Abstract
Background
Few studies have investigated functional capacity self‐assessment tools in either prediction of future major adverse cardiac outcomes beyond all‐cause mortality or direct comparisons with clinically available biomarkers.
Methods and Results
We estimated functional capacity using the Duke Activity Status Index (DASI) questionnaire in 8987 sequential stable patients without acute coronary syndrome who were undergoing elective diagnostic coronary angiography with 3‐year follow‐up of major adverse cardiac events (death, nonfatal myocardial infarction, or stroke). A low DASI score provided independent prediction of a 4.8‐fold increase in future risk of incident major adverse cardiac events at 3 years (quartiles 1 versus 4 hazard ratio [95% CI] 4.76 [4.03 to 5.61], P<0.001), and a 3.8‐fold increased risk after adjusting for traditional risk factors (3.77 [3.15 to 4.51], P<0.001). The prognostic value of the DASI score was evident in both primary and secondary prevention cohorts, with and without heart failure, as well as high and low C‐reactive protein and B‐type natriuretic peptide levels. The DASI score reclassified 15% of patients (P<0.001) beyond traditional risk factors in predicting future MACE.
Conclusion
A simple self‐assessment tool of functional capacity in stable patients undergoing elective diagnostic cardiac evaluation provides independent and incremental prognostic value for prediction of both significant coronary angiographic disease and long‐term adverse clinical events.
Keywords: functional capacity impairment, Duke activity status index, prognosis, coronary artery disease
Introduction
Risk stratification plays an increasing role in contemporary management of patients with cardiovascular disease. Traditionally, the presence of specific clinical signs and symptoms coupled with abnormalities in cardiac‐specific measurements and estimates of end‐organ dysfunction generates an integrated view of an individual patient's risk profile. Recently, the broad acceptance of novel blood‐based biomarkers has emerged in the arena of risk stratification, even though the true incremental value of such measures in routine clinical practice has been challenged in population‐based studies.1–2
Cardiovascular health relies heavily on maintaining appropriate dietary behaviors and physical activities.3 The ability to determine functional capacity has relied on subjective assessment based on direct patient interviews during the history and physical examination and objectively by quantifying measures identified from standardized exercise testing. Standardized instruments to assess functional capacity and/or health status have been developed,4–5 but their clinical adoption has been challenged by both logistical hurdles and an emphasis on “hard” clinical end points in the determination of treatment responses. Remarkably, few studies have undertaken the prospective validation of the functional capacity measures in the prediction of future major adverse cardiac outcomes beyond all‐cause mortality alone or direct comparisons with clinically available biomarkers. Here, we sought to determine the prognostic value of estimating functional capacity using a simple self‐administered assessment tool in a contemporary stable cardiac patient population.
Methods
Study Population
The Cleveland Clinic Institutional Review Board approved the study. The GeneBank study was conducted via prospective screening of patients aged ≥18 years who underwent elective coronary evaluation with coronary angiography or computed tomography between 2001 and 2007. Consecutive patients were screened and considered for enrollment without intentional exclusion. At the time of enrollment, we excluded patients who had known acute coronary syndrome within 30 days of enrollment and those who were unable to comply with or unwilling to follow study protocol. All potentially eligible patients were approached, and those who agreed to participate were provided with written informed consent.
Data Synthesis
All clinical characteristics were determined by patient self‐report and confirmed by clinical histories from Electronic Medical Record. Coronary artery disease (CAD) was defined as any clinical history of myocardial infarction (MI), percutaneous coronary intervention, or coronary artery bypass graft surgery. Peripheral artery disease (PAD) was defined as a self‐reported history of non‐CAD cardiovascular disease and/or history of or repair of aortic dissection/aneurysm. Self‐reported degree of angina was quantified by Canadian Cardiovascular Society (CCS) angina classification.6
Assessment of Functional Capacity: The Duke Activity Status Index Questionnaire
The Duke Activity Status Index (DASI) questionnaire is a self‐assessment tool to estimate functional capacity,7 which includes 12 activities representative of major aspects of physical function (personal care, ambulation, household tasks, sexual function, and recreational activities). It was developed to predict an individual's maximal exercise capacity. A score is calculated based on weighted answers from 12 questions related to daily activities of living, for which each item is weighted by its known metabolic cost, and weights of positive terms are summed to form the individual patient DASI score. The possible scores range from 0 (all “no” answers) to 58.2 (all “yes” answers). The DASI assessment was prospectively performed in this study as a predefined assessment of signs and symptoms related to activities of daily living at the time of enrollment and was performed by study personnel in a systematic manner whereby only definitive answers were scored. Specifically, trained research personnel approached each patient shortly after informed consent to administer the DASI questionnaire. Estimated peak oxygen consumption was calculated according the original formula from Hlatky and colleagues7: peak Vo2 (in mL×kg−1×min−1)=0.43×DASI+9.6, whereas metabolic equivalent (MET) was calculated as: 1 MET=3.5 mL×kg−1×min−1 Vo2.
