Abstract
Background
There are few data characterizing temporal changes in hospitalization for recurrent acute myocardial infarction (AMI) after AMI.
Methods and Results
Using a national sample of 2 305 441 Medicare beneficiaries hospitalized for AMI from 1999 to 2010, we evaluated changes in the incidence of 1‐year recurrent AMI hospitalization and mortality using Cox proportional hazards models. The observed recurrent AMI hospitalization rate declined from 12.1% (95% CI 11.9 to 12.2) in 1999 to 8.9% (95% CI 8.8 to 9.1) in 2010, a relative decline of 26.4%. The observed recurrent AMI hospitalization rate declined by a relative 27.7% in whites, from 11.9% (95% CI 11.8 to 12.1) to 8.6% (95% CI 8.5 to 8.8) versus a relative decline in blacks of 13.6% from 13.2% (95% CI 12.6 to 13.8) to 11.4% (95% CI 10.9 to 12.0). The risk‐adjusted rate of annual decline in recurrent AMI hospitalizations was 4.1% (HR 0.959; 95% CI 0.958 to 0.961), and whites experienced a higher rate of decline (HR 0.957, 95% CI 0.956 to 0.959) than blacks (HR 0.974, 95% CI 0.970 to 0.979).The overall, observed 1‐year mortality rate after hospitalization for recurrent AMI declined from 32.4% in 1999 to 29.7% in 2010, a relative decline of 8.3% (P<0.05). In adjusted analyses, 1‐year mortality after recurrent AMI hospitalization declined 1.8% per year (HR, 0.982; 95% CI 0.980 to 0.985).
Conclusions
In a national sample of Medicare beneficiaries hospitalized for AMI from 1999 to 2010, hospitalization for recurrent AMI decreased, as did subsequent mortality, albeit to a lesser extent. The risk of recurrent AMI hospitalization declined less in black patients than in whites, increasing observed racial disparities by the end of the study period.
Keywords: epidemiology, mortality, myocardial infarction
Introduction
Improvements in primary cardiovascular prevention, including control of hypertension, hyperlipidemia, and cigarette smoking, have translated into substantial declines in hospitalizations and deaths due to acute myocardial infarction (AMI) over the past decade.1 In contrast, there are few data characterizing the incidence of hospitalization for recurrent AMI. Previous studies of recurrent AMI have evaluated subjects before the introduction of modern cardiovascular prevention strategies2–3 or have been constrained to demographically and geographically homogeneous populations.4–8 Increasing use of routine revascularization for AMI9 and secondary prevention therapies10–15 may have reduced rates of hospitalization for recurrent AMI over the past decade. Conversely, improved AMI survival rates may have created a population at higher risk of hospitalization for recurrent AMI.16
Accordingly, we sought to examine recent national trends in the occurrence and outcomes (ie, mortality) of recurrent AMI hospitalization using a complete sample of fee‐for‐service Medicare beneficiaries hospitalized for AMI from 1999 to 2010. Medicare data provide sufficient numbers to precisely estimate changes in recurrent AMI hospitalization rates, and to examine differences between relevant demographic subgroups. Comparing trends in recurrent AMI hospitalization rates between age groups merits attention because older patients are at particularly high risk for recurrent cardiovascular events. In addition, previous work evaluating recent temporal trends in AMI hospitalization rates has shown that black men and women experienced a lower decline in AMI hospitalization rate than their white counterparts,1 but it is unknown whether such differences also exist in recurrent AMI hospitalization rates. These analyses can provide insight about whether contemporary management strategies for AMI have resulted in decreasing rates of recurrent AMI hospitalization, and whether these gains have been shared equally across demographic subgroups.
Methods
Data Source
We used Medicare Provider Analysis and Review inpatient data from the Centers for Medicare and Medicaid Services to identify a complete sample of fee‐for‐service Medicare beneficiaries who were hospitalized for AMI between January 1, 1999 and December 31, 2011. These administrative billing claims data include information on patient demographics (age, sex, and race), admission and discharge dates, and principal and secondary diagnosis codes as coded by the International Classification of Diseases, Ninth Revision, Clinical Modification. Medicare denominator files were used to ascertain beneficiary eligibility, and enrollment in fee‐for‐service Medicare. Institutional Review Board review and approval was obtained through the Yale University Human Investigation Committee. Medicare claims data were provided through a data use agreement with Centers for Medicare and Medicaid Services.
Study Sample
Subjects were included if they were aged 65 years or older and discharged alive from an acute care hospital with a principal discharge diagnosis of AMI (International Classification of Diseases, Ninth Revision, Clinical Modification code: 410.xx) between January 1, 1999 and December 31, 2010. We identified the first admission for AMI during the study period as the “index AMI” hospitalization and the first subsequent AMI admission as the “recurrent AMI hospitalization.” We excluded patients with principal discharge diagnosis International Classification of Diseases, Ninth Revision, Clinical Modification codes 410.x2, as these represent subsequent episodes of care related to the index AMI. Patients with a total length of stay of ≤1 day were excluded, as those hospitalizations are unlikely to represent true AMI. We also excluded (1) beneficiaries aged <65 years; (2) beneficiaries without ≥1 year of Medicare fee‐for‐service enrollment before and after their index AMI hospitalization, as data would be limited to assess comorbidity (see “Patient Characteristics” section below) and outcomes; (3) beneficiaries with conflicting dates of death and hospitalization; and (4) patients who were subsequently transferred to another acute care hospital for continuing care after an initial AMI. Patients who died during the 1‐year follow up period without experiencing hospitalization for recurrent AMI were censored at the time of death; all others were censored at the end of the follow‐up period.
