Abstract
Background
Galectin‐3 is a biomarker for prognostication and risk stratification of patients with heart failure (HF). It has been suggested that renal function strongly relates to galectin‐3 levels. We aimed to describe galectin‐3 renal handling in HF.
Methods and Results
In Sprague–Dawley rats, we infused galectin‐3 and studied distribution and renal clearance. Furthermore, galectin‐3 was measured in urine and plasma of healthy controls, HF patients and hemodialysis patients. To mimic the human situation, we measured galectin‐3 before and after the artificial kidney. Infusion in rats resulted in a clear increase in plasma and urine galectin‐3. Plasma galectin‐3 in HF patients (n=101; mean age 64 years; 93% male) was significantly higher compared to control subjects (n=20; mean age 58 years; 75% male) (16.6 ng/mL versus 9.7 ng/mL, P<0.001), while urinary galectin‐3 in HF patients was comparable (28.1 ng/mL versus 35.1 ng/mL, P=0.830). The calculated galectin‐3 excretion rate was lower in HF patient (2.3 mL/min [1.5 to 3.4] versus 3.9 mL/min [2.3 to 6.4] in control subjects; P=0.005). This corresponded with a significantly lower fractional excretion of galectin‐3 in HF patients (2.4% [1.7 to 3.7] versus 3.0% [1.9 to 5.5]; P=0.018). These differences, however, were no longer significant after correction for age, gender, diabetes, and smoking. HF patients who received diuretics (49%) showed significantly higher aldosterone and galectin‐3 levels. Hemodialysis patients (n=105; mean age 63 years; 65% male), without urinary galectin‐3 excretion, had strongly increased median plasma galectin‐3 levels (70.6 ng/mL).
Conclusions
In this small cross‐sectional study, we report that urine levels of galectin‐3 are not increased in HF patients, despite substantially increased plasma galectin‐3 levels. The impaired renal handling of galectin‐3 in patients with HF may explain the described relation between renal function and galectin‐3 and may account for the elevated plasma galectin‐3 in HF.
Keywords: heart failure, kidney, remodeling
Introduction
Galectin‐3 is a galactosidase‐binding lectin that is able to bind complex carbohydrates.1–3 In the heart, galectin‐3 is secreted by activated macrophages and binds several matricellular proteins such as laminin, fibronectin, and collagens. When ligand‐bound, galectin‐3 forms interstitial conjugates, which are thought to contribute to myocardial stiffness and cardiac dysfunction.4–5 Galectin‐3 is an emerging biomarker for prognostication and risk stratification of patients with acute and chronic heart failure (HF).4,6–7 The most recent American College of Cardiology/American Heart Association guidelines provided a level IIB recommendation for the use of galectin‐3 in risk stratification.8 In experimental models of HF, myocardial transcription and translation of galectin‐3 has been reported,9–10 and it is assumed that increased circulating levels of galectin‐3 in HF may be explained by excess cardiac production. In human subjects, galectin‐3 can be measured in plasma using an established ELISA.11 Circulating levels of galectin‐3 are in the 10 to 13 ng/mL range in the general population,11–13 but are substantially increased in HF,14–17 rising to 15 to 30 ng/mL, depending on the severity of HF and the presence of comorbidities, specifically kidney disease, as galectin‐3 levels in HF have a strong relation with renal function.16,18 Both in the general population12 and in patients with HF,16,18–19 decreased renal function is among the strongest correlates of increased circulating galectin‐3. It has also been observed that galectin‐3 levels precede the development of chronic kidney disease.20 Thus, renal function is an important factor to take into account in the interpretation of a given value of galectin‐3. Renal handling of galectin‐3 may be altered in patients with HF, a phenomenon that has been described for other biomarkers such as N‐terminal‐pro brain natriuretic peptide (NT‐proBNP) and neutrophil gelatinase‐associated lipocalin.21–22 We hypothesized that renal handling of galectin‐3 would be different in HF compared to healthy subjects. We studied this in a rat model, and by measuring plasma and urinary galectin‐3 in a well‐characterized cohort of 101 chronic HF patients, comparing them to 20 control subjects and 105 hemodialysis (HD) patients.
Methods
Experimental Studies of Galectin‐3 Clearance
Recombinant galectin‐3 production
Human recombinant galectin‐3 was produced by Escherichia coli (BL21[DE3] RIL) containing a specific plasmid (pet28b) encoding human full‐length galectin‐3. Recombinant galectin‐3 was collected and purified via binding to α‐lactose‐agarose beads (Sigma‐Aldrich). The purity of the obtained protein was studied by SDS‐PAGE followed by Coomassie blue staining and Western blot to exclude any contamination or protein degradation. We removed endotoxins via a column method (ActiClean Etox; Sterogene bioseparations, USA); in our experiments, the endotoxin levels were kept below 10 EU/mg. Quality control–endotoxin contamination was routinely checked by LAL assay (Lonza).
Animal studies
Animals were housed under standard conditions. All animal studies were approved by the Animal Ethical Committee of the University of Groningen, The Netherlands, and conducted in accordance with existing European Commission guidelines for the care and use of laboratory animals (reference number 6474).
First, we conducted an acute experiment, where we intravenously injected either a bolus of human recombinant galectin‐3 or saline in Sprague–Dawley rats (n=3/group). After infusion, we sampled blood at different time points over a period of 24 hours. Blood samples were centrifuged for 10 minutes at 2000g within 1 hour after collection, and plasma was obtained to measure levels of galectin‐3. The results were used to calculate the clearance and the volume of distribution of galectin‐3 in rats by Iterative Bayesian 2‐Stage analysis using the program MultiFit (written by J.H. Proost).23 These calculations were performed to estimate the continuous infusion rate of galectin‐3 resulting in a steady‐state plasma level of 25 ng/mL during long‐term infusion. Lastly, we conducted a long‐term experiment of galectin‐3 infusion to assess the effects of chronic galectin‐3 clearance in healthy animals. An osmotic minipump (Alzet, Cupertino CA, type 2ML4) filled with either recombinant galectin‐3 or saline was implanted intraperitoneally in Sprague–Dawley rats. After 4 days, we sampled blood and started to collect 24‐hour urine. The plasma and urine galectin‐3 concentrations were measured on both samples (control n=3; galectin‐3 infusion n=3). Inhalation of isoflurane 2% was used for all of the anesthesia throughout the study.
