Abstract
Background
Understanding the population‐level risk factor contribution to disease incidence is critical for effective allocation of resources for prevention. There are little data on the contribution of cardiovascular disease (CVD) risk factors in multiethnic elderly populations.
Methods and Results
The Northern Manhattan Study (n=3298) is a population‐based prospective cohort study of CVD outcomes in a multiethnic urban population. Multivariable Cox's models were used to calculate hazard ratios, population attributable risk (PAR), and 95% confidence intervals (CIs) for (1) combined vascular event (VE) endpoint of stroke/myocardial infarction/vascular death (n=835) and (2) stroke (n=347). The PAR resulting from hypertension (HTN) was 24.3% (95% CI, 13.2 to 35.4) for VE and 29.9% (95% CI, 12.5 to 47.4) for stroke; PAR resulting from diabetes was 12.7% (95% CI, 8.2 to 17.2) for VE and 19.5% (95% CI, 12.4 to 26.5) for stroke. The PAR resulting from HTN and diabetes for stroke differed by race‐ethnicity and age (P for differences <0.05). PAR for stroke reslting from HTN was greater among Hispanics (50.6%; 95% CI, 29.2 to 71.9) than non‐Hispanic whites (2.6%; 95% CI, −33.2 to 38.6) and in those <80 years of age (35.6%; 95% CI, 18.9 to 52.3) than in those ≥80 (−0.3%; 95% CI, −34.2 to 33.6). Similarly, the PAR for stroke resulting from diabetes was 23.6% among those <80 years of age (95% CI, 15.7 to 31.5) and 2.3% among those ≥80 (95% CI, −8.2 to 12.7; P for difference=0.001). The PAR for VE did not differ by age/sex/race‐ethnicity.
Conclusions
HTN and diabetes have important effects on the burden of stroke, particularly among those younger than age 80 and Hispanics. Public health campaigns targeted at specific risk factors in specific populations can lead to a greater reduction in CVD.
Keywords: Aging, attributable risk, diabetes, hypertension, stroke, vascular death
Introduction
The decline of stroke from the third to fourth leading cause of the death in the United States1 is an encouraging sign of the impact made by public health awareness and intervention campaigns. The causes of this decline are likely to be multifactorial, including better treatment of risk factors and improved access to acute stroke care.2 Despite these achievements, cardiovascular disease (CVD) as a whole remains the leading cause of death. The gains made in coronary heart disease and stroke mortality, however, have not affected all of the population equally.3 Non‐Hispanic blacks continue to be at higher risk of CVD than non‐Hispanic whites, and a partial explanation is likely to be a disproportionate burden of traditional CVD risk factors.1 Less is known about disparities in other segments of the population. The data reported for Hispanics in the United States has shown mixed results, with lower overall mortality associated with CVD compared to non‐Hispanic whites,4–7 despite a higher burden of risk factors8; this has been called the “Hispanic paradox.” The CVD epidemic has extended as well to the oldest old, in whom there is a large burden of multiple CVD risk factors. Overall, CVD remains the leading cause of mortality in the oldest old and Hispanics, and as these 2 segments of the population grow, so will the impact of CVD. On a public health level, therefore, there is a continued need to identify which risk factors have the greatest impact on CVD among Hispanics and the geriatric population. This information could guide public health interventions that have the greatest impact on reducing the burden of disease for 2 rapidly expanding groups for which is there is more‐limited data. In this analysis, we report on the population attributable risk (PAR) associated with CVD risk factors in the Northern Manhattan Study (NOMAS), a prospective cohort study with a multiethnic, urban‐dwelling, elderly population. We hypothesized a priori that diabetes and hypertension (HTN) would have different magnitudes of PAR by race‐ethnicity, sex, and age.
Methods
Study Design
Recruitment of the cohort
The NOMAS is a population‐based study designed to evaluate the impact of medical, socioeconomic, and other risk factors on the incidence of vascular disease in a stroke‐free cohort. Participants were identified by dual‐frame random digit dialing in Northern Manhattan as previously described9 and were eligible if they met the following criteria: (1) had never been diagnosed with a stroke; (2) were over the age of 39 years; and (3) resided in Northern Manhattan for ≥3 months in a household with a telephone. Pre‐existing coronary artery disease (CAD) was ascertained by questionnaires capturing self‐reported myocardial infarction (MI), angina, or previous cardiac revascularization. Participants with pre‐existing MI were excluded from the analysis of the combined vascular endpoint given the difference in risk between first and recurrent events. The study was approved by the institutional review boards at Columbia University Medical Center (New York, NY) and the University of Miami (Miami, FL). All participants gave informed consent to participate in the study.