Outcome Measures
All 8987 patients were followed prospectively over the ensuing 3 years by telephone contact, mailing, and medical record review by designated research personnel independent of study investigators. All major adverse cardiovascular events (MACE), defined as death, nonfatal MI, or nonfatal stroke after enrollment, were adjudicated with source documentation. Significantly obstructive CAD was defined as any clinical history of MI, percutaneous coronary intervention, coronary artery bypass graft surgery, or angiographic evidence of CAD (≥50% stenosis) in ≥1 major coronary artery. Subsequent MI was adjudicated from source documentation with supporting evidence including elevated cardiac enzymes, significant Q‐wave definitive electrocardiographic evidence of new infarction, the presence of myocardial wall akinesis or scar on imaging, or treatment with thrombolytic agents or direct percutaneous intervention. Stroke was defined as documented loss of neurologic function caused by an ischemic event with residual symptoms continuing ≥24 hours after onset (not to include transient ischemic attacks, microvascular infarcts, or amarosis fugax).
Biomarker Analyses
Blood samples were obtained either via venipuncture (in the case of coronary computed tomography patients) or during diagnostic coronary angiographic procedure right after arterial sheath access but before any heparin administration. Blood samples were immediately processed and frozen at −80°C until analysis. The biomarker subset included 4805 patients, for whom stored samples were randomly selected to undergo biomarker testing in a central core laboratory. Specifically, high‐sensitivity C‐reactive protein (hsCRP), B‐type natriuretic peptide (BNP), creatinine, fasting lipid profile, apolipoprotein A1 (apoA1), and apolipoprotein B (apoB) were all measured in the Architect ci8200 platform (Abbott Laboratories). Myeloperoxidase (MPO) was measured by using the CardioMPO assay kit (Cleveland Heart Lab). Total leukocyte count was measured by using the ADVIA 120 Hematology System (Siemens Medical Systems).
Statistical Analysis
We used the Student's t test or Wilcoxon–rank sum test for continuous variables and χ2 test for categorical variables for between‐group comparisons. Logistic regression was used to determine the association with cardiovascular phenotype. Cox proportional hazards regression was used for time‐to‐event analysis to determine hazard ratio (HR) and 95% confidence intervals (CIs) for MACE in comparing the highest with the lowest DASI quartiles for each cohort of interest. Adjustments were made for individual traditional cardiac risk factors (including age, sex, low‐density and high‐density lipoprotein cholesterol, systolic blood pressure, former or current cigarette smoking, and diabetes mellitus) to predict incident 3‐year MACE risk. The survival curve was derived using Kaplan–Meier survival analysis. A cubic spline term of DASI was fitted in the Cox model to assess how the spectrum of DASI scores related to the HR of MACE. A nomogram for MACE based on survival models was constructed (details of the nomogram construction methods are listed in Data S1 as previously published8) to visually display the predicted probability of a MACE event from a multivariate Cox model. Net reclassification analysis was performed to quantify improvement in models with and without DASI scores. In cutoff values for net reclassification index estimation,9 we used a ratio of 6:3:1 for low‐, medium‐ and high‐risk categories. Comparison between DASI score and Adult Treatment Panel III score, which estimates the 10‐year risk of developing CAD outcomes (MI or coronary death),10 was performed by resampling (250 bootstrap samples from the whole cohort [N=8987]). All data analyses, including receiver operator characteristic analyses and area under the curve (AUC) determinations, were separately recalculated at each resampling, and the AUCs calculated from the bootstrap samples were compared. All analyses were performed using R 2.15.1 software. Values of P<0.05 were considered statistically significant.
Results
Table 1 shows the baseline characteristics of the study population, which is representative of a contemporary patient population undergoing elective diagnostic coronary angiography (see Data S2). The reasons for angiography included history of positive or indeterminate stress test (45%), evaluation for possible ischemic causes of symptoms (63%), preoperative evaluation (15%), and history of cardiomyopathy (4%). The median DASI score was 38.2 (IQR 24.2 to 50.7), which corresponded to an estimated peak oxygen consumption of 26 mL/kg per minute (IQR 20 to 31 mL/kg per minute). As expected, patients with a lower DASI score were more likely to be older and female and to have an underlying history of HF, previous MI, or PAD (Table 1). We observed the association between lower DASI score with higher likelihood of underlying CAD (quartile 4 versus quartile 1 of DASI score, unadjusted odds ratio [OR] 2.99, 95% CI 2.59 to 3.46, P<0.01) or PAD (unadjusted OR 5.65, 95% CI 4.74 to 6.73, P<0.01). After adjustment for traditional risk factors, the association remained statistically significant (CAD: adjusted OR 2.65, .95% CI 2.22 to 3.15, P<0.01; PAD: adjusted OR 4.61, 95% CI 3.73 to 5.71, P<0.01).
