±3.3 | 24.5±3.6 | 0.005 | 24.2±3.6 | 24.3±3.7 | 0.959 |
| LA diameter, mm | 39.2±5.7 | 41.7±6.2 | 0.155 | 41.5±6.1 | 40.9±5.8 | 0.327 |
| LV ejection fraction, % | 66.8±6.6 | 66.7±6.9 | 0.964 | 66.7±6.9 | 66.0±7.7 | 0.356 |
| Estimated GFR, mL/min | 70.5±8.1 | 67.0±18.8 | 0.504 | 67.3±18.2 | 70.8±39.1 | 0.244 |
| High sensitive CRP, mg/dl | 0.45±0.63 | 0.32±0.58 | 0.708 | 0.33±0.58 | 0.43±1.07 | 0.392 |
| ANP, pg/mL | 53.4±70.5 (n=8) | 46.8±59.2 (n=77) | 0.770 | 474±59.9 (n=85) | 72.7±179.0 (n=67) | 0.224 |
| BNP, pg/mL | 27.9±28.5 (n=8) | 41.9±58.0 (n=77) | 0.502 | 40.6±55.9 (n=85) | 54.5±69.8 (n=67) | 0.176 |
| Pro‐BNP, pg/mL | 371±827 (n=88) | 341±1005 (n=309) | 0.797 | |||
| LA‐Eso distance, mm | 3.4±0.5 | 3.3±0.9 | 0.755 | |||
AF indicates atrial fibrillation; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CRP, C reactive protein; Eso, esophagus; GFR, glomerular filtration rate; LA, left atrium; LV, left ventricle.
Figure 4.

Representative axial images of the esophagus and posterior LA at the level of the left inferior PV. A, Cases without periesophageal vagal nerve injury. The distance between the LA and esophagus is 3.0 mm (left) and 3.7 mm (right), respectively. B, Cases with periesophageal vagal nerve injury (left image: case 2; right image: case 11). The distance between the LA and esophagus is 3.9 mm (left) and 3.1 mm (right), respectively. There was no significant difference in the LA–esophagus distance between the patients with and without periesophageal vagal nerve injury. Ao indicates descending aorta; Eso, esophagus; LA, left atrium; LIPV, left inferior PV.
Power Titration and Periesophageal Vagal Nerve Injury
Throughout the entire study period, there were no significant differences in the clinical characteristics of the patients undergoing AF ablation in the initial period and subsequently (Table 1). Although gastric hypomotility was observed in the initial period, no patients had any gastric hypomotility in the subsequent period. The prevalence of gastric hypomotility was significantly higher in the initial study period (13 of 165, 7.9%) than in the subsequent period, as shown by an unadjusted analysis (0 of 370, 0%; P<0.0001). The prevalence of gastric hypomotility was 12 of 347 healthy‐weight patients (3.46%), 1 of 156 overweight patients (0.64%), and 0 of 32 obese patients (0%). Multivariate logistic regression analysis using exact logistic analysis throughout the entire study period revealed that the baseline BMI was still an independent predictor for identifying patients with gastric hypomotility (odds ratio 0.736; 95% confidence interval 0.586 to 0.903; P=0.0021) in addition to the study period (odds ratio 6.930; 95% confidence interval 3.159 to infinity; P<0.0001). After adjustment for other clinical variables, each decrease of 1 in BMI was associated with a 35.9% increase in the occurrence of gastric hypomotility.
Clinical Outcome of Periesophageal Vagal Nerve Injury
Each patient fasted for 0 to 10 days, and panthotenic acid16 was intravenously administered during this period. After that, small low‐fat and low‐fiber meals were started gradually.11 Metoclopramide or mosapride were orally administered after patients were able to eat.11 All patients except 1 recovered completely with conservative treatment within 4 months (Table 2, case 5). Although the patient has had slight gastric symptoms without any difficulty in daily life, a gastric endoscopy revealed persistent gastric hypomotility even 28 months after the procedure (Figure 3B).
Table 2.
