Abstract
Background
Obstructive sleep apnea (OSA) is a common condition associated with cardiovascular disease. Its potential effect on progression of subclinical atherosclerosis is not well understood. We tested the hypothesis that self‐reported OSA is associated with progression of coronary artery calcium (CAC). We also evaluated whether traditional cardiovascular risk factors accounted for the association.
Methods and Results
In the Multi‐Ethnic Study of Atherosclerosis (MESA) prospective cohort, we studied 2603 participants who at baseline (2002–2004) completed a sleep questionnaire and underwent coronary computed tomography (CT) and, then 8 years later (2010–2011), a repeat coronary CT. Participants were categorized by symptoms of habitual snoring or reported physician diagnosis of OSA. At baseline, 102 (3.9%) reported diagnosed OSA; 666 (25.6%) reported diagnosed habitual snoring; and 1835 (70.5%) reported neither habitual snoring nor OSA (“normal”). At baseline, CAC prevalence was highest among those with OSA but similar for those with and without habitual snoring. During 8 years of follow‐up, greater progression of CAC was observed among those with OSA versus normal (mean increase of 204.2 versus 135.5 Agatston units; P=0.01), after accounting for demographics, behaviors, and body habitus. Modest attenuation was observed after adjustment for cardiovascular risk factors (188.7 versus 138.8; P=0.06). CAC progression among habitual snorers was similar to that observed in the normal group.
Conclusions
OSA was associated with CAC score progression after adjustment for demographics, behaviors, and body mass index. However, the association was not significant after accounting for cardiovascular risk factors, which may mediate the association between OSA and CAC.
Keywords: coronary artery calcium, obstructive sleep apnea, snoring, subclinical atherosclerosis risk factor
Introduction
Obstructive sleep apnea (OSA) is a common condition, estimated to be prevalent in at least 5% to 15% of the adult population in developed countries.1 Beyond poor quality of sleep and its directly related symptoms, an emerging body of evidence links OSA to a more adverse cardiovascular risk factor profile and increased risk of cardiovascular disease (CVD) events.2–3 Individuals who snore are known to have a disproportionately higher prevalence of undiagnosed OSA compared with those who do not snore,4 and snoring has also been associated with a higher risk of CVD risk factors and events.5–6
In an effort to better understand the relation between OSA and CVD, examining the association between OSA and subclinical coronary atherosclerotic change may be valuable.7 Coronary artery calcium (CAC) level assessed with the use of coronary computered tomography (CT) is a well‐established surrogate marker that has been shown in numerous studies to be predictive of future coronary events.8–9
Results have been inconsistent among the relatively few studies that have directly explored the association between OSA and CAC prevalence.10–14 To date, no studies have assessed the longitudinal association of OSA with change in CAC. In this study, we tested a hypothesis that during 8 years of follow‐up, participants with OSA would have a more pronounced increase in CAC, relative to participants who do not snore, in a racially/ethnically diverse population free of overt CVD. We anticipated that participants who self‐reported snoring would have an intermediate increase in CAC. The cross‐sectional association between self‐reported OSA and CAC prevalence is also reported.
Methods
Study Population
The Multi‐Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of 6814 community‐dwelling men and women aged 45 to 84 years without evidence of CVD at baseline (2000–2002). Participants were recruited from 6 US communities (Forsyth County, NC; northern Manhattan and the Bronx, NY; Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles County, CA) and self‐identified as white, Chinese, black, or Hispanic.15 A total of 6232 participants attended MESA exam 2 (September 2002–February 2004), of whom 5395 completed the sleep history questionnaire. Among those who completed the sleep questionnaire, 4919 underwent coronary CT at exam 2 or had coronary CT at both exam 1 (June 2000–August 2002) and exam 3 (March 2004–September 2005), thereby allowing us to derive interpolated exam 2 CAC for ≈50% of the cohort who did not have coronary CT at exam 2. These participants made up our “parent cohort.” We required that for the main analysis of CAC change, participants also attend a coronary CT exam at MESA visit 5 (April 2010–December 2011; attended by 55% of the parent cohort). After excluding participants with a history of revascularization and 1 participant with apparent CAC measurement error, our final analytic sample included 2603 participants (“final cohort”). The research protocols were approved by the institutional review board at each participating institution, and all participants gave written informed consent. Detailed descriptions of the study design and methods of MESA have been published previously.15
Sleep Disordered Breathing History
A self‐administered sleep history questionnaire conducted at exam 2 was used to construct 3 sleep disordered breathing (SDB) groups (OSA, habitual snoring, normal) using the algorithm illustrated in Figure 1. Because OSA (versus central sleep apnea) is by far the most common form of sleep apnea in the absence of heart failure or stroke, we considered self‐reported physician‐diagnosed sleep apnea to be OSA in this study. In addition, information on perception of excessive daytime sleepiness was extracted from the question: How often do you feel excessively (overly) sleepy during the day? (Those who responded “often” [5 to 15 days/month] or “almost always” [16 to 30 days/month] were considered to have excessive daytime sleepiness.) This information was used to test for potential effect modification.