Assessments at enrollment of the cohort
Baseline status and risk factors in the cohort were collected through interviews of participants by trained bilingual research assistants. Physical examinations, in‐person measurements, and analysis of fasting blood specimens were carried out by study physicians. Race‐ethnicity was determined by self‐identification in response to a questionnaire modeled after the 2000 U.S. census.10 Education was classified as completing high school versus not. Standardized questions were adapted from the Behavioral Risk Factor Surveillance System regarding the following conditions: HTN, diabetes, and cigarette smoking.11 Standard techniques were used to measure blood pressure, height, weight, and fasting glucose and lipid panels as previously described.12 HTN was defined as blood pressure ≥140/90 mm Hg, a physician diagnosis of HTN, or a patient's self‐report.13 Diabetes mellitus (DM) was defined as fasting blood glucose ≥126 mg/dL or the patient's self‐report. Fasting blood samples were obtained and lipid profile was measured as previously described.14 Physical activity and alcohol intake were ascertained with the use of previously validated questionnaires15–16; tobacco use was defined as a current smoker, past smoker, and never smoker.
Follow‐up and outcome measures
Participants are followed annually by phone screening to detect any new cardiac or neurological symptoms, interval hospitalizations, medical conditions, or death. Complete loss to follow‐up is present in <1% and is not associated with race‐ethnicity.17 Any participant who responded positively on phone screening for an incident event was scheduled for an in‐person assessment. Our primary prespecified outcome was a combined endpoint of MI, stroke, and vascular death (VD); we had an additional primary outcome of interest of all stroke. MI was defined by criteria adapted from the Cardiac Arrhythmia Suppression Trial and the Lipid Research Clinics Coronary Primary Prevention Trial and required at least 2 of the 3 following criteria: (1) ischemic cardiac pain determined to be typical angina; (2) cardiac marker abnormalities defined as abnormal CK‐MB fraction or troponin I values; and/or (3) ischemic EKG abnormalities.18–19 MI was adjudicated by study cardiologists independently after review of all the clinical data. VD was defined as all deaths resulting from underlying heart disease (MI, congestive heart failure (CHF), and other cardiac arrhythmias) or stroke. Cause of death was ascertained through phone discussion with the participant's family (including if in another country), review of medical records (from the United States and other countries), and, when available, a copy of the death certificate. Neurological events that were deemed to be possible strokes were adjudicated by 2 neurologists independently after review of all data. The final diagnosis of stroke (including hemorrhagic and ischemic subtypes) was decided by consensus of the 2 neurologists; any disagreements were adjudicated by a third neurologist.16
A total of 3298 participants were recruited between 1993 and 2001 and follow‐up is ongoing. Our primary exposures of interest were HTN and diabetes given their high prevalence in our community and a previously published important role in an international case‐control study of stroke risk factors.20 In order to explore interactions with age, we categorized age as greater than or equal to 80 versus less than 80 in keeping with previous cutoffs used in clinical trials and observational studies.21–22
Statistical Analysis
Baseline characteristics were compared by age, race‐ethnicity, and sex using Analysis of Variance or 2 sided t tests for continuous variables, and the χ2 test for proportions. The primary outcome was a combined vascular event (VE; stroke, MI, and VD), and the secondary outcome was stroke only. Cox's proportional hazard models were fitted to calculate hazard ratios (HRs) for the association of the risk factors of interest (HTN and diabetes) to the outcomes, and their PAR using the equation PAR=P (HR−1)/[1+P (HR−1)], where P is prevalence; the SE of PAR was calculated using the delta method. The models were fully adjusted for demographics (age, sex, and education) and vascular risk factors (waist circumference, tobacco use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, total cholesterol, moderate alcohol consumption, and physical inactivity). We tested for interactions to examine whether PAR for the outcomes resulting from HTN and diabetes differed by race‐ethnicity, sex, or age, by comparing PARs pairwise (ie, blacks vs. non‐Hispanic white, Hispanics vs. non‐Hispanic white, female vs. male, and age ≥80 vs. <80) as well as global comparison for any differences in PAR among 3 race‐ethnic groups. Stratified models were run when the P value for interaction was <0.05. Improvement of model fit for those interactions with >2 categorized groups, such as race‐ethnicity, as well as the global testing for the differences in PAR across race‐ethnicity were tested using chi‐squared test with 2 degrees of freedom. We chose a cutoff of 80 years for age interactions based on enrollment criteria for the Systolic Hypertension in the Elderly trial and lack of validity of the 2013 pooler risk calculator for atherosclerotic CVD.1,23 All statistical analyses were performed with SAS software (version 9.3; SAS Institute, Cary, NC).