Table 1.
Baseline Characteristics for Whole Cohort and Across Quartiles of Duke Activity Status Index (DASI) Score
| Whole Cohort (n=8987) | Quartile 1 (n=2193) | Quartile 2 (n=2219) | Quartile 3 (n=1597) | Quartile 4 (n=2978) | P Value* | |
|---|---|---|---|---|---|---|
| DASI score | <24 | 24 to 38 | 38 to 50 | ≥51 | ||
| Age, y | 64±11 | 68±11 | 66±11 | 63±10 | 59±10 | <0.001 |
| Male, % | 68 | 52 | 65 | 71 | 80 | <0.001 |
| Smoker, % | 66 | 66 | 66 | 68 | 65 | 0.214 |
| Diabetes mellitus, % | 38 | 51 | 42 | 37 | 26 | <0.001 |
| Hypertension, % | 74 | 81 | 77 | 74 | 65 | <0.001 |
| History of CAD, % | 68 | 77 | 72 | 66 | 59 | <0.001 |
| History of MI, % | 38 | 47 | 39 | 36 | 32 | <0.001 |
| History of HF, % | 21 | 38 | 22 | 16 | 9 | <0.001 |
| History of PAD, % | 26 | 38 | 32 | 23 | 15 | <0.001 |
| History of stroke, % | 7 | 12 | 8 | 5 | 3 | <0.001 |
| History of ventricular arrhythmia, % | 9 | 11 | 10 | 8 | 8 | 0.001 |
| LVEF <50% (%) | 26 | 35 | 28 | 24 | 19 | <0.001 |
| BMI, kg/m2 | 28.7 (25.7 to 32.7) | 29.4 (25.7 to 34.6) | 29 (25.9 to 33.1) | 28.7 (25.8 to 32.5) | 28.4 (25.5 to 31.6) | <0.001 |
| LDL cholesterol, mg/dL | 95 (76 to 118) | 92 (73 to 114) | 94 (75 to 117) | 95 (76 to 118) | 98 (79 to 120) | <0.001 |
| HDL cholesterol, mg/dL | 38 (31 to 47) | 37 (30 to 47) | 38 (31 to 47) | 38 (31 to 47) | 38 (31 to 48) | <0.001 |
| Total cholesterol, mg/dL | 165 (142 to 193) | 161 (138 to 191) | 164 (141 to 191) | 164 (143 to 193) | 168 (144 to 196) | <0.001 |
| Triglycerides, mg/dL | 122 (86 to 177) | 129 (91 to 187) | 122 (88 to 177) | 122 (86 to 174) | 117 (82 to 170) | <0.001 |
| hsCRP, mg/L | 2.40 (1.04 to 5.91) | 4.13 (1.72 to 10.26) | 2.58 (1.12 to 6.03) | 2.32 (1.02 to 5.22) | 1.58 (0.77 to 3.77) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 83 (67 to 95) | 72 (54 to 89) | 81 (65 to 93) | 86 (71 to 96) | 89 (76 to 99) | <0.001 |
| MPO, pmol/L | 111 (73 to 232) | 127 (81 to 277) | 112 (74 to 218) | 108 (70 to 234) | 100 (69 to 207) | <0.001 |
| BNP, pg/mL | 95 (38 to 242) | 176 (75 to 479) | 115 (46 to 281) | 83 (35 to 178) | 58 (26 to 131) | <0.001 |
| WBC, ×109/L | 6 (5 to 8) | 6 (5 to 8) | 6 (5 to 8) | 6 (5 to 7) | 6 (5 to 7) | <0.001 |
| apoA1 | 116 (103 to 132) | 115 (101 to 134) | 116 (103 to 132) | 115 (102 to 131) | 115 (104 to 131) | <0.001 |
| apoB | 81 (69 to 96) | 81 (69 to 96) | 82 (69 to 95) | 82 (69 to 96) | 81 (69 to 96) | <0.001 |
| Baseline medications | ||||||
| ACE inhibitors/ARBs, % | 50 | 57 | 52 | 52 | 43 | <0.001 |
| β‐Blockers, % | 62 | 66 | 64 | 61 | 57 | <0.001 |
| Statin, % | 59 | 59 | 60 | 58 | 57 | 0.11 |
| Aspirin, % | 72 | 69 | 72 | 72 | 73 | 0.005 |
CAD indicates coronary artery disease; MI, myocardial infarction; HF, heart failure; PAD, peripheral artery disease; LVEF, left ventricular ejection fraction; BMI, body mass index; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; eGFR, estimated glomerular filtration rate; MPO, myeloperoxidase; BNP, B‐type natriuretic peptide; WBC, total leukocyte count; apoA1, apolipoprotein A1; apoB, apolipoprotein B; ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker.