Figure 1.
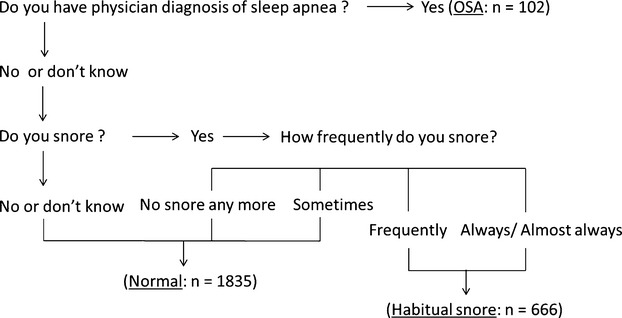
Algorithm for defining sleep disordered breathing history groups: MESA. Construction of 3 sleep breathing disorder history groups based on the self‐administered sleep history questionnaire in final cohort (n=2603), based on the following questions: (A) (Physician‐diagnosed sleep apnea question); Have you ever been told by a doctor that you had sleep apnea? (B) (Snoring question) Have you ever snored (now or at any time in the past)? (C) (Snoring frequency question) How often do you snore now? Snoring frequency response definitions: sometimes: snoring up to 2 nights a week; frequently: snoring 3 to 5 nights a week; always or almost always: snoring 6 to 7 nights a week. MESA indicates Multi‐Ethnic Study of Atherosclerosis; OSA, obstructive sleep apnea.
CAC Scores
Details of the technique and methodology of the scans and approach to quantifying CAC have been previously published.16 CAC scores were quantified by using the Agatston method17 from 2 consecutively obtained CT scans at each visit, and average CAC scores were obtained. CAC scores were subsequently adjusted with a standard calcium phantom that was scanned along with the participant.16 Interobserver and intraobserver agreements were high (κ statistics=0.93 and 0.90, respectively).18
The absolute difference in CAC score between MESA exam 2 (when the sleep survey took place) and exam 5 was used as our primary end point, representing change in CAC during an average of 8 years. By design, only 50% of the MESA sample underwent CT at exam 2. For those without available CT at exam 2, interpolated CAC was derived by averaging the CAC scores of exams 1 and 3, assuming a linear rate of change in CAC. Because approximately half of the participants who underwent exam 1 CT were randomly allocated to have a follow‐up CT at either exam 2 or 3 but not both, there was no overlap between the cohorts who underwent CT at exam 2 and exam 3. Notably, only one‐quarter of the full MESA cohort underwent CAC scans at exam 4 (September 2005–May 2007); to maximize the size of cohort and the time interval over which CAC progression might occur, exam 5 CAC was chosen for calculating CAC change instead of exam 4 CAC.
Covariates
Information on demographics, smoking, physical activity, and medical conditions were obtained via questionnaire at exam 2. Smoking status was categorized as never, former, or current smoker. Physical activity was determined by calculation of weekly MET‐minute of total walking, conditioning, and sports activity. Body mass index (BMI) obtained at exam 2 were categorized into normal (BMI <25 kg/m2), overweight (25≤BMI<30 kg/m2), and obese (BMI ≥30 kg/m2). Use of diabetes, hypertension, and cholesterol medications was determined via questionnaire and from medication containers that were brought to the exam 2 clinic visit. Blood samples collected after a 12‐hour fast were used to determine fasting glucose level, and low‐ and high‐density lipoprotein cholesterol (LDL‐C and HDL‐C, respectively). Diabetes was defined as a fasting glucose level of ≥7.0 mmol/L (126 mg/dL) or use of insulin or oral hypoglycemic medications. Hypertension was determined if seated systolic blood pressure was ≥140 mm Hg and/or diastolic blood pressure was ≥90 mm Hg.
Statistical Analysis
Characteristics of participants were compared based on the self‐reported SDB history, the main exposure predictor of interest (ie, normal, habitual snoring, and OSA), using χ2 test or 1‐way ANOVA for categorical and continuous variables, respectively. Mean (SD) and percentage were used to describe continuous and categorical variables, as appropriate.
Mean absolute CAC score and prevalence for CAC (CAC score >0 [ie, presence of CAC]) and CAC 400 (CAC score >400 [ie, high burden of CAC]) in the final cohort at baseline were compared according to SDB history characteristics using Kruskal–Wallis tests (with pairwise post‐hoc Wilcoxon test) and χ2 tests, respectively.
For multivariable comparison of the prevalence rates, Poisson regression with a robust error variance19 was used, with adjustment using the following nested models: model 1 included age, sex race/ethnicity, education level, site, smoking, and physical activity; model 2 included model 1 plus BMI category; and model 3 included model 2 plus several markers of potential intermediate variables in the pathway between OSA and CAC change (ie, prevalent diabetes, seated systolic blood pressure, HDL‐C, LDL‐C, and antihypertensive and lipid‐lowering medication).
