Abstract
Background
Glycosylated proteins partake in multiple cellular processes including inflammation. We hypothesized that GlycA, a novel biomarker of protein glycan N‐acetyl groups, is related to incident cardiovascular disease (CVD), and we compared it with high‐sensitivity C‐reactive protein (hsCRP).
Methods and Results
In 27 491 initially healthy women, baseline GlycA was quantified by nuclear magnetic resonance spectroscopy and hsCRP by an immunoturbidimetric assay. During median follow‐up of 17.2 years, 1648 incident CVD events occurred (myocardial infarction, ischemic stroke, coronary revascularization, and CVD death). GlycA and hsCRP were moderately correlated (Spearman r=0.61, P<0.0001). In Cox regression models that included age, ethnicity, smoking, blood pressure, medications, menopausal status, body mass index, and diabetes, hazard ratios for CVD across quartiles 1 to 4 of GlycA were 1.00, 1.10 (95% CI, 0.92 to 1.30), 1.34 (95% CI, 1.13 to 1.58), and 1.64 (95% CI, 1.39 to 1.93), similar to hsCRP, for which hazard ratios were 1.00, 1.18 (95% CI, 0.99 to 1.41), 1.35 (95% CI, 1.14 to 1.61), and 1.75 (95% CI, 1.47 to 2.09) (both Ptrend<0.0001). Associations were attenuated after additionally adjusting for lipids: the hazard ratio of quartile 4 versus 1 for GlycA was 1.23 (95% CI, 1.04 to 1.46; Ptrend=0.002) and for hsCRP was 1.44 (95% CI, 1.20 to 1.72; Ptrend<0.0001). Further adjustment for the other biomarker resulted in a hazard ratio of quartile 4 versus 1 for GlycA of 1.03 (95% CI, 0.85 to 1.24; Ptrend=0.41) and for hsCRP of 1.29 (95% CI, 1.06 to 1.56; Ptrend=0.001).
Conclusions
In this prospective study of initially healthy women, baseline GlycA was associated with incident CVD, consistent with a possible role for protein glycans in inflammation and CVD.
Clinical Trial Registration
URL: http//clinicaltrials.gov/. Unique identifier NCT00000479.
Keywords: cardiovascular, epidemiology, glycoproteins, inflammation
Introduction
Post‐translational modification of proteins enhances their functional heterogeneity and is important for many biological processes.1 The most common post‐translational modification process is protein glycosylation, which involves enzymatically adding a glycan (carbohydrate) moiety to the protein.1 Recent studies have established the relevance of glycosylated proteins to a variety of key biological processes, including cell adhesion, molecular trafficking and clearance, signal transduction, and, importantly, modulation of the innate immune system and control of immune cell homeostasis and inflammation.2–4 Indeed, the mammalian “glycome” may even be larger than the proteome and involves >1% of the genome (>100 genes).2,5 Examples of clinically relevant glycans include the human blood groups,4,6 the antithrombotic drug glycan heparin,7 and most inflammatory proteins.8–9 Elaboration of protein function via structural changes on their glycan attachments has led to interest in targeting glycans as early indicators of disease.9 To date, glycans have been difficult to measure in clinical populations because of their low plasma concentrations and the lack of sensitive, rapid, and high‐throughput technology.
Proton nuclear magnetic resonance (NMR) spectroscopy can detect circulating levels of plasma glycoproteins by suppressing resonance from high‐molecular‐mass plasma proteins.10 Through the initial work by Bell et al,10 proton NMR signals of human plasma were assigned to unique N‐acetyl methyl protons of N‐acetylated carbohydrate side chains that were covalently linked to acute‐phase plasma proteins. Furthermore, their work suggested that glycoproteins of acute‐phase reactants may be useful for the detection, prognosis, and therapeutic monitoring of tissue damage marked by inflammation. Recently, LipoScience Inc developed a research application called GlycA which quantifies the NMR signal that originates from the N‐acetyl methyl groups of the N‐acetylglucosamine residues located on specific glycan branches of particular plasma proteins (mainly α1‐acid glycoprotein, haptoglobin, α1‐antitrypsin, α1‐antichymotrypsin, and transferrin) (J. Otvos, PhD, LipoScience Inc, personal communication, 2014). The proton NMR spectra to which GlycA was assigned overlaps with the region previously identified by Bell et al;10 hence, GlycA is hypothesized to be a clinical marker of systemic inflammation and may also be a biomarker of cardiovascular risk.
To date, this hypothesis has not been tested in clinical populations. To address this question, GlycA was measured with NMR spectroscopy in 27 491 initially healthy women who were followed prospectively for 17.2 years for incident cardiovascular disease (CVD) events.11–12 We aimed to examine the association of baseline GlycA concentration with incident CVD events and to compare it with another commonly used clinical biomarker of chronic inflammation, high‐sensitivity C‐reactive protein (hsCRP). In addition, we aimed to evaluate the joint association of GlycA and hsCRP on the risk of future cardiovascular events to assess whether GlycA provided additional clinical utility for the risk of future cardiovascular events beyond the information conveyed by hsCRP.
Methods
Study Population
Study participants were from the Women's Health Study, a completed, randomized, double‐blinded, placebo‐controlled trial of low‐dose aspirin and vitamin E in the primary prevention of CVD and cancer in women.11 Participants were apparently healthy female health care professionals in the United States who were aged 45 years or older and free of self‐reported CVD or cancer at study entry. All participants provided written informed consent, and the institutional review board of the Brigham and Women's Hospital (Boston, MA) approved the study protocol. At enrollment, participants completed questionnaires on demographics, anthropometrics, medical history, and lifestyle behaviors. A blood sample was requested, but not required, from the 39 876 women who were randomized; 28 345 women provided a sample. Of the women who provided a blood sample at baseline, we obtained both GlycA and hsCRP measurements for 27 491 participants who were then included in the present study.
Laboratory Measurements
The blood samples obtained at enrollment were collected in EDTA tubes and stored in vapor‐phase liquid nitrogen (−170°C) until the time for laboratory analysis. GlycA signals were quantified at LipoScience Inc from plasma NMR spectra obtained from the automated NMR Profiler system. NMR signal amplitudes originating from the N‐acetyl methyl group protons of the N‐acetylglucosamine moieties located on the bi‐, tri‐, and tetra‐antennary branches of specific serum proteins (mainly α1‐acid glycoprotein, haptoglobin, α1‐antitrypsin, α1‐antichymotrypsin, and transferrin) were used to calculate the concentrations of GlycA (μmol/L of N‐acetyl methyl groups). The GlycA NMR signal is centered at 2.00±0.01 ppm in the NMR spectra of plasma, and only N‐acetylglucosamine with specific glycosidic linkage, namely, β (1→2) or β (1→6) with a preceding mannose residue, contribute to the GlycA signal (J. Otvos, PhD, LipoScience Inc, personal communication, 2014).