Kruskal–Wallis test for multigroup comparisons.
The relationship between DASI score and incident cardiovascular risk is illustrated in Figure 1, in which the Kaplan–Meier analysis revealed that lower DASI score (across quartiles of DASI score as well as estimated METs) is associated with a greater risk of future development of MACE. Over a 3‐year prospective follow‐up period, there were a total of 829 deaths, 404 MIs, and 178 strokes in our study cohort. We observed that a lower DASI score was associated with a higher risk of future death, MI, or stroke (quartile 4 versus quartile 1 of DASI score, unadjusted HR 4.76, 95% CI 4.03 to 5.61, P<0.01). The prognostic value of DASI score was preserved when adjusted for traditional risk factors (adjusted HR 3.77, 95% CI 3.15 to 4.51, P<0.01) or even plus history of heart failure and history of PAD (adjusted HR 2.89, 95% CI 2.39 to 3.50, P<0.01) and in cohorts with and without significantly obstructive CAD (Table 2). These findings were similar across previously reported METs categories11 (Table 3). As illustrated by the cubic spline curve, the risk for future MACE appeared to be steep and linear, with HR significantly above unity, particularly for DASI scores <38 (or the median, Figure 2). Lower DASI scores also predicted higher future risk of MACE at 3 years regardless of age, sex, body mass index, diabetes mellitus, hypertension, lipid status, hsCRP status, or prior MI (all P<0.01, Figure 3). The prognostic value of DASI score was also consistent regardless of the clinically assessed CCS angina class (see Data S3). Use of DASI score over traditional risk factors was also shown to reclassify patients (net reclassification index 15%, P<0.001; integrated discrimination improvement 13%, P<0.001; C‐statistics 66.5% versus 71.5%, P<0.001). Direct head‐to‐head comparison also demonstrated that DASI score provided more robust prediction of future development of MACE over a 3‐year period than did the Adult Treatment Panel III score10 (AUC: 0.67 [95% CI 0.66 to 0.69] versus 0.59 [0.57 to 0.60], P<0.001) in our study population. Combining traditional risk factors and comorbidities with DASI score, a nomogram is constructed with a point scoring system indicative of prospective risk for future MACE at 3 years (Figure 4).
Figure 1.
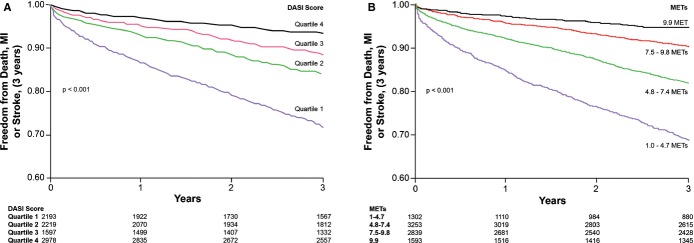
Kaplan–Meier analysis of Duke Activity Status Index (DASI) scores in predicting future risk of major adverse cardiac events in stable cardiac patients undergoing elective coronary angiography according to DASI quartiles (A) and estimated metabolic equivalents (METs, B). Ranges of DASI and METs are the same as for groups in Tables 2 and 3. MI indicates myocardial infarction.
Table 2.