The main outcome of interest, absolute CAC change continuously measured was determined by subtracting CAC score at exam 2 (measured or interpolated) from that at exam 5. Multiple linear regression was performed to calculate the mean CAC change between the SDB groups. The same covariates were used as for the baseline prevalence comparison. Because there are several different approaches to analyzing change,20 as a sensitivity analysis, we modeled change with absolute CAC at exam 5 as the dependent variable, without considering baseline CAC values. Possible effect modification of associations between SDB and CAC change by age, sex, race, BMI category, and reported excessive daytime sleepiness was tested by including cross‐product terms in the models. A P value <0.05 was considered significant. Sensitivity analysis was also performed after excluding participants with negative CAC change, which may represent measurement error. All statistical analyses were performed using SAS version 9.3 (SAS Institute).
Results
A total of 2603 participants were eligible for the main analysis evaluating change in CAC score between exams 2 and 5. This final cohort included 102 (3.9%) participants with OSA, 666 (25.6%) with habitual snoring, and 1835 (70.5%) without a history of habitual snoring or OSA (normal). This distribution was similar to the parent cohort (n=4919, OSA: n=3464 [70.4%], habitual snoring: n=1261 [25.6%], normal: n=194 [3.9%]).
The baseline characteristics are shown in Table 1. Participants with OSA, compared with those without OSA, were more likely to be white, male, have higher incomes and educational attainment, be obese, have prevalent diabetes, and be on medication for hypertension and hyperlipidemia.
Table 1.
Characteristics of the Participants in Final Cohort by Self‐Reported Sleep Disordered Breathing History (n=2603): MESA
| Variables | Normal (n=1835) | Habitual Snoring (n=666) | OSA (n=102) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (y), mean (SD) [range] | 61.8a (9.4) [46 to 86] | 59.6b (8.6) [46 to 83] | 59.5b (8.6) [46 to 81] | <0.0001 |
| Male, n (%) | 796 (43.4) | 401 (60.2) | 76 (74.5) | <0.0001 |
| Race, n (%) | 0.002 | |||
| White, Caucasian | 739 (40.3) | 234 (35.1) | 48 (47.1) | |
| Chinese American | 203 (11.1) | 97 (14.6) | 7 (6.9) | |
| Black, African American | 504 (27.5) | 162 (24.3) | 30 (29.4) | |
| Hispanic | 389 (21.2) | 173 (26.0) | 17 (16.7) | |
| Education, n (%) | 0.0499 | |||
| <High school degree | 226 (12.3) | 100 (15.0) | 6 (5.9) | |
| High school degree or some college | 883 (48.2) | 300 (45.1) | 47 (46.1) | |
| College graduate | 725 (39.5) | 265 (39.9) | 49 (48.0) | |
| Income, n (%) | 0.003 | |||
| <$20 000 | 328 (18.5) | 112 (17.3) | 8 (8.0) | |
| $20 000 to $50 000 | 679 (38.3) | 233 (36.0) | 30 (30.0) | |
| >$50 000 | 767 (43.2) | 302 (46.7) | 62 (62.0) | |
| Lifestyle | ||||
| Smoking, n (%) | 0.09 | |||
| Never | 889 (48.7) | 288 (43.7) | 39 (38.6) | |
| Former | 754 (41.3) | 295 (44.8) | 49 (48.5) | |
| Current | 184 (10.1) | 76 (11.5) | 13 (12.9) | |
| Physiologic characteristics | ||||
| BMI category (kg/m2), n (%) | <.0001 | |||
| <25 | 530 (28.9) | 137 (20.6) | 9 (8.8) | |
| 25 to 30 | 775 (42.3) | 254 (38.1) | 36 (35.3) | |
| ≥30 | 529 (28.8) | 275 (41.3) | 57 (55.9) | |
| Comorbidities | ||||
| Prevalent diabetes, n (%) | 209 (11.5) | 101 (15.3) | 21 (20.8) | 0.002 |
| Prevalent hypertension, n (%) | 737 (40.6) | 285 (43.3) | 48 (47.5) | 0.2 |
| Hypertension medication, n (%) | 655 (37.3) | 270 (42.7) | 47 (47.5) | 0.01 |
| SBP (mm Hg) mean (SD) [range] | 122.1a (19.7) [73 to 202] | 124.1b (18.9) [85.5 to 216] | 120.8ab (16.9) [87.5‐170.5] | 0.046 |
| HDL cholesterol (mg/dL) mean (SD) [range] | 52.9a (15.2) [21 to 161] | 48.0b (12.4) [24 to 111] | 46.8b (10.5) [24 to 77] | <.0001 |
| LDL cholesterol (mg/dL) mean (SD) [range] | 113.0a (31.0) [21 to 243] | 117.0b (30.3) [41 to 281] | 104.3c (30.1) [34 to 178] | <.0001 |
| Lipid‐lowering medications, n (%) | 380 (21.7) | 75 (20.9) | 31 (31.3) | 0.07 |
| Baseline CAC | ||||
| CAC score mean* (median) [range] | 108.5a (0) [0 to 4744.1] | 108.6a (1.2) [0 to 2963.9] | 163.2b (19.3) [0 to 1175.5] | 0.0006 |
| Prevalence CAC >0, n (%) | 900 (49.1) | 347 (52.1) | 66 (64.7) | 0.005 |
| Prevalence CAC >400, n (%) | 132 (7.2) | 48 (7.2) | 17 (16.7) | 0.002 |
For continuous variables, values without sharing common alphabet letters denote significant difference. MESA, Multi‐Ethnic Study of Atherosclerosis; OSA, obstructive sleep apnea; BMI, body mass index; SBP, systolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; CAC, coronary artery calcium.
