Abstract
Background
Obesity is associated with cardiometabolic disease, including insulin resistance (IR) and diabetes. Cyclic guanosine monophosphate (cGMP) signaling affects energy balance, IR, and glucose metabolism in experimental models. We sought to examine effects of phosphodiesterase‐5 inhibition with tadalafil on IR in a pilot study of obese nondiabetic individuals.
Methods and Results
We conducted a randomized, double‐blinded, placebo‐controlled trial of adults age 18 to 50 years with obesity and elevated fasting insulin levels (≥10 μU/mL). Participants were randomized to tadalafil 20 mg daily or placebo for 3 months. Oral glucose tolerance tests were performed, and the effect of tadalafil on IR was examined. A total of 53 participants (mean age, 33 years; body mass index [BMI], 38 kg/m2) were analyzed, 25 randomized to tadalafil and 28 to placebo. In the overall sample, measures of IR did not differ between tadalafil and placebo groups at 3 months. However, in individuals with severe obesity (BMI ≥36.2 kg/m2), tadalafil use was associated with improved IR (homeostatic model assessment for IR), compared to placebo (P=0.02, respectively). Furthermore, one measure of β‐cell compensation for IR (oral disposition index) improved with tadalafil in the overall sample (P=0.009) and in the subgroup with severe obesity (P=0.01).
Conclusion
Results of this pilot study did not show improvements in IR with tadalafil, compared to placebo. However, tadalafil may have favorable effects on β‐cell compensation, particularly in individuals with severe obesity. Future studies evaluating the potential metabolic benefits of cGMP modulation in obesity are warranted.
Clinical Trial Registration
URL: ClinicalTrials.gov. Unique Identifier: NCT01444651.
Keywords: cGMP, insulin resistance, obesity, phosphodiesterase type 5 inhibition
Introduction
One in 4 U.S. adults is obese, and predictions suggest that, by 2030, more than half of the population will be obese.1 With the obesity epidemic there has been a concomitant doubling in the incidence of diabetes mellitus (DM) over the last 30 years, a trend most pronounced in those with body mass index (BMI) ≥30 kg/m2,2 underscoring the close link between obesity and metabolic disease.
Interestingly, obese individuals have lower natriuretic peptide levels, which, in turn, appear to be associated with greater insulin resistance (IR), regardless of BMI.3 Natriuretic peptides drive lipolysis in human adipocytes,4 and higher circulating levels in humans are associated with favorable visceral adiposity.5 In experimental studies, B‐type natriuretic peptide (BNP) transgenic (Tg) mice were protected against obesity and IR, effects that are mediated by the cyclic guanosine monophosphate (cGMP)‐signaling cascade.6 Furthermore, modulation of nitric oxide (NO) upstream of cGMP also has been shown to affect insulin action.7 Taken together, these data suggest that cGMP signaling affects energy balance, IR, and glucose metabolism. The mechanisms underlying metabolic effects of cGMP remain uncertain, but may be related to both vasodilation, resulting in increased perfusion and substrate delivery to metabolically active skeletal muscle as well as direct effects on peripheral glucose handling.8
Sildenafil and tadalafil are phosphodiesterase type 5 (PDE5) inhibitors that prevent cGMP degradation and may represent a novel therapeutic approach in metabolic disease. In mice fed a high‐fat diet (HFD), sildenafil administration increased energy expenditure and reduced IR.9 In a study of 18 individuals with metabolic syndrome (MetS), tadalafil administration (10 mg every other day for 3 weeks) was associated with improved pancreatic β‐cell function, as assessed by oral glucose tolerance testing, when compared to placebo, and there were also modest trends toward reduced IR.10 However, changes in IR and other metabolic characteristics may take longer than 3 weeks to become evident. Furthermore, it has been shown that higher doses of tadalafil lead to greater plasma concentrations of tadalafil11 and thus may have greater therapeutic effects. Accordingly, we performed a randomized, double‐blind, controlled trial to examine the effects of 3 months of high‐dose tadalafil treatment on IR and secretion in obese individuals.