Patient Characteristics
We used the Medicare Provider Analysis and Review data set to collect information on patient characteristics, including age, sex, race, and comorbidities. Race was determined from the Medicare denominator files, which use patient‐reported data from the Social Security Administration. Coexisting illnesses were classified according to the categorization used by Centers for Medicare and Medicaid Services for the AMI 30‐day mortality measure.17 We identified comorbidities from both primary and secondary diagnosis codes of all patient hospitalizations up to 1 year before the initial hospitalization for AMI. For example, the inpatient data from 1998 were used to obtain comorbidity information on patients who were hospitalized for AMI in 1999.
Outcomes
Our first study outcome was recurrent AMI within 1 year of (admission for) the index AMI. Our second outcome was 1‐year all‐cause mortality rates among patients who were hospitalized for recurrent AMI.
Statistical Analysis
Baseline characteristics of patients hospitalized for AMI during the study period were collapsed into 3‐year intervals to simplify presentation, and we used the Cochran–Armitage trend test to examine the significance of trends. We used Cox proportional hazards regression models to assess annual trends in recurrent AMI hospitalization rates, adjusting for age, sex, race, and all comorbidities shown in Table. We fitted separate Cox models to analyze trends in recurrent AMI hospitalization rates among subgroups (ie, age, sex, and race). All Cox models included an ordinal time variable, ranging from 0 to 11, corresponding to years 1999 (time=0) through 2010 (time=11) to represent the risk‐adjusted annual trend in 1‐year recurrent AMI hospitalizations. Similarly, Cox proportional hazards regression models were used to analyze 1‐year mortality among patients who experienced recurrent AMI hospitalization.
Table 1.
Patient Characteristics
| Characteristics | 1999–2001 | 2002–2004 | 2005–2007 | 2008–2010 | Cumulative |
|---|---|---|---|---|---|
| Total | 592 255 | 631 114 | 565 082 | 516 990 | 2 305 441 |
| Age in years, mean (SD) | 78.8 (7.8) | 78.8 (8.1) | 78.9 (8.3) | 78.9 (8.5) | 78.8 (8.2) |
| Female, n (%) | 303 715 (51.3) | 321 297 (50.9) | 282 310 (50.0) | 254 543 (49.2) | 1 161 865 (50.4) |
| White, n (%) | 525 818 (88.8) | 555 212 (88.0) | 496 067 (87.8) | 452 153 (87.5) | 2 029 250 (88.0) |
| Black, n (%) | 42 645 (7.2) | 47 188 (7.5) | 43 024 (7.6) | 40 325 (7.8) | 173 182 (7.5) |
| Other race, n (%) | 23 792 (4.0) | 28 714 (4.5) | 25 991 (4.6) | 24 512 (4.7) | 103 009 (4.5) |
| Comorbidity, n (%) | |||||
| Coronary artery disease | 413 276 (69.8) | 459 239 (72.8) | 415 081 (73.5) | 383 472 (74.2) | 1 671 068 (72.5) |
| Hypertension | 326 468 (55.1) | 373 051 (59.1) | 344 592 (61.0) | 343 818 (66.5) | 1 387 929 (60.2) |
| Diabetes | 182 534 (30.8) | 199 071 (31.5) | 177 807 (31.5) | 164 339 (31.8) | 723 751 (31.4) |
| COPD | 135 963 (23.0) | 152 168 (24.1) | 139 223 (24.6) | 106 540 (20.6) | 533 894 (23.2) |
| Congestive heart failure | 95 656 (16.1) | 102 466 (16.2) | 90 212 (16.0) | 79 159 (15.3) | 367 493 (15.9) |
| Pneumonia | 73 783 (12.5) | 87 483 (13.9) | 81 442 (14.4) | 79 956 (15.5) | 322 664 (14.0) |
| Dementia | 56 303 (9.5) | 66 764 (10.6) | 60 434 (10.7) | 58 597 (11.3) | 242 098 (10.5) |
| Renal failure | 27 923 (4.7) | 38 572 (6.1) | 54 477 (9.6) | 66 316 (12.8) | 187 288 (8.1) |
| Peripheral vascular disease | 38 715 (6.5) | 44 107 (7.0) | 39 733 (7.0) | 35 977 (7.0) | 158 532 (6.9) |
| Cancer | 37 070 (6.3) | 40 736 (6.5) | 37 047 (6.6) | 34 393 (6.7) | 149 246 (6.5) |
| Trauma | 31 268 (5.3) | 38 071 (6.0) | 36 030 (6.4) | 32 167 (6.2) | 137 536 (6.0) |
| Depression | 27 372 (4.6) | 34 267 (5.4) | 30 676 (5.4) | 28 552 (5.5) | 120 867 (5.2) |
| History of MI | 29 546 (5.0) | 32 848 (5.2) | 27 353 (4.8) | 26 107 (5.0) | 115 854 (5.0) |