Clinical Outcome in Patients With Periesophageal Vagal Nerve Injury
| No | Age | Sex | AF Type | Ht | Wt | BMI | LAD | LVEF | Fasting Period | Recovery | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | M | PAF | 160 | 38 | 14.8 | 48 | 65 | No | 1 day | Full recovery |
| 2 | 60 | M | PAF | 166 | 62 | 22.5 | 38 | 71 | No | 1 day | Full recovery |
| 3 | 58 | F | PAF | 171 | 66 | 22.6 | 33 | 64 | No | 10 days | Full recovery |
| 4 | 65 | M | PAF | 168 | 63 | 22.3 | 40 | 69 | No | 4 months | Full recovery |
| 5 | 60 | M | PAF | 167 | 53 | 19.0 | 37 | 58 | 10 days | 28 months follow‐up | Partial recovery |
| 6 | 69 | M | PAF | 161 | 53 | 20.4 | 29 | 69 | 4 days | 2 weeks | Full recovery |
| 7 | 51 | M | PAF | 162 | 58 | 22.1 | 40 | 74 | 6 days | 5 days | Full recovery |
| 8 | 70 | F | PAF | 151 | 48 | 21.1 | 44 | 65 | No | 1 month | Full recovery |
| 9 | 61 | M | PAF | 164 | 57 | 21.2 | 46 | 59 | No | 2 weeks | Full recovery |
| 10 | 74 | F | PAF | 143 | 60 | 29.3 | 41 | 66 | 3 days | 1 week | Full recovery |
| 11 | 66 | F | PAF | 161 | 63 | 24.3 | 44 | 76 | 3 days | 10 days | Full recovery |
| 12 | 67 | F | PAF | 149 | 47 | 21.2 | 31 | 76 | 7 days | 2 weeks | Full recovery |
| 13 | 59 | M | PAF | 171 | 56 | 19.2 | 38 | 56 | 3 days | 2 weeks | Full recovery |
BMI indicates body mass index; Ht, height; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; PAF, paroxysmal atrial fibrillation; Wt, weight.
Discussion
The results of this study demonstrated the following findings. First, in clinical practice, PNI is more common collateral damage than direct esophageal injury in PVAI using RF energy. Second, BMI is an independent preprocedural predictor for identifying the occurrence of symptomatic PNI after PVAI. Third, no PNI was observed after PVAI, regardless of BMI, under power titration at a specific small area of the posterior LA where the ablation line transversed the esophagus. That complication was evaluated in 535 consecutive patients who underwent uniform ablation therapy (sole PVAI without any additional LA ablation, using the same irrigated‐tip RF ablation catheter with the same 3‐dimensional mapping system guidance, without esophageal temperature monitoring) because this was the most widely accepted AF ablation strategy based on previous evidence. In addition, uniform ablation therapy was used to eliminate the influence of any additional LA ablation on the periesophageal vagal nerve. These findings emphasized the recognition of this unnoticeable complication and the importance of energy titration at a specific area while taking BMI into account in AF ablation procedures.
Direct Esophageal Injury and Periesophageal Vagal Nerve Injury
Because Pappone et al reported cases with atrioesophageal fistulae as a lethal complication of LA ablation in 2004,8 great attention has been paid to avoid direct esophageal injury. It has been recognized that the esophagus itself is vulnerable during AF ablation due to thermal injury from the ablation on the posterior LA. However, PNI was largely ignored until a seminal report by Shah et al in 2005.9 In fact, a worldwide survey of catheter ablation of AF involving 182 centers reported an incidence of atrioesophageal fistulae of 0.04%, with no reports of PNI.6 Consequently, most electrophysiologists have not paid attention to this complication, in contrast to direct esophageal injury. This can occur not only with RF energy delivery but also with cryothermal energy delivery.17
The esophageal course is variable because it descends through the mediastinum posterior to the LA, although generally it lies closer to the left PV ostia than the right. Two branches descending on the anterior surface form the anterior esophageal plexus.18–19 The posterior and anterior esophageal plexuses enter the abdomen through the esophageal diaphragmatic opening. The distance between the anterior esophageal vagal plexus and the posterior LA is an average of 4.1±1.4 mm. The periesophageal vagal nervous plexus controls gastric peristalsis, the pyloric sphincter, and gastric motility. Injury to the vagal nerve above these organs can result in gastroparesis and pyloric spasms manifested as abdominal bloating, pain, nausea, early or easy satiety, and weight loss and has been described. The course of this structure cannot be visualized by current modalities, which makes it difficult to avoid this complication completely.