Results
The baseline characteristics of the NOMAS cohort are described in Table 1. The prevalence of HTN, diabetes, physical inactivity, and active tobacco use were higher in non‐Hispanic blacks and Hispanics, compared to non‐Hispanic whites. Women had a higher prevalence of HTN, but not diabetes, in comparison to men, whereas those ≥80 years had a lower prevalence of DM and no difference in HTN, compared to those <80 years.
Table 1.
Baseline Demographics of the Northern Manhattan Study Cohort (n=3298)
| Overall (n=3298) | Hispanic (n=1727) | Non‐Hispanic Black (n=803) | Non‐Hispanic White (n=690) | Women (n=2071) | Men (n=1227) | Age <80 (n=2736) | Age ≥80 (n=562) | |
|---|---|---|---|---|---|---|---|---|
| Age, y | 69.2 (10.3) | 66.3 (9.4)* | 71.7 (10.4)* | 73.6 (10.0)* | 70.1 (10.6)* | 67.8 (9.6)* | 66.0 (7.9) | 85.0 (4.3) |
| Diabetes | 716 (21.8) | 408 (23.7)* | 196 (24.4)* | 100 (14.6)* | 438 (21.2) | 278 (22.7) | 632 (23.13)* | 84 (15.0)* |
| Hypertension | 2429 (73.7) | 1296 (75.0)* | 638 (79.5)* | 449 (65.1)* | 1572 (75.9)* | 857 (69.9)* | 2005 (73.3) | 424 (75.4) |
| Coronary artery disease | 463 (14.0) | 243 (14.1)* | 91 (11.3)* | 121 (17.5)* | 283 (13.7) | 180 (14.7) | 377 (13.8) | 86 (15.3) |
| Active tobacco use | 560 (17.0) | 276 (16.0)* | 180 (22.4)* | 91 (13.2)* | 289 (14.0)* | 271 (22.1)* | 506 (18.5)* | 54 (9.6)* |
| Former tobacco user | 1191 (36.1) | 574 (33.2)* | 292 (36.4)* | 299 (43.3)* | 608 (29.4)* | 583 (47.5)* | 983 (35.9)* | 208 (37.1)* |
| Physically inactive | 1389 (42.1) | 860 (49.8)* | 280 (34.5)* | 218 (31.6)* | 918 (44.3)* | 471 (38.4)* | 1152 (42.1) | 237 (42.1) |
| Moderate alcohol use* | 1086 (32.9) | 510 (29.5)* | 256 (31.9)* | 293 (42.5)* | 557 (26.9)* | 529 (43.1)* | 950 (34.7)* | 136 (24.2)* |
| Body mass index, kg/m2 | 27.8 (5.5) | 28.4 (5.0)* | 28.3 (6.4)* | 26.2 (5.4)* | 28.4 (6.0)* | 26.9 (4.6)* | 28.4 (5.5)* | 25.3 (4.7)* |
| Low‐density lipoprotein cholesterol, mg/dL | 129.2 (35.8) | 129.5 (35.4)* | 126.5 (37.0)* | 131.6 (34.9)* | 133.0 (36.0)* | 122.9 (34.4)* | 129.2 (36.0) | 129.5 (34.3) |
| High‐density lipoprotein cholesterol, mg/dL | 47.8 (14.6) | 43.7 (13.1)* | 51.8 (16.1)* | 48.1 (14.6)* | 50.1 (14.7)* | 41.1 (12.6)* | 46.0 (14.3)* | 50.6 (15.4)* |
Results displayed as means (±SD) or number (proportion).
P value for difference in mean or proportions <0.05.
Moderate alcohol use: between 1 serving per month and 2 servings per day.
PAR for the Combined Outcome of Stroke, MI, and VD
Among 3054 of those without history of MI at baseline, 835 subjects developed VE over a median follow‐up of 12 years. Diabetes (HR, 1.70; 95% CI, 1.43 to 2.00), HTN (HR, 1.44; 95% CI, 1.20 to 1.74), and CAD (HR, 1.46; 95% CI, 1.22 to 1.74) were associated with a greater risk of VE.
The PAR for VE point resulting from HTN was 24.3% (95% CI, 13.2 to 35.4) and resulting from diabetes was 12.7% (95% CI 8.2 to 17.2; Table 2; Figure). The combination of these 2 risk factors accounted for 34.5% (95% CI, 23.5 to 45.5) of PAR for VE. The PAR for VE resulting from diabetes and HTN did not differ by race‐ethnicity, sex, or age ≥80 versus <80 (P value for all interactions, >0.05), though the PARs remained statistically significant across the entire cohort. For illustrative purposes, however, we carried out stratified models for the PAR of diabetes and HTN for VE (Table 3). We calculated the PAR for physical inactivity, tobacco use, and dyslipidemia and found no statistically significant results.