Methods
Study Design
We conducted a randomized, double‐blinded, placebo‐controlled trial at Massachusetts General Hospital (ClinicalTrials.gov Identifier NCT01444651; Boston, MA). Adults ages 18 to 50 years with obesity, defined as BMI ≥27 kg/m2 and elevated fasting insulin levels (≥10 μU/mL) were included. Participants were excluded if they had a history of DM, hypertension, cardiac, renal, or liver disease, or any contraindications to receiving tadalafil, including screening systolic blood pressure <100 mm Hg, current therapy with nitrates or medications altering cytochrome P450 3A4 (CYP3A4), or history of nonarteritic ischemic optic neuropathy (please see Table 1 for full inclusion and exclusion criteria). Participants were randomized in a 1:1 fashion to receive either tadalafil 20 mg orally once a day or matching placebo for a total of 3 months, and randomization was stratified by sex. Medical history, anthropometrics, physical exam including seated blood pressure, and fasting blood draws were obtained at baseline, 6 weeks, and 3 months. All participants provided written informed consent, and the study was approved by the Partners Human Research Committee.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria |
| Adults ages 18 to 50 years |
| Obesity (BMI ≥27 kg/m2) |
| Fasting insulin levels ≥10 μU/mL |
| Exclusion criteria |
| Systolic blood pressure <100, >150 mm Hg |
| Current antihypertensive medication use |
| Current use of organic nitrates |
| Current use of phosphodiesterase 5 inhibitors (sildenafil, tadalafil, vardenafil) |
| History of reaction to phosphodiesterase 5 inhibitors |
| Known HIV infection |
| Use of medications that strongly alter CYP3A4 activity |
| History of myocardial infarction, angina, uncontrolled cardiac arrhythmia, stroke, transient ischemic attack, or seizure |
| Known nonarteritic ischemic optic neuropathy |
| History of hearing loss |
| Estimated glomerular filtration rate <60 mL/min per 1.73 m2 |
| Hepatic transaminase (AST and ALT) levels greater than 3 times the upper limit of normal |
| Known pregnancy or those unwilling to avoid pregnancy during the course of the study |
| History of priapism |
| Use in excess of 4 alcoholic drinks daily |
| History of diabetes mellitus or use of antidiabetic medications |
| Known anemia (men, hematocrit <38%; women, hematocrit <36%) |
| Fasting insulin levels ≥100 μU/mL at time of oral glucose tolerance test |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CYP3A4, cytochrome P450 3A4; HIV, human immunodeficiency virus.
Laboratory Measurements
Participants underwent a standard 2‐hour 75‐g oral glucose tolerance test (OGTT) after a 12‐hour overnight fast at the baseline and 3‐month visits. Blood samples were obtained at 0‐, 30‐, 60‐, 90‐, and 120‐minute time points. Plasma glucose measurements were obtained immediately using the hexokinase/glucose‐6‐phosphate dehydrogenase method (Roche Diagnostics GmbH, Mannheim, Germany) using a fully automated chemistry analyzer (Roche e501), with an interassay coefficient of variation of <2% at both low and high concentrations (60 and 338 mg/dL, respectively). The remainder of fasting EDTA plasma specimens were obtained and immediately processed and frozen at −80°C until assayed. Free insulin concentrations were measured on frozen EDTA plasma specimens using an immunoelectrochemiluminometric assay (Roche E170),12 with an interassay coefficient of variation (CV) of <3% at both low and high concentrations (32.6 and 170.9 μU/mL, respectively). cGMP was measured on plasma specimens using a GMP enzyme immunoassay kit according to the protocol for acetylated samples, with a CV of 13% (Alfa Aesar, Ward Hill, MA).
Assessments of IR and β‐Cell Function
The primary outcome measure of the study was change in IR, as assessed by the homeostatic model assessment of insulin resistance (HOMA‐IR) in the fasting state.13 We examined a second measure of IR, the Matsuda14 index, which utilizes measurements from the fasting state and 4 additional time points following an oral glucose load. Additional outcome measures in this pilot study included estimates of the insulinogenic index,15 which utilizes measurements from the fasting state and 30 minutes following the oral glucose load; the oral disposition index, which corrects the insulinogenic index by fasting insulin (thereby assessing the β‐cell ability to compensate for IR),16 and the Matsuda disposition index, which corrects the insulinogenic index by the Matsuda sensitivity index.17 Details of calculations are summarized in Table 2.
Table 2.
Calculation of Insulin Resistance and Secretion
| Biological Relevance | Glycemic Trait | Formula |
|---|---|---|
| Insulin resistance | HOMA‐IR | [Fasting glucose×fasting insulin]/405 |
| Insulin resistance | Matsuda sensitivity index | 10 000/SQRT [fasting glucose×fasting insulin×(mean glucose from time 30, 60, 90, 120)×(mean insulin at time 30, 60, 90, 120)] |
| Insulin secretion | Insulinogenic index | [Fasting insulin−insulin at time 30]/[fasting glucose−glucose at time 30] |
| Composite | Oral disposition index | Insulinogenic index/fasting insulin |
| Composite | Disposition index | Matsuda sensitivity index×insulinogenic index |
Glucose expressed in mg/dL, insulin expressed in μU/mL. HOMA‐IR indicates homeostatic model assessment of insulin resistance.
