Fogo Selvagem (FS) is an endemic form of pemphigus foliaceus (PF) which is prevalent in certain regions of Brazil (Diaz et al., 1989). Genetic and environmental factors such as insect bites may contribute to the development of FS (Aoki et al., 2004; Diaz et al., 1989; Moraes et al., 1997). Blister formation in FS is mediated by predominantly IgG4 anti-desmoglein 1 (Dsg1) antibodies (Rock et al., 1989), which are predictors of FS (Qaqish et al., 2009). Our recent studies demonstrate that FS IgG4 antibodies cross-react with LJM11, a salivary protein from sand fly Lutzomyia longipalpis (Qian et al., 2012), suggesting that sand fly bites may deliver the antigen(s) that play a role in driving the development of these IgG4 autoantibodies in FS.
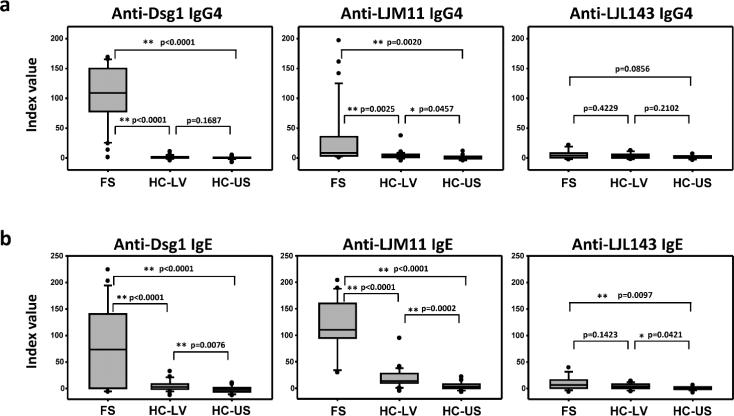
The concomitant development of IgE and IgG4 has been well documented in allergy (Lichtenstein et al., 1968; Muller, 2005). In addition, insect bites are known to induce IgE responses. IgE response in PV and FS has been described by Nagel et al (Nagel et al., 2009) and us (Qian et al., 2012; Qian et al., 2011). We have demonstrated that FS patients have significantly higher IgG4 and IgE against sand fly salivary gland antigens (SGLL) and monoclonal IgG4 autoantibodies derived from FS patients cross-react with LJM11, a major immunogenic component from SGLL (Qian et al., 2012). In this study, we seek to determine whether IgE antibodies to either autoantigen (Dsg1) and/or environmental antigen (LJM11) are present among individuals living in FS endemic regions. All serum samples from controls and patients in this investigation were obtained from individuals studied during the last 25 years and kept in a bank of sera at the University of North Carolina Dermatology Research Laboratories. These studies are approved by the institutional review boards from the University of North Carolina, Chapel Hill, and the University of Sao Paulo, Sao Paulo, Brazil. Randomly selected serum samples (n=30) from FS patients, as well as serum samples of normal control individuals (HC) from FS endemic region (n=32), Limao Verde in Brazil (HC-LV) and non-FS endemic region, United States (n=32) (HC-US) were tested by ELISA for their reactivity with Dsg1, LJM11, and LJL143 which is another major component of SGLL. As expected, FS patients have significantly higher level of IgG4 anti-Dsg1 autoantibodies compared to HC-LV and HC-US (Fig 1a, left panel). Similarly, FS patients also have significantly higher IgG4 anti-LJM11 antibodies than HC-LV and HC-US groups (Fig 1a, middle panel). There is no significant difference between the levels of anti-LJL143 IgG4 antibodies in the sera of FS patients and the two control groups (Fig 1a, right panel), suggesting that LJM11 is the main component from SGLL recognized by IgG4 antibodies from FS patients. Because of the close association of the IgE and IgG4 development and our previous finding that FS patients have significant levels of IgE and IgG4 anti-SGLL (Qian et al., 2012), it is expected that FS patients may also have higher IgE anti-Dsg1 and anti-LJM11 compared to HC-LV and HC-US. As shown in Fig 1b, FS patients have significantly higher IgE anti-Dsg1 (Fig 1b, left panel) as we previously reported (Qian et al., 2012; Qian et al., 2011), and also have significantly higher level of IgE anti-LJM11 antibodies (Fig 1b, middle panel) than both HC-LV and HC-US. Importantly, HC-LV sera have higher levels of IgE anti-LJM11 antibodies than those from HC-US (Fig 1b, middle panel). The anti-LJL143 IgE levels are overall generally much lower (about 10 times lower) in FS, HC-LV and HC-US groups as compared to anti-LJM11 IgE levels in the same group (Fig 1b, middle and right panel). These findings suggest that the FS endemic area of LV where the sera of FS and HC-LV originated from, and where bites by Lutzomya longipalpis are prevalent, may harbor environmental factors that direct the development of these antibodies in FS endemic regions.