Table 2.
Population Attributable Risk and 95% Confidence Interval of a Combined EndPoint of Stroke, Myocardial Infarction, and Vascular Death (Vascular Endpoint; VE), and Stroke Alone, in the Northern Manhattan Study
| Prevalence | Hazard Ratio* | 95% Confidence Interval of HR | Population Attributable Risk | 95% Confidence Interval of PAR | |||
|---|---|---|---|---|---|---|---|
| Stroke, MI or vascular deaths* | |||||||
| Hypertension | 72.6% | 1.44 | 1.20 | 1.73 | 24.3% | 13.2% | 35.4% |
| Diabetes | 20.9% | 1.70 | 1.44 | 2.00 | 12.7% | 8.2% | 17.2% |
| All stroke | |||||||
| Hypertension | 73.7% | 1.58 | 1.16 | 2.14 | 29.9% | 12.5% | 47.3% |
| Diabetes | 21.6% | 2.11 | 1.66 | 2.68 | 19.5% | 12.4% | 26.5% |
HR indicates hazard ratio; MI, myocardial infarction; PAR, population attributable risk.
Adjusted for age, race‐ethnicity, education, sex, insurance status, alcohol use, physical activity, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, tobacco use, and coronary artery disease.
Excludes previous history of myocardial infarction.
Figure 1.
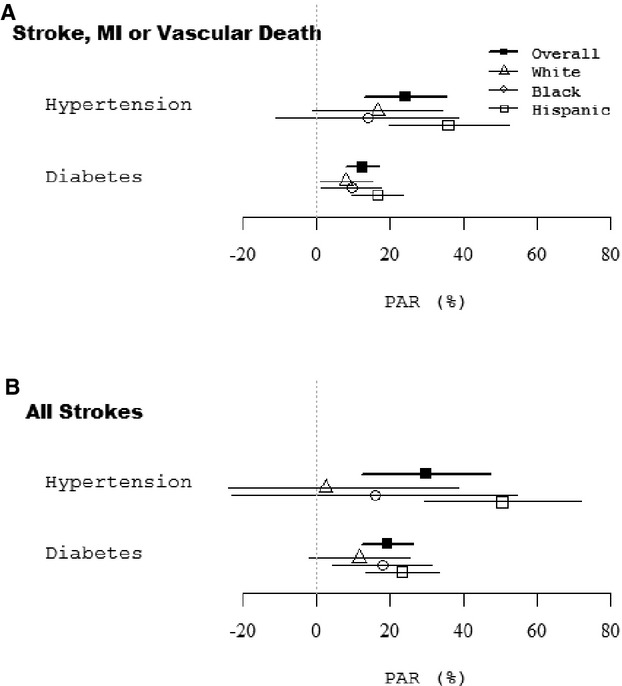
Population attributable risk for (A) stroke, myocardial infarction, and vascular death; (B) stroke.
Table 3.
Population Attributable Risk and 95% Confidence Interval for Stroke, Myocardial Infarction, and Vascular Death by Race‐Ethnicity and Age*
| Hypertension | Diabetes | |||||
|---|---|---|---|---|---|---|
| Prevalence % | Population Attributable Risk %* (95% CI) | P Value* | Prevalence % | Population Attributable Risk %* (95% CI) | P Value* | |
| Race‐ethnicity* | ||||||
| White | 63.2 | 16.9 (−0.7, 34.5) | Ref. | 13.5 | 8.2 (1.1, 15.3) | Ref. |
| Black | 79.1 | 14.0 (−10.7, 38.8) | 0.85 | 23.5 | 9.7 (1.6, 17.9) | 0.78 |
| Hispanic | 74.1 | 36.0 (19.6, 52.3) | 0.12 | 22.7 | 16.8 (9.8, 23.7) | 0.09 |
| Age, y | ||||||
| <80 | 72.3 | 26.1 (14.6, 37.6) | 0.5 | 22.1 | 14.4 (9.2, 19.7) | 0.06 |
| ≥80 | 74.7 | 19.4 (3.0, 35.7) | 14.6 | 6.5 (0.3, 12.8) | ||
Negative values in population attributable risk reflect the hazard ratios used for this calculation crossing the null and the lack of increased risk in the population.
Excluding history of myocardial infarction.
Adjusted for age, race‐ethnicity, education, sex, insurance status, alcohol use, physical activity, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, tobacco use, and coronary artery disease.
P value for testing for the differences in population attributable risk.