Assessment of Vascular Function
Participants underwent noninvasive assessment of endothelial function at baseline and 3 months using reactive hyperemia peripheral arterial tonometry (EndoPAT device; Itamar Medical Ltd., Tokyo, Japan). This technique allows measurement of changes in digital pulse volume during reactive hyperemia and has been validated against invasive measures of endothelial function.18 In brief, participants were asked to refrain from smoking or caffeine consumption for ≥8 hours, and testing was conducted in the fasting state. Peripheral arterial tonometry probes were placed on both index fingers. After a 5‐minute equilibration period, a blood pressure cuff was inflated to 200 mm Hg and kept inflated for 5 minutes. The cuff was then rapidly deflated in order to record the reactive hyperemic response, and reactive hyperemia index was calculated using the post‐/preocclusion pulse volume ratio.
Applanation tonometry was used to determine arterial stiffness noninvasively (SphygmoCor device; AtCor Medical, Sydney, Australia). In brief, pulse wave analysis was performed on the right radial artery, and 10 sequential waveforms were acquired. Central aortic pressures and wave forms were obtained using a validated generalized transfer function.19 Ten waveforms were averaged to obtain augmentation pressure, the augmentation index (the ratio of augmentation pressure to pulse pressure), the augmentation index normalized to a heart rate of 75 bpm, and time to return of the reflected wave.
Statistical Analyses
Baseline characteristics in the tadalafil and placebo groups were summarized and compared using 2‐sample t tests for normally distributed variables, Wilcoxon's rank‐sum tests for non‐normally distributed variables, or the chi‐square test statistic, as appropriate. We noted 3 extreme insulin outliers with insulin levels >6 standard deviations above the group mean (≥100 μU/mL). Because OGTT results cannot be interpreted in the nonfasting state, participants with a pre‐OGTT insulin level ≥100 μU/mL were deemed nonfasting and excluded from the final analysis (n=3; Figure 1).
Figure 1.
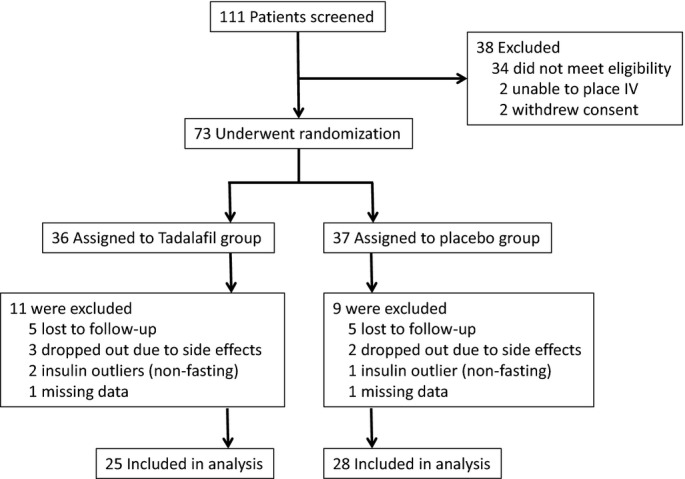
Participant recruitment, screening, and randomization chart.
The primary outcome of this pilot study was the difference in IR, as measured by HOMA‐IR, in the tadalafil versus placebo groups. Assuming a sample size of 75 randomized participants, we would have 80% power to detect a 0.7 standard deviation difference in the primary outcome between groups at P=0.05.
In order to evaluate the primary outcome of change in IR in the tadalafil compared to placebo groups, the change in HOMA‐IR (“delta”) over the treatment course was calculated as the 3‐month minus the baseline value. The effect of tadalafil versus placebo on the delta was examined using multivariable linear regression, adjusting for baseline values of a given trait. In secondary analyses, models were further adjusted for age and sex. Similar analyses were performed to assess the effect of tadalafil on β‐cell function estimates, blood pressure, BMI, and vascular measures. In further exploratory analyses, we stratified our sample by median BMI and examined the effect of tadalafil on estimates of IR and β‐cell function in participants with BMI ≥36.2 versus <36.2 kg/m2. We tested for sex by treatment as well as weight category by treatment interactions. We examined the area under the curve (AUC) for glucose and insulin response curves following OGTT using the trapezoidal method. We calculated both the total AUC, as well as the incremental AUC, after accounting for baseline values. Treatment group‐specific differences between 3‐month and baseline AUC were examined using repeated‐measures ANOVA. Because of outliers for the oral and Matsuda disposition indices, a sensitivity analysis was conducted after exclusion values outside of ±3 standard deviations around the mean.