| Cerebrovascular disease | 33 581 (5.7) | 32 471 (5.1) | 25 257 (4.5) | 22 256 (4.3) | 113 565 (4.9) |
| Unstable angina | 36 748 (6.2) | 31 937 (5.1) | 22 021 (3.9) | 17 102 (3.3) | 107 808 (4.7) |
| Respiratory failure | 15 475 (2.6) | 17 774 (2.8) | 20 445 (3.6) | 25 121 (4.9) | 78 815 (3.4) |
| Malnutrition | 13 212 (2.2) | 16 266 (2.6) | 17 528 (3.1) | 23 703 (4.6) | 70 709 (3.1) |
| Functional disability | 15 897 (2.7) | 16 246 (2.6) | 12 807 (2.3) | 13 409 (2.6) | 58 359 (2.5) |
| Stroke | 12 731 (2.1) | 12 653 (2.0) | 10 802 (1.9) | 9649 (1.9) | 45 835 (2.0) |
| Psychiatric disease | 11 536 (1.9) | 12 202 (1.9) | 10 029 (1.8) | 10 877 (2.1) | 44 644 (1.9) |
| Liver disease | 2980 (0.5) | 3730 (0.6) | 3566 (0.6) | 3291 (0.6) | 13 567 (0.6) |
| Length of stay, mean (SD) | 7.1 (6.2) | 6.8 (6.2) | 6.4 (5.9) | 5.9 (5.4) | 6.5 (5.4) |
| Discharged to home, n (%) | 367 198 (62.0) | 365 415 (57.9) | 314 186 (55.6) | 286 929 (55.5) | 1 332 545 (57.8) |
| Discharged to ICF/SNF, n (%) | 114 897 (19.4) | 126 223 (20.0) | 115 842 (20.5) | 102 881 (19.9) | 461 088 (20.0) |
| Discharged to home care, n (%) | 76 993 (13.0) | 89 618 (14.2) | 89 283 (15.8) | 81 684 (15.8) | 336 594 (14.6) |
| Discharged to hospice, n (%) | 148 064 (0.25) | 8836 (1.4) | 14 692 (2.6) | 17 061 (3.3) | 41 498 (1.8) |
COPD indicates chronic obstructive pulmonary disease; MI, myocardial infarction; ICF, intermediate care facility; SNF, skilled nursing facility.
Y.W. conducted all analyses using SAS 9.3 64‐bit version (SAS Institute Inc, Cary, NC), and takes responsibility for the accuracy of the results. Hazard ratios are reported with 95% CI. Statistical tests were 2‐sided at a significance level of 0.05. The Yale University Human Investigation Committee approved the study and waived the requirement for participant informed consent.
Results
Patient Characteristics
During the 12‐year study period, 3 067 263 patients experienced an index AMI hospitalization. The number of patients who experienced an index AMI hospitalization decreased from 1283 (95% CI 1278 to 1287) per 100 000 person‐years in 1999 to 830 (95% CI 827 to 833) per 100 000 person‐years in 2010. Among patients who experienced an index AMI hospitalization, 2 305 441 were enrolled continuously in the Medicare fee‐for‐service program for at least 12 months before and 12 months following (in the absence of death) the index AMI hospitalization, were discharged alive after at least 1 day, and were not transferred to another short‐term acute‐care hospital.
The characteristics of the patients in the final study sample are presented in Table. The mean age was 78.8 years (SD 8.2 years), 50.4% were women, and 12.0% were of nonwhite race. The most common comorbidities were coronary artery disease (72.5%), hypertension (60.2%), diabetes (31.4%), chronic obstructive pulmonary disease (COPD) (23.2%), and heart failure (15.9%).
There were several notable (and statistically significant, P<0.05) changes in the characteristics of patients hospitalized for AMI during the 12‐year study period. The percentage of females decreased from 51.1% in 1999 to 48.8% in 2010, and the percentage of nonwhites increased from 11.0% to 12.7%. The prevalence of a diagnosis of coronary artery disease prior to the index MI and hypertension both increased over the study period, from 68.3% to 74.1% and 53.1% to 66.9%, respectively. The prevalence of renal failure also increased notably from 4.2% to 13.5%. The mean length of stay decreased from 7.2 days (SD 6.2 days) in 1999 to 5.6 days (SD 5.3 days) in 2010. The percentage of patients discharged to home decreased over the study period (62.6% to 56.0%), while the percentage of patients discharged to home care increased (13.4% to 15.9%). Among participants who did not experience a recurrent AMI hospitalization during the year following an initial AMI hospitalization, the 1‐year mortality declined from 21.7% (95% CI, 21.6 to 21.9) in 1999 to 21.1% (95% CI, 20.9 to 21.3) in 2010.
