Abstract
Background
It is uncertain whether sex and race affect thrombogenicity in patients with coronary artery disease. We evaluated the effects of sex and race on thrombogenicity in patients with coronary artery disease treated with aspirin.
Methods and Results
Patients on aspirin therapy for 1 week or longer with known or suspected coronary artery disease undergoing nonurgent cardiac catheterization (n=1172), of whom 924 were on aspirin and clopidogrel therapy, were studied. The primary end point was thrombin‐induced platelet‐fibrin clot strength (MAKH) measured by thrombelastography. Secondary end points included coagulation index, a measure of overall coagulation; G, another measure of clot strength; and maximal platelet aggregation. Women had greater MAKH, G, and coagulation index than men, both with and without clopidogrel therapy (with clopidogrel: 68.3±6 versus 65.8±6 mm, P<0.0001; 11.4±3 versus 9.5±4 dyne/cm2, P<0.0001; and 0.12±3 versus −0.7±3, P=0.003, respectively). Platelet aggregation (induced by ADP, thrombin receptor activating peptide, or collagen) did not differ between sexes. Black patients had greater MAKH and G than white patients (with clopidogrel: 67.8±7 versus 66.4±6 mm, P=0.005; 11±4 versus 10±3 dyne/cm2, P=0.02, respectively). Black women had the highest MAKH levels. By multivariate analysis, sex, race, diabetes, platelet count, and hemoglobin level were independently associated with MAKH. Sex, but not race, was also associated with the frequency of MAKH ≥72 mm (a threshold related to ischemic event occurrence in patients undergoing coronary intervention).
Conclusions
Sex and race independently influence platelet‐fibrin clot strength. Black women appear to have the highest thrombogenicity profile, potentially conferring a high‐risk phenotype for thrombotic event occurrence.
Keywords: epidemiology, platelets, sex, thrombelastography, thrombosis
Introduction
Sex‐ and race‐specific disparities in the prevalence, clinical presentation, and outcomes (including mortality) of coronary artery disease (CAD) have been reported.1–5 These disparities may be explained, in part, by sex‐ and race‐based differences in the management and health care of patients.4–5 Differences in platelet function, the response to antiplatelet therapy, and other factors affecting thrombosis may also contribute to clinical outcome disparities.
Sex‐related differences in platelet reactivity have been reported, with women having more reactive platelets than men.6–9 Greater platelet reactivity in women has been demonstrated by higher platelet aggregation, both spontaneously and in response to various agonists, including ADP, collagen, epinephrine, and arachidonic acid.6–9 In addition, platelets from women have been shown to express more activated glycoprotein IIb/IIIa receptors.10 The heightened platelet reactivity among women may explain the observations of a reduced response to aspirin when evaluated by pathways that are not directly related to cyclooxygenase 1 (eg, collagen‐ and ADP‐induced aggregation).7 Limited information is available about other measurements of thrombogenicity. Small studies from our group and others suggested that women have greater platelet‐fibrin clot strength, when evaluated by thrombelastography (TEG).11–12
Effects of race on platelet reactivity and other thrombotic parameters have been less studied. Black participants have been shown to have higher markers of platelet activation than white participants, as assessed by flow cytometry, in the Atherosclerosis Risk in Communities (ARIC) population study.13 Smaller studies reported that black participants and particularly, black women, had greater platelet‐fibrin clot strength than white participants11 and higher platelet reactivity during clopidogrel treatment.14 In patients with CAD, however, the evidence in support of racial disparities in platelet function and other thrombogenicity parameters is quite limited. Given the lack of data, we aimed to evaluate the effects of sex and race on thrombogenicity in a large cohort of patients with CAD treated with aspirin.
Methods
The study cohort included 1172 patients who underwent elective cardiac catheterization and had blood samples taken for thrombogenicity evaluation. Data were gathered from studies performed at the Sinai Center for Thrombosis Research (Baltimore, Maryland). All patients in the study cohort had known or suspected CAD. Known CAD was defined as a previous percutaneous coronary intervention (PCI), previous coronary artery bypass grafting, or known coronary stenosis of at least 50% from a prior angiography. Indications for the index cardiac catheterization were stable anginal syndrome, acute coronary syndrome that did not require urgent catheterization, or a positive stress test or cardiac computed tomography. Patients with ST‐segment elevation myocardial infarction were excluded because they frequently receive early treatment with anticoagulants, which may affect thrombogenicity tests. In addition, increased platelet reactivity typical of the acute ST‐segment elevation myocardial infarction setting may affect thrombogenicity evaluation. All patients were treated with aspirin for at least 1 week prior to catheterization and received 325 mg aspirin on the day of the procedure. Clopidogrel treatment varied according to the study protocol; however, the majority of patients (79%) received clopidogrel and had thrombogenicity determined either on chronic clopidogrel treatment (n=614) or 18 to 24 hours after a clopidogrel loading dose of 300 to 600 mg (n=310). The current analysis was performed separately for samples taken while patients were not receiving clopidogrel treatment (clopidogrel naïve, n=558) and during clopidogrel treatment (on clopidogrel, n=924). In 310 patients, blood samples were taken at 2 time points: before clopidogrel treatment and 18 to 24 hours after clopidogrel loading. These 310 patients are included in both clopidogrel‐naïve and clopidogrel‐treated analyses.
Data for the current cohort were derived from the following studies: the Multi Analyte Genetic Markers of Atherosclerosis study (performed 2011–2013, with samples drawn before cardiac catheterization),15 the Clopidogrel Loading with Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS 1 and 2 studies; performed 2003–2004 and 2006–2007, respectively; the current cohort included only patients from the control groups that did not receive eptifibatide therapy),16–17 the Timing Based on Platelet Function Strategy to Reduce Clopidogrel‐Associated Bleeding Related to Coronary Artery Bypass Grafting study (TARGET‐CABG; performed 2008–2011; the current cohort included only blood samples drawn before cardiac catheterization),18 and the study of time dependence of clopidogrel effect (platelet activation versus platelet aggregation; performed 2009–2010).19
All studies were approved by the institutional review board at Sinai Hospital of Baltimore. All patients meeting criteria for eligibility provided written informed consent prior to inclusion in the respective study.