Measurement of hsCRP was performed with a high‐sensitivity immunoturbidimetric assay on the Hitachi 917 autoanalyzer (Roche Diagnostics), with reagents and calibrators from Denka Seiken, as described previously.13 Standard lipids were measured in a laboratory certified by the National Heart, Lung, and Blood Institute and Centers for Disease Control and Prevention Lipid Standardization Program, as described previously.12,14
Assessment of Other Variables
Covariables of interest were self‐reported on the questionnaire administered at study entry and included age, race or ethnicity, smoking, menopausal status, postmenopausal hormone use, parental history of premature myocardial infarction, use of cholesterol medication, history of diabetes mellitus, and hypertension (physician diagnosis of hypertension, antihypertensive treatment, or self‐reported systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg). Body mass index was calculated as the weight in kilograms divided by the height in square meters.
Ascertainment of Cardiovascular Events
The primary end point of total CVD was first cardiovascular event as defined in the Women's Health Study protocol.11 This was a composite of nonfatal coronary heart disease (CHD; nonfatal myocardial infarction and coronary revascularization procedures), nonfatal ischemic stroke, and death from cardiovascular causes. We separately examined the composite end points of incident CHD (nonfatal myocardial infarction, coronary revascularization, or coronary death) and ischemic stroke. Information on the occurrence of these end points was ascertained via annual follow‐up questionnaires, letters, and telephone calls. Following written informed consent, medical records were obtained and reviewed by a blinded end points committee for the adjudication of all reported end points based on predefined criteria, as described previously.11
Statistical Analysis
Statistical analyses were performed using SAS version 9.3 (SAS Institute). Baseline characteristics of participants across the quartile distribution of GlycA were summarized as medians (25th to 75th percentiles) for quantitative variables and as percentages for qualitative variables. Comparisons across quartiles were assessed with the Wilcoxon rank sum test for quantitative variables and with χ2 tests for qualitative variables. Spearman coefficients were used to correlate GlycA and risk factors.
Person‐years of follow‐up were calculated, and Kaplan–Meier survival curves were obtained according to quartiles of GlycA and hsCRP. Cox proportional hazards regression was used to determine hazard ratios (HRs) and corresponding 95% CIs for incident events by quartiles and by 1 SD for each biomarker. Multivariable adjusted models were built in 3 stages. First we built a basic model adjusting for age, race or ethnicity, smoking, systolic blood pressure, hypertensive medication, cholesterol treatment, menopausal status, hormone use, body mass index, diabetes, and randomized trial assignments. The second model included the basic‐model variables plus standard lipids (low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and natural logarithm [log]‐transformed triglycerides). The third model evaluated the potential role of inflammation on the association between GlycA and events by additionally adjusting the second model for hsCRP. The P value for linear trend was computed by fitting a continuous variable that assigned the median value for each quartile in regression models. Following satisfactory assessment of a linear relation between GlycA and events by visual inspection of a plot of martingale residuals, we refitted the models by 1‐SD increments in the biomarkers. Log‐transformed hsCRP values were used when analyzed as a continuous variable.
Next, the joint association of GlycA and hsCRP on cardiovascular events was evaluated by dividing participants into 4 prespecified groups of high or low GlycA (greater than top tertile or less than or equal to top tertile, ie, 399 μmol/L) and high or low hsCRP (>3 or ≤3 mg/L) and was based on 9 prespecified groups according to low, medium, and high concentrations, using tertiles of GlycA and the American Heart Association and Centers for Disease Control and Prevention cut points for hsCRP (<1, 1 to 3, and >3 mg/L). Repeated analyses were also performed by applying the tertile distribution of hsCRP. Interaction between GlycA and hsCRP groups on incident events was performed by including the cross product for GlycA (categorical) and hsCRP (categorical). The proportional hazard assumption was tested by including an interaction term between the follow‐up time (log transformed) and the biomarkers in relation to events. All probability tests were 2‐tailed, with values <0.05 considered statistically significant.
Results
Baseline Characteristics and Correlations
The mean age of the study population at baseline was 54.7 years (SD: 7.1 years). Median concentrations for GlycA and hsCRP were 369 μmol/L (25th to 75th percentile: 326 to 416 μmol/L) and 2.03 mg/L (25th to 75th percentile: 0.81 to 4.38 mg/L), respectively. Increasing quartiles of GlycA were associated with a higher prevalence of traditional CVD risk factors and higher concentrations of hsCRP (Table 1). GlycA correlated positively with hsCRP (Spearman r=0.61, P<0.0001). GlycA also correlated positively in increasing magnitude with age, low‐density lipoprotein cholesterol, body mass index, and triglycerides (Spearman r=0.12 to 0.47, P for all <0.0001) and negatively with high‐density lipoprotein cholesterol (Spearman r=−0.27, P<0.0001).
Table 1.