P value for global testing for the differences in PAR across the race‐ethnicity with chi‐squared test with 2 degrees of freedom=0.2 for hypertension and =0.2 for diabetes.
PAR for Stroke
Among 3298 stroke‐free individuals, there were 347 strokes during follow‐up. Risk factors for total stroke (ischemic and hemorrhagic) were HTN (HR, 1.58; 95% CI, 1.16 to 2.15), diabetes (HR, 2.11; 95% CI, 1.66 to 2.68), and CAD (HR, 1.46; 95% CI, 1.14 to 1.88). The PAR for stroke resulting from HTN was 30.0% (95% CI, 12.5 to 47.4) and resulting from diabetes was 19.5% (95% CI, 12.4 to 26.5; Table 4; Figure). The combination of HTN and diabetes accounted for 49.5% (95% CI, 33.7 to 65.4) of the PAR for stroke.
Table 4.
Population Attributable Risk and 95% Confidence Interval for Stroke by Race‐Ethnicity and Age
| Hypertension | Diabetes | |||||
|---|---|---|---|---|---|---|
| Prevalence % | Population Attributable Risk %*(95% CI) | P Value* | Prevalence % | Population Attributable Risk %* (95% CI) | P Value* | |
| Race‐ethnicity* | ||||||
| White | 65.1 | 2.6 (−33.3, 38.65) | Ref. | 14.6 | 11.7 (−1.9, 25.3) | Ref. |
| Black | 79.5 | 16.0 (−22.7, 54.7) | 0.62 | 24.4 | 18.0 (4.5, 31.4) | 0.52 |
| Hispanic | 75.0 | 50.6 (29.2, 71.9) | 0.02 | 23.6 | 23.4 (13.4, 33.4) | 0.17 |
| Age, y | ||||||
| <80 | 73.3 | 35.6 (18.9, 52.3) | 0.06 | 23.1 | 23.6 (15.7, 31.5) | 0.001 |
| ≥80 | 75.4 | −0.2 (−34.2, 33.6) | 15.0 | 2.3 (−8.2, 15.7) | ||
Adjusted for age, race‐ethnicity, education, sex, insurance status, alcohol use, physical activity, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, tobacco use, and coronary artery disease.
P value for testing for the difference in population attributable risk. Note: negative values in population attributable risk reflect the hazard ratios used for this calculation crossing the null and the lack of increased risk in the population.
P value for global testing for the differences in PAR across the race‐ethnicity with chi‐squared test with 2 degrees of freedom=0.048 for hypertension and =0.19 for diabetes.
Effect Modification of Attributable Risk for Stroke by Race‐Ethnicity and Age
The attributable risk for stroke resulting from HTN was modified by race‐ethnicity (P value for likelihood ratio test with 2 df=0.048) and age (P value for interaction by age <80 versus ≥80 years=0.06); the PAR for stroke resulting from HTN was greater among Hispanics (50.6%; 95% CI, 29.2 to 71.9) than non‐Hispanic whites (2.6%; 95% CI, −33.2 to 38.6; P for difference of PAR=0.02). The PAR for stroke resulting from HTN was greater in those less than 80 years of age (35.6%; 95% CI, 18.9 to 52.3) than in those age 80 or older (−0.2%; 95% CI, −34.2 to 33.6). Similarly, the PAR for stroke resulting from diabetes differed by age groups (P for difference=0.001); the PAR of diabetes for stroke was 23.6% among those less than age 80 (95% CI, 15.7 to 31.5) and 2.3% among those 80 or older (95% CI, −8.2 to 12.7). Table 5 outlines PAR for HTN and diabetes using other age cutoffs: None of the differences were statistically significant.
Table 5.