Kruskal–Wallis test with pairwise post‐hoc Wilcoxon test was used.
Levels of both HDL and LDL were lower among the OSA group; however, after restricting the analysis to participants not taking lipid‐lowering medications, LDL levels were similar across SDB categories. Overall, characteristics of the habitual snoring group were similar to those of the normal group. More than a third of participants with OSA (38.6%) reported having excessive daytime sleepiness compared with 27.9% and 18.5% in the habitual snoring and normal groups, respectively (P<0.0001). Unadjusted baseline CAC score and CAC prevalence were significantly different across the groups (Table 1).
Prevalence of CAC at Baseline From Parent Cohort (n=4919)
At baseline, the overall prevalence of positive CAC (CAC >0) was high in all groups but was highest in the OSA group (OSA: 64.4%; habitual snoring: 55.0%; normal: 55.6%, P=0.04). The prevalence of high CAC burden (CAC >400) showed a similar pattern (OSA: 19.1%, habitual snoring: 10.4%, normal: 12.0%, P=0.002). In multivariable analyses, for CAC >0 the association was no longer present when adjusted for BMI (Table 2A). For CAC >400, the association persisted after adjustment for BMI and CVD risk factors (Table 2B). For both CAC >0 and CAC >400, the habitual snoring group behaved similar to the normal group.
Table 2.
Prevalence Ratio of Positive CAC at Baseline in Parent Cohort by Sleep Disordered Breathing History (n=4919): MESA
| Habitual Snoring vs Normal | OSA vs Habitual Snoring | OSA vs Normal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PR | 95% CI | P Value | PR | 95% CI | P Value | PR | 95% CI | P Value | |
| A: CAC >0 | |||||||||
| Model 1 | 1.05 | 0.99 to 1.11 | 0.1 | 1.11 | 1.00 to 1.23 | 0.05 | 1.16 | 1.06 to 1.29 | 0.002 |
| Model 2 | 1.02 | 0.96 to 1.08 | 0.6 | 1.07 | 0.96 to 1.19 | 0.2 | 1.08 | 0.98 to 1.20 | 0.1 |
| Model 3 | 1.02 | 0.97 to 1.08 | 0.4 | 1.03 | 0.93 to 1.16 | 0.5 | 1.06 | 0.96 to 1.18 | 0.3 |
| B: CAC >400 | |||||||||
| Model 1 | 1.09 | 0.90 to 1.31 | 0.4 | 1.51 | 1.11 to 2.06 | 0.009 | 1.64 | 1.24 to 2.17 | 0.0005 |
| Model 2 | 1.02 | 0.84 to 1.24 | 0.8 | 1.38 | 1.01 to 1.88 | 0.04 | 1.41 | 1.06 to 1.87 | 0.02 |
| Model 3 | 1.05 | 0.87 to 1.27 | 0.6 | 1.33 | 0.97 to 1.81 | 0.08 | 1.40 | 1.05 to 1.86 | 0.02 |
Model 1 adjusted for age, race, sex, site, income level, educational level, smoking status, and physical activity level (n=4703). Model 2 adjusted for model 2 plus BMI category (n=4702). Model 3 adjusted for model 2 plus diabetes, systolic blood pressure, hypertension medication, and LDL, HDL, and cholesterol medication (n=4401). CAC indicates coronary artery calcium; MESA, Multi‐Ethnic Study of Atherosclerosis; PR, prevalence ratio; OSA, obstructive sleep apnea; BMI, body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Longitudinal Change in CAC (n=2603)
During 8 years of follow‐up, CAC change in the final cohort ranged from −362 to 3261, and its mean and median were 140.4 and 25.9, respectively. Unadjusted change in CAC was significantly more pronounced in the OSA group compared with normal or habitual snoring groups (Agatston units mean [95% CI] OSA: 227.7 [173.3 to 282.1]; habitual snoring: 140.4 [119.1 to 161.6], normal: 135.6 [95% CI: 122.8 to 148.4]). The pattern persisted in multivariable analysis adjusting for baseline demographic, life style factors (model 1), and BMI (model 2, OSA: 204.2 [152.8 to 255.5]; habitual snoring: 132.6 [112.2 to 153.0], normal: 135.5 [123.3 to 147.6]) (Table 3). Figure 2 shows CAC scores at visit 2 and at visit 5, stratified by SDB category, with model 2 adjustments. With further adjustment for CVD risk factors (model 3), the association between OSA and the normal group was modestly attenuated and no longer significant (P=0.06). In sensitivity analyses modeling CAC at exam 5 (the follow‐up CAC) without taking into account baseline CAC, the results were slightly attenuated (Table 4). At exam 5, the prevalence of CAC >0 was 67.6%, 70.3%, and 73.5 for the no snoring, habitual snoring, and OSA groups, respectively (P=0.24).