One‐Year Recurrent AMI Hospitalization Rate
The overall (during the entire study period) 1‐year recurrent AMI hospitalization rate was 10.1% (95% CI 10.0 to 10.1). The mean number of days until readmission for recurrent AMI increased from 108 in 1999 to 117 in 2010 (P<0.05).
The observed (ie, unadjusted) annual recurrent AMI hospitalization rate declined from 12.1% (95% CI 11.9 to 12.2) in 1999 to 8.9% (95% CI 8.8 to 9.1) in 2010, a relative decline of 26.4% (Figure 1). The mean, relative annual decline was 2.4%. The decline in recurrent AMI hospitalization rate was observed among all demographic subgroups (Figure 1). Throughout the study period, the lowest recurrent AMI hospitalization rate was observed in those 65 to 74 years old (9.5%, 95% CI 9.2 to 9.7). Although a decline in recurrent AMI hospitalization rate was observed in both males and females, females consistently had a higher rate of recurrent AMI hospitalization than males over the course of the study period. The recurrent AMI hospitalization rate declined by a relative 27.0% in females, from 12.6% (95% CI 12.4 to 12.9) in 1999 to 9.2% 95% CI 9.0 to 9.5) in 2010, and by 25.2% in males, from 11.5% (95% CI 11.3 to 11.7) in 1999 to 8.6% (95% CI 8.4 to 8.8) in 2010. When compared by race, the reduction in recurrent AMI hospitalization rate was larger in whites than blacks. The recurrent AMI hospitalization rate declined by a relative 27.7% in whites, from 11.9% (95% CI 11.8 to 12.1) to 8.6% (95% CI 8.5 to 8.8) versus a relative decline in blacks of 13.6% from 13.2% (95% CI 12.6 to 13.8) to 11.4% (95% CI 10.9 to 12.0). The discrepancy between recurrent AMI hospitalization rates in whites and blacks was therefore higher by the end of the study period (ie, 8.6% versus 11.4%) compared with the beginning (11.9% versus 13.2%).
Figure 1.
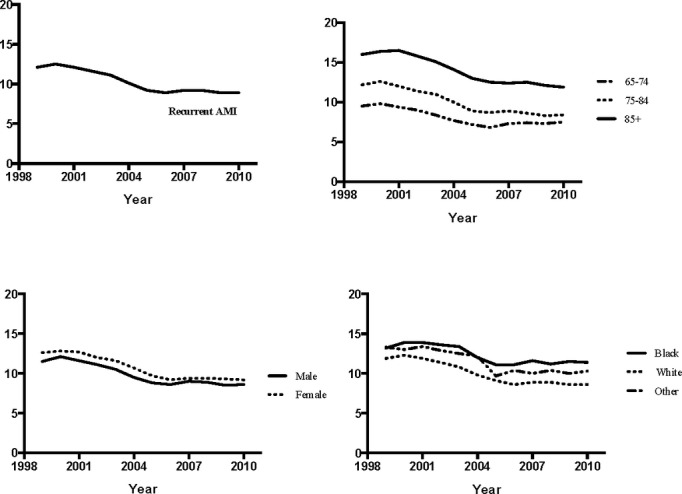
Observed 1‐year rates of recurrent acute myocardial infarction (AMI) hospitalization.
As shown in Figure 2, after adjusting for demographic and clinical covariates, the overall risk‐adjusted rate of annual decline in recurrent AMI hospitalizations was 4.1%, corresponding to a HR of 0.959 (95% CI 0.958 to 0.961). The rate of annual decline was highest among those 75 to 84 years of age, HR 0.953 (95% CI 0.951 to 0.955). While males and females had similar adjusted rates of decline in recurrent AMI hospitalization, whites experienced a statistically significantly higher rate of decline (HR 0.957, 95% CI 0.956 to 0.959) than black patients (HR 0.974, 95% CI 0.970 to 0.979).
Figure 2.
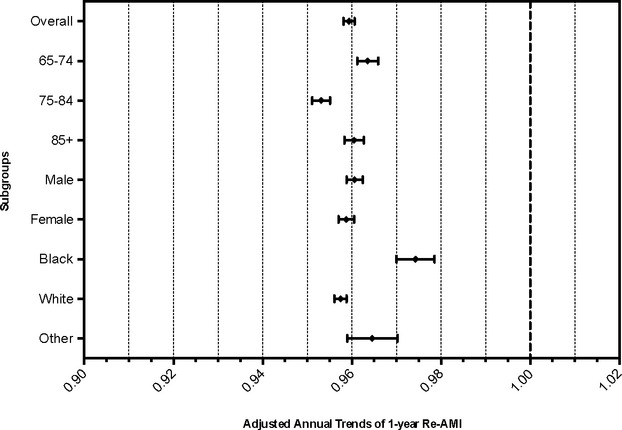
Risk‐adjusted annual trends in reinfarction rates. Cox proportional hazards regression models were used to assess annual trends in recurrent acute myocardial infarction (AMI) hospitalization rates, adjusting for age, sex, race, and all comorbidities shown in Table.