Unadjusted and Adjusted Hazard Ratio (HR) for Major Adverse Cardiac Events at 3 Years Stratified According to Quartiles of DASI Score
| Quartile 4 | Quartile 3 | Quartile 2 | Quartile 1 | |
|---|---|---|---|---|
| All patients (n=8 987) | ||||
| Range | ≥51 | 38 to 50 | 24 to 38 | <24 |
| Unadjusted HR | 1 | 1.78 (1.45 to 2.19)* | 2.54 (2.12 to 3.03)* | 4.76 (4.03 to 5.61)* |
| Adjusted HR (1) | 1 | 1.58 (1.29 to 1.95)* | 2.16 (1.80 to 2.59)* | 3.77 (3.15 to 4.51)* |
| Adjusted HR (2) | 1 | 1.53 (1.24 to 1.89)* | 1.87 (1.54 to 2.25)* | 2.89 (2.39 to 3.50)* |
| Event rate | 187/2978 | 176/1597 | 341/2219 | 587/2193 |
| Significantly obstructive CAD (n=6 520) | ||||
| Range | ≥51 | 37 to 51 | 23 to 37 | <23 |
| Unadjusted HR | 1 | 1.63 (1.32 to 2.03)* | 2.37 (1.94 to 2.89)* | 4.21 (3.51 to 5.06)* |
| Adjusted HR (1) | 1 | 1.44 (1.16 to 1.78)* | 2.06 (1.68 to 2.52)* | 3.48 (2.86 to 4.25)* |
| Adjusted HR (2) | 1 | 1.38 (1.11 to 1.73)* | 1.76 (1.43 to 2.17)* | 2.66 (2.16 to 3.27)* |
| Event rate | 151/1876 | 185/1431 | 287/1584 | 485/1629 |
| Not significantly obstructive CAD (n=2 467) | ||||
| Range | ≥58 | 45 to 58 | 30 to 45 | <30 |
| Unadjusted HR | 1 | 1.62 (0.88 to 2.99) | 3.32 (1.95 to 5.65)* | 5.63 (3.38 to 9.37)* |
| Adjusted HR (1) | 1 | 1.71 (0.91 to 3.23) | 3.58 (2.05 to 6.28)* | 5.37 (3.06 to 9.42)* |
| Adjusted HR (2) | 1 | 1.76 (0.92 to 3.35) | 3.42 (1.92 to 6.08)* | 4.66 (2.59 to 8.40)* |
| Event rate | 18/676 | 24/558 | 56/645 | 85/588 |
Model 1: adjusted for traditional risk factors including age, sex, systolic blood pressure, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, smoking, and diabetes mellitus. Model 2: adjusted for Model 1 plus history of heart failure and peripheral artery disease. CAD indicates coronary artery disease; DASI, Duke Activity Status Index.
P<0.01.
Table 3.
Unadjusted and Adjusted Hazard Ratio (HR) for Major Adverse Cardiac Events at 3 Years Stratified According to Previously Reported Metabolic Equivalents (METs) Categories11
| Range | 9.9 METs | 7.5 to 9.8 METs | 4.8 to 7.4 METs | 1.0 to 4.7 METs |
|---|---|---|---|---|
| Unadjusted HR | 1 | 1.88 (1.46 to 2.42)* | 3.63 (2.87 to 4.6)* | 6.86 (5.38 to 8.75)* |
| Adjusted HR (1) | 1 | 1.63 (1.25 to 2.11)* | 2.86 (2.23 to 3.66)* | 5.21 (4.00 to 6.79)* |
| Adjusted HR (2) | 1 | 1.64 (1.25 to 2.14)* | 2.47 (1.91 to 3.21)* | 3.97 (3.00 to 5.26)* |
| Event rate | 79/1593 | 264/2839 | 562/3253 | 386/1302 |
Model 1: adjusted for traditional risk factors including age, sex, systolic blood pressure, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and smoking, diabetes mellitus. Model 2: adjusted for model 1 plus history of heart failure and peripheral artery disease.
P<0.01.
Figure 2.
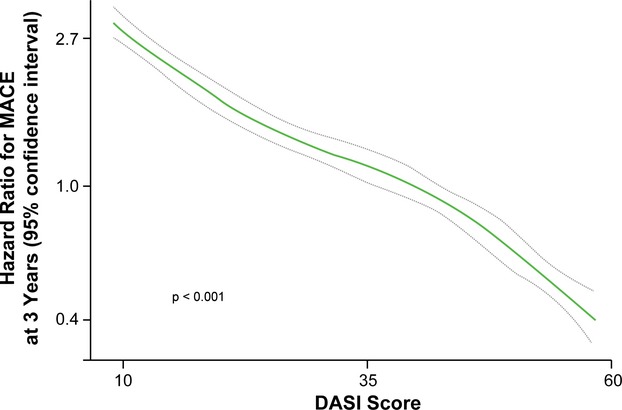
Cubic spline curve for hazard ratios for major adverse clinical events (MACE) at 3 years with Duke Activity Status Index (DASI) scores.
Figure 3.
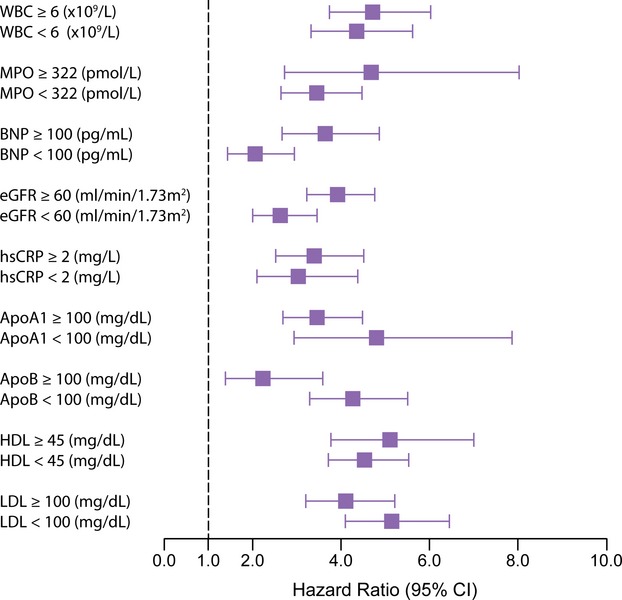
Subgroup analysis of Duke Activity Status Index (DASI) scores and future risk of major adverse cardiac events (MACE) according to standard cardiac biomarkers. Hazard ratio (x‐axis) of 3‐year MACE similar to that presented in Table 2 (quartile 4 vs quartile 1 of DASI score) but across subgroups of different biomarker cutoffs. apoA1 indicates apolipoprotein A1; apoB, apolipoprotein B; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; MPO, myeloperoxidase; WBC, white blood cell count.