Table 3.
Comparison of Absolute CAC Score Change Over 8 Years of Follow‐up by Sleep Disordered Breathing History in Final Cohort (n=2603): MESA
| Normal (n=1835) | Habitual Snoring (n=666) | OSA (n=102) | Habitual Snoring vs Normal | OSA vs Habitual Snoring | OSA vs Normal | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | P Value | |||
| Model 1 | 132.3 | 120.1 to 144.4 | 138.9 | 118.5 to 159.3 | 220.6 | 169.2 to 272.0 | 0.6 | 0.004 | 0.001 |
| Model 2 | 135.5 | 123.3 to 147.6 | 132.6 | 112.2 to 153.0 | 204.2 | 152.8 to 255.5 | 0.8 | 0.01 | 0.01 |
| Model 3 | 138.8 | 126.8 to 150.8 | 127.6 | 107.2 to 148.0 | 188.7 | 137.7 to 239.6 | 0.4 | 0.03 | 0.06 |
Model 1 adjusted for age, race, sex, site, income level, educational, smoking status, and physical activity level (n=2502). Model 2 adjusted for model 1 plus BMI category (n=2501). Model 3 adjusted for model 2 plus diabetes, systolic blood pressure, hypertension medication, and LDL, HDL, and cholesterol medication (n=2344). CAC indicates coronary artery calcium; MESA, Multi‐Ethnic Study of Atherosclerosis; OSA, obstructive sleep apnea; BMI, body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Figure 2.
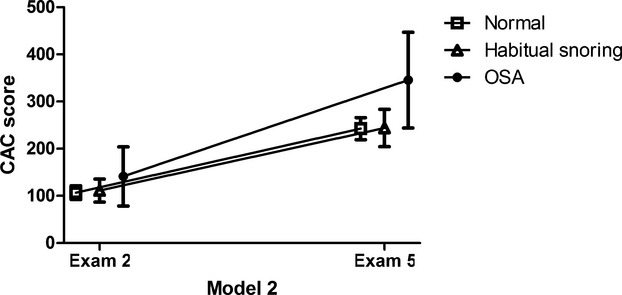
CAC progression during an 8‐year period: MESA. Adjusted* absolute CAC scores (mean and 95% CI) and CAC progression over 8 year period stratified by sleep disordered breathing history. *Model 2 adjusted for age, race, sex, site, income level, educational, smoking status, physical activity level, and BMI category (n=2501). P values comparing CAC change between the groups. OSA vs normal: 0.01; OSA vs habitual snoring: 0.01; habitual snoring vs normal: 0.8. BMI indicates body mass index; CAC, coronary artery calcium; MESA indicates Multi‐Ethnic Study of Atherosclerosis; OSA, obstructive sleep apnea.
Table 4.
Absolute CAC Score at Exam 5 Without Accounting for Baseline CAC Values by Sleep Disordered Breathing Category in Final Cohort (n=2603): MESA
| Normal (n=1835) | Habitual Snoring (n=666) | OSA (n=102) | Habitual Snoring vs Normal | OSA vs Habitual Snoring | OSA vs Normal | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | P Value | |||
| Model 1 | 238.5 | 214.9 to 262.1 | 251.7 | 212.2 to 291.3 | 365.6 | 265.9 to 465.3 | 0.6 | 0.04 | 0.02 |
| Model 2 | 242.5 | 218.9 to 266.1 | 243.9 | 204.2 to 283.5 | 345.5 | 245.5 to 445.5 | 1.0 | 0.06 | 0.051 |
| Model 3 | 250.7 | 226.6 to 274.8 | 239.3 | 198.5 to 280.2 | 316.9 | 214.8 to 419.0 | 0.6 | 0.2 | 0.2 |
Model 1 adjusted for age, race, sex, site, income level, educational, smoking status, and physical activity level (n=2502). Model 2 adjusted for model 1 plus BMI category (n=2501). Model 3 adjusted for model 2 plus diabetes, systolic blood pressure, hypertension medication, and LDL, HDL, and cholesterol medication (n=2344). BMI indicates body mass index; CAC, coronary artery calcium; MESA, Multi‐Ethnic Study of Atherosclerosis; OSA, obstructive sleep apnea; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Effect modification was not present by age, sex, BMI category, or excessive daytime sleepiness but was present by race. Subsequent stratified analysis showed that in blacks, no difference in CAC change was observed across the groups. This result should be viewed cautiously, however, given the number of comparisons and the limited sample size for race‐specific analyses.