Baseline Characteristics According to Quartiles of GlycA
| Quartiles of GlycA, μmol/L | ||||
|---|---|---|---|---|
| ≤326 n=6983 | 327 to 369 n=6797 | 370 to 416 n=6866 | ≥417 n=6845 | |
| Age, y | 51 (48 to 57) | 53 (49 to 59) | 54 (49 to 60) | 54 (50 to 60) |
| Race or ethnicity | ||||
| White | 94.5 | 95.6 | 95.7 | 95.0 |
| Hispanic | 1.0 | 1.2 | 1.1 | 1.0 |
| Black | 1.7 | 1.7 | 1.6 | 2.5 |
| Other | 2.8 | 1.59 | 1.6 | 1.1 |
| Postmenopausal, % | 46.0 | 53.8 | 58.0 | 60.7 |
| Hormone use, % | 36.8 | 41.7 | 46.4 | 49.9 |
| Diabetes, % | 1.1 | 1.7 | 2.4 | 5.5 |
| Hypertension, % | 14.5 | 20.8 | 27.0 | 38.5 |
| Parental history of premature myocardial infarction, % | 11.9 | 12.4 | 12.8 | 14.3 |
| Current smoking, % | 7.7 | 10.6 | 12.6 | 15.8 |
| Cholesterol treatment, % | 1.3 | 2.3 | 3.2 | 6.0 |
| Body mass index, kg/m2 | 22.9 (21.2 to 25.1) | 24.2 (22.2 to 27.2) | 25.7 (23.2 to 29.1) | 27.5 (24.6 to 31.8) |
| hsCRP, mg/L | 0.72 (0.35 to 1.54) | 1.51 (0.72 to 2.89) | 2.6 (1.4 to 4.6) | 5.1 (2.8 to 8.3) |
| Hemoglobin A1c, % | 4.9 (4.8 to 5.1) | 5.0 (4.8 to 5.2) | 5.0 (4.9 to 5.2) | 5.1 (4.9 to 5.3) |
| Total cholesterol, mg/dL | 197 (175 to 221) | 207 (184 to 232) | 213 (189 to 239) | 219 (193 to 247) |
| LDL cholesterol, mg/dL | 113 (94 to 133) | 121 (101 to 144) | 126 (104 to 148) | 129 (106 to 152) |
| HDL cholesterol, mg/dL | 57 (48 to 68) | 53 (45 to 64) | 50 (42 to 60) | 47 (39 to 56) |
| Triglycerides, mg/dL | 86 (65 to 116) | 111 (82 to 154) | 133 (97 to 186) | 168 (120 to 236) |
Values shown are medians (25th to 75th percentile) or percentages. P values were obtained from Wilcoxon rank sum test for quantitative variables and χ2 tests for qualitative variables. All P values for trend across quartiles were <0.001. HDL indicates high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein.
Associations With Incident CVD
Over a median follow‐up of 17.2 years, a total of 1648 CVD events occurred (1089 CHD and 462 ischemic strokes). Kaplan–Meier curves for CVD event–free survival diverged according to quartiles of GlycA and hsCRP (Plog‐rank<0.0001 for both) (Figure 1).
Figure 1.
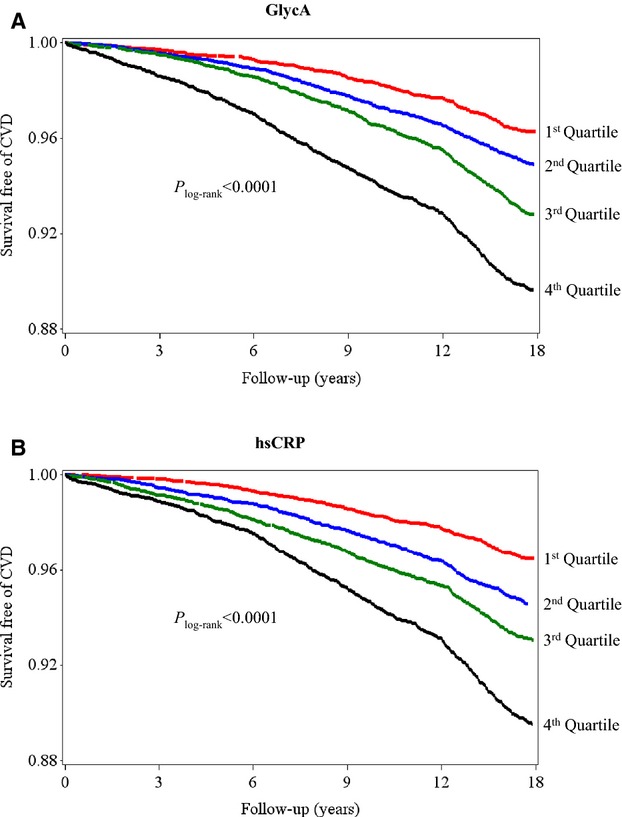
Kaplan–Meier curves of incident CVD according to quartiles of GlycA (A) and hsCRP (B). Quartile concentrations were ≤326, 327 to 369, 370 to 416, and ≥417 μmol/L for GlycA and ≤0.81, 0.82 to 2.03, 2.04 to 4.38, and ≥4.39 mg/L for hsCRP. CVD indicates cardiovascular disease; hsCRP, high‐sensitivity C‐reactive protein.
In Cox regression models that included age, ethnicity, smoking, blood pressure, medications, menopausal status, body mass index, diabetes, HRs for CVD across quartiles 1 to 4 of GlycA were 1.00, 1.10 (95% CI, 0.92 to 1.30), 1.34 (95% CI, 1.13 to 1.58), and 1.64 (95% CI, 1.39 to 1.93), similar to hsCRP, for which HRs were 1.00, 1.18 (95% CI, 0.99 to 1.41), 1.35 (95% CI, 1.14 to 1.61), and 1.75 (95% CI, 1.47 to 2.09) (both Ptrend<0.0001) (Table 2). Associations were attenuated after additionally adjusting for lipids: the quartile 4 versus 1 GlycA HR was 1.23 (95% CI, 1.04 to 1.46; Ptrend=0.002), and the hsCRP HR was 1.44 (95% CI, 1.20 to 1.72; Ptrend<0.0001). Further adjustment for the other biomarker resulted in quartile 4 versus 1 HRs for GlycA of 1.03 (95% CI, 0.85 to 1.24; Ptrend=0.41) and for hsCRP of 1.29 (95% CI, 1.06 to 1.56; Ptrend=0.001). Similar results were obtained when the biomarkers were examined as continuous variables per 1 SD, except that GlycA remained significantly associated with CVD after adjusting for hsCRP (HR 1.08; 95% CI, 1.01 to 1.15; P=0.02); the corresponding value for hsCRP was 1.16 (95% CI, 1.08 to 1.25; P<0.0001).
Table 2.