In all studies, patients had baseline whole blood samples drawn prior to catheterization and before administration of parenteral antithrombotic therapy. In the 310 patients with baseline and postclopidogrel samples, the baseline sample was taken as described above, and the second sample was taken 18 to 24 hours after clopidogrel loading (with exclusion of patients receiving glycoprotein IIb/IIIa inhibitors). Patients were tested at resting conditions. After discarding the first 2 to 3 mL of free‐flowing blood, samples were transferred to vacutainer blood‐collecting tubes containing 45 United States Pharmacopeia lithium heparin and tubes containing 3.8% trisodium citrate (Becton‐Dickinson). The vacutainer tube was filled to capacity and gently inverted 3 to 5 times to ensure complete mixing with the anticoagulant. Thrombogenicity assays were performed within 2 hours of blood collection.
Assessment of Thrombogenicity
Thrombelastography
Platelet‐fibrin clot strength and kinetics of the clotting process were determined by TEG (TEG 5000 Thrombelastograph Hemostasis Analyzer system; Haemonetics), as described previously.18,20
In the TEG assay, a stationary pin is suspended into an oscillating cup containing the whole blood sample. As the blood clots, fibrin strands produced during coagulation, stimulated by kaolin, link a rotating sample cup with a stationary pin suspended by a torsion wire. The torque of the rotating cup is transmitted to the immersed pin. Pin movement is converted to an electrical signal by a transducer and is interpreted by a computer to create a tracing. The degree of platelet contribution to the clot strength through platelet‐fibrin binding directly influences the magnitude of pin movement and, ultimately, the amplitude of the tracing. Clot strength is determined by measuring the amplitude of the rotation of the pin, which increases proportionally with clot strength. Maximum amplitude (MA; millimeters) reflects maximum platelet‐fibrin clot strength (MAKH). Reaction time (R; minutes), a representative of the initiation phase of enzymatic clotting, is the time from the start of the sample run to the point of the first significant clot formation corresponding to an amplitude of a 2‐mm reading on the TEG tracing. G (dyne/cm2) is another representation of clot strength and is inclusive of both platelet and enzymatic contributions to clot strength. G is a computer‐generated value calculated from MAKH as follows: G=(5000×MAKH)/(100−MAKH). Coagulation index represents the overall coagulation. It is derived from the R; K, a measure of the speed to reach 20‐mm clot strength from R; MA; and angle of native or kaolin‐activated whole blood tracings. Angle is reflective of fibrinogen activity and is the size in degrees of the angle formed by the tangent line to TEG tracing measure at R (Figure 1).
Figure 1.
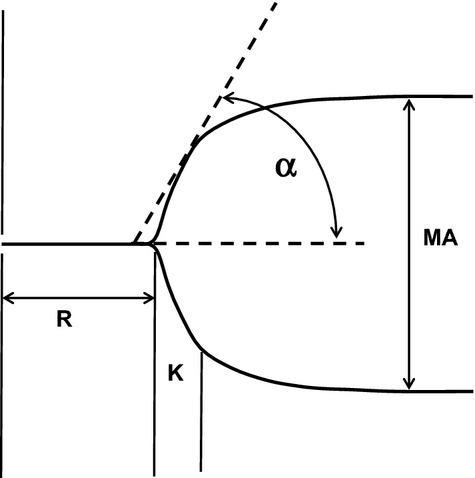
Thrombelastography tracing parameters studied. K indicates the time from R to reach 20 mm of clot strength; MA, maximum amplitude of the platelet fibrin clot; R, reaction time in minutes; α, angle.
Light transmittance aggregometry
Citrated blood samples were centrifuged at 120g for 5 minutes to recover platelet‐rich plasma and were further centrifuged at 850g for 10 minutes to recover platelet‐poor plasma. Platelet count was not normalized. Maximal platelet aggregation following stimulation with 5 and 20 μmol/L ADP, 10 μmol/L thrombin receptor activating peptide, and 4 μg/mL collagen was assessed using a Chrono‐log Lumi‐Aggregometer (Model 490‐4D) with the Aggrolink software package (Chrono‐log), as described previously.16–17 Complete platelet aggregation data were available in 75% to 80% of patients depending on the agonist, whereas TEG data were available for all patients.
Statistical Analysis
Continuous variables are presented as mean±SD, and categorical variables are expressed as number and percentage. All continuous variables were normally distributed (by the Kolmogorov–Smirnov test) and compared using Student t tests, as appropriate. Chi‐square tests were used for comparison of categorical variables. The primary end point of the study was platelet‐fibrin clot strength (MAKH). All analyses were performed separately for samples on and off clopidogrel (ie, clopidogrel naïve). To examine which variables independently affect MAKH, multivariate analysis of variance was used for the categorical scaled variables, and multivariate linear regression analysis (stepwise method) for the continuous scaled variables. In total, 20 categorical variables and 6 continuous variables were included in the model. A separate analysis was performed to compare the proportion of patients with MAKH ≥72 mm (a threshold previously demonstrated to be associated with an increased thrombotic risk in patients undergoing PCI21). Multiple logistic regression analysis (with stepwise method) was performed to examine the factors related to MAKH as a categorical variable (with ≥72‐mm threshold). Based on previous analysis, 9 variables were entered in the logistic regression model. Analyses were performed with SPSS Statistics 21 software (IBM Corp), and P≤0.05 was considered statistically significant.
Results
Clinical Characteristics According to Sex and Race
The cohort comprised 694 men (59.2%) and 478 women (40.8%) (Table 1). Women were older; had greater body mass index; and, more frequently, had diabetes, hypertension, and treatment with a proton pump inhibitor. In addition, more women than men had nonsignificant CAD (13.6% versus 7.8%, P=0.001), and women less often had multivessel disease. Men more often had a family history of CAD and a history of coronary artery bypass grafting and were more often treated with an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker. In addition, hemoglobin level was higher and platelet count was lower among men.