We checked for a hyperbolic relationship between insulin sensitivity (as measured by Matsuda sensitivity index) and measures of β‐cell function (fasting insulin and insulinogenic index), as previously reported.16,20 In brief, measures were natural log‐transformed and linear regression used to determine the slope of the regression line. The association was deemed hyperbolic if the 95% confidence interval (CI) of slope of the regression line included −1.16,20 All analyses were conducted using SAS statistical software (version 9.1.3; SAS Institute Inc., Cary, NC).
Results
We screened 111 individuals, of whom 73 underwent randomization to receive tadalafil 20 mg daily versus matching placebo for the duration of 3 months (Figure 1). Of these, a total of 53 participants were included in the analysis, including 25 participants assigned to receive tadalafil and 28 to placebo. The 20 randomized participants excluded from the final analysis met the following criteria for early termination: 10 participants were lost to follow‐up, 5 withdrew because of side effects (3 in the tadalafil group and 2 in the placebo group), 3 were excluded because of nonphysiological fasting insulin levels (defined as insulin ≥100 μU/mL, thought to be a result of the nonfasting state, rendering OGTT data uninterpretable), and 2 were excluded because of missing data.
Baseline characteristics were well balanced in both groups (Table 3), with a mean age of 33±9 years and 38% women. Participants were obese with a mean BMI of 37.7±6.8 kg/m2, with 27% individuals classified as severely obese (BMI between 35 and 40 kg/m2) and 35% morbidly obese (BMI >40 kg/m2). BMI ranges were 27.7 to 53.5 kg/m2 in the tadalafil group and 27.4 to 50.8 kg/m2 in the placebo group. Participants had elevated HOMA‐IR (3.7±3.4 mg*μU/dL*mL) and normal fasting glucose concentrations (85±11 mg/dL).
Table 3.
Baseline Characteristics by Treatment Group
| Total (n=53) | Tadalafil (n=25) | Placebo (n=28) | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y | 33 (9) | 33 (9) | 34 (9) |
| Women, n (%) | 20 (38) | 7 (28) | 13 (46) |
| Systolic blood pressure, mm Hg | 115 (13) | 114 (11) | 116 (14) |
| Diastolic blood pressure, mm Hg | 75 (8) | 74 (8) | 75 (9) |
| Heart rate, bpm | 75 (9) | 76 (10) | 73 (6) |
| Body mass index, kg/m2 | 37.7 (6.8) | 38.7 (6.8) | 36.8 (6.8) |
| Creatinine, mg/dL | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) |
| Current smoker, n (%) | 4 (8) | 3 (12) | 1 (4) |
| Indices of insulin resistance and β‐cell function | |||
| Hemoglobin A1c, % | 5.3 (0.3) | 5.3 (0.3) | 5.3 (0.3) |
| Fasting glucose, mg/dL | 85 (11) | 83 (11) | 86 (10) |
| Fasting insulin, μU/mL | 17 (13) | 17 (10) | 17 (16) |
| HOMA‐IR, mg*μU/dL*mL | 3.7 (3.4) | 3.6 (2.9) | 3.8 (3.8) |
| Insulinogenic index | 1.33 (5.73) | 0.39 (8.04) | 2.17 (2.06) |
| AUC (insulin) | 11870 (7632) | 12121 (7355) | 11659 (8003) |
| Incremental AUC (insulin) | 9732 (7053) | 10029 (7198) | 9483 (7068) |
Values represent means (standard deviations), unless otherwise noted. AUC indicates area under the curve; HOMA‐IR, homeostatic model assessment of insulin resistance.
Changes in IR and β‐Cell Function With Tadalafil Treatment
There were no differences in the AUCs or corrected AUCs for insulin and glucose between the 3‐month and baseline values for either the tadalafil or placebo groups (P>0.05 for all).
Baseline and 3‐month values of IR and secretion traits are presented by treatment group in Table 4. Measures of IR were not statistically different between tadalafil and placebo groups (P>0.05 for all; Table 4), though trends appeared to favor tadalafil therapy. For example, HOMA‐IR worsened in the placebo group, whereas there was relatively little change in the tadalafil group. Similarly, IR by the assessment of Matsuda index increased in the tadalafil group, whereas it decreased in the placebo group. Participants in the tadalafil group had improved overall β‐cell function, as estimated by the oral disposition index, which assesses the ability of the β‐cell to compensate for IR, improved in the tadalafil group compared to the placebo group (Figure 2; P=0.009). Similarly, the Matsuda disposition index supported a trend toward benefit in the tadalafil group (P=0.05; Table 4). The insulinogenic index, an estimate of β‐cell function that did not assess insulin secretion in the context of IR, was not different in the treatment groups. Secondary analyses further adjusting for age and sex did not materially alter the results. There were no significant sex by treatment interactions (P>0.05 for all). A sensitivity analysis excluding outliers ±3 standard deviations outside the mean demonstrated improved oral disposition index and Matsuda disposition index in the tadalafil, compared to the placebo, group (P=0.003 for both).