Population Attributable Risk and 95% Confidence Interval for a Combined EndPoint (Stroke, Myocardial Infarction, and Vascular Death) and Stroke by Age
| Age, y | Hypertension | Diabetes | ||||
|---|---|---|---|---|---|---|
| Prevalence % | Population Attributable Risk % (95% CI) | P Value* | Prevalence % | Population Attributable Risk % (95% CI) | P Value* | |
| Combined vascular endpoint | ||||||
| <60 | 61.2 | 21.8% (−0.7 to 44.3) | 0.8 | 14.4 | 5.9% (−2.4 to 17.2) | 0.06 |
| 60 to 79 | 75.5 | 27.0% (15.3 to 38.7) | 24.4 | 16.1% (10.4 to 21.8) | ||
| ≥80 | 74.7 | 19.5% (2.9 to 36.2) | 14.6 | 6.6% (0.3 to 12.9) | ||
| All stroke | ||||||
| <60 | 62.1 | 29.3% (−1.1 to 59.8) | 0.02 | 14.8 | 12.3% (−4.2 to 28.7) | 0.07 |
| 60 to 79 | 76.4 | 36.7% (19.8 to 53.6) | 25.4 | 25.8% (17.4 to 34.3) | ||
| ≥80 | 75.4 | 0.6% (−34.2 to 35.3) | 15.0 | 2.4% (−8.1 to 12.9) | ||
P value for global testing for the difference in population attributable risk with chi‐squared test with 2 degrees of freedom. All P values for differences comparing age categories with <60 years old as reference >0.1
Discussion
In our prospective cohort study of a multiethnic, urban‐dwelling, predominantly older population, we found that the PAR resulting from the combination of diabetes and HTN for stroke, MI, and VD was 35%, whereas for stroke alone it was ≈50%. We focused on HTN and diabetes because they are highly prevalent in our community, are associated with our outcomes, and are modifiable with population‐level behavioral changes (such as reduction in salt or processed carbohydrates in the food supply), lifestyle changes, and pharmacological therapy. While we concentrated on these 2 major risk factors, however, we fully adjusted our analyses for other sociodemographic and cardiovascular risk (CVR) factors, including education, physical activity, obesity, tobacco and alcohol use, and lipids. We are likely underestimating the overall public health impact of HTN and diabetes in populations with a high prevalence such as ours given the additional association of these risk factors with vascular cognitive impairment, peripheral arterial disease, and renal disease.1 The high PAR of diabetes and HTN for vascular endpoints indicates that public health campaigns targeted at modifying those risk factors could have a substantial impact on the burden of CVDs and stroke. Notably, our analyses also suggested that the PAR for stroke resulting from HTN and diabetes differs by race‐ethnicity and age, but this was not the case for a combined endpoint. In particular, we found HTN attributed a 50% risk to stroke in Hispanics. We also found that PARs of HTN and diabetes for stroke were significant only among those age <80.
The explanations for why we found a differential effect by race‐ethnicity for stroke, but not the combined endpoint, could be artifact or result from a true effect. One possible explanation for our findings is that non‐Hispanic blacks and whites could suffer MI and VDs at an earlier age than stroke. In standard Cox's models therefore, participants with VD would be censored before they could experience stroke, creating a competing risk scenario that would mask the true impact of a particular risk factor. The competing risk of other events needs to be considered in particular for epidemiological studies in older adults.24 Second, the proportion of non‐Hispanic blacks, who also have a higher prevalence of HTN, is lower in our cohort study, compared to Hispanics, and we may have been underpowered to detect important effects of HTN on risk of stroke in that population. Third, the mean age of non‐Hispanic blacks and whites at enrollment was also higher than for Hispanics with similar prevalence of HTN. Considering that age is strongly associated with VD in particular, the different age distributions across race‐ethnicity may be one of explanation. Last, there are scant data on different genetic profiles and life/neighborhood stressors in populations such as ours that may differentially modify the effect of HTN and diabetes on different CVD outcomes.25
The differential effect of diabetes and HTN by age also raises important questions. In our cohort, we had a reasonable proportion of participants who were older than 80 and had survived to this age without developing a stroke and are at higher risk of dementia than their younger counterparts. These older participants may have thus had other unmeasured factors, such as a genetic profile, which may have mitigated the deleterious effect of HTN and diabetes. In previous studies, this has been termed “resilience”; for example, in a recent analysis of the Leiden‐85 cohort, elevated blood pressure was associated with a paradoxically slower decline in physical and cognitive function in those older than 85.26 In this same cohort, investigators found that the effect of blood pressure on risk of stroke was modified by baseline cognitive function, whereby increased systolic blood pressure was associated with a higher risk of stroke among those who were cognitively intact.27 The findings in the Leiden‐85 cohort may also be in keeping with nonsignificant reductions on risk of CVD with treatment of HTN noted in a prespecified analysis of those ≥80 enrolled in the Systolic Hypertension in the Elderly Program.28 Our findings are consistent with recommendations from JNC‐8 emphasizing the importance of antihypertensive treatment in the elderly, albeit to a less‐strict target than in younger persons, to prevent CD.29 The trial and epidemiological data on risk of stroke among those over the age of 80, however, is limited. In a multinational clinical trial using indapamide (and perindopril as needed) in participants over 80 and with a sustained blood pressure greater than 160 mm Hg, there was a reduced risk of CHF and death, as well as a nearly significant effect on stroke.30 In analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) study, investigators found that disparities in stroke incidence resulting from HTN between non‐Hispanic blacks and whites were significantly blunted in those ≥75.31 Whether HTN needs to be as aggressively treated in the elderly, as suggested by recent reports of greater decline in total brain volume32 and increased risk of falls in this population,33 remains to be answered.