Association of GlycA and hsCRP With Incident Cardiovascular Disease (n=1648 Events)
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Linear Trend | Per 1 SD* | P Value | |
|---|---|---|---|---|---|---|---|
| GlycA | |||||||
| Range, μmol/L | ≤326 | 327 to 369 | 370 to 416 | ≥417 | |||
| Incidence rate per 1000 person‐years | 2.1 | 2.9 | 4.1 | 6.2 | |||
| Basic model | 1.00 | 1.10 (0.92 to 1.30) | 1.34 (1.13 to 1.58) | 1.64 (1.39 to 1.93) | <0.0001 | 1.26 (1.19 to 1.32) | <0.0001 |
| Basic model plus lipids | 1.00 | 0.97 (0.81 to 1.16) | 1.08 (0.91 to 1.28) | 1.23 (1.04 to 1.46) | 0.002 | 1.15 (1.08 to 1.21) | <0.0001 |
| Basic model plus lipids plus hsCRP | 1.00 | 0.91 (0.77 to 1.09) | 0.97 (0.81 to 1.15) | 1.03 (0.85 to 1.24) | 0.41 | 1.08 (1.01 to 1.15) | 0.02 |
| hsCRP | |||||||
| Range, mg/L | ≤0.81 | 0.82 to 2.03 | 2.04 to 4.38 | ≥4.39 | |||
| Incidence rate per 1000 person‐years | 2.0 | 3.1 | 4.0 | 6.2 | |||
| Basic model | 1.00 | 1.18 (0.99 to 1.41) | 1.35 (1.14 to 1.61) | 1.75 (1.47 to 2.09) | <0.0001 | 1.28 (1.20 to 1.36) | <0.0001 |
| Basic model plus lipids | 1.00 | 1.07 (0.89 to 1.27) | 1.13 (0.95 to 1.35) | 1.44 (1.20 to 1.72) | <0.0001 | 1.21 (1.13 to 1.29) | <0.0001 |
| Basic model plus lipids plus GlycA | 1.00 | 1.04 (0.87 to 1.24) | 1.06 (0.89 to 1.28) | 1.29 (1.06 to 1.56) | 0.001 | 1.16 (1.08 to 1.25) | <0.0001 |
Basic model for each biomarker is adjusted for age, ethnicity, smoking, systolic blood pressure, hypertensive medications, cholesterol treatment, postmenopausal status, hormone use, body mass index, diabetes, and trial treatment assignments. Lipids include low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and log triglycerides. SD is 68 μmol/L for GlycA and 5.7 mg/L for hsCRP. hsCRP indicates high‐sensitivity C‐reactive protein.
hsCRP was log transformed.
Results of secondary analyses for GlycA and the individual end points of CHD and ischemic stroke generally mirrored the main results for CVD (Table 3).
Table 3.
Association of GlycA and hsCRP With Incident Events
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Linear Trend | Per 1 SD* | P Value | |
|---|---|---|---|---|---|---|---|
| Coronary heart disease (no. events=1089) | |||||||
| GlycA | |||||||
| Range, μmol/L | ≤326 | 327 to 369 | 370 to 416 | ≥417 | |||
| Incidence rate per 1000 person‐years | 1.2 | 1.8 | 2.9 | 4.2 | |||
| Basic model | 1 | 1.13 (0.90 to 1.41) | 1.59 (1.29 to 1.96) | 1.78 (1.44 to 2.20) | <0.0001 | 1.28 (1.20 to 1.36) | <0.0001 |
| Basic model plus lipids | 1 | 0.95 (0.76 to 1.20) | 1.19 (0.96 to 1.47) | 1.22 (0.98 to 1.52) | 0.02 | 1.13 (1.05 to 1.21) | 0.0006 |
| Basic model plus lipids plus hsCRP | 1 | 0.89 (0.71 to 1.12) | 1.05 (0.85 to 1.31) | 1.00 (0.79 to 1.26) | 0.69 | 1.05 (0.97 to 1.13) | 0.23 |
| hsCRP | |||||||
| Range, mg/L | ≤0.81 | 0.82 to 2.03 | 2.04 to 4.38 | ≥4.39 | |||
| Incidence rate per 1000 person‐years | 1.3 | 2.0 | 2.7 | 4.2 | |||
| Basic model | 1 | 1.17 (0.94 to 1.46) | 1.36 (1.09 to 1.69) | 1.76 (1.42 to 2.19) | <0.0001 | 1.32 (1.22 to 1.43) | <0.0001 |
| Basic model plus lipids | 1 | 1.01 (0.81 to 1.26) | 1.06 (0.85 to 1.33) | 1.36 (1.08 to 1.70) | 0.0002 | 1.23 (1.13 to 1.33) | <0.0001 |
| Basic model plus lipids plus GlycA | 1 | 0.98 (0.79 to 1.23) | 1.01 (0.81 to 1.27) | 1.23 (0.97 to 1.57) | 0.01 | 1.19 (1.09 to 1.31) | 0.0002 |
| Ischemic stroke (no. events=462) | |||||||
| GlycA | |||||||
| Range, μmol/L | ≤326 | 327 to 369 | 370 to 416 | ≥417 | |||
| Incidence rate per 1000 person‐years | 0.7 | 0.9 | 1.1 | 1.6 | |||
| Basic model | 1 | 1.19 (0.87 to 1.62) | 1.21 (0.89 to 1.64) | 1.50 (1.11 to 2.03) | 0.008 | 1.22 (1.11 to 1.35) | <0.0001 |
| Basic model plus lipids | 1 | 1.13 (0.83 to 1.54) | 1.10 (0.80 to 1.50) | 1.31 (0.95 to 1.80) | 0.09 | 1.17 (1.06 to 1.30) | 0.003 |
| Basic model plus lipids plus hsCRP | 1 | 1.08 (0.79 to 1.48) | 1.01 (0.73 to 1.40) | 1.14 (0.81 to 1.61) | 0.48 | 1.13 (1.00 to 1.28) | 0.04 |
| hsCRP | |||||||
| Range, mg/L | ≤0.81 | 0.82 to 2.03 | 2.04 to 4.38 | ≥4.39 | |||
| Incidence rate per 1000 person‐years | 0.6 | 0.9 | 1.1 | 1.6 | |||
| Basic model | 1 | 1.16 (0.84 to 1.60) | 1.33 (0.97 to 1.83) | 1.72 (1.24 to 2.38) | 0.0003 | 1.21 (1.08 to 1.36) | 0.001 |
| Basic model plus lipids | 1 | 1.11 (0.80 to 1.53) | 1.22 (0.88 to 1.69) | 1.54 (1.10 to 2.15) | 0.004 | 1.17 (1.03 to 1.32) | 0.01 |
| Basic model plus lipids plus GlycA | 1 | 1.07 (0.77 to 1.48) | 1.14 (0.82 to 1.59) | 1.35 (0.94 to 1.93) | 0.07 | 1.09 (0.95 to 1.25) | 0.20 |
Basic model for each biomarker is adjusted for age, ethnicity, smoking, systolic blood pressure, hypertensive medications, cholesterol treatment, postmenopausal status, hormone use, body mass index, diabetes, and trial treatment assignments lipids include low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and log triglycerides. SD is 68 μmol/L for GlycA and 5.7 mg/L for hsCRP. hsCRP indicates high‐sensitivity C‐reactive protein.
hsCRP was log transformed.