Table 1.
Clinical Characteristics and Medical Treatment in Relation to Sex and Race
| Men (n=694) | Women (n=478) | P Value | White (n=742) | Black (n=430) | P Value | |
|---|---|---|---|---|---|---|
| Age, y | 63.8±11 | 65.3±12 | 0.03 | 64.6±11 | 64.2±11 | 0.5 |
| BMI | 29.9±6.5 | 31.4±7 | 0.001 | 30.6±6 | 31.0±6 | 0.3 |
| Diabetes mellitus | 220 (31.7%) | 189 (39.5%) | 0.005 | 244 (32.8%) | 165 (38.4%) | 0.07 |
| Hypertension | 533 (76.8%) | 389 (81.4%) | 0.06 | 578 (77.9%) | 344 (80%) | 0.4 |
| History of smoking | 609 (87.8%) | 410 (85.8%) | 0.3 | 674 (90.8%) | 345 (80.2%) | 0.0001 |
| Hyperlipidemia | 562 (81%) | 385 (80.5%) | 0.9 | 600 (80.9%) | 347 (80.7%) | 0.9 |
| Significant CAD* | 641 (92.4%) | 412 (86.2%) | 0.0006 | 661 (89.1%) | 392 (91.2%) | 0.3 |
| Multivessel disease* | 554 (79.8%) | 334 (69.9%) | <0.0001 | 549 (74%) | 339 (78.8%) | 0.07 |
| Family history of CAD | 467 (67.3%) | 289 (60.5%) | 0.02 | 518 (69.8%) | 238 (55.3%) | <0.0001 |
| History of PCI | 231 (33.3%) | 155 (32.4%) | 0.8 | 241 (32.5%) | 145 (33.7%) | 0.7 |
| History of CABG | 145 (20.9%) | 60 (12.6%) | 0.0002 | 123 (16.6%) | 82 (19.1%) | 0.3 |
| History of MI | 200 (28.8%) | 127 (26.6%) | 0.4 | 193 (26%) | 134 (31.2%) | 0.06 |
| History of stroke | 54 (7.8%) | 33 (6.9%) | 0.5 | 41 (5.5%) | 46 (10.7%) | 0.002 |
| PAD | 60 (8.6%) | 37 (7.7%) | 0.5 | 59 (8%) | 38 (8.8%) | 0.6 |
| Creatinine, mg/dL | 1.1±0.4 | 1.1±0.5 | 0.6 | 1.1±0.4 | 1.2±0.5 | 0.0002 |
| Hemoglobin, g/dL | 13.9±2 | 13±2 | <0.0001 | 13.7±2 | 13.4±3 | 0.04 |
| Hematocrit, % | 41.2±5 | 39±5 | 0.01 | 40.6±5 | 40.3±5 | 0.3 |
| Platelets (×1000/μL) | 224.5±65 | 243.5±68 | 0.0002 | 231.1±66 | 236.2±66 | 0.2 |
| White blood cells (×1000/μL) | 7.4±4 | 7.3±3 | 0.7 | 7.3±2 | 7.5+4 | 0.3 |
| Medications | ||||||
| Clopidogrel* | 541 (77.9%) | 383 (80.1%) | 0.3 | 572 (77.1%) | 352 (81.9%) | 0.05 |
| Beta blockers | 453 (65.3%) | 294 (61.5%) | 0.2 | 456 (61.5%) | 291 (67.7%) | 0.04 |
| Statins | 528 (76.1%) | 345 (72.2%) | 0.1 | 538 (72.5%) | 335 (77.9%) | 0.04 |
| ACE inhibitors or ARBs | 489 (70.5%) | 306 (64%) | 0.02 | 470 (63.3%) | 325 (75.6%) | <0.0001 |
| Calcium blockers | 150 (21.6%) | 113 (23.6%) | 0.5 | 148 (19.9%) | 115 (26.7%) | 0.009 |
| PPIs | 395 (56.9%) | 318 (66.5%) | 0.001 | 484 (65.2%) | 229 (53.3%) | <0.0001 |
| Nitrates | 149 (21.4%) | 88 (18.4%) | 0.2 | 140 (18.9%) | 97 (22.6%) | 0.1 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor.
At least 1 coronary artery with ≥50% stenosis according to current angiogram.
At least 2 coronary arteries with ≥50% stenosis according to current angiogram.
Either chronic clopidogrel treatment or 18 to 24 hours following loading.
The total cohort comprised 742 white participants (63.3%) and 430 black participants (36.7%) (Table 1). White participants more often had a history of smoking and familial history of CAD, whereas black participants more often had a previous myocardial infarction or stroke. Black participants were more often treated with clopidogrel, beta blockers, statins, angiotensin‐converting enzyme inhibitors, or angiotensin receptor blockers, whereas white participants were more often treated with proton pump inhibitors. In addition, hemoglobin level was higher and creatinine was lower among white participants.
Thrombogenicity According to Sex and Race
Women had significantly higher MAKH, G, and coagulation index than men, regardless of clopidogrel treatment status (Table 2). Platelet aggregation in response to various agonists did not differ between sexes with or without clopidogrel treatment. Black patients had significantly higher MAKH both on and off clopidogrel treatment and higher G on clopidogrel treatment than did white patients. Platelet aggregation did not differ between races either with or without clopidogrel treatment (Table 2).
Table 2.