Patient Populations
We analyzed baseline plasma and urine samples from 3 cohorts: (1) 101 stable chronic HF patients; (2) 105 stable patients with end‐stage renal disease who were on HD; and (3) 20 control subjects. The HF patient cohort has been described in detail previously (Clinical trial identifier NC T01092130).24 In brief, 101 HF patients ≥18 years of age and a left ventricular ejection fraction <45% were included, who received optimal HF medication (including angiotensin‐converting enzyme inhibitor [ACEi] or angiotensin receptor blocker, β‐blocker, and mineralocorticoid‐receptor antagonist, when indicated), and randomized to vitamin D (2000 IU daily) or control for 6 weeks. The HD patient cohort has also been described in detail elsewhere.25 These patients received HD for at least 3 months on a 3‐time‐per‐week schedule and had a left ventricular ejection fraction of 50% (±10). Additionally, 20 healthy subjects were randomly selected from a large population cohort12 and served as controls. Healthy was defined as the absence of the self‐reported cardiovascular disease or cancer, and currently not being prescribed medication.
These studies and the current analyses have been performed to conform with the Declaration of Helsinki; all 3 study protocols were reviewed and approved by the local Institutional Review Board, and all study subjects provided written informed consent.
Biochemical Measurements
Hematology, chemistry, and urinalysis were performed by routine clinical chemistry on the day of the visit. Blood of the HD patients was drawn prior to hemodialysis after a period of 2 days prior to HD treatment. Plasma and urine galectin‐3 levels were determined by an ELISA developed by BG Medicine (Galectin‐3 assay™, BG Medicine, Inc., Waltham, USA). This assay is US Food and Drug Administration approved, and has a high sensitivity (lower limit of detection 1.13 ng/mL) and has no cross‐reactivity with collagens or other members of the galectin family. Calibration of the assay was performed according to the manufacturer's recommendation and values were normalized to a standard curve.11 Commonly used medication such as ACEi, β‐blockers, aldosterone antagonist, diuretics, acetylsalicylic acid, warfarin, coumarines, and digoxin have no interference with the assay. Two standard controls were included in all plasma runs: a lower control (expected value 13.0 to 23.1 ng/mL) and an upper control (expected value 48.9 to 81.5 ng/mL). The average lower control values were 21.9±0.65 ng/mL, and the average upper control values were 71.3±1.33 ng/mL. We observed in our plasma control samples an intravariability in coefficient of variation of 6.3% and an intervariability in coefficient of variation of 2.4%. Plasma aldosterone concentration was measured using an ELISA kit (Alpco, Salem, NH) and the results are reported in pg/mL. The lower detection limit was 10 pg/mL. Intra‐assay precision has been reported as 6.6% and interassay precision has been reported as 9.6%.26
Renal Galectin‐3 Parameters
Twenty‐four‐hour urine was collected from all HF patients and control subjects. We calculated creatinine clearance (expressed as mL/min) using the formula: ((Ucreat×Vurine)/Pcreat), where Ucreat stands for urine creatinine concentration, Vurine for 24‐hour urinary volume, and Pcreat for plasma creatinine concentration. This value is used as the glomerular filtration rate (GFR). We also calculated estimated GFR with the simplified modification of diet in renal disease formula and the chronic kidney disease‐epidemiology formula,27 to assess whether the use of different formulae would alter the study results. Creatinine clearance of HD patients was by definition 0 mL/min, because these patients were anuric. We expressed urine galectin‐3 concentrations as per gram urinary creatinine to account for differences in concentration due to urine dilution or concentration.
Various calculation methods to assess different stages in the filtration process of the kidney were used. These methods have previously been used by our department regarding NT‐proBNP.21 The theoretical amount of freely filtered galectin‐3 by the glomerulus to the tubules was calculated by: GFR×Pgalectin‐3 (=filtered load, expressed as μg/24 hours). The actual urinary galectin‐3 excretion was calculated as: Ugalectin‐3×Vurine (expressed as μg/24 hours). The renal clearance of galectin‐3 was calculated as: ((Ugalectin‐3×Vurine)/Pgalectin‐3 (expressed as mL/min). To examine the proportion of the filtered load that was excreted, we calculated the fractional galectin‐3 clearance: (renal clearance of galectin‐3/GFR)×100%.
Since it has been reported that aldosterone is closely linked with galectin‐3,28 and aldosterone antagonists play a central role in treatment of HF,29 we evaluated whether plasma aldosterone modulates the renal handling of galectin‐3 in HF.
Galectin‐3 Levels Before and After the Dialyzer
To provide further clarification of the percentage of galectin‐3 filtered by the kidney, we conducted an experiment using bicarbonate hemodialysis with a low‐flux polysulfone hollow‐fiber dialyzer (F8; Fresenius Medical Care) during a standard HD session after a 2‐day break of HD treatment. Two different samples were taken from 16 HD patients after 30 minutes of hemodialysis: (1) unfiltered blood flowing towards the artificial kidney; and (2) filtered blood immediately after the artificial kidney, in which galectin‐3 and creatinine concentrations were measured.
Statistical Analyses
Continuous variables with a normal distribution are expressed as means±SEM. Nominal variables are expressed as n (%).Variables that are not normally distributed are expressed as medians with interquartile ranges. Galectin‐3 levels were correlated with other variables using Spearman's rank correlation coefficient. Data with a normal distribution were compared with Student t tests, while data with skewed distribution were compared by means of the Mann–Whitney U tests, and categorical clinical variables were compared with the Fisher exact test. One‐way ANOVA was performed to analyze differences for multiple‐group comparisons, followed by Bonferroni post hoc analysis. Analysis on repeated measurements was performed using the Student paired 2‐tailed t test. All reported P values are 2‐tailed, and values of P<0.05 were considered statistically significant. Analyses were performed with Statistical Package for Social Sciences software (SPSS version 20.0.0.1 for Windows, SPPS Inc, Chicago, IL). The authors had full access to and take full responsibility for the integrity of the data.