Table 4.
Differences in Treatment Effect Between Tadalafil and Placebo Groups After 3 Months
| Tadalafil (n=25) | Placebo (n=28) | Beta Estimate*(SE) | P Value* | |||
|---|---|---|---|---|---|---|
| Baseline (SE) | 3 Months (SE) | Baseline (SE) | 3 Months (SE) | |||
| Indices of insulin resistance and β‐cell function | ||||||
| Fasting insulin, μU/mL | 17 (10) | 16 (14) | 17 (16) | 22 (15) | −5.65 (4.07) | 0.17 |
| HOMA‐IR | 3.57 (2.86) | 3.81 (5.14) | 3.84 (3.80) | 5.06 (3.95) | −1.25 (1.29) | 0.34 |
| Matsuda sensitivity index | 3.60 (2.54) | 4.27 (2.83) | 3.51 (2.15) | 3.28 (2.42) | 0.96 (0.68) | 0.18 |
| Insulinogenic index | 0.39 (8.04) | 2.62 (3.03) | 2.17 (2.06) | 1.04 (2.32) | 1.48 (0.77) | 0.06 |
| Oral disposition index | 1.74 (11.56) | 4.48 (4.62) | 2.87 (3.67) | 0.58 (5.21) | 3.76 (1.38) | 0.009 |
| Matsuda disposition index | 2.23 (24.19) | 9.22 (8.51) | 6.67 (9.45) | 0.19 (18.42) | 8.09 (4.05) | 0.05 |
| Clinical traits | ||||||
| Systolic blood pressure, mm Hg | 114 (11) | 111 (12) | 116 (14) | 117 (11) | −4.72 (2.62) | 0.08 |
| Diastolic blood pressure, mm Hg | 74 (8) | 71 (9) | 75 (9) | 74 (8) | −3.60 (2.11) | 0.09 |
| Pulse pressure, mm Hg | 40 (11) | 40 (10) | 42 (12) | 42 (12) | −1.13 (2.65) | 0.67 |
| Body mass index, kg/m2 | 38.6 (6.8) | 37.4 (6.0) | 37.1 (6.8) | 36.3 (6.5) | −0.30 (0.66) | 0.65 |
| Heart rate, bpm | 76 (10) | 77 (12) | 73 (6) | 74 (9) | 1.45 (2.61) | 0.58 |
| Vascular traits | ||||||
| Reactive hyperemia index | 2.1 (0.5) | 2.1 (2.7) | 2.3 (0.6) | 2.2 (0.3) | −0.18 (0.58) | 0.76 |
| Augmentation pressure, mm Hg | 3.7 (4.9) | 4.4 (5.8) | 4.9 (5.9) | 3.5 (5.4) | 1.04 (1.58) | 0.51 |
| Augmentation index, % | 8.3 (11.8) | 10.4 (10.1) | 11.1 (8.9) | 8.7 (10.4) | 3.12 (2.00) | 0.13 |
| Augmentation index @ 75, % | 8.0 (11.3) | 10.1 (8.2) | 9.7 (10.0) | 8.0 (10.4) | 3.43 (2.20) | 0.13 |
| Central systolic BP, mm Hg | 100 (9) | 98 (9) | 103 (13) | 102 (10) | −3.05 (2.27) | 0.19 |
| Central diastolic BP, mm Hg | 74 (8) | 71 (9) | 75 (9) | 75 (8) | −4.18 (2.24) | 0.07 |
| Central pulse pressure, mm Hg | 26 (7) | 27 (6) | 28 (8) | 27 (9) | 1.33 (1.78) | 0.46 |
| Time to return, ms | 144 (6) | 143 (8) | 147 (11) | 145 (13) | −0.19 (2.55) | 0.65 |
Values represent means (standard deviations). BP indicates blood pressure; HOMA‐IR, homeostatic model assessment of insulin resistance.
Beta estimates and P values from linear regression model, predicting change in the trait and comparing tadalafil versus placebo groups, adjusted for baseline value of a given clinical trait.
Figure 2.