How to Avoid Periesophageal Vagal Nerve Injury
Despite the close anatomic proximity of vagal fibers, it is unclear whether any reduction in the delivered energy will be effective in avoiding PNI. Although knowledge of the position of the underlying esophagus may be of help, it is not clear to what extent this correlates with the location of relevant nerve fibers. In fact, nerve fibers innervating the pyloric sphincter and gastric antrum are thought to travel within the left vagal trunk but also may course within intercommunicating fibers; however, each person has distinctive anatomy, making any predictions impossible. In contrast to many proposed strategies for avoiding direct esophageal injury, there are few proposed strategies for avoiding periesophageal nerve injury due to the lack of visualization of this nervous system and understanding of the precise mechanism of this complication.
In the present study, no symptomatic direct esophageal injury was observed without esophageal temperature monitoring, regardless of the different power settings used throughout the study period. This is probably because energy power was limited at the posterior LA (<30 W throughout the study period), minimal anesthesia was used so as to recognize any early evoked pain during the procedure, and the prophylactic administration of proton pump inhibitors might have prevented any progression from an asymptomatic injury. General anesthesia itself showed a significant trend of increasing the risk of direct esophageal injury.20–21 A strong relationship between an esophageal temperature rise and pain has been shown by prior study.22 Use of a proton pump inhibitor might facilitate ulcer healing and prevent the progression from esophageal injury to an atrioesophageal fistula.23–24 In contrast, PNI was observed in 7.9% of the patients during the period when 25 to 30 W were applied to the posterior LA, suggesting that the periesophageal vagal nervous system was much more fragile than the esophagus itself to thermal injury. This complication was not observed after a reduction of power only at specific small areas of the posterior LA where the ablation line transversed the esophagus (Figure 2, yellow points). This result suggested that this area where the ablation line transversed the esophagus was the critical site where thermal injury could damage the periesophageal vagal nervous system. Considering the course of the periesophageal nerves, this result seems to be reasonable. Excessive transmural injury along the transverse line could completely interrupt the periesophageal nerve‐mediated parasympathetic stimulation of downstream organs. A recent study reported that, despite using maximum energy of 20 W at the LA posterior wall near the esophagus, morphological changes in the periesophageal connective tissue were observed by endosonography in 27% of the patients.25 The clinical course of this complication is considered to be dependent on the extent of the injury, and high‐energy settings may create irreversible complications. In the present study, BMI was also a predictor for identifying this complication, and higher BMI was protective for this complication, which was in line with the report by Yamasaki et al.26 They reported 10 asymptomatic excessive transmural injuries (4 esophageal injuries and 6 periesophageal vagal nerve injuries) among 104 patients and showed the relationship between BMI and asymptomatic excessive transmural injuries. It should be noted that this complication was identified even in obese patients. In the present study, the LA–esophageal distance did not predict the occurrence of PNI and thus suggested the difficulty in estimating the LA–vagal nerve distance from the LA–esophageal distance with current modalities. Maximal attention should be paid to avoid this unnoticeable complication during PVAI.
Clinical Implications
An esophageal fistula is one of the most feared complications after LA ablation because of its lethality; however, PNI is more commonly observed in AF ablation in clinical practice. This complication is not lethal, but it could considerably decrease the patient's quality of life. Although there is no established approach for avoiding injury to the periesophageal vagal nerves, simple energy titration at specific small areas might reduce the occurrence of this complication. In addition, it seems reasonable to determine the power settings after taking the individual BMI into account. Special attention should be paid when ablating in this area.
Study Limitations
The study design was not a prospective randomized trial. Asymptomatic esophageal injury and PNI were not assessed in this study. The contact force, which is an important factor associated with lesion depth, was not evaluated in this study. It is possible that the esophagus moved during the procedure; however, we always start RF delivery from the posterior part of the PVs on the left side just after the esophagography, and then proceed to the anterior part of the left‐side PVs, and right‐side PVs are ablated later. Consequently, we believe that the impact of the mobilization might be minimized. It might be possible that the symptoms are not only due to PNI because they are not specific for this complication; however, the symptoms were prolonged, and the diagnosis was confirmed by abdominal x‐ray in all cases.