Results
Galectin‐3 Clearance Experiment
We first ascertained that the US Food and Drug Administration–approved ELISA detected human recombinant galectin‐3, whereas it should not detect endogenous rat galectin‐3, thus allowing reliable detection of the infused recombinant human galectin‐3 in rats. The course of plasma galectin‐3 concentration in rats over a period of 24 hours after an intravenous bolus infusion of human recombinant galectin‐3 is displayed in Figure 1A. We did not detect any human recombinant galectin‐3 in the plasma of saline‐infused rats. On the basis of these results, we determined that galectin‐3 plasma clearance is 0.92 mL/min with a volume of distribution of 90 mL. We then conducted an experiment in which we infused human recombinant galectin‐3 continuously for the duration of 4 days. After 4 days, steady‐state galectin‐3 levels were 23.1 (±4.9) ng/mL. The mean concentration of galectin‐3 measured in 24‐hour urine was 27.2 (±8.1) ng/mL (Figure 1B). From these experiments, we conclude that galectin‐3 can be cleared by the kidney, albeit incompletely. We controlled for purity with Coomassie blue staining and Western blot staining regarding the infused recombinant human galectin‐3 (Figure 1C).
Figure 1.
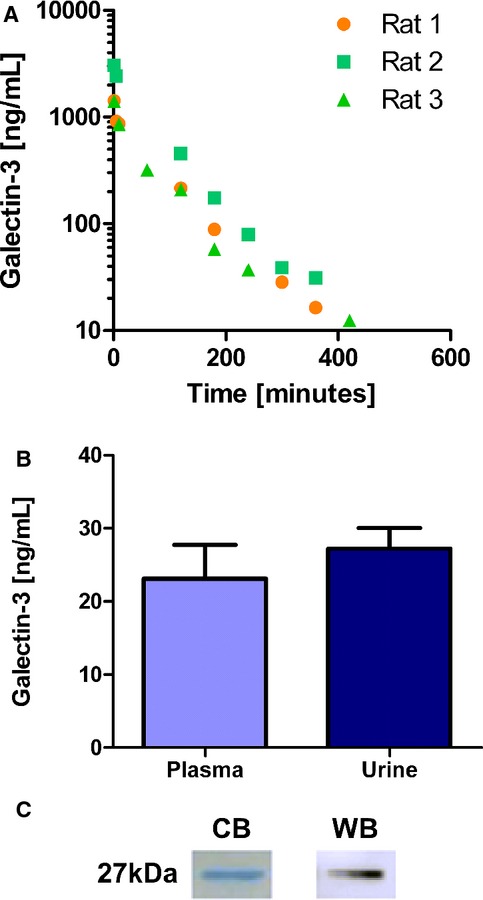
Experimental data. A, Displays the galectin‐3 concentration over time after a single bolus IV injection (3 rats/group; saline‐infused rats are not displayed because they did not show any recombinant‐human‐galectin‐3). B, Displays the plasma and urine galectin‐3 level of rats having received a continuous infusion of galectin‐3 via an osmotic minipump (n=3); not significant. C, Staining of recombinant human galectin‐3. CB indicates Coomassie blue staining; WB, Western blot.
Patient Populations
Baseline characteristics of the HF patients, HD patients, and control subjects are presented in Table 1. The healthy controls differed substantially and significantly from HF and HD patients, with respect to coefficient of variation risk factors such as smoking and diabetes, the use of medication, and biochemical variables. Overall, the mean age of all study participants was between 55 and 75 years and 60% were male. The HF patients were mostly categorized in New York Heart Association class II (90%) and had a mean left ventricular ejection fraction of 35% (±8). All HF patients received background therapy according to the current European Society of Cardiology guidelines: ACE‐i/angiotensin receptor blocker: n=101 (100%), β–blockers: n=98 (97%), mineralocorticoid‐receptor antagonists: n=29 (29%), and diuretics (mostly furosemide): n=49 (49%). The HD patients received ACEi/angiotensin receptor blocker (n=10, 9.5%) and β‐blockers (n=60, 57%). By protocol, the control subjects received no medication. GFR was significantly lower in HF patients compared to controls (96 versus 124 mL/min; P=0.002).
Table 1.
Baseline Characteristics of the Study Patients
| Variables | HF Patients (n=101) | Hemodialysis Patients (n=105) | Control Subjects (n=20) | P Value |
|---|---|---|---|---|
| Age, y (mean, SD) | 64±10 | 63±16 | 58±4 | 0.17 |
| Male, n (%) | 93 (93) | 68 (65) | 15 (75) | 0.039 |
| NYHA class, II/III | 89/11 | NA | NA | N/A |
| Diabetes mellitus, n (%) | 14 (14) | 23 (22) | 0 | <0.001 |
| Current smoking, n (%) | 22 (22) | NA | 0 | <0.001 |
| LVEF, % (mean, SD) | 35±8 | 50±10 | NA | <0.001 |
| Systolic blood pressure, mm Hg (mean, SD) | 118±18 | 141±25 | 119±11 | <0.001 |
| Diastolic blood pressure, mm Hg (mean, SD) | 72±12 | 81±18 | 71±8 | <0.001 |
| Serum creatinine, μmol/L (mean, SD) | 90±18 | NA | 78±13 | 0.005 |
| Creatinine clearance, mL/min (mean, SD) | 96±16 | 0 | 124±11 | <0.001 |
| Plasma NGAL, ng/mL (mean, SD) | 93±74 | NA | 43±40 | 0.004 |
| Hematocrit, % (mean, SD) | 43±4 | 35±4 | 41±4 | <0.001 |
| Medication | ||||
| ACEi/ARB, n (% use) | 101 (100) | 10 (10) | 0 | <0.001 |
| β‐Blocker, n (% use) | 98 (97) | 60 (57) | 0 | <0.001 |
| Loop diuretic, n (% use) | 49 (49) | 0 | 0 | <0.001 |
| Mineralocorticoid receptor antagonist, n (% use) | 29 (29) | NA | 0 | <0.001 |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HF, heart failure; LVEF, left ventricular ejection fraction; n, number of subjects; NGAL, neutrophil gelatinase‐associated lipocalin; NA, not available; N/A, not applicable; NYHA, New York Heart Association.