Joint Analysis of GlycA and hsCRP With Incident CVD
Kaplan–Meier curves for the joint analyses based on the 4 prespecified groups of high and low GlycA or hsCRP are shown in Figure 2. The lowest rates of CVD‐free survival was significantly associated with high levels for both GlycA and hsCRP, and the highest rates of CVD‐free survival were significantly associated with low levels of both biomarkers (Plog‐rank<0.0001). Of note, the survival rates for women who had high levels of GlycA was indistinguishable by low or high hsCRP levels until after 6 years of follow‐up, when the survival rates became evidently lower in women with high GlycA and high hsCRP compared with those with high GlycA but low hsCRP levels.
Figure 2.
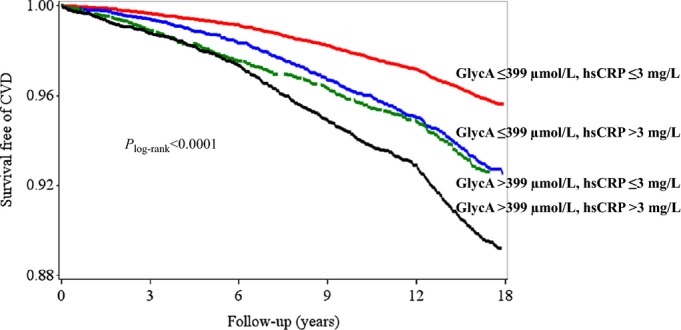
Kaplan–Meier curve of incident CVD according to joint levels of GlycA and hsCRP. High levels of GlycA were defined as greater than top tertile (>399 μmol/L). High levels of hsCRP were defined as >3 mg/L, according to clinical guidelines, which corresponded approximately to the top‐tertile value in this study. CVD indicates cardiovascular disease; hsCRP, high‐sensitivity C‐reactive protein.
Joint analyses according to the 9 prespecified groups based on GlycA tertiles and hsCRP clinical cut points are shown in Figure 3. Compared with the referent group (GlycA <341 μmol/L and hsCRP <1 mg/L), participants with GlycA >399 μmol/L and hsCRP >3 mg/L had a multivariable‐adjusted HR of 1.34 (95% CI, 1.08 to 1.65; P=0.007). In comparison, when examined separately based on these cut points, the adjusted HR for GlycA was 1.19 (95% CI, 1.03 to 1.37; P=0.02), and for hsCRP was 1.30 (95% CI, 1.11 to 1.52; P=0.001). There was no evidence of multiplicative interaction between GlycA and hsCRP groups on incident CVD (Pinteraction=0.13). Repeat analysis based on cut points derived from hsCRP tertiles yielded essentially the same result. Results of secondary joint analysis on the individual end points of CHD and ischemic stroke were similar.
Figure 3.
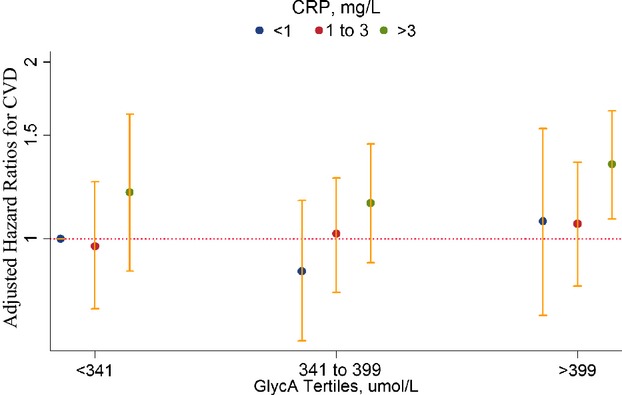
Hazard ratios for incident CVD events are shown on the y‐axis (log scale) for categories of GlycA and high‐sensitivity CRP (hsCRP). The hazard ratios are adjusted for age, ethnicity, smoking, systolic blood pressure, hypertensive medications, cholesterol treatment, postmenopausal status, use of hormone replacement therapy, body mass index, history of diabetes, trial treatment assignments, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and log triglycerides. Categories of GlycA are based on tertile cut points (<341, 341 to 399, and >399 μmol/L). Categories of hsCRP are based on clinical cut points (<1, 1 to 3, and >3 mg/L), as recommended by clinical guidelines. CRP indicates C‐reactive protein; CVD, cardiovascular disease.
Sensitivity Analysis by Follow‐Up Time
Because of a statistical interaction with time of follow‐up (P<0.05), we performed 2 additional sensitivity analyses by follow‐up time at or greater than 6 years, informed by a somewhat different divergence pattern of the Kaplan–Meier curves at 6 years versus later (Figures 1 and 2). In the analysis restricted to 6 years of follow‐up (422 CVD events; Table 4), the association for quartile 4 versus 1 of GlycA with CVD after adjusting for risk factors and hsCRP was HR 1.49 (95% CI, 1.01 to 2.19; Plinear trend=0.009). After adjusting for risk factors and GlycA, the corresponding value for hsCRP and CVD was HR 1.29 (95% CI, 0.85 to 1.96; Plinear trend=0.58). Results were similar in models examining each biomarker as a continuous variable. For participants with >6 years of follow‐up (n=26 697; 1226 CVD events), results were similar to that of the main analysis.
Table 4.