1‐Year All‐Cause Mortality After Recurrent AMI Hospitalization
The overall, observed 1‐year mortality rate after hospitalization for recurrent AMI declined from 32.4% in 1999 to 29.7% in 2010, a relative decline of 8.3% (P<0.05) (Figure 3). Within age subgroups, the decline in 1‐year mortality was greatest among those 75 to 84 years of age, declining relatively 16.4%, from 31.7% in 1999 to 26.5% in 2010 (P<0.05). Declines in 1‐year mortality were observed across both sexes and all races. Consistently higher 1‐year mortality rates were observed in females compared with males: 30.8% versus 28.5% by 2010 (P<0.0001).
Figure 3.
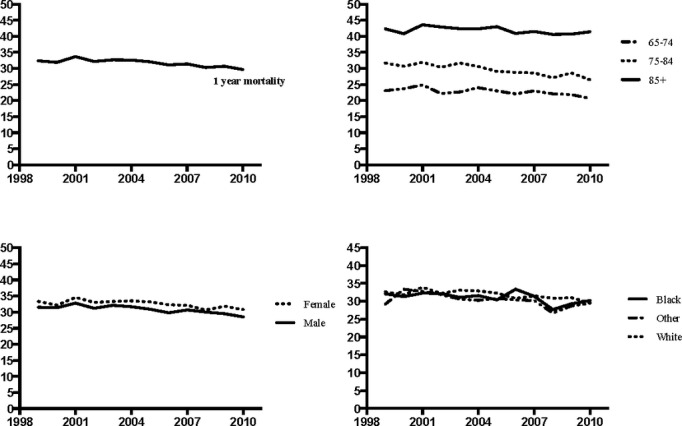
Observed 1‐year rates of mortality.
In adjusted analyses, (Figure 4) the 1‐year mortality declined 1.8% per year (HR 0.982 [95% CI 0.980 to 0.985]). Among the age subgroups, the lowest rate of decline was observed in those over 85 years of age (HR 0.988 [95% CI 0.984 to 0.993]). Adjusted rates of mortality decline were similar in both sexes and in all racial groups.
Figure 4.
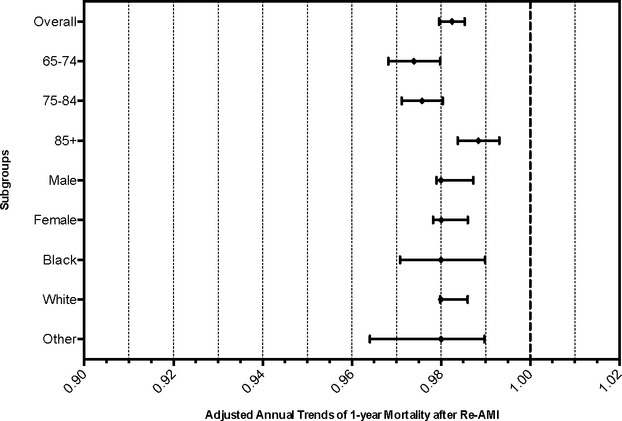
Risk‐adjusted annual trends in 1‐year mortality. Cox proportional hazards regression models were used to assess annual trends in mortality rates, adjusting for age, sex, race, and all comorbidities shown in Table. AMI indicates acute myocardial infarction.
Discussion
In a national sample of Medicare fee‐for‐service beneficiaries, we found that the incidence of recurrent AMI hospitalization declined substantially from 1999 to 2010. The overall risk‐adjusted rate of annual decline in recurrent AMI hospitalizations was 4.1%. Declines were seen in all demographic groups, but blacks had less decline in recurrent AMI hospitalizations as compared with whites. Females and those in the oldest age group (85 years and older) remained at higher risk for recurrent AMI hospitalization throughout the study period, compared with males and younger persons, respectively. When considering risk‐adjusted mortality after hospitalization for recurrent AMI, there was an annual decline of 1.8%, and females again had persistently higher mortality than males.
Our analysis extends prior work examining temporal trends in recurrent AMI hospitalization rates. A Swedish study found an adjusted decrease in average risk of 2.5% per calendar year for women and 3.1% for men for recurrent AMI from 1972 to 2001.4 A decline of 5.9% per year in the rate of recurrent AMI was observed in the Danish MONICA population from 1982 to 1991,5 and similar decreases in the rate of recurrent AMI were observed during this time period in Iceland6 and Finland.7–8 In the United States, a decline in annual rate of recurrent AMI in men (2.6% per year) and women (1.9% per year) was reported between 1987 and 1994 in 4 different United States communities in the ARIC study.18–19 Notably, these previous studies do not include contemporary data from a racially diverse population. To our knowledge, our study is the first to examine changes in the rate of hospitalization for recurrent AMI after AMI over the last decade using US‐based national data.