Renal Handling
Plasma galectin‐3 in HF patients was significantly higher compared to control subjects (16.6 ng/mL [14.5 to 19.3] versus 9.7 ng/mL [8.9 to 12.4], respectively, P<0.001). In contrast, the median urine galectin‐3 levels in HF patients were similar to controls (28.1 ng/mL [19.7 to 49.5] in controls versus 35.1 ng/mL [21.2 to 50.3] in HF, P=0.830). After correction for urinary creatinine, the difference in urine galectin‐3 remained nonsignificant (P=0.983, Figure 2). As a consequence, the calculated renal galectin‐3 clearance was higher in control subjects compared to HF patients (3.9 mL/min [2.3 to 6.4] versus 2.3 mL/min [1.5 to 3.4], P=0.005). We observed that creatinine clearance was inversely correlated with plasma galectin‐3 levels in all subjects combined (r=−0.315, P=0.001). Fractional galectin‐3 clearance was significantly lower in HF patients compared to the control subjects (2.4% [1.7 to 3.7] versus 3.0% [1.9 to 5.5], P=0.018) (Figure 3A and 3B) (Table 2). The same results were observed when calculations were made with the simplified modification of diet in renal disease and the chronic kidney disease‐epidemiology formulas (not shown).
Figure 2.
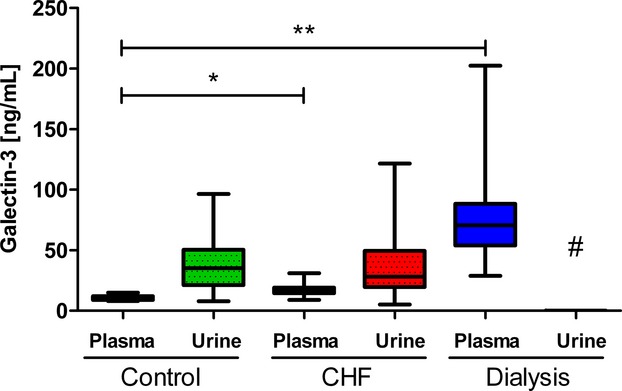
Plasma and urine galectin‐3 concentrations in 3 different cohorts. Overview of the plasma and urine galectin‐3 concentrations in the 3 cohorts (control [n=20], CHF [n=101], and hemodialysis [n=105]). Data are displayed as medians with IQR. Statistical test: Mann–Whitney U test. *P<0.05 control galectin‐3 plasma compared to CHF galectin‐3 plasma. **P<0.05 control galectin‐3 plasma compared to dialysis galectin‐3 plasma. #Hemodialysis patients were not obtained so no values could be measured. CHF indicates chronic heart failure; IQR, interquartile ranges.
Figure 3.
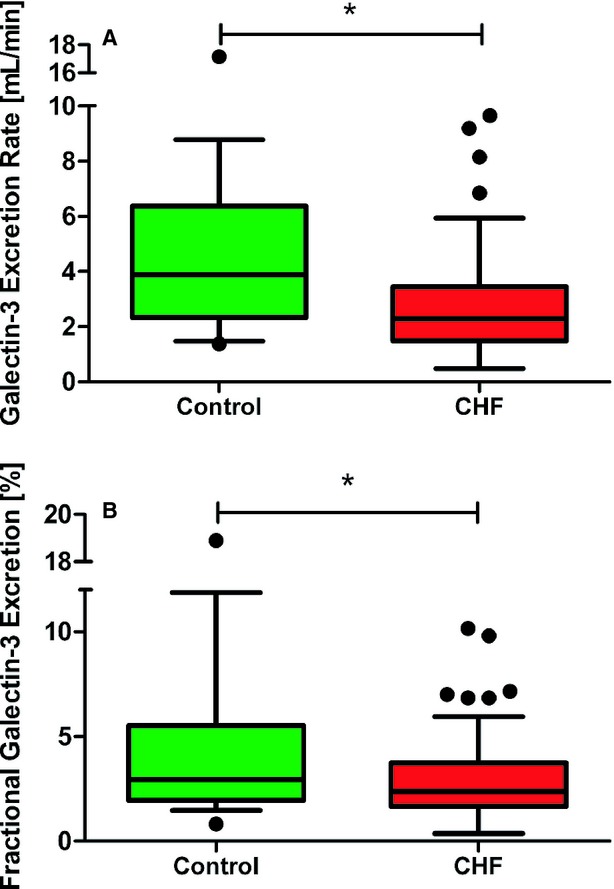
Renal handling of galectin‐3. A, Shows the galectin‐3 excretion rate difference between control subjects and heart failure patients. B, Displays the difference in the proportion of actually excreted galectin‐3 in percentage between control subjects and heart failure patients. *P<0.05 control subjects compared to heart failure patients (n=20 vs n=101). Data are displayed as medians with IQR. Statistical test: Mann–Whitney U test. CHF indicates chronic heart failure; IQR, interquartile ranges.
Table 2.
Parameters of Plasma and Urine Galectin‐3
| Variables | HF Patients (n=101) | Control Subjects (n=20) | P Value |
|---|---|---|---|
| Plasma galectin‐3, ng/mL | 16.6 (14.5 to 19.3) | 9.7 (8.9 to 12.4) | <0.001 |
| Urinary galectin‐3, ng/mL | 28.1 (19.7 to 49.5) | 35.1 (21.2 to 50.3) | 0.830 |
| Urinary galectin‐3, μg/g Cr | 38.3 (27.9 to 65.5) | 29.7 (19.3 to 79.1) | 0.983 |
| Urinary galectin‐3, μg/24 h | 51.4 (34.3 to 78.1) | 47.5 (31.6 to 109.0) | 0.958 |
| Theoretical filtered load, μg/24 h | 1543 (1228 to 1909) | 1297 (839 to 1580) | 0.016 |
| Galectin‐3 clearance, mL/min | 2.3 (1.5 to 3.4) | 3.9 (2.3 to 6.4) | 0.005 |
| Fractional galectin‐3 excretion, % | 2.4 (1.7 to 3.7) | 3.0 (1.9 to 5.5) | 0.018 |
Median values with (25th to 75th percentile). Cr indicates urinary creatinine.
To address the issue of potential confounding, we conducted further analyses. We analyzed the differences between HF patients and controls by stepwise correcting for age, diabetes, smoking status, and gender. We observed that correction for these individual factors appears to attenuate the numerical and statistical differences between HF patients and controls; however, in all subanalyses we always observed that in HF patients, plasma galectin‐3 is elevated, while urinary galectin‐3 is not, resulting in decreased galectin‐3 clearance (Tables S1 through S7). A final analysis in which we corrected for age, gender, diabetes, and smoking resulted in a comparison between 26 HF patients versus 15 healthy controls, shown in Table 3. In this subset of HF patients and controls, plasma galectin‐3 remained elevated in HF patients, and urinary galectin‐3 was comparable between HF patients and controls, while the difference in galectin‐3 clearance and fractional galectin‐3 excretion was no longer significant between HF patients and controls.