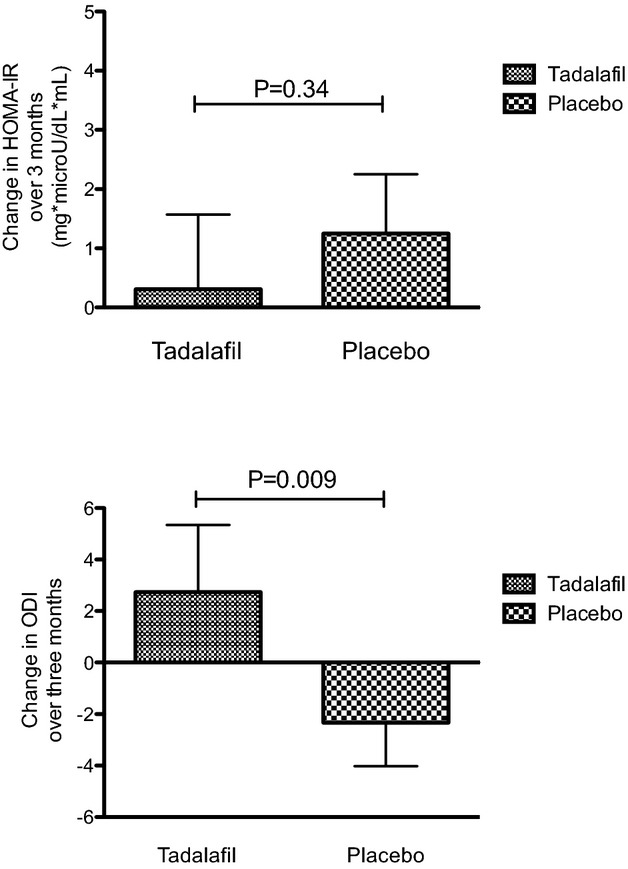
Change in HOMA‐IR and oral disposition index after 3 months of treatment with tadalafil vs placebo groups. Data represent means±standard deviations. HOMA‐IR indicates homeostatic model of insulin resistance, ODI indicates oral disposition index..
We evaluated the association between measures of insulin sensitivity and β‐cell function. Specifically, in a linear regression model with log‐transformed Matsuda sensitivity index as the independent variable, and log‐transformed fasting insulin as the dependent variable, the slope of the regression line was −0.87 (95% CI, −1.03 to −0.71), suggesting a hyperbolic relationship.
Tadalafil Effects on IR and β‐Cell Function Were Most Pronounced in the Severely Obese
In exploratory analyses, we stratified our sample by median BMI. Participants with BMI ≥36.2 kg/m2 were more insulin resistant at baseline with a mean HOMA‐IR of 4.6±3.2, compared with 2.9±3.4 mg*μU/dL*mL in participants with BMI <36.2 kg/m2. In the severely obese, tadalafil treatment was associated with improved IR, as estimated by HOMA‐IR (P=0.02) and the Matsuda sensitivity index (P=0.04; Table 5). Tadalafil also appeared to have a beneficial effect on β‐cell function when corrected for level of IR, compared to placebo, in the severely obese (P=0.01 for oral disposition index; P=0.007 for Matsuda disposition index). In contrast, no significant differences were noted between the tadalafil and placebo groups among participants with BMI <36.2 kg/m2. There were no significant interactions of BMI subgroup and treatment assignment (P>0.10 for all).
Table 5.
Effect of Tadalafil on Indices of Insulin Resistance and β‐Cell Function According to Degree of Obesity
| BMI <36.2 kg/m2 (n=26) | BMI ≥36.2 kg/m2 (n=27) | |||
|---|---|---|---|---|
| Beta Estimate (SE)* | P Value | Beta Estimate (SE)* | P Value | |
| HOMA‐IR | 0.59 (2.41) | 0.81 | −2.99 (1.16) | 0.02 |
| Matsuda sensitivity index | −0.08 (1.02) | 0.94 | 2.05 (0.92) | 0.04 |
| Insulinogenic index | 0.78 (1.08) | 0.48 | 1.30 (0.71) | 0.08 |
| Oral disposition index | 1.43 (1.50) | 0.35 | 3.98 (1.50) | 0.01 |
| Matsuda disposition index | 6.62 (6.16) | 0.29 | 7.16 (2.43) | 0.007 |
BMI indicates body mass index; HOMA‐IR, homeostatic model assessment of insulin resistance.
Beta estimates represent difference in (3‐month/baseline) trait between the tadalafil and placebo group, adjusted for baseline value.
Tadalafil Effect on Clinical, Vascular, and Laboratory Measures
Individuals assigned to tadalafil had a trend toward lower systolic and diastolic blood pressures, when compared to placebo (P=0.08 and P=0.09, respectively). There were no significant differences in change in BMI or vascular measures between treatment groups (Table 4). There were no differences in plasma cGMP concentrations between individuals treated with tadalafil, compared to placebo, either at baseline or 3‐month follow‐up.