Figure 4.
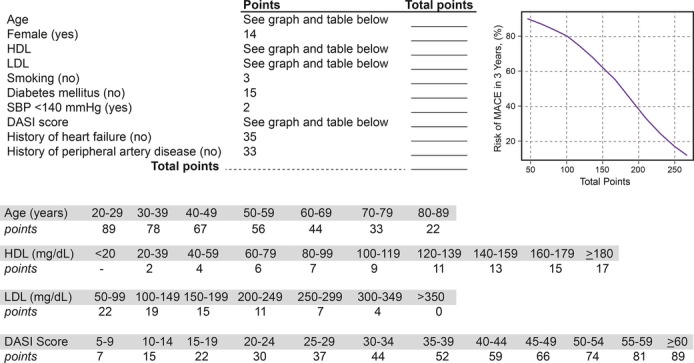
Nomogram for estimating risk of major adverse cardiac events (MACE) incorporating Duke Activity Status Index (DASI) score with traditional risk factors. Methodology as described in Data S1. HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
In the subset of patients with biomarkers measured (n=4805), there were no significant differences in baseline characteristics compared with those without biomarker assessment. Lower DASI scores remained prognostically significant in both high and low levels of fasting lipid profiles, hsCRP, and BNP, as well as total leukocyte count and MPO. In fact, the predictive value of DASI score in predicting future MACE (AUC: 0.67 [95% CI 0.66 to 0.69]) appears to be comparable with commonly used prognostic cardiac biomarkers such as BNP (AUC: 0.68 [95% CI 0.66 to 0.70] or hsCRP (AUC: 0.61 [95% CI 0.59 to 0.64]). When hsCRP, BNP, and estimated glomerular filtration rate were all added to the multivariate model that included traditional risk factors, lower DASI score (quartile 4 versus quartile 1) still demonstrated a significant 2‐fold increased risk in future MACE at 3 years (adjusted HR 2.00, 95% CI 1.55 to 2.58, P<0.01).
Discussion
The key finding of this study is the strong prognostic value of functional capacity estimated by using a simple 12‐question evaluation tool in a large, contemporary cohort of stable cardiac patients undergoing coronary angiography. We observed that the functional capacity estimated with the DASI score is incremental and superior to that of traditional cardiovascular risk factors, with or without underlying heart failure. We further established the association between functional capacity estimated by DASI score and markers of inflammation and oxidative stress in a subset of patients. Taken together, our findings underscore the relative importance of objectively determining the functional capacity for purposes of risk stratification in cardiac patients.
Although the DASI questionnaire was developed over 2 decades ago, the primary focus in using this self‐assessment tool thus far has been to estimate functional capacity in the setting of preoperative evaluation or prediction of underlying ischemia.7,12–13 While the original objective of DASI was to estimate functional capacity, the responses can also be used to assess physical limitations relevant to an individual's quality of life and to potentially uncover significant problems related to patients who experienced CAD, PAD, or heart failure. The importance of prognostication of DASI in our study, particularly with its strong incremental value to traditional risk factors and cardiac biomarkers, underscores the need to systematically assess how DASI score can be of clinical utility. Indeed, the findings of our study may not be limited to DASI or even to CAD patients, in that other instruments that assess daily physical activity such as the RAND Physical Limitation Scale14 or the Kansas City Cardiomyopathy Questionnaire for heart failure15–16 have also demonstrated their incremental prognostic utilities and responses to therapeutic interventions. These latter instruments aim to assess health status and have not been evaluated as to whether they correlate directly with functional capacity.17 Interestingly, the design of DASI queries an individual's self‐perception of his or her functional capacity (ie, what an individual is able to do) rather than a direct recall of prior physical activities and/or limitations. Therefore, the DASI questionnaire may potentially overestimate the actual functional capacity as illustrated in the validation studies,18 yet this further underscores an even greater importance for a standardized instrument such as the DASI questionnaire to estimate functional capacity when the ability to reclassify risk may be up to 15% as shown in our study.