Table 3.
Parameters of Plasma and Urine Galectin‐3 After Correction for Potential Confounders
| Variables | HF Patients (n=26) | Control Subjects (n=15) | P Value |
|---|---|---|---|
| Age, y (mean, SD) | 58±4 | 57±4 | 0.462 |
| Gender male, n (%) | 26 (100) | 15 (100) | N/A |
| Diabetes, n (%) | 0 (0) | 0 (0) | N/A |
| Smoking, n (%) | 0 (0) | 0 (0) | N/A |
| Plasma galectin‐3, ng/mL | 15.1 (13.6 to 18.0) | 9.7 (8.7 to 12.1) | <0.001 |
| Urinary galectin‐3, ng/mL | 28.0 (21.5 to 44.6) | 39.7 (22.5 to 52.3) | 0.376 |
| Urinary galectin‐3, μg/g Cr | 34.4 (25.9 to 53.9) | 53.3 (23.6 to 81.6) | 0.347 |
| Urinary galectin‐3, μg/24 h | 53.0 (31.7 to 90.5) | 65.6 (37.4 to 115.9) | 0.392 |
| Theoretical filtered load, μg/24 h | 1609 (1325 to 1915) | 1164 (750 to 1529) | 0.001 |
| Galectin‐3 clearance, mL/min | 2.4 (1.4 to 3.9) | 2.8 (2.0 to 5.7) | 0.142 |
| Fractional galectin‐3 excretion, % | 2.2 (1.6 to 3.2) | 2.6 (1.0 to 4.8) | 0.900 |
Median values with (25th to 75th percentile). Cr indicates urinary creatinine; HF, heart failure; N/A, not applicable.
All biochemical analyses were repeated 6 weeks after baseline assessment to confirm a steady state of galectin‐3 handling, and we further examined whether renal clearance could be based upon stratification of GFR. These results are presented in Figures 4 and 5. We observed a moderate correlation between plasma aldosterone and plasma galectin‐3 levels in HF patients (r=0.360, P<0.001). Furthermore, increased plasma aldosterone showed a significant association with lower urinary galectin‐3 excretion (P=0.028; r=−0.311). Additionally, HF patients who received diuretics (49%) had significantly higher plasma aldosterone levels and plasma galectin‐3 levels; 44% of the patients on diuretics showed both aldosterone and galectin‐3 levels above median (Figure 6).
Figure 4.
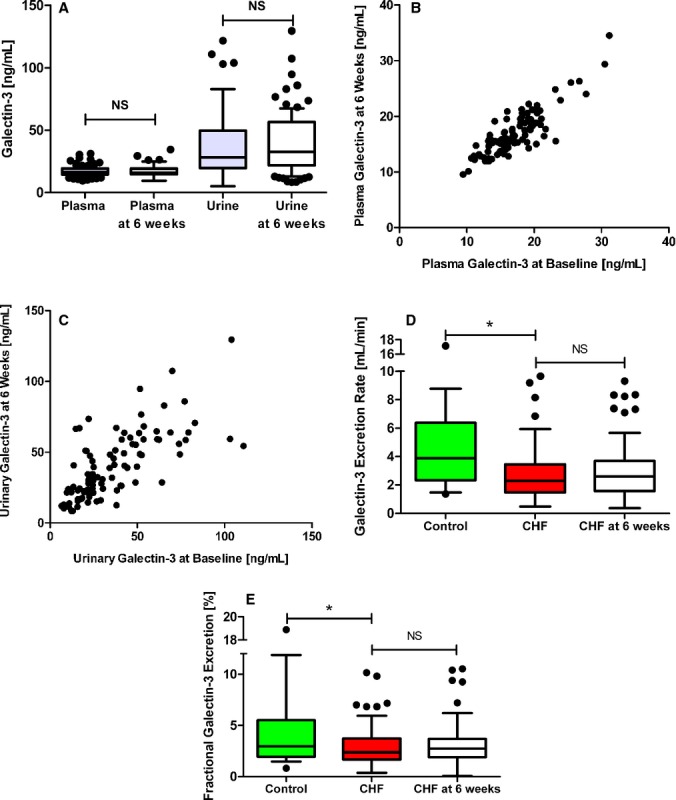
Repeated analysis in samples from the same patients 6 weeks after baseline assessment. A, Plasma and urinary galectin‐3 at baseline and at 6 weeks. B, Scatterplot of plasma galectin‐3 levels at baseline and at 6 weeks. C, Scatterplot of urinary galectin‐3 levels at baseline and at 6 weeks. D, Galectin‐3 excretion rate in controls and chronic heart failure patients at baseline and 6 weeks. E, Fractional galectin‐3 excretion in controls and chronic heart failure patients at baseline and 6 weeks. *P<0.05. NS, non‐significant.
Figure 5.
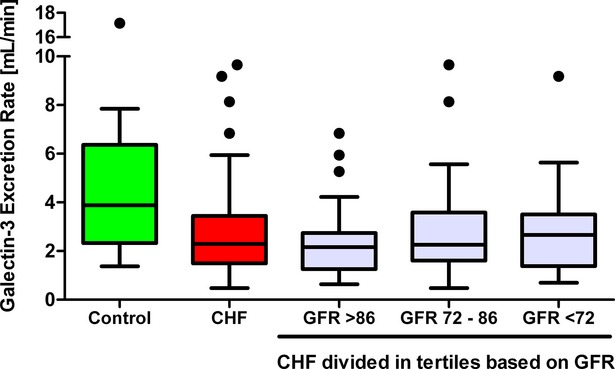
Galectin‐3 excretion rate in control and CHF patients stratified by GFR. Excretion rate of galectin‐3 in control subjects and CHF patients. Stratification of the CHF patients was based upon GFR. CHR indicates chronic heart failure; GFR, glomerular filtration rate.
Figure 6.