Tadalafil was tolerated well by most participants. Five participants in the tadalafil group (20%) and 1 participant in the placebo group complained of headaches during the course of the study, and 3 participants in the tadalafil group reported back or leg pain (12%), whereas none in the placebo group reported back or leg pain. A total of 3 participants withdrew from the tadalafil group (1 because of headache and 2 because of back/leg aches), and 2 participants withdrew from the placebo group (1 because of blurry vision and 1 because of arm pain).
Discussion
We conducted a randomized, placebo‐controlled pilot study to examine the effect of PDE5 inhibition on glucose metabolism in obese individuals. Oral tadalafil at 20 mg daily was reasonably well tolerated for the 3‐month study. Though we found no significant improvements in estimates of IR after 3 months of tadalafil treatment, compared to placebo, estimates of β‐cell function, when viewed in the context of IR, improved in the tadalafil group, when compared to placebo.
Notably, when stratified by BMI in subgroup analyses, we found that tadalafil treatment was associated with improved measures of IR, compared to placebo, among severely obese participants. Though the results of subgroup analyses in our modest sample have to be viewed as exploratory, this finding warrants further study in the future.
The oral disposition index estimates pancreatic β‐cell function adjusted for a given background of insulin sensitivity.16 In prospective studies, abnormalities in the disposition index precede the development of clinical diabetes21 and reflect inadequate insulin release early in the disease process. Our study findings suggest that tadalafil may have favorable metabolic effects in obese insulin‐resistant individuals even before the development of clinical diabetes, particularly in the severely obese.
The effect of tadalafil on β‐cell function has previously been studied in a small human trial of 18 patients with MetS. In that study, tadalafil improved β‐cell function in women, but not men, and no effects on IR were noted.10 In the current study, targeting participants with evidence of IR, we found trends both in improved IR and β‐cell function. These differences may be the result of higher dose and longer duration of tadalafil treatment, compared to the previous study, reflecting short‐ versus long‐term effects of phosphodiesterase inhibition.
The cGMP pathway appears to regulate glucose metabolism, both through upstream activation of NO or the natriuretic peptide receptor. Previous animal studies have demonstrated that inhibition or deficiency of NO leads to IR, and that NO itself augments insulin release.7,22–24 Similarly, BNP Tg mice fed an HFD were protected against IR.6 Furthermore, administration of sildenafil improved energy expenditure and reduced IR in a mouse model.9 Taken together, these data suggest that PDE5 inhibitors may improve glucose metabolism by preventing cGMP degradation within metabolically active tissues. The mechanisms by which cGMP improve glucose metabolism are not completely understood, and mechanistic inferences cannot be drawn from our pilot study. Previous experimental studies have implicated vasodilatory effects that may improve substrate delivery to skeletal muscle25–26 or direct effects on cellular glucose transport in skeletal muscle and vascular IR.22,27 Though we did not observe changes in plasma concentrations of cGMP, this may not fully reflect changes within metabolically active tissues.
Several limitations deserve mention. This was a pilot study, and our sample size may have limited our power to detect more modest effects. We observed favorable trends in IR, derived from correlated indices, but these were not statistically significant, and our findings need to be confirmed in larger studies in the future. Furthermore, we used estimates of β‐cell function and IR derived from fasting and post–oral glucose load blood samples, rather than measures from more‐intensive physiologic assessment. The hyperinsulinemic euglycemic clamp may have provided a more‐precise estimate of insulin sensitivity. Still, the estimates used in this study are correlated with clamp‐derived measures of IR.13–14,28 Future studies should undertake more‐precise measurements of IR. More than half of our study sample was severely obese, and exploratory subgroup analyses suggested that potential benefits of tadalafil on glucose handling were more pronounced in the severely obese. This may limit the generalizability of our findings to broader populations, although the mean BMI in our study was comparable to a previous human study examining metabolic effects of tadalafil.10 We did not detect differences in plasma cGMP concentrations, which may be because of our modest sample size. It also may be that plasma cGMP concentrations do not necessarily reflect cGMP activity at the tissue level, or that cGMP concentrations need to be understood within the context of other signaling molecules, such as natriuretic peptides. Last, dose‐response effects of tadalafil on metabolic traits are not known because only one dose was tested, and long‐term effects are not known given the 3‐month duration of the study.