While the prognostic value for functional capacity as determined by standardized exercise testing has been well established, the requirements of testing skills, equipment, and experience often hinder broad adoption. At the other end of the spectrum, simple grading systems such as New York Heart Association Classification and CCS angina classification have aided physicians in communicating the degrees of symptomatic severity, yet they are often subjective and imprecise. Hence, the ability for a series of questions that can be collected at the bedside (or adapted to remote monitoring devices or portals) to risk stratify patients for future MACE above and beyond established prognostic cardiac biomarkers speaks to the enduring importance of bedside evaluation of patient‐centered reporting of functional limitations in the era of biomarker testing. Our findings also highlight the limitations of any clinical or biochemical measure to adequately quantify functional limitations that may be more meaningful to patients. Based on our findings, the quest is to examine what treatment strategies (eg, the ability to detect impaired functional capacity as a target of physical training and cardiac rehabilitation) can be effectively incorporated in those patients with low DASI scores to improve their perceived or objective functional capacity and to determine whether such interventions can modify the natural history of the disease.
Few studies have directly examined the potential use of DASI to predict long‐term adverse cardiovascular risk in a broad patient population with stable cardiac disease. Indeed, the Women's Ischemia Syndrome Evaluation (WISE) study was one of the first studies to identify that functional impairment estimated by the DASI correlates with indeterminate exercise test results and is associated with an adverse prognosis among women with suspected myocardial ischemia.11 Surgical studies also illustrated the finding that poor functional capacity estimated by DASI following cardiac surgery identifies patients who are at risk for reduced long‐term survival.19 Of note, the relationship between DASI score and risk was linear and extended to the higher functional capacity end (higher score). Thus, those who achieved a maximum baseline DASI demonstrated better risk‐adjusted long‐term survival (HR 0.64; 95% CI 0.50 to 0.83; P=0.0005).19 Such a finding is consistent with improved overall survival conferred by improved functional capacity achieved during cardiac rehabilitation in the post cardiothoracic surgical setting.19 In the setting of heart failure or chronic kidney disease, the ability to assess functional status and prognosis has been demonstrated.18,20 Recent data from a large heart failure cohort further implied that those with changes in DASI scores over 1 year demonstrated stronger association with long‐term outcomes than objective assessment (6‐minute walk distance).21 These findings are supportive of the ability of improving DASI score as a potential therapeutic target. Future investigations into potential benefits of using DASI scores (especially those with DASI <38) to triage cardiac evaluation and guide therapeutic interventions, such as more in‐depth evaluation of disability, more aggressive secondary prevention strategies, more intensive cardiac rehabilitation, or closer outpatient follow‐up, are therefore warranted for this higher‐risk population.
Despite one of the largest studies conducted to date on the prognostic value of DASI and functional capacity in the cardiovascular population, our study has several limitations. The large sample size is reassuring regarding how the results can be generalized, although it may not be completely generalizable beyond the setting of coronary evaluation due to referral and ascertainment bias at the point of recruitment and study (ie, cardiac catheterization laboratory). There are also several notable limitations. First, while DASI has been validated in the past as a measure of metabolic capacity and function, there is no objective validation of functional capacity measurement in our large cohort besides angina class, and the ability to respond to the questions is required (thus excluding those that were unable to complete the questionnaires due to their underlying diseases or functional or communication limitations). We did not systematically collect baseline or interim information regarding history of atrial fibrillation and stroke in those enrolled. Second, we only have biochemical analyses on roughly half of the study cohort, although there is still adequate power to determine the incremental prognostic value of the DASI score. Third, although we have provided subgroup assessment in the non–heart failure and non‐PAD cohorts (potential confounding effect due to underlying cardiac and vascular insufficiency), we cannot exclude other reasons that affect an individual's functional limitations. Nevertheless, this also speaks to the power of this simple questionnaire that can depict risks that are otherwise unaccounted for, such as frailty, deconditioning, or psychosocial issues. Last, the fact that there is only a single time‐point assessment may require further studies to determine if improvement in DASI score can be associated with improvement in short‐ and long‐term prognosis as implied in the postsurgical or heart failure literature or with treatment strategies such as cardiac rehabilitation.19 We would also like to emphasize that the nomogram is largely derived to highlight the relative clinical contributions of DASI to the overall prognostic value when other factors of functional capacity are under consideration. All patients were used in deriving the score, and further validation of this score is warranted in an independent study population.
Conclusion
The DASI, a self‐assessment tool of functional capacity, provides strong independent and incremental prognostic value for long‐term adverse clinical events that is comparable to cardiac biomarkers in stable cardiac patients undergoing coronary evaluation.
Supplementary Material
Appendix Data supplements S1 to S3.