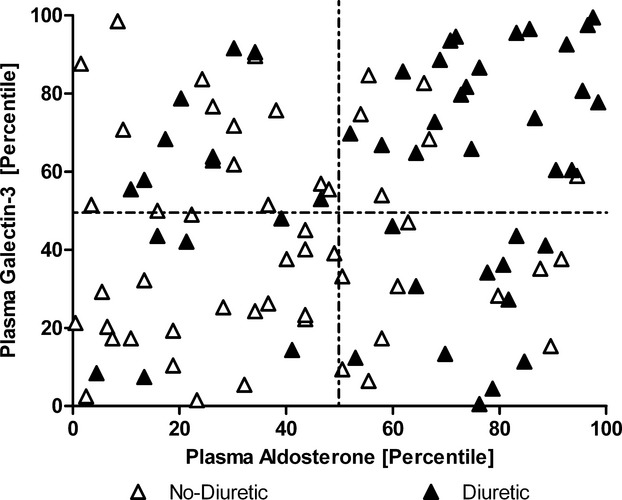
Distribution of plasma aldosterone and galectin‐3 and further stratified by diuretic and nondiuretic users. Displays the distribution of the heart failure patients based upon plasma galectin‐3 level (percentile) and plasma aldosterone level (percentile). The cohort is divided based upon the use of diuretics. As shown in the upper right quadrant, 45% of the patients on diuretics are present and only 8% of the patients without diuretics.
In HD patients, who by definition have no galectin‐3 clearance via the urine, we observed elevated plasma galectin‐3 levels before HD (median 70.6 ng/mL [54.0 to 88.3]) compared to plasma galectin‐3 levels of HF patients and healthy subjects (16.6 and 9.7 ng/mL, respectively).
Galectin‐3 Filtration in HD Patients
To study whether galectin‐3 could potentially be filtered by human glomeruli, we measured galectin‐3 before and after a dialyzer. The median galectin‐3 concentration of blood before the artificial kidney was 49.7 ng/mL [33.2 to 60.4], while after the dialyzer this was 34.3 [27.6 to 47.3] (−26%); P<0.001. To normalize our results, and obtain a reference, we also measured creatinine in samples before (719±263 μmol/L) and after the dialyzer (160±68 μmol/L (−449%). We conclude that galectin‐3 can be filtered by the dialyzer, albeit at a lesser rate than creatinine (Figure 7).
Figure 7.
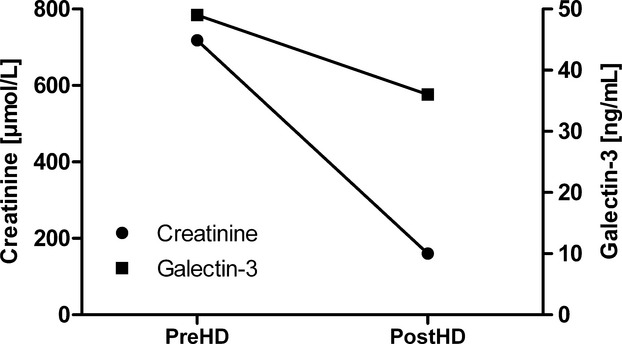
Galectin‐3 and creatinine levels before and after the dialyzer. Displayed are the results of the values of galectin‐3 and creatinine before and after the dialyzer. PostHD indicates after the artificial kidney; PreHD, prior to the artificial kidney.
Discussion
This study is the first to describe that galectin‐3 can be measured in the urine of HF patients, and provides novel insights in renal handling of galectin‐3 in healthy and diseased human subjects. Renal handling of galectin‐3 appears to be altered in HF, as plasma galectin‐3 is clearly elevated, while urinary levels are comparable to control subjects. As a consequence, we demonstrate that fractional galectin‐3 clearance is reduced in HF. HD patients, with no residual clearance, showed strongly elevated plasma galectin‐3 levels. Although we clearly have to consider that differences between the HF and HD patients and the controls may have confounded our findings, these data shed new light on the reported relation between renal function and plasma galectin‐3, and the altered renal handling of galectin‐3 could explain, at least in part, why galectin‐3 accumulates in HF patients with concomitant renal disease.
Galectin‐3 is a relatively new biomarker that may be used for risk stratification in HF. However, several studies reported that the predictive value of galectin‐3 might be mitigated when renal function is taken into account. Gopal et al. analyzed the relation between estimated GFR and galectin‐3 in control subjects and HF patients, both with preserved and reduced ejection fraction, and showed that in HF patients, levels of galectin‐3 have a strong correlation with renal function.18 However, these studies were descriptive only, and it remains unclear whether galectin‐3 elevations are a consequence of renal function decline, or that rather the elevated levels of galectin‐3 themselves lead to further loss of kidney function (and dysfunction of other organs, such as the heart). The latter hypothesis is supported by observations in the general population. de Boer et al. reported that plasma galectin‐3 bears a close relation with several parameters of renal function, such as serum creatinine, estimated GFR, and cystatin‐C.12 In the Framingham Heart Study, galectin‐3 was shown to predict new‐onset HF, and a recent article from the same group reported that elevated plasma galectin‐3 preceded the development of renal disease.20 From this, it has been suggested that galectin‐3 is not merely a marker of disease, but rather may contribute to the development and progression of heart and renal disease.4,12–13,20 Experimental studies provided evidence that high levels of galectin‐3 have detrimental effects on the kidneys (fibrosis and inflammation),30 and this also applies to the heart (fibrosis, cardiac dysfunction),10 the liver (fibrosis),31 and the lungs (fibrosis).32 Therefore, it is important to understand how galectin‐3 is regulated and handled, because sustained elevations may not just be a marker for outcome, but may perpetuate disease progression.
Renal dysfunction is very common in HF, and this is accompanied by renal structural and functional changes that have proven impact on the HF progression and prognosis.33 Our data suggest that renal function also affects galectin‐3 handling in patients with HF. We speculate that reduced renal clearance of galectin‐3 may lead to accumulation of plasma galectin‐3 levels. The precise details of renal handling are difficult to study in human subjects. Due to the molecular size of the galectin‐3 molecule—around 27 kDa—it is questionable whether galectin‐3 is easily filtered by the glomeruli, but this could be facilitated by accurate folding of the protein before filtration. We have conducted several experiments to study this. First, we infused human recombinant galectin‐3 in rats, and were able, once a steady state developed after several days, to measure galectin‐3 release in the urine. Second, we measured galectin‐3 in samples before and after HD, assuming that the dialyzer has specifications with regard to galectin‐3 that resemble the physiological situation. We show that galectin‐3 seemed to be filtered, however, far less than creatinine. However, given the relatively high circulating levels—galectin‐3 is about a 1000‐fold more abundant than BNP—we would have expected higher urinary levels of galectin‐3 if the protein would had been freely filtered. We hypothesize that other mechanisms must play a role. It may be that circulating galectin‐3 is bound to polysaccharides, which augments the size of the complexes and thus further decreases free filtering. However, ancillary renal mechanisms likely play a role also (for instance, reabsorption, tubular degradation, or active tubular handling). We could speculate that renal injury occurring in HF may attenuate adequate tubular reabsorption. However, calculated from the theoretical filtered load, we would have expected more galectin‐3 to be present in urine. To further elucidate this, further detailed mechanistic studies will be required.