In alternative analyses, when the OSA group was compared with combined “no OSA group” (habitual snoring+normal group), the OSA group experienced a significantly greater increase in CAC score, regardless of degree of adjustment (Table 5). Because negative change in CAC might be due to measurement error rather than a true regression, we performed analysis by excluding subjects (6%) with any negative change. The results were similar before and after the exclusion (data not shown). Finally because CAC change did not exhibit normally distributed pattern owing to large portion of cohort without significant change, we also performed the analysis using log‐transformed CAC change. However, results were largely unchanged (data not shown).
Table 5.
Comparison of Absolute CAC Score Change Based on the Presence of OSA in Final Cohort (n=2603): MESA
| No OSA (n=2501) | OSA (n=102) | P Value | |||
|---|---|---|---|---|---|
| Mean | CI | Mean | CI | ||
| Model 1 | 134.0 | 123.7 to 144.4 | 220.3 | 169.0 to 271.7 | 0.001 |
| Model 2 | 134.7 | 124.4 to 145.0 | 204.3 | 153.0 to 255.7 | 0.009 |
| Model 3 | 135.8 | 125.7 to 146.0 | 189.3 | 138.4 to 240.3 | 0.04 |
Model 1 adjusted for age, race, sex, site, income level, educational, smoking status, and physical activity level (n=2502). Model 2 adjusted for model 1 plus BMI category (n=2501). Model 3 adjusted for model 2 plus diabetes, systolic blood pressure, hypertension medication, and LDL, HDL, and cholesterol medication (n=2344). CAC indicates coronary artery calcium; OSA, obstructive sleep apnea; MESA, Multi‐Ethnic Study of Atherosclerosis; BMI, body mass index.
Discussion
In this large racially and ethnically diverse cohort without evidence of CVD at baseline, we found that having OSA based on self‐reported physician diagnosis versus being a habitual snorer or reporting a normal SDB pattern was associated with a greater progression of CAC, as measured by absolute change in CAC scores over an average of 8 years. These associations remained significant even after adjusting for BMI but were modestly attenuated after additionally accounting for key CVD risk factors, such as hypertension, diabetes, and hyperlipidemia. Although this attenuation may reflect confounding, current etiological understanding suggests that these traditional CVD risk factors may mediate the association between OSA and CAC progression.
Indeed OSA has been linked to the development of these cardiovascular risk factors,21–22 and these risk factors have been associated with CAC incidence and progression.23 Perhaps the strongest evidence exists for hypertension. In observational data, a dose–response association has been observed between SDB and incidence of hypertension,22 and randomized controlled trials of OSA patients have demonstrated improved blood pressure after treatment with continuous positive airway pressure.24–25
Relatively few cross‐sectional studies have explored the association between OSA and CAC prevalence, and they have yielded conflicting results, in both clinical and population‐based settings. One clinic‐based study of patients who were referred for both a sleep study and coronary CT showed a significantly higher prevalence of positive CAC and a higher CAC score in patients with OSA in a dose‐dependent manner according to the severity.13 Such a finding, however, was not replicated in a similarly designed clinic‐based study.12 One population‐based study showed an independent linear association of OSA severity with CAC score in women of all ages and in men <65 years old.11 To the contrary, other studies involving a community cohort failed to show a significant independent association between OSA and prevalence or burden of CAC after adjusting for BMI.10,14 Likewise, in the present study, the association between OSA and presence of CAC >0 at baseline was not significant after adjusting for BMI. When CAC >400 was considered, however, the association did persist after accounting for BMI, as well as traditional CVD risk factors.
A major advantage of our analysis over prior work is the prospective design, assessing the relation of OSA and CAC change over 8 years of follow‐up. While baseline CAC score offers independent incremental information over and above traditional risk factors in the prediction of all‐cause mortality,26 progression of CAC has been shown to further increase the risk for coronary heart disease events in the MESA cohort.27 Also within MESA, self‐reported OSA was found to be predictive of increased risk of incident CVD events and all‐cause mortality.28 Since OSA is considered a treatable condition, this provides an important basis for future studies evaluating the treatment of OSA on the development and progression of cardiovascular risk factors, which may mediate the association between OSA and CAC progression.