Conclusions
PNI is more common collateral damage than direct esophageal injury during PVAI. The energy setting and BMI were significantly associated with the occurrence of PNI after PVAI. Maximal attention should be paid to avoid this underreported complication during PVAI.
Disclosures
None.
Acknowledgments
The authors thank John Martin for extending his assistance in the preparation of this article and T. Hamasaki for support with the statistical analysis.
References
- 1.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins. N Engl J Med. 1998; 339:659-666. [DOI] [PubMed] [Google Scholar]
- 2.Nault I, Miyazaki S, Forclaz A, Wright M, Jadidi A, Jaïs P, Hocini M, Haïssaguerre M. Drugs vs. ablation for the treatment of atrial fibrillation: the evidence supporting catheter ablation. Eur Heart J. 2010; 31:1046-1054. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi A, Iesaka Y, Takahashi Y, Takahashi R, Kobayashi K, Takagi K, Kuboyama O, Nishimori T, Takei H, Amemiya H, Fujiwara H, Hiraoka M. Electrical connections between pulmonary veins: implication for ostial ablation of pulmonary veins in patients with paroxysmal atrial fibrillation. Circulation. 2002; 105:2998-3003. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Dong J, Mavrakis HE, Hu F, Long D, Fang D, Yu R, Tang R, Hao P, Lu C, He X, Liu X, Vardas PE, Ma C. Achievement of pulmonary vein isolation in patients undergoing circumferential pulmonary vein ablation: a randomized comparison between two different isolation approaches. J Cardiovasc Electrophysiol. 2006; 17:1263-1270. [DOI] [PubMed] [Google Scholar]
- 5.Arentz T, Weber R, Bürkle G, Herrera C, Blum T, Stockinger J, Minners J, Neumann FJ, Kalusche D. Small and large isolation areas around the pulmonary veins for treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007; 115:3057-3063. [DOI] [PubMed] [Google Scholar]
- 6.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:32-38. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012; 9:632-696. [DOI] [PubMed] [Google Scholar]
- 8.Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso F, Torracca L, Benussi S, Alfieri O, Hong R, Lau W, Hirata K, Shikuma N, Hall B, Morady F. Atrio‐esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004; 109:2724-2726. [DOI] [PubMed] [Google Scholar]
- 9.Shah D, Dumonceau JM, Burri H, Sunthorn H, Schroft A, Gentil‐Baron P, Yokoyama Y, Takahashi A. Acute pyloric spasm and gastric hypomotility: an extracardiac adverse effect of percutaneous radiofrequency ablation for atrial fibrillation. J Am Coll Cardiol. 2005; 46:327-330. [DOI] [PubMed] [Google Scholar]
- 10.Bunch TJ, Ellenbogen KA, Packer DL, Asirvatham SJ. Vagus nerve injury after posterior atrial radiofrequency ablation. Heart Rhythm. 2008; 5:1327-1330. [DOI] [PubMed] [Google Scholar]
- 11.Kuwahara T, Takahashi A, Takahashi Y, Kobori A, Miyazaki S, Takei A, Fujino T, Okubo K, Takagi K, Fujii A, Takigawa M, Watari Y, Hikita H, Sato A, Aonuma K. Clinical characteristics and management of periesophageal vagal nerve injury complicating left atrial ablation of atrial fibrillation: lessons from eleven cases. J Cardiovasc Electrophysiol. 2013; 24:847-851. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki S, Kuwahara T, Kobori A, Takahashi Y, Takei A, Sato A, Isobe M, Takahashi A. Long‐term clinical outcome of extensive pulmonary vein isolation‐based catheter ablation therapy in patients with paroxysmal and persistent atrial fibrillation. Heart. 2011; 97:668-673. [DOI] [PubMed] [Google Scholar]