Although we cannot identify the specific reasons for the decline in recurrent AMI hospitalizations and 1‐year mortality rates, several theories might explain these observations. One possible explanation is that the improvements may reflect the implementation of secondary prevention as recommended by the American College of Cardiology and American Heart Association, including increasing use of aspirin, β‐blockers, angiotensin converting enzyme inhibitors, thienopyridines, and statins.20–25 A study based on the Global Registry of Acute Coronary Events, which includes data from 113 hospitals across 14 countries, reported that use of these medications increased in AMI patients from 23% in 2000 to 58% in 2005,25 findings similar to the increases reported in the Worcester, Mass study from 1995 to 2005.23 Furthermore, the use of percutaneous coronary intervention has increased substantially over the last decade, which may also have contributed to improved outcomes. A recent study based on the United States Nationwide Inpatient Sample database, an all‐payer inpatient care database from 1000 hospitals, reported that percutaneous coronary intervention for ST‐segment elevation myocardial infarction increased by 33.5% among patients aged 65 to 79 and 22% in patients >80 years of age in the United States from 2001 to 2010.26 Of note, the observed decrease in recurrent AMI hospitalizations occurred despite the increasing use of high‐sensitivity troponin assays to diagnose AMI over the study period. It is possible that the observed decrease would have been even greater if laboratory assays for AMI diagnosis had not changed.
While the overall declines in recurrent AMI hospitalization and subsequent mortality rates over the past decade are encouraging, not all groups benefitted equally from improvements in recurrent AMI hospitalizations. When compared by race, the reduction in observed, recurrent AMI hospitalization rate in whites was double that seen in blacks, with a relative decline of 27.7% versus 13.6% from 1999 to 2010 (P<0.05). Racial disparities in recurrent AMI hospitalization rates thereby actually widened over the study period. The explanation for the attenuated decline observed in black patients is likely multifactorial. A higher prevalence of cardiac risk factors (hypertension, diabetes, smoking, obesity), as well as socioeconomic factors (ie, healthcare access, insurance) leading to differences in quality of care have been linked to worse outcomes in blacks as compared to whites.27–30 As multivariable models were constructed separately for each demographic subgroup, it is possible that differences in cardiac risk factors contributed to the differences observed between blacks and whites in recurrent AMI hospitalization rates. Furthermore, some evidence suggests that nonwhite race is a risk factor for nonadherence to recommended American College of Cardiology/American Heart Association guidelines for treatment of AMI.31–34 A study of AMI patients in the 1992–2003 Medicare Current Beneficiary Survey reported that while overall combination drug therapy increased over the study period, nonwhite race was independently associated with suboptimal treatment.34 Notably, mortality rates were similar in blacks and whites, making differential censoring an unlikely explanation for the differences in recurrent AMI hospitalization.
There are several potential limitations in our study. Our data are based on the Medicare fee‐for‐service population, and trends in recurrent AMI hospitalizations may differ in younger patients or those with different health insurance. Because we relied on administrative data, we were also not able to obtain detailed clinical information about risk factor profiles (eg, cigarette smoking), mechanism of recurrent MI (eg, stent thrombosis), and cause of death. Comorbidities were ascertained from administrative codes and not clinically confirmed, and hospitals may have changed patterns of medical coding over time. Finally, we used a linear model to examine changes in recurrent AMI rates. The decline in recurrent AMI appears to diminish in the final years of the study period, and future studies are needed to determine whether there is continuing decline in recent years.
Conclusions
In a national cohort of Medicare patients, the risk‐adjusted rate of annual decline in recurrent AMI hospitalizations was 4.1% from 1999 to 2010. The risk‐adjusted rate of annual decline in mortality within 1 year after recurrent AMI hospitalization was 1.8% during this period. Improvements in recurrent AMI hospitalization rates were significantly attenuated in black patients, resulting in widened racial disparities by the end of the study period. Future work should examine strategies to ensure that gains realized in post‐AMI outcomes can be shared equally among all patients, regardless of race.
Sources of Funding
This study was supported by grant 1 U01 HL105270‐04 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute (NHBLI). In addition, Dr Chaudhry is supported by the National Institutes of Health (NIH) NHBLI grant R01HL115295 (Risk Stratification in Older Persons with Acute Myocardial Infarction: SILVER‐AMI) and a Beeson Career Development Award from the NIH/ National Institute of Aging (NIA) (K23 AG030986); Dr Chen is supported by the Agency for Healthcare and Quality Career Development Award (1K08HS018781‐01); Dr Dodson is also supported by the NIH NHBLI grant R01HL115295 (Risk Stratification in Older Persons with Acute Myocardial Infarction: SILVER‐AMI) and the NIH NIA grant R03AG045067, a T. Franklin Williams Scholarship Award (funded by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Alliance for Academic Internal Medicine‐Association of Specialty Professors, and the American College of Cardiology), and is the recipient of a Clinical Research Loan Repayment award from the NHLBI; Dr Dharmarajan is supported by a NIH T32 training grant in cardiovascular disease (2T32HL007854‐16A1) from Columbia University; and Dr Krumholz is supported by grant U01 HL105270‐02 (Center for Cardiovascular Outcomes Research at Yale University) from the NHLBI.