Platelet Reactivity and Thrombelastography‐Derived Parameters in Relation to Sex and Race
| Men (n=694) | Women (n=478) | P Value | White (n=742) | Black (n=430) | P Value | |
|---|---|---|---|---|---|---|
| Clopidogrel‐naïve | n=315 | n=243 | n=401 | n=157 | ||
| 5 μmol/L ADP‐induced aggregation, % | 59.1±15 | 59.8±15 | 0.7 | 59±16 | 60.3±14 | 0.6 |
| 20 μmol/L ADP‐induced aggregation, % | 71.5±17 | 71.1±16 | 0.8 | 71.7±17 | 70.3±17 | 0.6 |
| 10 μmol/L TRAP‐induced aggregation, % | 71±17 | 72.1±13 | 0.7 | 71.6±14 | 71.2±15 | 0.8 |
| 4 μg/mL‐induced collagen aggregation, % | 36.7±26 | 36.1±25 | 0.8 | 35.7±25 | 38.5±25 | 0.3 |
| MAKH, mm | 64.7±6 | 67.6±5 | <0.0001 | 65.6±5 | 67.1±6 | 0.01 |
| CI | −0.4±3 | 0.26±3 | 0.01 | −0.2±3 | −0.1±3 | 0.9 |
| G, dyne/cm2 | 9.6±4 | 11.4±3 | 0.0002 | 10.1±3 | 10.7±3 | 0.2 |
| R, mm | 7.3±2 | 7.0±2 | 0.2 | 7.1±2 | 7.3±3 | 0.3 |
| On clopidogrel treatment | n=541 | n=383 | n=557 | n=367 | ||
| 5 μmol/L ADP‐induced aggregation, % | 35.9±19 | 36.3±20 | 0.8 | 36.4±20 | 35.6±18 | 0.7 |
| 20 μmol/L ADP‐induced aggregation, % | 48.2±22 | 46±24 | 0.3 | 46.7±23 | 48.1±21 | 0.6 |
| 10 μmol/L TRAP‐induced aggregation, % | 49.8±23 | 47.9±23 | 0.6 | 47.6±24 | 51.4±20 | 0.15 |
| 4 μg/mL‐induced collagen aggregation, % | 29.5±24 | 27.7±25 | 0.5 | 28.4±24 | 29.1±24 | 0.7 |
| MAKH, mm | 65.8±6 | 68.3±6 | <0.0001 | 66.4±6 | 67.8±7 | 0.005 |
| CI | −0.7±3 | 0.12±3 | 0.003 | −0.5±3 | −0.1±3 | 0.15 |
| G, dyne/cm2 | 9.5±4 | 11.4±3 | <0.0001 | 10±3 | 11±4 | 0.02 |
| R, mm | 7.4±2 | 7.1±3 | 0.1 | 7.2±3 | 7.3±3 | 0.8 |
CI indicates coagulation index; G, another representation of clot strength; MAKH, maximum thrombin‐induced platelet‐fibrin clot strength; R, reaction time, a representative of the initiation phase of clot formation; TRAP, thrombin receptor activating peptide.
Multivariate analysis of variance (for categorical scaled variables) and multiple linear regression analysis (for continuous variables) were performed to test the effects of various variables on MAKH for patients on and off clopidogrel (Tables 3 and 4). In both clopidogrel‐naïve and clopidogrel‐treated patients, sex and diabetes were strongly associated with MAKH levels (P<0.0001). Race and hypertension were also significantly associated with MAKH, although the association was weaker (P=0.02 to 0.04). Treatment with calcium blockers was associated with MAKH in clopidogrel‐naïve patients, and treatment with beta blockers was associated with MAKH in clopidogrel‐treated patients (Table 3). In the linear regression analysis, platelet count and hemoglobin level and, to a lesser extent, white blood cells were all significantly associated with MAKH in clopidogrel‐naïve and clopidogrel‐treated patients (P=0.02 for white blood cells, and P<0.0001 for platelets and hemoglobin) (Table 4).
Table 3.
Effect of Categorical Variables on Maximum Thrombin‐Induced Platelet‐Fibrin Clot Strength
| Variable | Clopidogrel‐Naïve | On Clopidogrel | ||
|---|---|---|---|---|
| F | P Value | F | P Value | |
| Sex | 46 | <0.0001 | 43 | <0.0001 |
| Race | 4.3 | 0.04 | 5.4 | 0.02 |
| Diabetes mellitus | 23.2 | <0.0001 | 15.2 | <0.0001 |
| Hypertension | 5.6 | 0.02 | 5.1 | 0.02 |
| History of smoking | 0.4 | 0.7 | 0.3 | 0.8 |
| Hyperlipidemia | 0.1 | 0.8 | 0 | 1 |
| Significant CAD* | 0.7 | 0.5 | 0.1 | 0.9 |
| Family history of CAD | 1.4 | 0.3 | 0.1 | 0.9 |
| History of PCI | 0.8 | 0.4 | 0.2 | 0.7 |
| History of CABG | 0.02 | 0.9 | 0.2 | 0.6 |
| History of MI | 0.7 | 0.4 | 0.4 | 0.5 |
| History of stroke | 0.1 | 0.8 | 0 | 1 |
| PAD | 1.1 | 0.3 | 0.4 | 0.5 |
| Chronic renal insufficiency* | 3.5 | 0.07 | 1.3 | 0.2 |
| Beta blockers | 0.9 | 0.4 | 5.6 | 0.02 |
| Statins | 1 | 0.4 | 0.4 | 0.8 |
| ACE inhibitors or ARBs | 0.4 | 0.6 | 1.3 | 0.25 |
| Calcium blockers | 8.6 | 0.001 | 2.5 | 0.08 |
| PPIs | 0.9 | 0.5 | 0.5 | 0.8 |
| Nitrates | 0.7 | 0.5 | 0.1 | 0.9 |
Analyses performed by multivariate analysis of variance. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor.
At least 1 coronary artery with ≥50% stenosis according to current angiogram.
†Creatinine ≥1.5 mg/dL.
Table 4.
Effect of Continuous Variables on Maximum Thrombin‐Induced Platelet‐Fibrin Clot Strength
| Variable | Clopidogrel‐Naïve | On Clopidogrel | ||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Platelets | 0.46 | <0.0001 | 0.44 | <0.0001 |
| Hemoglobin | −0.32 | <0.0001 | −0.23 | 0.001 |
| White blood cells | 0.21 | 0.02 | 0.17 | 0.02 |
| Age | 0.12 | 0.2 | 0.08 | 0.25 |
| BMI | 0.1 | 0.2 | 0.12 | 0.08 |
| Creatinine | −0.08 | 0.5 | −0.05 | 0.6 |
Analyses performed by multivariate linear regression analysis (stepwise method). BMI indicates body mass index.