Our study has some important strengths, including one of the first assessments of attributable risk of CVD and stroke within a prospective multiethnic, elderly cohort with very low loss to follow‐up. In addition, our cohort comprises a high proportion of first‐generation Caribbean Hispanic immigrants, who have been infrequently studied in epidemiological studies of CVD. There are also important limitations to consider in our study. We had a low proportion of non‐Hispanic blacks and whites and were thus likely underpowered to detect differences by race‐ethnicity. We did not collect information on risk factor control, such as glycosylated hemoglobin or ambulatory blood pressure monitoring, nor did we analyze for interval development of risk factors after enrollment, which are likely to influence the risk of CVD.34 The control of systolic HTN may be particularly important in the elderly,35 as well as the potential benefit beyond blood pressure of certain antihypertensives. Without repeated measures of blood pressure, we cannot comment on the recent relaxation of blood pressure targets up to 150/90 mm Hg.29 We also did not collect data on important confounders, such as interval development of atrial fibrillation, or objective measures of physical functioning, such as walking speed, that could be significant predictors of stroke and vascular death in the elderly.36 We also did not include in our analyses information on novel CVR factors, such as inflammatory markers,37 though, in previous studies, they did not have a clinically significant additive effect on prediction of disease and there are limited treatment options for inflammatory markers. Last, we did not collect information on important social determinants of health, including the built environment and socioeconomic status,38–39 which are likely to modify the effect of traditional CVD risk factors on outcomes.
In summary, we found that the PAR on a combined CVD endpoint from HTN and diabetes in a multiethnic, urban‐dwelling cohort was close to 50% for stroke and 35% for CVD. These findings are notable because CVD remains the leading source of death in the United States, while stroke remains the fourth leading cause of death and the leading cause of serious disability. Furthermore, much of the public health success in mitigating the impact of these diseases has not been as notable in Hispanics and non‐Hispanic blacks. While additional research is required to characterize the driving force behind the continued high impact of CVD and stroke in developed counties, our findings support that, in high‐risk populations, public health interventions to control diabetes and HTN will lead to a substantial public health impact. Though we found differential effects by race‐ethnicity and age for stroke, the overall effect on a combined cardiovascular endpoint emphasizes the importance of treating both modifiable risk factors.
Sources of Funding
Funding for this project was provided by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NINDS) R37 NS 29993 and the Evelyn F. McKnight Brain Institute (University of Miami). Willey was funded by NINDS K23 NS 073104. The first author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The others report no other financial conflicts of interest.
Disclosures
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013; 127:143-152. [DOI] [PubMed] [Google Scholar]
- 2.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014; 45:315-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng‐Mak DS, Dohrenwend BP, Abraido‐Lanza AF, Turner JB. A further analysis of race differences in the National Longitudinal Mortality Study. Am J Public Health. 1999; 89:1748-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crimmins EM, Kim JK, Alley DE, Karlamangla A, Seeman T. Hispanic paradox in biological risk profiles. Am J Public Health. 2007; 97:1305-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, Hazuda HP. All‐cause and cardiovascular mortality among Mexican‐American and non‐Hispanic White older participants in the San Antonio Heart Study‐ evidence against the “Hispanic paradox”. Am J Epidemiol. 2003; 158:1048-1057. [DOI] [PubMed] [Google Scholar]
- 6.Patel KV, Eschbach K, Ray LA, Markides KS. Evaluation of mortality data for older Mexican Americans: implications for the Hispanic paradox. Am J Epidemiol. 2004; 159:707-715. [DOI] [PubMed] [Google Scholar]
- 7.Willey JZ, Rodriguez CJ, Moon YP, Paik MC, Di Tullio MR, Homma S, Sacco RL, Elkind MS. Coronary death and myocardial infarction among Hispanics in the Northern Manhattan Study: exploring the Hispanic paradox. Ann Epidemiol. 2012; 22:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraido‐Lanza AF, Dohrenwend BP, Ng‐Mak DS, Turner JB. The Latino mortality paradox: a test of the “salmon bias” and healthy migrant hypotheses. Am J Public Health. 1999; 89:1543-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacco RL, Anand K, Lee HS, Boden‐Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the Northern Manhattan Study. Stroke. 2004; 35:2263-2269. [DOI] [PubMed] [Google Scholar]
- 10.Budget OoMa. Race and ethnic standards for federal statistics and administrative reporting (directive no 15). 1978:43 Federal Register 19269. [PubMed]