Association of GlycA and hsCRP With Incident Cardiovascular Disease by Follow‐Up Time
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Linear Trend | Per 1 SD* | P Value | |
|---|---|---|---|---|---|---|---|
| Follow‐up during 1st 6 years (no. events=422) | |||||||
| GlycA | |||||||
| Range, μmol/L | ≤326 | 327 to 369 | 370 to 416 | ≥417 | |||
| Incidence rate per 1000 person‐years | 1.2 | 1.8 | 2.4 | 5.1 | |||
| Basic model | 1 | 1.18 (0.80 to 1.72) | 1.36 (0.95 to 1.95) | 2.17 (1.54 to 3.07) | <0.0001 | 1.34 (1.22 to 1.48) | <0.0001 |
| Basic model plus lipids | 1 | 1.06 (0.72 to 1.55) | 1.13 (0.78 to 1.63) | 1.68 (1.17 to 2.40) | 0.0003 | 1.24 (1.12 to 1.38) | <0.0001 |
| Basic model plus lipids plus hsCRP | 1 | 1.02 (0.69 to 1.49) | 1.05 (0.72 to 1.53) | 1.49 (1.01 to 2.19) | 0.009 | 1.20 (1.06 to 1.35) | 0.004 |
| hsCRP | |||||||
| Range, mg/L | ≤0.81 | 0.82 to 2.03 | 2.04 to 4.38 | ≥4.39 | |||
| Incidence rate per 1000 person‐years | 1.1 | 2.0 | 3.1 | 4.1 | |||
| Basic model | 1 | 1.41 (0.96 to 2.07) | 1.92 (1.33 to 2.77) | 2.02 (1.38 to 2.95) | 0.001 | 1.29 (1.14 to 1.46) | <0.0001 |
| Basic model plus lipids | 1 | 1.26 (0.85 to 1.85) | 1.61 (1.11 to 2.34) | 1.65 (1.12 to 2.43) | 0.03 | 1.22 (1.07 to 1.38) | 0.003 |
| Basic model plus lipids plus GlycA | 1 | 1.19 (0.81 to 1.75) | 1.42 (0.97 to 2.08) | 1.29 (0.85 to 1.96) | 0.58 | 1.10 (0.95 to 1.27) | 0.21 |
| Follow‐up >6 years (no. events=1226)* | |||||||
| GlycA | |||||||
| Range, μmol/L | ≤326 | 327 to 369 | 370 to 416 | ≥417 | |||
| Incidence rate per 1000 person‐years | 1.7 | 2.3 | 3.2 | 4.3 | |||
| Basic model | 1 | 1.07 (0.88 to 1.31) | 1.33 (1.11 to 1.61) | 1.48 (1.23 to 1.79) | <0.0001 | 1.22 (1.15 to 1.30) | <0.0001 |
| Basic model plus lipids | 1 | 0.95 (0.78 to 1.15) | 1.07 (0.88 to 1.29) | 1.11 (0.91 to 1.34) | 0.14 | 1.11 (1.04 to 1.19) | 0.002 |
| Basic model plus lipids plus hsCRP | 1 | 0.89 (0.73 to 1.08) | 0.95 (0.78 to 1.16) | 0.91 (0.73 to 1.12) | 0.55 | 1.04 (0.97 to 1.12) | 0.31 |
| hsCRP | |||||||
| Range, mg/L | ≤0.81 | 0.82 to 2.03 | 2.04 to 4.38 | ≥4.39 | |||
| Incidence rate per 1000 person‐years | 1.6 | 2.4 | 2.9 | 4.6 | |||
| Basic model | 1 | 1.13 (0.93 to 1.38) | 1.21 (0.99 to 1.47) | 1.69 (1.38 to 2.06) | <0.0001 | 1.27 (1.19 to 1.37) | <0.0001 |
| Basic model plus lipids | 1 | 1.02 (0.84 to 1.25) | 1.01 (0.83 to 1.24) | 1.39 (1.13 to 1.71) | <0.0001 | 1.20 (1.11 to 1.29) | <0.0001 |
| Basic model plus lipids plus GlycA | 1 | 1.00 (0.82 to 1.23) | 0.97 (0.79 to 1.20) | 1.30 (1.04 to 1.62) | 0.0006 | 1.18 (1.08 to 1.28) | 0.0002 |
Basic model for each biomarker is adjusted for age, ethnicity, smoking, systolic blood pressure, hypertensive medications, cholesterol treatment, postmenopausal status, hormone use, body mass index, diabetes, and trial treatment assignments. Lipids include low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and log triglycerides. SD is 68 μmol/L for GlycA and 5.7 mg/L for hsCRP. hsCRP indicates high‐sensitivity C‐reactive protein.
hsCRP was log transformed.
Late follow‐up, n=26 697.
Discussion
This prospective study of 27 491 initially healthy women followed for a median of 17.2 years provides the first population‐based evidence for the potential application of protein glycan side‐chain biomarkers to convey information on incident CVD events. Specifically, we found that baseline concentrations of GlycA, an NMR‐measured protein glycan biomarker, was associated with a graded increase in the risk of incident CVD events in a manner and magnitude that was comparable to that of hsCRP. Furthermore, the association of GlycA with CVD was attenuated after adjusting for hsCRP, consistent with a possible role for protein glycans in inflammation and CVD.
An altered protein glycosylation pattern has been described as a significant event that occurs during the transition from healthy to diseased tissue. Expansion of biological information from the human genome into complex cellular function occurs in part by post‐translational modification of proteins. Protein glycosylation is the most ubiquitous of these processes.1 The diverse forms of protein glycans enable a range of physiological and pathological processes, including inflammation, which is known to promote atherosclerosis.2,15–16 Harnessing clinical utility from the evolving field of glycobiology has been hampered by analytical measurement difficulties related in part to the non–template‐driven nature of glycan biosynthesis, unlike biopolymer counterparts (eg, proteins and nucleic acids). This challenge is further complicated by the diversity of monosaccharide structure, sequence, and linkage that is present in the human glycome repertoire.17 NMR spectroscopy identifies unique protein glycans1,10 and thus may provide a high‐throughput and readily available technology for the quantitative analysis of protein glycans.