To further analyze the effects of sex and race on thrombogenicity, we divided the total cohort into 4 subgroups: white men (n=439), black men (n=255), white women (n=303), and black women (n=175). Black women had higher platelet‐fibrin clot strength (MAKH) than the other groups, regardless of clopidogrel treatment status (Figure 2).
Figure 2.
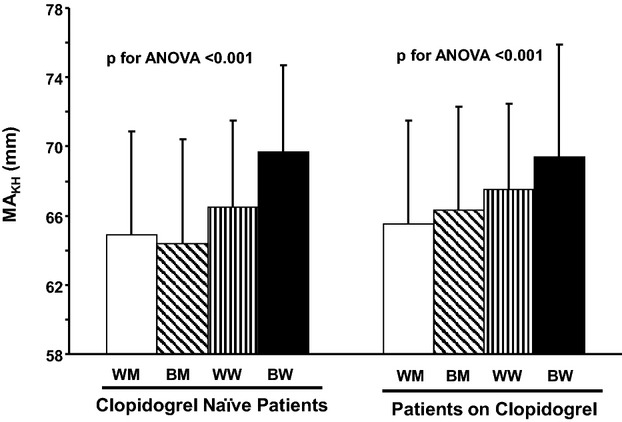
Maximum thrombin induced‐platelet fibrin clot strength according to sex and race in clopidogrel‐naïve patients and patients on clopidogrel therapy. Results presented as mean±SD. BM indicates black men; BW, black women; WM, white men; WW, white women; MAKH, maximum thrombin‐induced platelet fibrin clot strength.
Analysis According to MAKH ≥72‐mm Threshold
Analysis of MAKH according to a threshold ≥72 mm21 showed that a higher proportion of women exceeded the threshold than men, with or without clopidogrel treatment (clopidogrel naive: 42 of 243 women [17.3%] versus 34 of 315 men [10.8%], P=0.03; on clopidogrel: 84 of 383 women [21.9%] versus 84 of 541 men [15.5%], P=0.01) (Figure 3A). In addition, a higher proportion of black participants than white participants had MAKH ≥72 mm (clopidogrel naïve: 30 of 157 black participants [19.1%] versus 46 of 401 white participants [11.5%], P=0.02; on clopidogrel: 77 of 366 black participants [21%] versus 91 of 558 white participants [16.3%], P=0.07) (Figure 3B).
Figure 3.
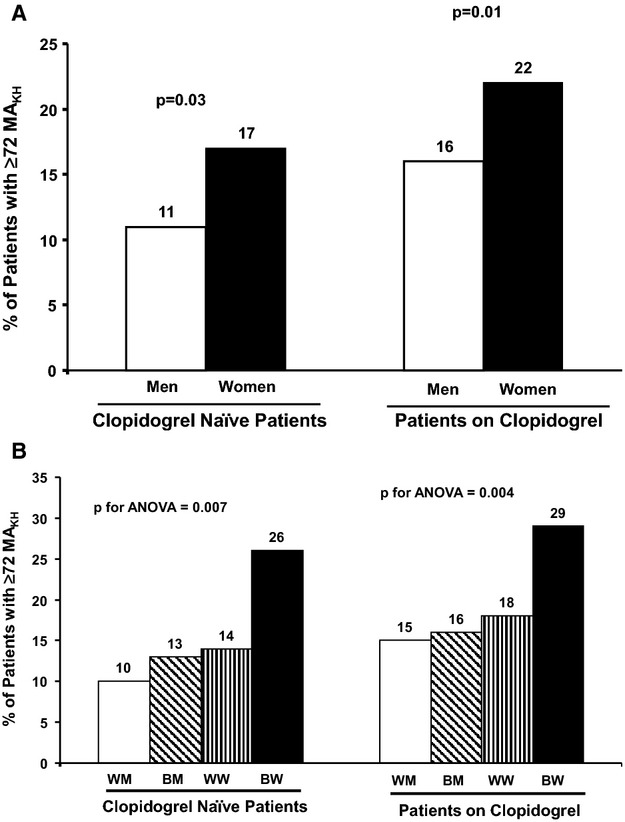
A, Proportion of patients with MAKH ≥72 mm according to sex. B, Proportion of patients with MAKH ≥72 mm according to sex and race. BM indicates black men; BW, black women; WM, white men; WW, white women; MAKH, maximum thrombin‐induced platelet‐fibrin clot strength.
A multiple logistic regression analysis was performed to examine the factors related to MAKH as a categorical variable (with ≥72‐mm threshold) (Table 5). Nine variables were included in the logistic regression model based on the results presented in Tables 3 and 4: sex, race, diabetes, hypertension, beta blockers, calcium blockers, platelets, hemoglobin, and white blood cells. For clopidogrel‐naïve patients, sex (P=0.05), diabetes (P=0.01), treatment with calcium blockers (P=0.04) and platelet count (P=0.001) were related to MAKH; women and patients with diabetes and higher platelet counts were more likely to have MAKH ≥72 mm. For clopidogrel‐treated patients, sex (P=0.03), diabetes (P=0.03), treatment with beta blockers (P=0.02), and platelet count (P=0.0001) were all associated with MAKH ≥72 mm (Table 5). Race and other factors entered into the model were not associated with MAKH ≥72 mm either on or off clopidogrel treatment.
Table 5.
Factors Affecting the Frequency of MAKH ≥72 mm
| Clopidogrel‐Naïve | On Clopidogrel | |||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Sex | 0.30 | 0.05 | 0.38 | 0.03 |
| Diabetes mellitus | 0.42 | 0.01 | 0.35 | 0.03 |
| Beta blockers | 0.2 | 0.1 | 0.36 | 0.02 |
| Calcium blockers | 0.28 | 0.04 | 0.2 | 0.07 |
| Platelets | 0.65 | 0.001 | 0.67 | 0.0001 |
Multiple logistic regression analysis (stepwise method) performed to examine the factors related to MAKH as a categorical variable (with ≥72‐mm threshold). Nine variables were included in the logistic regression model based on the results presented in Tables 3 and 4: sex, race, diabetes, hypertension, beta blockers, calcium blockers, platelets, hemoglobin, and white blood cells. Shown in the table are only factors that were significantly associated with MAKH ≥72 mm either on or off clopidogrel treatment. Race, hypertension, hemoglobin level, and white blood cell count were not significantly associated with the frequency of MAKH ≥72 mm (P≥0.1). MAKH indicates maximum thrombin‐induced platelet‐fibrin clot strength.