Given that people who habitually snore are more likely to have undiagnosed OSA4 and the body of evidence relating habitual snoring to cardiovascular morbidities, we hypothesized that those reporting “habitual snoring” would have greater CAC progression than those reporting no snoring (“normal” group). However, our analysis showed that, overall, CAC progression in the habitual snoring group was similar to that of the normal group. Habitual snoring likely identifies individuals with a very wide range of OSA severity, including individuals with snoring without significant airflow limitation to those with frequent apnea, hypopnea, and oxyhemoglobin desaturation. The stronger finding with self‐reported physician‐diagnosed OSA, which likely identifies the more severely affected (and hypoxemic individuals), provides support to the notion that intermittent hypoxemia contributes to the pathogenesis of atherosclerosis as measured by CAC progression. Although the underlying mechanism of OSA's effect on CAC progression is uncertain, it likely acts through repetitive airway obstruction and intermittent hypoxemia resulting in endothelial dysfunction29 and inflammation30–31 via oxidative stress32 or heightened sympathetic activity.33 Through these mechanisms, OSA may exert its deleterious effect on the progression of subclinical atherosclerosis either directly or indirectly via mediators such as hypertension,34 diabetes,35 and metabolic syndrome,36 which can all lead to hard CVD events such as coronary heart disease, stroke, and heart failure as shown in recent prospective studies.3,37 Although not feasible in this study, relating our findings to simultaneously obtained biomarkers of endothelial dysfunction or inflammation will provide valuable insights into the underlying mechanism. Key strengths of this study are the prospective design that included an average of 8 years of follow‐up, objectively measured CAC, and a diverse study sample. A limitation of our study is the relatively small number of participants with OSA, which at times resulted in wide confidence intervals and perhaps imprecise estimates. OSA is known to be underdiagnosed in the community, particularly as many individuals with OSA lack classic symptoms.38 Because in our sample diagnosis of OSA relied solely on self‐reported history but not on the objective measurement, the prevalence of OSA (4%) reported in our study is almost certainly an underestimate. The resultant misclassification may have biased our results toward the null. Conversely, the participants with OSA in our study may represent highly symptomatic individuals with more severe OSA, thus possibly biasing our results away from the null. Likelihood of receiving an OSA diagnosis may also have been dependent on the access to health care; in our sample those self‐reporting OSA tended to have higher incomes and greater educational attainment. In addition, it is possible that a number of participants in the OSA group may have been receiving treatment for OSA, which may have weakened the association, though to date there is no supporting evidence of a beneficial effect of therapy on the progression of CAC. However, a graded relationship has been noted between adherence to OSA treatment using continuous positive airway pressure and total mortality.39 Residual confounding resulting from unmeasured confounders in our hypothesized casual model or imprecisely measured covariates should be also considered. Finally, several caveats need to be explained in regard to the methodology of quantifying and analyzing CAC progression in our study. First, we observed negative CAC change in varying degrees in a small number of subjects (6% of the final cohort). This likely represents measurement error, though true regression of coronary calcification is not impossible. Analysis removing participants with negative CAC progression did not change the results. Second, the absolute CAC score used to measure progression in our study can introduce an overestimation of the actual progression of CAC in subjects with higher baseline CAC compared with those with low baseline CAC due to higher interscan variability.40 Third, to enhance power, interpolated data based on an assumption of linear progression was used to estimate baseline CAC in approximately half of the cohort (those who had CAC measurement at exams 1 and 3 but not exam 2). Because our focus was on the absolute CAC change rather than an incidence of CAC, we believe interpolation was an acceptable approach.
In summary, in this large and racially/ethnically diverse cohort, participants who self‐reported physician diagnosis of OSA experienced greater CAC progression during 8 years of follow‐up, relative to those who were habitual snoring or reported a normal SDB pattern. CAC progression was similar among participants who reported habitual snoring and those classified as normal. Furthermore, despite being free of clinical CVD at baseline, individuals with OSA had higher levels of severe CAC at baseline. Our finding that participants with OSA experienced greater CAC progression provides an important rationale for further studies focusing on the effect of objectively measured OSA on the development of cardiovascular risk factors and subclinical cardiovascular disease.