Disclosures
Dr Krumholz works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; he is also a recipient of research grants from Medtronic and from Johnson & Johnson, through Yale University, to develop methods of clinical data sharing. Dr Krumholz is the chair of a cardiac scientific advisory board for UnitedHealth. Dr Masoudi has contracts with the Oklahoma Foundation for Medical Quality and the American College of Cardiology Foundation. All other authors (Chaudhry, Khan, Dharmarajan, Wang, and Dodson)—none.
References
- 1.Chen J, Normand SL, Wang Y, Drye EE, Schreiner GC, Krumholz HM. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee‐for‐service beneficiaries: progress and continuing challenges. Circulation. 2010; 121:1322-1328. [DOI] [PubMed] [Google Scholar]
- 2.Buch P, Rasmussen S, Gislason GH, Rasmussen JN, Kober L, Gadsboll N, Stender S, Madsen M, Torp‐Pedersen C, Abildstrom SZ. Temporal decline in the prognostic impact of a recurrent acute myocardial infarction 1985 to 2002. Heart. 2007; 93:210-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shotan A, Gottlieb S, Goldbourt U, Boyko V, Reicher‐Reiss H, Arad M, Mandelzweig L, Hod H, Kaplinsky E, Behar SSecondary Prevention Reinfarction Israeli Nifedipine Trial Study G, Israeli Thrombolytic Survey G. Prognosis of patients with a recurrent acute myocardial infarction before and in the reperfusion era–a national study. Am Heart J. 2001; 141:478-484. [DOI] [PubMed] [Google Scholar]
- 4.Gulliksson M, Wedel H, Koster M, Svardsudd K. Hazard function and secular trends in the risk of recurrent acute myocardial infarction: 30 years of follow‐up of more than 775,000 incidents. Circ Cardiovasc Qual Outcomes. 2009; 2:178-185. [DOI] [PubMed] [Google Scholar]
- 5.Davidsen M, Bronnum‐Hansen H, Jorgensen T, Madsen M, Gerdes LU, Osler M, Schroll M. Trends in incidence, case‐fatality and recurrence of myocardial infarction in the Danish MONICA population 1982–1991. Eur J Epidemiol. 2001; 17:1139-1145. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdsson G, Sigfusson N, Gudmundsdottir II, Agnarsson U, Sigvaldason H, Gudnason V. The absolute risk of recurrent myocardial infarction is similar amongst both sexes: MONICA Iceland Study 1981–1999. Eur J Cardiovasc Prev Rehabil. 2004; 11:121-124. [DOI] [PubMed] [Google Scholar]
- 7.Salomaa V, Ketonen M, Koukkunen H, Immonen‐Raiha P, Jerkkola T, Karja‐Koskenkari P, Mahonen M, Niemela M, Kuulasmaa K, Palomaki P, Arstila M, Vuorenmaa T, Lehtonen A, Lehto S, Miettinen H, Torppa J, Tuomilehto J, Kesaniemi YA, Pyorala K. Trends in coronary events in Finland during 1983–1997. The FINAMI study. Eur Heart J. 2003; 24:311-319. [DOI] [PubMed] [Google Scholar]
- 8.Salomaa V, Miettinen H, Kuulasmaa K, Niemela M, Ketonen M, Vuorenmaa T, Lehto S, Palomaki P, Mahonen M, Immonen‐Raiha P, Arstila M, Kaarsalo E, Mustaniemi H, Torppa J, Tuomilehto J, Puska P, Pyorala K. Decline of coronary heart disease mortality in Finland during 1983 to 1992: roles of incidence, recurrence, and case‐fatality. The FINMONICA MI Register Study. Circulation. 1996; 94:3130-3137. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SR, Cannon CP, Fox KAA, Wallentin L, Boden WE, Spacek R, Widimsky P, McCullough PA, Hunt D, Braunwald E, Yusuf S. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta‐analysis of randomized trials. JAMA. 2005; 293:2908-2917. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001; 345:494-502. [DOI] [PubMed] [Google Scholar]
- 11.Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, Boulenc JM, Morice MC, Maillard L, Pansieri M, Choussat R, Pinton P. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001; 344:1895-1903. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001; 285:1711-1718. [DOI] [PubMed] [Google Scholar]
- 13.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004; 350:1495-1504. [DOI] [PubMed] [Google Scholar]
- 14.Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid‐lowering statin therapy compared with moderate lipid‐lowering statin therapy after acute coronary syndromes from the PROVE IT‐TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol. 2009; 54:2358-2362. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J. High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005; 294:2437-2445. [DOI] [PubMed] [Google Scholar]
- 16.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Long‐term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008; 118:2057-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand S‐LT. An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with an acute myocardial infarction. Circulation. 2006; 113:1683-1692. [DOI] [PubMed] [Google Scholar]