Discussion
To our knowledge, this is the largest study assessing the effects of sex and race on thrombogenicity determined by TEG and platelet aggregation in patients with very high prevalence of CAD. The main findings of our study were that sex was strongly associated with platelet‐fibrin clot strength, with women having higher MAKH levels than men, but sex was not associated with platelet aggregation. Race was also significantly associated with platelet‐fibrin clot strength, with black participants having higher MAKH levels than white participants, but was not associated with platelet aggregation. Black women appear to have the highest platelet‐fibrin clot strength among the various sex and race subgroups examined.
The greater platelet‐fibrin clot strength and other TEG‐derived parameters indicating high thrombogenicity in women in our study confirm previous smaller reports.11–12 Unexpectedly, and contrary to some of the previous studies,6–9,22 we did not find significant differences in platelet aggregation between women and men. The differences between the studies may be related to different concentrations of agonists used, different methodologies to determine platelet aggregation, and other confounding factors such as treatment with clopidogrel in the majority of patients in our study. Prior studies that have detected differences in platelet aggregation between the sexes generally used lower agonist concentrations than we did,7,9 frequently used whole blood platelet aggregation7–8 or a laser light‐scattering method,9 and evaluated volunteers8–9 or healthy subjects before and after aspirin therapy.7 Despite the lack of differences in platelet aggregation, our study reinforces the concept of elevated thrombogenicity in women, as demonstrated by increased platelet‐fibrin clot strength and coagulation index in addition to increased expression of platelet activation markers shown in previous studies.10
The mechanism of sex‐based differences in thrombogenicity has been reported to be influenced by sex hormones. Megakaryocytes and platelets have been shown to express the estrogen receptor beta and androgen receptor.23 Furthermore, estradiol exerted inhibitory effects on platelet activation, through the activation of platelet nitric oxide synthase, and via enhancement of nitric oxide synthesis and release from endothelial cells.24–25 Estrogen has also been shown to affect the coagulation and fibrinolysis pathways. Estrogen therapy has been associated with decreased levels of fibrinogen; however, estrogen has also been associated with a reduction in plasma concentrations of the anticoagulant proteins antithrombin III and protein S and the antifibrinolytic protein plasminogen‐activator inhibitor type 1, a net effect that increases prothrombotic tendency.26 Another mechanism possibly mediating the sex‐based differences in thrombogenicity are genetic polymorphisms in platelet glycoproteins,27–28 although less is known about the exact association of these polymorphisms with sex‐based differences in platelet biology.
There is limited information about the independent effects of race on platelet function and other thrombotic parameters. In a large cohort of asymptomatic subjects reported by Farday et al, baseline platelet aggregation and antiplatelet response to aspirin appeared to be similar in white and black participants.29 However, the ARIC study demonstrated higher values of platelet activation markers among black subjects.13 Furthermore, black patients undergoing PCI appear to have a higher prevalence of high‐on‐treatment platelet reactivity and reduced response to clopidogrel14 that may be a result of higher cytochrome (CYP) 2C19*2 allele carriage compared with white patients.14,30 In our study, platelet aggregation did not differ between races at baseline or during clopidogrel treatment. Differences in the methodologies for assessment of platelet reactivity during clopidogrel treatment and in the timing of blood samples may explain the discordant findings from previous studies.14 Significant differences, however, were observed in MAKH between the races, with black patients exhibiting higher platelet‐fibrin clot strength than white patients. Although race was significantly associated with MAKH levels, the association was not as strong as the effect of sex and diabetes on MAKH.
Several other factors apart from sex and race, such as diabetes, platelet count, and hemoglobin level, were independently associated with platelet‐fibrin clot strength (MAKH) in our study. These findings are consistent with previous studies that have reported higher thrombogenicity parameters in patients with diabetes, higher platelet count, and reduced hematocrit level.31–34 Diabetes has been shown to be strongly associated with increased coagulability, as assessed by rotational TEG,31 and with platelet hyperreactivity.32 Both hematocrit and platelet count have been shown to influence TEG‐derived parameters including MA.33–34
The current study has important implications. TEG‐derived parameters, such as platelet‐fibrin–mediated clot strength, have been correlated with adverse ischemic events in patients with CAD.11,21 Specifically, increased platelet‐fibrin clot strength and rapid fibrin formation have been reported to be independent predictors of ischemic event occurrence after PCI.11,21 Thus the differences in MAKH between sexes, and especially increased MAKH among black women, identify a potentially high‐risk group for thrombotic event occurrence. Previous large‐scale reports have shown that women (compared with men) and black patients (compared with white patients) generally have higher risk of cardiovascular events.1–2 In addition, women and black patients have an increased risk of adverse ischemic events after coronary intervention,35–36 although the sex‐based disparity of post‐PCI outcomes has narrowed in the drug‐eluting stent era.36 Despite the abundance of sex‐ and race‐related outcome data, only a few studies have focused on the specific risk profile of black women. We have previously reported that this group of patients was at increased risk of ischemic events following PCI,11 but further research is required to characterize and identify the underlying causes, including potential genetic and epigenetic variables, of their higher thrombotic risk.