In general, urinary excretion and renal handling of commonly used biomarkers in HF is an important topic of interest. For instance, plasma and urinary NT‐proBNP have been measured before in control subjects, and a chronic HF cohort and a decreased urinary concentration of NT‐proBNP was reported in chronic HF patients. The reduced urinary NT‐proBNP excretion was not related to concomitant impairment of GFR, but was associated with impaired renal perfusion. It was speculated that the reduced excretion could be related to altered tubular handling.21
To provide further insights of correlates of urinary excretion of galectin‐3 in HF, we studied and identified an interesting observation between galectin‐3, plasma aldosterone, and the use of diuretics. A close relationship between aldosterone and galectin‐3 has been suggested in experimental studies.28 Chronic use of diuretics is accompanied by reciprocal activation of the renin‐angiotensin‐aldosterone system, and this leads to elevated circulating levels of aldosterone.34 However, chronic use of diuretics has been linked to progressive glomerulosclerosis and may not always be of benefit to the patient.35 Clearly, we cannot exclude that the associations between the use of diuretics, elevated aldosterone, and elevated galectin‐3 levels may be confounded by severity of disease in this post‐hoc analysis. It has however been shown before, that chronic use of diuretics is associated with increases in aldosterone levels. From our data, we hypothesize that this may be associated with persistent elevations of galectin‐3 as well. Elevated galectin‐3 has been linked to HF development and progression, but also to development of renal disease. We herein provide evidence that renal clearance of galectin‐3 is impaired in HF. We captured the potential interactions between galectin‐3, aldosterone, and cardiorenal disease in a hypothetical scheme (Figure 8).
Figure 8.
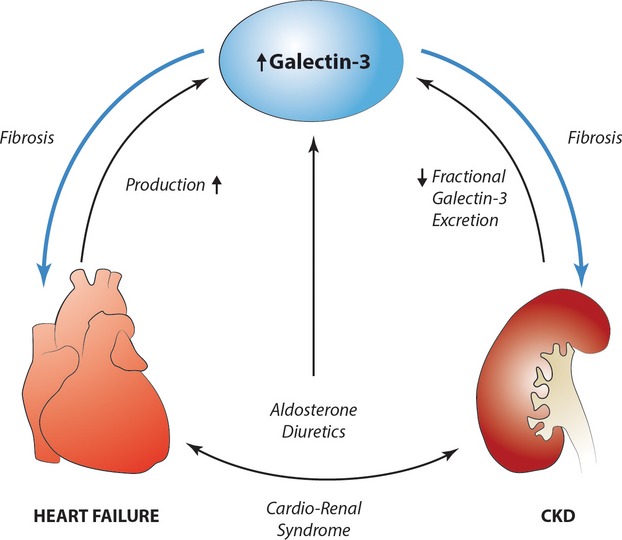
The role of galectin‐3 in the cardiorenal syndrome. Hypothetical scheme showing the possible interactions between galectin‐3 and aldosterone in cardiorenal disease. CKD indicates chronic kidney disease.
Strengths and Limitations
This is a relatively small study, and we could not extensively model the statistical correlations of urine galectin‐3. Furthermore, the absolute levels of urine galectin‐3 cannot be compared to other data. We are aware of 1 other report in prostate cancer patients where urinary galectin‐3 levels were measured. Unfortunately, a different ELISA was used in this study.36 It is, however, the first study that evaluated plasma and urine galectin‐3 in the same patient cohorts. We studied control subjects, HF patients, and also end‐stage renal disease patients who were on HD, spanning different degrees of renal (dys)function. There is an imbalance in clinical characteristics between the 3 different study groups, which could have led to potential confounding. We made an effort to fully correct for most potential confounders, but this resulted in a very small subgroup with limited power.
Perspectives
Renal handling of galectin‐3 appears to be different in HF. Plasma galectin‐3 is clearly elevated, while urinary levels are similar to control subjects. This may be explained by a reduced renal clearance of galectin‐3 and a lower fractional galectin‐3 clearance, and this is associated with high aldosterone levels and the use of diuretics. This is supported by extremely elevated galectin‐3 concentration in HD patients. We conclude that reduced renal clearance of galectin‐3 in HF patients may explain, in part, the accumulation and increase in plasma galectin‐3 in HF. These data help to explain the observed relation between renal dysfunction and galectin‐3 in HF, but may also provide a novel mechanism as to why renal dysfunction has detrimental long‐term consequences for HF patients.
Supplementary Material
Appendix Supplementary Tables S1 to S7.
Sources of Funding
This work was supported by the Netherlands Heart Foundation (grant 2007T046) and the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (VENI grant 916.10.117 and VIDI grant 917.13.350), all to Dr Boer; and the Netherlands Foundation for Cardiovascular Excellence (grant 2009‐02 to Dr Ruifrok).
Disclosures
BG Medicine Inc. (BGM, Waltham, MA) holds certain rights with respect to the use of galectin‐3 in HF. The University Medical Center Groningen, which employs the authors, received research grants from BGM. Prof Dr Boer received consultancy and speaker fees from BGM, Abbott, Biomérieux, Novartis, and Medcon.
Acknowledgments
We would like to thank Dr J.H. Proost for providing help with the clearance, volume of distribution, and infusion rate calculations. We thank L. van Genne for her assistance in the development of recombinant human galectin‐3.