- 13.Miller MA, d'Avila A, Dukkipati SR, Koruth JS, Viles‐Gonzalez J, Napolitano C, Eggert C, Fischer A, Gomes JA, Reddy VY. Acute electrical isolation is a necessary but insufficient endpoint for achieving durable PV isolation: the importance of closing the visual gap. Europace. 2012; 14:653-660. [DOI] [PubMed] [Google Scholar]
- 14.Arentz T, Macle L, Kalusche D, Hocini M, Jais P, Shah D, Haissaguerre M. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004; 15:1041-1047. [DOI] [PubMed] [Google Scholar]
- 15.Shah D, Haissaguerre M, Takahashi A, Jais P, Clementy J. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation. 2000; 102:1517-1522. [DOI] [PubMed] [Google Scholar]
- 16.Giraldi G, De LdE, Mannocci A, Vecchione V, Martinoli L. A pilot study of the effect of pantothenic acid in the treatment of post‐operative ileus: results from an orthopedic surgical department. Clin Ter. 2012; 163:e121-e126. [PubMed] [Google Scholar]
- 17.Guiot A, Savoure A, Godin B, Anselme F. Collateral nervous damages after cryoballoon pulmonary vein isolation. J Cardiovasc Electrophysiol. 2012; 23:346-351. [DOI] [PubMed] [Google Scholar]
- 18.Doubilet H, Shafiroff BGP, Mulholland JH. The anatomy of the peri‐oesophageal vagi. Ann Surg. 1948; 127:128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho SY, Cabrera JA, Sanchez‐Quintana D. Vagaries of the vagus nerve: relevance to ablationists. J Cardiovasc Electrophysiol. 2006; 17:330-331. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa H, Seres KA, Yokoyama K, Collier J, Katari V, Lockwood DJ, Po SS, Slobodova A, Herring L, Olsen J, Lazzara R, Harty RF, Beckman KJ, Jackman WM. High incidence of asymptomatic esophageal ulceration after pulmonary vein antrum isolation in patients with atrial fibrillation. Heart Rhythm. 2007; 14:S61 [Google Scholar]
- 21.Di Biase L, Saenz LC, Burkhardt DJ, Vacca M, Elayi CS, Barrett CD, Horton R, Bai R, Siu A, Fahmy TS, Patel D, Armaganijan L, Wu CT, Kai S, Ching CK, Phillips K, Schweikert RA, Cummings JE, Arruda M, Saliba WI, Dodig M, Natale A. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol. 2009; 2:108-112. [DOI] [PubMed] [Google Scholar]
- 22.Aryana A, Heist EK, d'Avila A, Holmvang G, Chevalier J, Ruskin JN, Mansour MC. Pain and anatomical locations of radiofrequency ablation as predictors of esophageal temperature rise during pulmonary vein isolation. J Cardiovasc Electrophysiol. 2008; 19:32-38. [DOI] [PubMed] [Google Scholar]
- 23.Martinek M, Bencsik G, Aichinger J, Hassanein S, Schoefl R, Kuchinka P, Nesser HJ, Purerfellner H. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J Cardiovasc Electrophysiol. 2009; 20:726-733. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa H, Kenneth AS, Jackman WM. Limitations of esophageal temperature‐ monitoring to prevent esophageal injury during atrial fibrillation ablation. Circ Arrhythmia Electrophysiol. 2008; 1:1150-1152. [DOI] [PubMed] [Google Scholar]
- 25.Zellerhoff S, Ullerich H, Lenze F, Meister T, Wasmer K, Mönnig G, Köbe J, Milberg P, Bittner A, Domschke W, Breithardt G, Eckardt L. Damage to the esophagus after atrial fibrillation ablation: just the tip of the iceberg? High prevalence of mediastinal changes diagnosed by endosonography. Circ Arrhythm Electrophysiol. 2010; 3:155-159. [DOI] [PubMed] [Google Scholar]
- 26.Yamasaki H, Tada H, Sekiguchi Y, Igarashi M, Arimoto T, Machino T, Ozawa M, Naruse Y, Kuroki K, Tsuneoka H, Ito Y, Murakoshi N, Kuga K, Hyodo I, Aonuma K. Prevalence and characteristics of asymptomatic excessive transmural injury after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2011; 8:826-832. [DOI] [PubMed] [Google Scholar]