Sources of Funding
This research was supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute, by grants UL1‐TR‐000040, UL1‐RR‐025005 from NCRR, R01HL098433 (MESA Sleep), and T32‐HL069764. This publication was also developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the US Environmental Protection Agency, which has not been formally reviewed by the EPA. The views expressed in this document are those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Disclosures
None.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep‐disordered breathing in adults. Am J Epidemiol. 2013; 177:1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008; 118:1080-1111. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener‐West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010; 122:352-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliwise DL, Nekich JC, Dement WC. Relative validity of self‐reported snoring as a symptom of sleep apnea in a sleep clinic population. Chest. 1991; 99:600-608. [DOI] [PubMed] [Google Scholar]
- 5.Sands M, Loucks EB, Lu B, Carskadon MA, Sharkey K, Stefanick M, Ockene J, Shah N, Hairston KG, Robinson J, Limacher M, Hale L, Eaton CB. Self‐reported snoring and risk of cardiovascular disease among postmenopausal women (from the women's health initiative). Am J Cardiol. 2013; 111:540-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu FB, Willett WC, Manson JE, Colditz GA, Rimm EB, Speizer FE, Hennekens CH, Stampfer MJ. Snoring and risk of cardiovascular disease in women. J Am Coll Cardiol. 2000; 35:308-313. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013; 127:e6-e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, Agatston A, Blumenthal RS, Nasir K. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J. 2014; 35:2232-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, Pohost GM, Shaw LJ, Weintraub WS, Winters WL, Jr, Forrester JS, Douglas PS, Faxon DP, Fisher JD, Gregoratos G, Hochman JS, Hutter AM, Jr, Kaul S, Wolk MJ. American College of Cardiology/American Heart Association Expert Consensus Document on electron‐beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation. 2000; 102:126-140. [DOI] [PubMed] [Google Scholar]
- 10.Matthews KA, Strollo PJ, Jr, Hall M, Mezick EJ, Kamarck TW, Owens JF, Buysse DJ, Reis SE. Associations of Framingham risk score profile and coronary artery calcification with sleep characteristics in middle‐aged men and women: Pittsburgh SleepSCORE study. Sleep. 2011; 34:711-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinreich G, Wessendorf TE, Erdmann T, Moebus S, Dragano N, Lehmann N, Stang A, Roggenbuck U, Bauer M, Jockel KH, Erbel R, Teschler H, Mohlenkamp S. Association of obstructive sleep apnoea with subclinical coronary atherosclerosis. Atherosclerosis. 2013; 231:191-197. [DOI] [PubMed] [Google Scholar]
- 12.Kepez A, Niksarlioglu EY, Hazirolan T, Hayran M, Kocabas U, Demir AU, Aytemir K, Tokgozoglu L, Nazli N. Evaluation of association between obstructive sleep apnea and coronary risk scores predicted by tomographic coronary calcium scoring in asymptomatic patients. Anadolu Kardiyol Derg. 2011; 11:428-435. [DOI] [PubMed] [Google Scholar]
- 13.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia‐Touchard A, Lopez‐Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008; 133:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Cho GY, Baik I, Kim J, Kim SJ, Lee JB, Lim HE, Lim SY, Park J, Shin C. Association of coronary artery calcification with obstructive sleep apnea and obesity in middle‐aged men. Nutr Metab Cardiovasc Dis. 2010; 20:575-582. [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871-881. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JC, Kronmal RA, Carr JJ, McNitt‐Gray MF, Wong ND, Loria CM, Goldin JG, Williams OD, Detrano R. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005; 235:403-414. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827-832. [DOI] [PubMed] [Google Scholar]
- 18.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt‐Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility–mesa study. Radiology. 2005; 236:477-484. [DOI] [PubMed] [Google Scholar]
- 19.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004; 159:702-706. [DOI] [PubMed] [Google Scholar]
- 20.Shahar E, Shahar DJ. Causal diagrams and change variables. J Eval Clin Pract. 2012; 18:143-148. [DOI] [PubMed] [Google Scholar]
- 21.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009; 5:15-20. [PMC free article] [PubMed] [Google Scholar]
- 22.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000; 342:1378-1384. [DOI] [PubMed] [Google Scholar]
- 23.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation. 2007; 115:2722-2730. [DOI] [PubMed] [Google Scholar]
- 24.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007; 176:706-712. [DOI] [PubMed] [Google Scholar]
- 25.Drager LF, Polotsky VY, Lorenzi‐Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011; 140:534-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all‐cause mortality. Radiology. 2003; 228:826-833. [DOI] [PubMed] [Google Scholar]
- 27.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013; 61:1231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeboah J, Redline S, Johnson C, Tracy R, Ouyang P, Blumenthal RS, Burke GL, Herrington DM. Association between sleep apnea, snoring, incident cardiovascular events and all‐cause mortality in an adult population: MESA. Atherosclerosis. 2011; 219:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007; 3:409-415. [PMC free article] [PubMed] [Google Scholar]
- 30.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM‐1, IL‐8, and MCP‐1. J Appl Physiol. 2003; 94:179-184. [DOI] [PubMed] [Google Scholar]
- 31.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005; 112:2660-2667. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006; 40:1683-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Investig. 1995; 96:1897-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population‐based study of sleep‐disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997; 157:1746-1752. [PubMed] [Google Scholar]
- 35.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008; 133:496-506. [DOI] [PubMed] [Google Scholar]
- 36.Drager LF, Togeiro SM, Polotsky VY, Lorenzi‐Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013; 62:569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, Diener‐West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea‐hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010; 182:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002; 165:1217-1239. [DOI] [PubMed] [Google Scholar]
- 39.Campos‐Rodriguez F, Pena‐Grinan N, Reyes‐Nunez N, De la Cruz‐Moron I, Perez‐Ronchel J, De la Vega‐Gallardo F, Fernandez‐Palacin A. Mortality in obstructive sleep apnea‐hypopnea patients treated with positive airway pressure. Chest. 2005; 128:624-633. [DOI] [PubMed] [Google Scholar]
- 40.Hokanson JE, MacKenzie T, Kinney G, Snell‐Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004; 182:1327-1332. [DOI] [PubMed] [Google Scholar]