The positive association between GlycA, a nonspecific marker of systemic inflammation, and CVD risk factors, such as smoking, body mass index, hypertension, and dyslipidemia, suggest a role for modifiable lifestyle risk factors in the expression of protein glycans that produce the GlycA signal. Similar relationships with CVD risk factors were observed in a prior study examining human plasma N‐glycans measured with another technique (high‐performance liquid chromatography).18 Specific glycosylation changes have also been described for lipids. Low‐density lipoprotein particles in patients with CHD, for example, have reduced sialylation (a type of glycosylation), which may make these low‐density lipoprotein particles more susceptible to subendothelial retention, oxidative modification, and atherosclerotic plaque formation.19
Our observation that hsCRP attenuates the association of GlycA, especially in the long term, suggests that GlycA may convey CVD risk information, at least in part, through inflammation. It is well described that glycan processing is distinctly controlled during inflammation.2,15–16,20 Endothelial cell dysfunction occurring as a result of a proinflammatory stimuli, for instance, leads to pronounced changes in glycan‐processing mechanisms, which in turn direct specific glycan expression patterns on isolated human endothelial cells.20 In particular, proinflammatory stimuli have been shown to induce distinct changes in N‐glycan processing mechanisms.21–24 Adhesion molecules have also been shown to be heavily glycosylated, and the associated glycans modulate the interaction of adhesion molecules with their ligands.15,20 Furthermore, changes in endothelial cell glycosylation patterns regulate the functions of adhesion molecules at sites of early atherosclerotic plaque development. Indeed, proteins associated with chronic inflammation not only experience changes in their serum concentrations as a result of an inflammatory stimulus but also are marked by alterations in their glycan structures.8
It is worthy of note that GlycA is a composite NMR signal arising from a subset of acute‐phase reactants. As such, GlycA signals originating from different glycoproteins or from the same glycoprotein are indistinguishable by NMR. In other words, measurement of GlycA from a given volume of plasma does not provide information about the numbers of glycoproteins, the particular glycoform emitting the signal (given that glycan conjugates may vary dynamically), or the contribution of each glycoprotein to the GlycA signal. Based on known plasma concentrations of each abundant glycoprotein and the number of N‐glycosylation sites present on each, Otvos and colleagues estimated the relative contribution of known acute‐phase glycoproteins to the GlycA signal (J. Otvos, PhD, LipoScience Inc, personal communication, 2014). This estimate yielded 5 major acute‐phase glycoproteins that were then shown to produce GlycA signals in proportion to their glycan N‐acetyl glucosamine concentrations (J. Otvos, PhD, LipoScience Inc, personal communication, 2014). Their findings indicate that α1‐acid glycoprotein, haptoglobin, α1‐antitrypsin, α1‐antichymotrypsin, and transferrin—all of which have been implicated in the development of CVD18,25–30—make major contributions to the GlycA signal. Biomarker profiling by NMR spectroscopy in an Estonian Biobank study found α1‐acid glycoprotein to be significantly predictive of cardiovascular mortality, a finding that was validated in a replication cohort.18 In another recent study, investigators from the Framingham Heart Study used a systems approach to biomarker discovery and also found α1‐acid glycoprotein to be 1 of 4 multimarker proteins that significantly predicted incident atherosclerotic CVD.25 Regarding haptoglobin, functional genotypes have been linked to CVD risk, with more limited data on the association of circulating levels of haptoglobin with CVD.26–27 Furthermore, the serine protease inhibitor α1‐antitrypsin has also been linked to CVD.28–29 However, the roles of α1‐antichymotrypsin (another serine protease inhibitor) and transferrin (an iron‐binding protein) are less well characterized with respect to CVD. Other notable inflammatory proteins that have been implicated in the development and manifestation of CVD (fibrinogen, CRP and interleukin‐6)31 contribute negligibly to the GlycA signal (J. Otvos, PhD, LipoScience Inc, personal communication, 2014). Although it does not contribute much to the GlycA signal, the CRP protein is glycosylated with glycan attachments that exhibit disease specific changes.9
Our finding of a time‐dependent association of GlycA on CVD risk was not prespecified and hence may be due to chance. Additional studies will be needed to verify whether GlycA conveys short‐ to medium‐term CVD risk (up to 6 years) independent of hsCRP. Nonetheless, glycosylation is a post‐translational process that modifies protein function and is exquisitely responsive to the cellular milieu, which may provide a biological explanation of this observation. In this regard, it is possible that the glycan conjugates of acute‐phase glycoproteins that are captured as GlycA provide a better signal of imminent events than longer term events when compared with hsCRP. In addition, the small additive association of GlycA and hsCRP with CVD risk suggests that these biomarkers may play both overlapping and distinct roles in the development of CVD. Noninflammatory proteins implicated in CVD may also bear the glycan side chain that is captured by the GlycA NMR signal. Apolipoprotein B, for example, the major protein of atherogenic lipoproteins, presumably contributes (albeit negligibly) to the novel GlycA signal (J. Otvos, PhD, LipoScience Inc, personal communication, 2014).
Given the branched structure of glycan polymers, a combination of a finite number of monosaccharides can give rise to an exponential number of different glycan structures. As such the NMR method may offer a unique technology to harness important clinical information conveyed by the expression of specific protein glycans, as shown in our study. The molecular structure for any protein glycan with >1 glycan attachment has yet to be defined, given the inherent difficulties in studying glycan structures.17 Enrichment of the GlycA signal sites for molecular characterization will be important for future insight into the diagnostic and therapeutic potential of the related protein glycans.
Strengths of this study include the long prospective follow‐up (median 17.2 years) of a large number of participants (N=27 491) with well‐characterized cardiovascular health markers and available biomarker phenotyping. The prospective nature of our observation deserves particular mention, given that glycan conjugates may undergo dynamic structural changes that are responsive to the phenotypic and metabolic state of a cell.17 The standardized prospective ascertainment of CVD events is also an important strength of our study. Limitations of our study include the availability of blood only at baseline, thus measurements of GlycA or hsCRP may not reflect levels over a long period of time. In addition, generalizability of our findings may be limited because our study sample comprised female health professionals, who may have different health behaviors than women in the general population or men. Finally, the observational nature of this study precludes our ability to rule out chance, residual confounding, or confounding due to unmeasured variables. The results presented are thus best viewed as hypothesis‐generating data and, as such, should be validated in other studies.
In conclusion, we have shown a significant positive association between baseline levels of N‐acetyl methyl groups of specific moieties of O‐ and N‐linked protein glycans and incident CVD events, an association which was attenuated after adjusting for hsCRP, a biomarker of systemic inflammation. Although the results presented show promise for the clinical application of glycobiology in CVD prevention, the advent of new tools for glycoprotein analysis will aid the structural characterization of glycans. Such research may unlock the variety of prognostic, diagnostic, and therapeutic opportunities that are yet untapped in the emerging field of glycobiology.
Sources of Funding
The research for this article was supported by the American Heart Association and by grants HL43851, HL 080467, and CA 47988 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, National Institutes of Health, a charitable gift from the Molino Family Trust and with additional support from U01 HL108630 (Mechanism‐Associated Phenotypes for Genetic analyses of Heart, Lung, Blood, and Sleep Diseases [MAPGen for HLBS]). LipoScience Inc supplied the GlycA information at no additional cost. The funding agencies played no role in the design, conduct, data management, analysis, or manuscript preparation related to this study. Dr Akinkuolie was also supported by the National Heart, Lung, and Blood Institute (T32 HL007575).