- 18.Rosamond WD, Folsom AR, Chambless LE, Wang CHCommunities AIARi. Coronary heart disease trends in four United States communities. The Atherosclerosis Risk in Communities (ARIC) study 1987–1996. Int J Epidemiol. 2001; 30suppl 1:S17-S22. [DOI] [PubMed] [Google Scholar]
- 19.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998; 339:861-867. [DOI] [PubMed] [Google Scholar]
- 20.Spencer FA, Lessard D, Yarzebski J, Gore JM, Goldberg RJ. Decade‐long changes in the use of combination evidence‐based medical therapy at discharge for patients surviving acute myocardial infarction. Am Heart J. 2005; 150:838-844. [DOI] [PubMed] [Google Scholar]
- 21.Rogers WJ, Canto JG, Lambrew CT, Tiefenbrunn AJ, Kinkaid B, Shoultz DA, Frederick PD, Every N. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: the National Registry of Myocardial Infarction 1, 2 and 3. J Am Coll Cardiol. 2000; 36:2056-2063. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RH, Montoye CK, Gallogly M, Baker P, Blount A, Faul J, Roychoudhury C, Borzak S, Fox S, Franklin M, Freundl M, Kline‐Rogers E, LaLonde T, Orza M, Parrish R, Satwicz M, Smith MJ, Sobotka P, Winston S, Riba AA, Eagle KACardiology GAPSCotACo. Improving quality of care for acute myocardial infarction: the Guidelines Applied in Practice (GAP) Initiative. JAMA. 2002; 287:1269-1276. [DOI] [PubMed] [Google Scholar]
- 23.Fornasini M, Yarzebski J, Chiriboga D, Lessard D, Spencer FA, Aurigemma P, Gore JM, Goldberg RJ. Contemporary trends in evidence‐based treatment for acute myocardial infarction. Am J Med. 2010; 123:166-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burwen DR, Galusha DH, Lewis JM, Bedinger MR, Radford MJ, Krumholz HM, Foody JM. National and state trends in quality of care for acute myocardial infarction between 1994–1995 and 1998–1999: the Medicare health care quality improvement program. Arch Intern Med. 2003; 163:1430-1439. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg RJ, Spencer FA, Steg PG, Flather M, Montalescot G, Gurfinkel EP, Kennelly BM, Goodman SG, Dedrick R, Gore JMGlobal Registry of Acute Coronary Events I. Increasing use of single and combination medical therapy in patients hospitalized for acute myocardial infarction in the 21st century: a multinational perspective. Arch Intern Med. 2007; 167:1766-1773. [DOI] [PubMed] [Google Scholar]
- 26.Khera S, Kolte D, Palaniswamy C, Mujib M, Aronow WS, Singh T, Gotsis W, Silverman G, Frishman WH. ST‐elevation myocardial infarction in the elderly–temporal trends in incidence, utilization of percutaneous coronary intervention and outcomes in the United States. Int J Cardiol. 2013; 168:3683-3690. [DOI] [PubMed] [Google Scholar]
- 27.Mehta RH, Marks D, Califf RM, Sohn S, Pieper KS, Van de Werf F, Peterson ED, Ohman EM, White HD, Topol EJ, Granger CB. Differences in the clinical features and outcomes in African Americans and whites with myocardial infarction. Am J Med. 2006; 119:70.e1-70.e8. [DOI] [PubMed] [Google Scholar]
- 28.Taylor HA, Chaitman BR, Rogers WJ, Kern MJ, Terrin ML, Aguirre FV, Sopko G, McMahon R, Ross RN, Bovill EC. Race and prognosis after myocardial infarction: results of the thrombolysis in myocardial infarction (TIMI) phase II trial. Circulation. 1993; 88:1484-1494. [DOI] [PubMed] [Google Scholar]
- 29.Cooper RS, Simmons B, Castaner A, Prasad R, Franklin C, Ferlinz J. Survival rates and prehospital delay during myocardial infarction among black persons. Am J Cardiol. 1986; 57:208-211. [DOI] [PubMed] [Google Scholar]
- 30.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA. 1994; 271:1175-1180. [PubMed] [Google Scholar]
- 31.Tang L, Patao C, Chuang J, Wong ND. Cardiovascular risk factor control and adherence to recommended lifestyle and medical therapies in persons with coronary heart disease (from the National Health and Nutrition Examination Survey 2007–2010). Am J Cardiol. 2013; 112:1126-1132. [DOI] [PubMed] [Google Scholar]
- 32.Spencer F, Scleparis G, Goldberg RJ, Yarzebski J, Lessard D, Gore JM. Decade‐long trends (1986 to 1997) in the medical treatment of patients with acute myocardial infarction: a community‐wide perspective. Am Heart J. 2001; 142:594-603. [DOI] [PubMed] [Google Scholar]
- 33.Lee HY, Cooke CE, Robertson TA. Use of secondary prevention drug therapy in patients with acute coronary syndrome after hospital discharge. J Manag Care Pharm. 2008; 14:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjia J, Briesacher B, Xie D, Fu J, Goldberg RJ. Disparities in combination drug therapy use in older adults with coronary heart disease: a cross‐sectional time‐series in a nationally representative US sample. Drugs Aging. 2010; 27:149-158. [DOI] [PMC free article] [PubMed] [Google Scholar]