- 11.Gentry EM, Kalsbeek WD, Hogelin GC, Jones JT, Gaines KL, Forman MR, Marks JS, Trowbridge FL. The behavioral risk factor surveys: ii. Design, methods, and estimates from combined state data. Am J Prev Med. 1985; 1:9-14. [PubMed] [Google Scholar]
- 12.Rodriguez CJ, Lin F, Sacco RL, Jin Z, Boden‐Albala B, Homma S, Di Tullio MR. Prognostic implications of left ventricular mass among Hispanics: the Northern Manhattan Study. Hypertension. 2006; 48:87-92. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden‐Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study). J Am Coll Cardiol. 2009; 54:2303-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paultre F, Tuck CH, Boden‐Albala B, Kargman DE, Todd E, Jones J, Paik MC, Sacco RL, Berglund L. Relation of Apo(a) size to carotid atherosclerosis in an elderly multiethnic population. Arterioscler Thromb Vasc Biol. 2002; 22:141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkind MS, Sciacca R, Boden‐Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2006; 37:13-19. [DOI] [PubMed] [Google Scholar]
- 16.Willey JZ, Moon YP, Paik MC, Boden‐Albala B, Sacco RL, Elkind MS. Physical activity and risk of ischemic stroke in the Northern Manhattan Study. Neurology. 2009; 73:1774-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willey JZ, Rodriguez CJ, Carlino RF, Moon YP, Paik MC, Boden‐Albala B, Sacco RL, DiTullio MR, Homma S, Elkind MS. Race‐ethnic differences in the association between lipid profile components and risk of myocardial infarction: the Northern Manhattan Study. Am Heart J. 2011; 161:886-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984; 251:351-364. [DOI] [PubMed] [Google Scholar]
- 19. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med. 1989; 321:406-412. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez‐Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010; 376:112-123. [DOI] [PubMed] [Google Scholar]
- 21.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002; 360:1903-1913. [DOI] [PubMed] [Google Scholar]
- 22.Willey JZ, Ortega‐Gutierrez S, Petersen N, Khatri P, Ford AL, Rost NS, Ali LK, Gonzales NR, Merino JG, Meyer BC, Marshall RS. Impact of acute ischemic stroke treatment in patients >80 years of age: the specialized program of translational research in acute stroke (SPOTRIAS) consortium experience. Stroke. 2012; 43:2369-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O'Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997; 350:757-764. [DOI] [PubMed] [Google Scholar]
- 24.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010; 58:783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005; 111:1332-1336. [DOI] [PubMed] [Google Scholar]
- 26.Sabayan B, Oleksik AM, Maier AB, van Buchem MA, Poortvliet RK, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85‐plus study. J Am Geriatr Soc. 2012; 60:2014-2019. [DOI] [PubMed] [Google Scholar]
- 27.Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85‐plus study. Stroke. 2013; 44:15-20. [DOI] [PubMed] [Google Scholar]
- 28.Staessen JA, Fagard R, Thijs L, Celis H, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Fletcher AE, Babarskiene MR, Forette F, Kocemba J, Laks T, Leonetti G, Nachev C, Petrie JC, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Zanchetti A. Subgroup and per‐protocol analysis of the randomized european trial on isolated systolic hypertension in the elderly. Arch Intern Med. 1998; 158:1681-1691. [DOI] [PubMed] [Google Scholar]
- 29.James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311:507-520. [DOI] [PubMed] [Google Scholar]
- 30.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008; 358:1887-1898. [DOI] [PubMed] [Google Scholar]
- 31.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013; 173:46-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson JD, Launer LJ, Bryan RN, Coker LH, Lazar RM, Gerstein HC, Murray AM, Sullivan MD, Horowitz KR, Ding J, Marcovina S, Lovato L, Lovato J, Margolis KL, Davatzikos C, Barzilay J, Ginsberg HN, Linz PE, Miller ME. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014; 174:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP, Zhou B, Lin H. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014; 174:588-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banerjee C, Moon YP, Paik MC, Rundek T, Mora‐McLaughlin C, Vieira JR, Sacco RL, Elkind MS. Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2012; 43:1212-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleg JL, Forman DE, Berra K, Bittner V, Blumenthal JA, Chen MA, Cheng S, Kitzman DW, Maurer MS, Rich MW, Shen WK, Williams MA, Zieman SJ. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation. 2013; 128:2422-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5‐year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998; 279:585-592. [DOI] [PubMed] [Google Scholar]
- 37.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet. 2010; 375:132-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. JAMA. 2005; 294:2879-2888. [DOI] [PubMed] [Google Scholar]
- 39.Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi‐Ethnic Study of Atherosclerosis). Am J Hypertens. 2011; 24:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]