Our study has several limitations. First, the data were gathered from 5 different studies, and although the platelet reactivity and TEG‐based parameters were assessed in an identical manner in all studies, the different study designs can introduce biases. The main difference between the studies is in the timing of blood samples in clopidogrel‐treated patients (chronic treatment versus 18 to 24 hours after loading), which may affect platelet‐reactivity measurements; however, the main end point of the study, platelet‐fibrin clot strength (MAKH), has been shown to be unaffected by clopidogrel treatment.21 Second, full platelet‐aggregation data in response to all agonists were not available for all patients in the cohort (only 75% to 80% of the patients), possibly limiting the strength of the platelet‐aggregation analysis. Third, all of the studies of this cohort were performed at a single center. Although the total cohort is large, it reflects the demographics of a specific region in the United States. Finally, there were many significant imbalances between the sex‐ and race‐based subgroups. Although we attempted to control for all confounding demographic and laboratory factors with multivariate analyses, it is still possible that additional factors may have contributed to the differences in platelet‐fibrin clot strength observed between sexes and between the sex–race subgroups.
In conclusion, from this large cohort of patients with CAD treated with oral antiplatelet therapy, sex and, to a lesser extent, race are significantly associated with platelet‐fibrin clot strength. Black women with CAD appear to have the greatest thrombogenicity, potentially conferring a high‐risk phenotype for thrombotic event occurrence.
Sources of Funding
This study was supported by a grant from Robert and Irene Russel to the Sinai Center for Thrombosis Research, Sinai Hospital of Baltimore, Baltimore, MD, USA.
Disclosures
Dr Gurbel reports serving as a consultant for Daiichi Sankyo, Lilly, Bayer, AstraZeneca, Boehringer Ingelheim, Merck, Medtronic, CSL, and Haemonetics; receiving grants from the National Institutes of Health, Daiichi Sankyo, Lilly, CSL, AstraZeneca, Sanofi‐Aventis, Arastasis, Harvard Clinical Research Institute, and Duke Clinical Research Institute; receiving payment for lectures, including service on speakers' bureaus, from Lilly, Daiichi Sankyo, Sanofi‐Aventis, and Merck; receiving payment for development of educational presentations from Schering‐Plough, the Discovery Channel, and Pri‐Med. Dr Lev received honoraria from Eli Lilly, Astra Zeneca, and Pfizer and is member of advisory boards for Eli Lilly, Astra Zeneca, and Pfizer. Other authors report no disclosures.
Acknowledgments
We would like to acknowledge Dr. Keren Or‐Chen from the University of Haifa, Israel for her excellent statistical assistance.
References
- 1.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012; 366:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard GREGARDS Investigators. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012; 308:1768-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd‐Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong YAmerican Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008; 117:e25-e146. [DOI] [PubMed] [Google Scholar]
- 4.Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX, Boden WE, Roe MT, Ohman EM, Gibler WB, Newby LKCRUSADE Investigators. Gender disparities in the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes: large‐scale observations from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol. 2005; 45:832-837. [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, Blake GJ, Drazner MH, Morrow DA, Scirica BM, Murphy SA, McCabe CH, Weintraub WS, Gibson CM, Cannon CP. Influence of race on death and ischemic complications in patients with non‐ST‐elevation acute coronary syndromes despite modern, protocol‐guided treatment. Circulation. 2005; 111:1217-1224. [DOI] [PubMed] [Google Scholar]
- 6.Johnson M, Ramey E, Ramwell PW. Sex and age differences in human platelet aggregation. Nature. 1975; 253:355-357. [DOI] [PubMed] [Google Scholar]
- 7.Becker DM, Segal J, Vaidya D, Yanek LR, Herrera‐Galeano JE, Bray PF, Moy TF, Becker LC, Faraday N. Sex differences in platelet reactivity and response to low‐dose aspirin therapy. JAMA. 2006; 295:1420-1427. [DOI] [PubMed] [Google Scholar]
- 8.Zwierzina WD, Kunz F, Kogelnig R, Herold M. Sex‐related differences in platelet aggregation in native whole blood. Thromb Res. 1987; 48:161-171. [DOI] [PubMed] [Google Scholar]
- 9.Haque SF, Matsubayashi H, Izumi S, Sugi T, Arai T, Kondo A, Makino T. Sex difference in platelet aggregation detected by new aggregometry using light scattering. Endocr J. 2001; 48:33-41. [DOI] [PubMed] [Google Scholar]
- 10.Faraday N, Goldschmidt‐Clermont PJ, Bray PF. Gender differences in platelet GPIIb‐IIIa activation. Thromb Haemost. 1997; 77:748-754. [PubMed] [Google Scholar]
- 11.Gurbel PA, Bliden KP, Cohen E, Navickas IA, Singla A, Antonino MJ, Fissha M, Kreutz RP, Bassi AK, Tantry US. Race and sex differences in thrombogenicity: risk of ischemic events following coronary stenting. Blood Coagul Fibrinolysis. 2008; 19:268-275. [DOI] [PubMed] [Google Scholar]
- 12.Hobson AR, Qureshi Z, Banks P, Curzen N. Gender and responses to aspirin and clopidogrel: insights using short thrombelastography. Cardiovasc Ther. 2009; 27:246-252. [DOI] [PubMed] [Google Scholar]
- 13.Folsom AR, Aleksic N, Sanhueza A, Boerwinkle E. Risk factor correlates of platelet and leukocyte markers assessed by flow cytometry in a population‐based sample. Atherosclerosis. 2009; 205:272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pendyala LK, Torguson R, Loh JP, Devaney JM, Chen F, Kitabata H, Minha S, Barbash IM, Suddath WO, Satler LF, Pichard AD, Waksman R. Racial disparity with on‐treatment platelet reactivity in patients undergoing percutaneous coronary intervention. Am Heart J. 2013; 166:266-272. [DOI] [PubMed] [Google Scholar]