Disclosures
Dr Ridker is listed as a co‐inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease that have been licensed to AstraZeneca and Seimens. Dr Mora has received institutional research support from AstraZeneca, Atherotech Diagnostics, and NHLBI; served as a consultant to Genzyme, Quest Diagnostics, Lilly, and Cerenis Therapeutics. The other authors report no conflicts.
References
- 1.Marino K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: A path through the maze. Nat Chem Biol. 2010; 6:713-723. [DOI] [PubMed] [Google Scholar]
- 2.van Kooyk Y, Rabinovich GA. Protein‐glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008; 9:593-601. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Ann Rev Immuno. 2007; 25:21-50. [DOI] [PubMed] [Google Scholar]
- 4.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006; 126:855-867. [DOI] [PubMed] [Google Scholar]
- 5.Schachter H, Freeze HH. Glycosylation diseases: quo vadis? Biochim Biophys Acta. 2009; 1792:925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landsteiner K. Individual differences in human blood. Science. 1931; 73:403-409. [DOI] [PubMed] [Google Scholar]
- 7.Shriver Z, Raguram S, Sasisekharan R. Glycomics: a pathway to a class of new and improved therapeutics. Nat Rev Drug Discov. 2004; 3:863-873. [DOI] [PubMed] [Google Scholar]
- 8.Arnold JN, Saldova R, Hamid UM, Rudd PM. Evaluation of the serum n‐linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics. 2008; 8:3284-3293. [DOI] [PubMed] [Google Scholar]
- 9.Gornik O, Lauc G. Glycosylation of serum proteins in inflammatory diseases. Dis Markers. 2008; 25:267-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell JD, Brown JC, Nicholson JK, Sadler PJ. Assignment of resonances for ‘acute‐phase’ glycoproteins in high resolution proton nmr spectra of human blood plasma. FEBS Lett. 1987; 215:311-315. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005; 352:1293-1304. [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009; 119:931-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of c‐reactive protein and low‐density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002; 347:1557-1565. [DOI] [PubMed] [Google Scholar]
- 14.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006; 295:1412-1419. [DOI] [PubMed] [Google Scholar]
- 15.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008; 8:874-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dube DH, Bertozzi CR. Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005; 4:477-488. [DOI] [PubMed] [Google Scholar]
- 17.Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010; 143:672-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer K, Kettunen J, Wurtz P, Haller T, Havulinna AS, Kangas AJ, Soininen P, Esko T, Tammesoo ML, Magi R, Smit S, Palotie A, Ripatti S, Salomaa V, Ala‐Korpela M, Perola M, Metspalu A. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all‐cause mortality: an observational study of 17,345 persons. PLoS Med. 2014; 11:e1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varki A, Freeze HH. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. (eds.). Glycans in acquired human diseases. Essentials of Glycobiology. 2009Cold Spring Harbor, NY: Cold Spring Harbor; http://www.ncbi.nlm.nih.gov/books/NBK1946/ [PubMed] [Google Scholar]
- 20.Scott DW, Patel RP. Endothelial heterogeneity and adhesion molecules n‐glycosylation: implications in leukocyte trafficking in inflammation. Glycobiology. 2013; 23:622-633. [DOI] [PubMed] [Google Scholar]
- 21.Chacko BK, Scott DW, Chandler RT, Patel RP. Endothelial surface n‐glycans mediate monocyte adhesion and are targets for anti‐inflammatory effects of peroxisome proliferator‐activated receptor gamma ligands. J Biol Chem. 2011; 286:38738-38747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia‐Vallejo JJ, Van Dijk W, Van Het Hof B, Van Die I, Engelse MA, Van Hinsbergh VW, Gringhuis SI. Activation of human endothelial cells by tumor necrosis factor‐alpha results in profound changes in the expression of glycosylation‐related genes. J Cell Physiol. 2006; 206:203-210. [DOI] [PubMed] [Google Scholar]
- 23.Peng Y, Li J, Geng M. The glycan profile of endothelial cells in the present of tumor‐conditioned medium and potential roles of beta‐1,6‐glcnac branching on huvec conformation. Mol Cell Bbiochem. 2010; 340:143-152. [DOI] [PubMed] [Google Scholar]
- 24.Scott DW, Chen J, Chacko BK, Traylor JG, Jr, Orr AW, Patel RP. Role of endothelial n‐glycan mannose residues in monocyte recruitment during atherogenesis. Arterioscler Thromb Vasc Biol. 2012; 32:e51-e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin X, Subramanian S, Hwang SJ, O'Donnell CJ, Fox CS, Courchesne P, Muntendam P, Gordon N, Adourian A, Juhasz P, Larson MG, Levy D. Protein biomarkers of new‐onset cardiovascular disease: prospective study from the systems approach to biomarker research in cardiovascular disease initiative. Arterioscler Thromb Vasc Biol. 2014; 34:939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cahill LE, Levy AP, Chiuve SE, Jensen MK, Wang H, Shara NM, Blum S, Howard BV, Pai JK, Mukamal KJ, Rexrode KM, Rimm EB. Haptoglobin genotype is a consistent marker of coronary heart disease risk among individuals with elevated glycosylated hemoglobin. J Am Coll Cardiol. 2013; 61:728-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CW, Cheng TM, Lin CP, Pan JP. Plasma haptoglobin concentrations are elevated in patients with coronary artery disease. PLoS One. 2013; 8:e76817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correale M, Totaro A, Abruzzese S, Di Biase M, Brunetti ND. Acute phase proteins in acute coronary syndrome: an up‐to‐date. Cardiovasc Hematol Agents Med Chem. 2012; 10:352-361. [DOI] [PubMed] [Google Scholar]
- 29.Nordestgaard BG, Adourian AS, Freiberg JJ, Guo Y, Muntendam P, Falk E. Risk factors for near‐term myocardial infarction in apparently healthy men and women. Clin Chem. 2010; 56:559-567. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed MS, Jadhav AB, Hassan A, Meng QH. Acute phase reactants as novel predictors of cardiovascular disease. ISRN Inflamm. 2012; 2012:953461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Hennekens CH, Buring JE, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000; 342:836-843. [DOI] [PubMed] [Google Scholar]