References
- 1.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin‐3. Glycoconj J. 2004; 19:527-535. [DOI] [PubMed] [Google Scholar]
- 2.Dumic J, Dabelic S, Flogel M. Galectin‐3: an open‐ended story. Biochim Biophys Acta. 2006; 1760:616-635. [DOI] [PubMed] [Google Scholar]
- 3.Liu FT. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol. 2005; 136:385-400. [DOI] [PubMed] [Google Scholar]
- 4.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin‐3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009; 11:811-817. [DOI] [PubMed] [Google Scholar]
- 5.de Boer RA, Edelmann F, Cohen‐Solal A, Mamas MA, Maisel A, Pieske B. Galectin‐3 in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013; 15:1095-1101. [DOI] [PubMed] [Google Scholar]
- 6.McCullough PA, Olobatoke A, Vanhecke TE. Galectin‐3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med. 2011; 12:200-210. [DOI] [PubMed] [Google Scholar]
- 7.Yanavitski M, Givertz MM. Novel biomarkers in acute heart failure. Curr Heart Fail Rep. 2011; 8:206-211. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013; 15:240-327. [DOI] [PubMed] [Google Scholar]
- 9.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin‐3 marks activated macrophages in failure‐prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004; 110:3121-3128. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, van Veldhuisen DJ, Bank RA, van Gilst WH, Sillje HH, de Boer RA. Genetic and pharmacological inhibition of galectin‐3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013; 6:107-117. [DOI] [PubMed] [Google Scholar]
- 11.Christenson RH, Duh SH, Wu AH, Smith A, Abel G, de Filippi CR, Wang S, Adourian A, Adiletto C, Gardiner P. Multi‐center determination of galectin‐3 assay performance characteristics: anatomy of a novel assay for use in heart failure. Clin Biochem. 2010; 43:683-690. [DOI] [PubMed] [Google Scholar]
- 12.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ, van der Harst P. The fibrosis marker galectin‐3 and outcome in the general population. J Intern Med. 2012; 272:55-64. [DOI] [PubMed] [Google Scholar]
- 13.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin‐3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012; 60:1249-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. Utility of amino‐terminal pro‐brain natriuretic peptide, galectin‐3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006; 48:1217-1224. [DOI] [PubMed] [Google Scholar]
- 15.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin‐3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL‐HF study. Clin Res Cardiol. 2010; 99:323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin‐3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011; 43:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, Adourian A, Bohm M, van Veldhuisen DJ, Komajda M, Cleland JG, Wikstrand J, McMurray JJ, Aukrust PCORONA Study Group. Galectin‐3 predicts response to statin therapy in the controlled rosuvastatin multinational trial in heart failure (CORONA). Eur Heart J. 2012; 33:2290-2296. [DOI] [PubMed] [Google Scholar]
- 18.Gopal DM, Kommineni M, Ayalon N, Koelbl C, Ayalon R, Biolo A, Dember LM, Downing J, Siwik DA, Liang CS, Colucci WS. Relationship of plasma galectin‐3 to renal function in patients with heart failure: effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J Am Heart Assoc. 2012; 1:e000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, Shirolkar SC, Donahue M, Kitzman DW, Zannad F, Pina IL, O'Connor CM. Galectin‐3 in ambulatory patients with heart failure: results from the HF‐ACTION study. Circ Heart Fail. 2012; 5:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin‐3 precedes the development of CKD. J Am Soc Nephrol. 2013; 24:1880-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linssen GC, Damman K, Hillege HL, Navis G, van Veldhuisen DJ, Voors AA. Urinary N‐terminal prohormone brain natriuretic peptide excretion in patients with chronic heart failure. Circulation. 2009; 120:35-41. [DOI] [PubMed] [Google Scholar]
- 22.Park M, Vittinghoff E, Liu KD, Shlipak MG, Hsu CY. Urine biomarkers neutrophil gelatinase‐associated lipocalin (NGAL) and kidney injury molecule‐1 (KIM‐1) have different patterns in heart failure exacerbation. Biomark Insights. 2013; 8:15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proost JH, Eleveld DJ. Performance of an iterative two‐stage bayesian technique for population pharmacokinetic analysis of rich data sets. Pharm Res. 2006; 23:2748-2759. [DOI] [PubMed] [Google Scholar]
- 24.Schroten NF, Ruifrok WP, Kleijn L, Dokter MM, Sillje HH, Lambers HH, Bakker SJ, Kema IP, van Gilst WH, van Veldhuisen DJ, Hillege HL, de Boer RA. Short‐term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: An open‐label, blinded end point, randomized prospective trial (VitD‐CHF trial). Am Heart J. 2013; 166:357.e2-364.e2. [DOI] [PubMed] [Google Scholar]
- 25.Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, de Jong PE, Franssen CF. Hemodialysis‐induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment‐related factors, and prognostic significance. Clin J Am Soc Nephrol. 2012; 7:1615-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Boer RA, Schroten NF, Bakker SJ, Mahmud H, Szymanski MK, van der Harst P, Gansevoort RT, van Veldhuisen DJ, van Gilst WH, Hillege HL. Plasma renin and outcome in the community: data from PREVEND. Eur Heart J. 2012; 33:2351-2359. [DOI] [PubMed] [Google Scholar]
- 27.Valente MA, Hillege HL, Navis G, Voors AA, Dunselman PH, van Veldhuisen DJ, Damman K. The chronic kidney disease epidemiology collaboration equation outperforms the modification of diet in renal disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail. 2014; 16:86-94. [DOI] [PubMed] [Google Scholar]
- 28.Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez‐Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, Lopez‐Andres N. Galectin‐3 mediates aldosterone‐induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013; 33:67-75. [DOI] [PubMed] [Google Scholar]
- 29.Hu LJ, Chen YQ, Deng SB, Du JL, She Q. Additional use of an aldosterone antagonist in patients with mild to moderate chronic heart failure: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2013; 75:1202-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolatsi‐Joannou M, Price KL, Winyard PJ, Long DA. Modified citrus pectin reduces galectin‐3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011; 6:e18683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin‐3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA. 2006; 103:5060-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, Simpson AJ, Forbes SJ, Hirani N, Gauldie J, Sethi T. Regulation of transforming growth factor‐beta1‐driven lung fibrosis by galectin‐3. Am J Respir Crit Care Med. 2012; 185:537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000; 102:203-210. [DOI] [PubMed] [Google Scholar]
- 34.Felker GM, O'Connor CM, Braunwald EHeart Failure Clinical Research Network Investigators. Loop diuretics in acute decompensated heart failure: necessary? evil? A necessary evil? Circ Heart Fail. 2009; 2:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah BN, Greaves K. The cardiorenal syndrome: a review. Int J Nephrol. 2010; 2011:920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balasubramanian K, Vasudevamurthy R, Venkateshaiah SU, Thomas A, Vishweshwara A, Dharmesh SM. Galectin‐3 in urine of cancer patients: stage and tissue specificity. J Cancer Res Clin Oncol. 2009; 135:355-363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Supplementary Tables S1 to S7.