- 15.Singla A, Bliden KA, Tantry US, Tabrizchi A, Ens G, Guyer K, Antonino M, Gurbel PA. First report of dose dependent effect of statin therapy on urinary 11‐dehydrothromboxane B2 levels in patients treated with aspirin: a new marker for personalizing statin therapy. Circulation. 2012; 126:A15997 [Google Scholar]
- 16.Gurbel PA, Bliden KP, Zaman KA, Yoho JA, Hayes KM, Tantry US. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation. 2005; 111:1153-1159. [DOI] [PubMed] [Google Scholar]
- 17.Gurbel PA, Bliden KP, Saucedo JF, Suarez TA, DiChiara J, Antonino MJ, Mahla E, Singla A, Herzog WR, Bassi AK, Hennebry TA, Gesheff TB, Tantry US. Bivalirudin and clopidogrel with and without eptifibatide for elective stenting: effects on platelet function, thrombelastographic indexes, and their relation to periprocedural infarction results of the CLEAR PLATELETS‐2 (Clopidogrel with Eptifibatide to Arrest the Reactivity of Platelets) study. J Am Coll Cardiol. 2009; 53:648-657. [DOI] [PubMed] [Google Scholar]
- 18.Mahla E, Suarez TA, Bliden KP, Rehak P, Metzler H, Sequeira AJ, Cho P, Sell J, Fan J, Antonino MJ, Tantry US, Gurbel PA. Platelet function measurement‐based strategy to reduce bleeding and waiting time in clopidogrel‐treated patients undergoing coronary artery bypass graft surgery: the timing based on platelet function strategy to reduce clopidogrel‐associated bleeding related to CABG (TARGET‐CABG) study. Circ Cardiovasc Interv. 2012; 5:261-269. [DOI] [PubMed] [Google Scholar]
- 19.Gurbel PA, Bliden KP, Antonino MJ, Gesheff T, Cummings CC, Dubois BV, Herzog WR, Tantry US. Time dependence of clopidogrel loading effect: platelet activation versus platelet aggregation. Thromb Res. 2012; 129:1-2. [DOI] [PubMed] [Google Scholar]
- 20.Gurbel PA, Bliden KP, Guyer K, Aggarwal N, Tantry US. Delayed thrombin‐induced platelet‐fibrin clot generation by clopidogrel: a new dose‐related effect demonstrated by thrombelastography in patients undergoing coronary artery stenting. Thromb Res. 2007; 119:563-570. [DOI] [PubMed] [Google Scholar]
- 21.Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, Bassi AK, Tantry US. Platelet reactivity in patients and recurrent events post‐stenting: results of the PREPARE POST‐STENTING Study. J Am Coll Cardiol. 2005; 46:1820-1826. [DOI] [PubMed] [Google Scholar]
- 22.Bobbert P, Stellbaum C, Steffens D, Schütte C, Bobbert T, Schultheiss HP, Rauch U. Postmenopausal women have an increased maximal platelet reactivity compared to men despite dual antiplatelet therapy. Blood Coagul Fibrinolysis. 2012; 23:723-728. [DOI] [PubMed] [Google Scholar]
- 23.Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, Bray PF. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood. 2000; 95:2289-2296. [PubMed] [Google Scholar]
- 24.Wu GJ, Lee JJ, Chou DS, Jayakumar T, Hsiao G, Chen WF, Sheu JR. Inhibitory signaling of 17β‐estradiol in platelet activation: the pivotal role of cyclic AMP‐mediated nitric oxide synthase activation. Eur J Pharmacol. 2010; 649:140-149. [DOI] [PubMed] [Google Scholar]
- 25.Caulin‐Glaser T, García‐Cardeña G, Sarrel P, Sessa WC, Bender JR. 17 beta‐estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997; 81:885-892. [DOI] [PubMed] [Google Scholar]
- 26.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999; 340:1801-1811. [DOI] [PubMed] [Google Scholar]
- 27.Bray PF. Platelet glycoprotein polymorphisms as risk factors for thrombosis. Curr Opin Hematol. 2000; 7:284-289. [DOI] [PubMed] [Google Scholar]
- 28.Kunicki TJ, Williams SA, Salomon DR, Harrison P, Crisler P, Nakagawa P, Mondala TS, Head SR, Nugent DJ. Genetics of platelet reactivity in normal, healthy individuals. J Thromb Haemost. 2009; 7:2116-2122. [DOI] [PubMed] [Google Scholar]
- 29.Faraday N, Yanek LR, Mathias R, Herrera‐Galeano JE, Vaidya D, Moy TF, Fallin MD, Wilson AF, Bray PF, Becker LC, Becker DM. Heritability of platelet responsiveness to aspirin in activation pathways directly and indirectly related to cyclooxygenase‐1. Circulation. 2007; 115:2490-2496. [DOI] [PubMed] [Google Scholar]
- 30.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p‐450 polymorphisms and response to clopidogrel. N Engl J Med. 2009; 360:354-362. [DOI] [PubMed] [Google Scholar]
- 31.Feuring M, Wehling M, Burkhardt H, Schultz A. Coagulation status in coronary artery disease patients with type II diabetes mellitus compared with non‐diabetic coronary artery disease patients using the PFA‐100® and ROTEM®. Platelets. 2010; 21:616-622. [DOI] [PubMed] [Google Scholar]
- 32.Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011; 123:798-813. [DOI] [PubMed] [Google Scholar]
- 33.Bowbrick VA, Mikhailidis DP, Stansby G. Influence of platelet count and activity on thromboelastography parameters. Platelets. 2003; 14:219-224. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald SG, Luddington RJ. Critical factors contributing to the thromboelastography trace. Semin Thromb Hemost. 2010; 36:712-722. [DOI] [PubMed] [Google Scholar]
- 35.Gaglia MA, Steinberg DH, Pinto Slottow TL, Roy PK, Bonello L, Delabriolle A, Lemesle G, Okabe T, Torguson R, Kaneshige K, Xue Z, Suddath WO, Kent KM, Satler LF, Pichard AD, Lindsay J, Waksman R. Racial disparities in outcomes following percutaneous coronary intervention with drug‐eluting stents. Am J Cardiol. 2009; 103:653-658. [DOI] [PubMed] [Google Scholar]
- 36.Kornowski R, Vaknin‐Assa H, Assali A, Lev EI, Porter A, Battler A, Bental T. A comparative analysis of major clinical outcomes with drug‐eluting stents versus bare metal stents in male versus female patients. Eurointervention. 2012; 7:1051-1059. [DOI] [PubMed] [Google Scholar]