Sources of Funding
This research was supported by National Institutes of Health grants P01HL076491, P01HL103453, P01HL098055, R01HL103866, R01HL103931, and P20HL113452 and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439). Dr Hazen is also partially supported by a gift from the Leonard Krieger Endowment and by the Foundaton LeDucq.
Disclosures
Dr Hazen reports being listed as co‐inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr Hazen reports having been paid as a consultant or speaker for the following companies: Abbott Diagnostics, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc, Merck & Co, Inc, Pfizer Inc, and Proctor & Gamble. Dr Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc, Pfizer Inc, Proctor & Gamble, & Takeda Pharmaceuticals. Dr Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from these companies: Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc, and Siemens. All other authors reported no relationships to disclose.
References
- 1.Melander O, Newton‐Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009; 302:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton‐Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006; 355:2631-2639. [DOI] [PubMed] [Google Scholar]
- 3.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd‐Jones DM. Status of cardiovascular health in us adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003‐2008. Circulation. 2012; 125:45-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010; 122:191-225. [DOI] [PubMed] [Google Scholar]
- 5.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J. EACPR/AHA scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012; 126:2261-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letter CL. Grading of angina pectoris. Circulation. 1976; 54:522-523. [PubMed] [Google Scholar]
- 7.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self‐administered questionnaire to determine functional capacity (the duke activity status index). Am J Cardiol. 1989; 64:651-654. [DOI] [PubMed] [Google Scholar]
- 8.Harrell FE. Rms: Regression modeling strategies. R package version 4.1‐0, 2013. Available at: http://cran.R-project.Org/package=rms. Accessed September 17, 2014.
- 9.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med. 2008; 27:157-172. [DOI] [PubMed] [Google Scholar]
- 10. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III. JAMA. 2001; 285:2486-2497. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Olson MB, Kip K, Kelsey SF, Johnson BD, Mark DB, Reis SE, Mankad S, Rogers WJ, Pohost GM, Arant CB, Wessel TR, Chaitman BR, Sopko G, Handberg E, Pepine CJ, Bairey Merz CN. The value of estimated functional capacity in estimating outcome: results from the NHBLI‐Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol. 2006; 47:S36-S43. [DOI] [PubMed] [Google Scholar]
- 12.Alonso J, Permanyer‐Miralda G, Cascant P, Brotons C, Prieto L, Soler‐Soler J. Measuring functional status of chronic coronary patients. Reliability, validity and responsiveness to clinical change of the reduced version of the Duke Activity Status Index (DASI). Eur Heart J. 1997; 18:414-419. [DOI] [PubMed] [Google Scholar]
- 13.Handberg E, Johnson BD, Arant CB, Wessel TR, Kerensky RA, von Mering G, Olson MB, Reis SE, Shaw L, Bairey Merz CN, Sharaf BL, Sopko G, Pepine CJ. Impaired coronary vascular reactivity and functional capacity in women: results from the NHLBI Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2006; 47:S44-S49. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O'Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008; 359:677-687. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006; 47:752-756. [DOI] [PubMed] [Google Scholar]
- 16.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004; 110:546-551. [DOI] [PubMed] [Google Scholar]
- 17.Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Pina IL, Weinfurt KP. Relationships between changes in patient‐reported health status and functional capacity in outpatients with heart failure. Am Heart J. 2012; 163:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravani P, Kilb B, Bedi H, Groeneveld S, Yilmaz S, Mustata S. The duke activity status index in patients with chronic kidney disease: a reliability study. Clin J Am Soc Nephrol. 2012; 7:573-580. [DOI] [PubMed] [Google Scholar]
- 19.Koch CG, Li L, Lauer M, Sabik J, Starr NJ, Blackstone EH. Effect of functional health‐related quality of life on long‐term survival after cardiac surgery. Circulation. 2007; 115:692-699. [DOI] [PubMed] [Google Scholar]
- 20.Parissis JT, Nikolaou M, Birmpa D, Farmakis D, Paraskevaidis I, Bistola V, Katsoulas T, Filippatos G, Kremastinos DT. Clinical and prognostic value of Duke's activity status index along with plasma B‐type natriuretic peptide levels in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2009; 103:73-75. [DOI] [PubMed] [Google Scholar]
- 21.Spruit MA, Maeder MT, Knackstedt C, Ammann P, Jeker U, Uszko‐Lencer NH, Kiencke S, Pfisterer ME, Rickli H, Brunner‐La Rocca HP. Prognostic value of self‐reported versus objectively measured functional capacity in patients with heart failure: results from the TIME‐CHF (Trial of Intensified Versus Standard Medical Therapy in Elderly Patients With Congestive Heart Failure). J Am Coll Cardiol. 2012; 60:2125-2126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Data supplements S1 to S3.