In conclusion, we did not find a significant improvement in IR with tadalafil in obese individuals in our study. However, exploratory findings suggest that PDE5 inhibition may have favorable metabolic effects with regard to β‐cell function when accounting for IR in obese individuals, particularly those with severe obesity. Though weight loss remains the mainstay of treatment for severe obesity, in light of rising rates of obesity and concomitant cardiometabolic disease, additional, well‐powered studies evaluating potential metabolic benefits of cGMP modulation in obese individuals are warranted.
Acknowledgment
The authors thank Jonathan S. Williams, MD, of Brigham and Women's Hospital for serving as the data safety monitor of the study.
Sources of Funding
This study was supported, in part, by the National Institutes of Health (K23‐HL116780 to Dr Ho; R01‐HL098283 and R01‐HL113933 to Dr Newton‐Cheh; and R01‐HL086875 to Dr Wang), the American Heart Association (10SDG2610313 to Dr Buys), a Boston University School of Medicine Department of Medicine Career Investment Award to Dr Ho, and a Massachusetts General Hospital Research Scholars Award to Dr Florez.
Disclosures
Dr Wang has served as a consultant for Pfizer, Inc.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013; 127:e6-e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D'Agostino RB., Sr Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation. 2006; 113:2914-2918. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007; 115:1345-1353. [DOI] [PubMed] [Google Scholar]
- 4.Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000; 14:1345-1351. [PubMed] [Google Scholar]
- 5.Cheng S, Fox CS, Larson MG, Massaro JM, McCabe EL, Khan AM, Levy D, Hoffmann U, O'Donnell CJ, Miller KK, Newton‐Cheh C, Coviello AD, Bhasin S, Vasan RS, Wang TJ. Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am J Cardiol. 2011; 108:979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cGMP/cGMP‐dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009; 58:2880-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001; 104:342-345. [DOI] [PubMed] [Google Scholar]
- 8.Young ME, Leighton B. Evidence for altered sensitivity of the nitric oxide/cGMP signalling cascade in insulin‐resistant skeletal muscle. Biochem J. 1998; 329Pt 1:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat‐fed conscious mice. Diabetes. 2007; 56:1025-1033. [DOI] [PubMed] [Google Scholar]
- 10.Hill KD, Eckhauser AW, Marney A, Brown NJ. Phosphodiesterase 5 inhibition improves beta‐cell function in metabolic syndrome. Diabetes Care. 2009; 32:857-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE, Mitchell MI. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006; 61:280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem. 2007; 53:922-932. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412-419. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999; 22:1462-1470. [DOI] [PubMed] [Google Scholar]
- 15.Yoneda H, Ikegami H, Yamamoto Y, Yamato E, Cha T, Kawaguchi Y, Tahara Y, Ogihara T. Analysis of early‐phase insulin responses in nonobese subjects with mild glucose intolerance. Diabetes Care. 1992; 15:1517-1521. [DOI] [PubMed] [Google Scholar]
- 16.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2‐h glucose levels. Diabetes Care. 2009; 32:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Coletta DK, Mandarino LJ, Shaibi GQ. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care. 2012; 35:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004; 44:2137-2141. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000; 525Pt 1:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D Jr. Quantification of the relationship between insulin sensitivity and beta‐cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993; 42:1663-1672. [DOI] [PubMed] [Google Scholar]
- 21.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic beta‐cell function in Latino women at high risk for type 2 diabetes. Diabetes. 2006; 55:1074-1079. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo NO, Maloney E, Pham M, Luttrell I, Wessells H, Tateya S, Daum G, Handa P, Schwartz MW, Kim F. Reduced NO‐cGMP signaling contributes to vascular inflammation and insulin resistance induced by high‐fat feeding. Arterioscler Thromb Vasc Biol. 2010; 30:758-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron AD, Zhu JS, Marshall S, Irsula O, Brechtel G, Keech C. Insulin resistance after hypertension induced by the nitric oxide synthesis inhibitor L‐NMMA in rats. Am J Physiol. 1995; 269:E709-E715. [DOI] [PubMed] [Google Scholar]
- 24.Smukler SR, Tang L, Wheeler MB, Salapatek AM. Exogenous nitric oxide and endogenous glucose‐stimulated beta‐cell nitric oxide augment insulin release. Diabetes. 2002; 51:3450-3460. [DOI] [PubMed] [Google Scholar]
- 25.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990; 85:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes. 2000; 49:768-774. [DOI] [PubMed] [Google Scholar]
- 27.Kapur S, Bedard S, Marcotte B, Cote CH, Marette A. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes. 1997; 46:1691-1700. [DOI] [PubMed] [Google Scholar]
- 28.Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care. 2001; 24:796-797. [DOI] [PubMed] [Google Scholar]