Figure 1. FS patients have significantly higher IgG4 (A) and IgE (B) anti-Dsg1 and anti-LJM11 antibodies than normal control individual from FS and non-FS endemic regions.
Anti-Dsg1 (a) and anti-LJM11 (b) ELISA were conducted as described (Qian et al., 2007; Qian et al., 2012; Qian et al., 2011) using SuperSignal ELISA Pico (for IgG4) and Femto (for IgE) (Thermo Fisher, Rockford, IL) as substrate. The Relative Light Units (RLUs) were measured by LMax II 384 Luminometer (Molecular Devices, Sunnyvale, CA). Horseradish peroxidase conjugated mouse anti-human IgE (clone HP6029, (Hamilton and Morrison, 1993), SouthernBiotech, Birmingham, AL) was used as secondary antibody for IgE ELISA. The levels of IgG4 or IgE anti-Dsg1 or anti-LJM11 are expressed as index value (Amagai et al., 1999; Qaqish et al., 2009). The horizontal lines within the box plots indicate the median values. The p values of the comparisons between each group pair are indicated. Statistical significance between any two groups is assessed by t-test. Significant difference is marked by * (p<0.05) or ** (p<0.01), and p values are indicated.
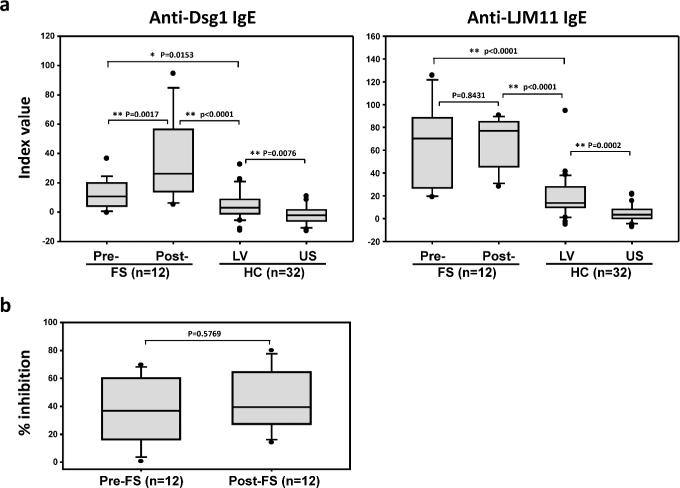
Our findings that LV inhabitants and FS patients show significant levels of IgE anti-LJM11 antibodies suggest that FS patients during the pre-clinical stage of the disease (pre-FS) may also exhibit elevated levels of these IgE antibodies. It is known that pre-FS would eventually develop pathogenic IgG4 autoantibodies and clinical FS (post-FS) (Qaqish et al., 2009), and IgG4 antibody development lags behind the IgE response. Hence, individuals at risk to develop FS may develop IgE and IgG4 responses when exposed to LJM11 during the repeated bites of sand flies. To test this hypothesis, 12 FS patients whose serum samples were collected prior to the onset of clinical FS (1 to 4 years) and after their onset of FS were studied for anti-Dsg1 and anti-LJM11 IgE activity. The HC-LV and HC-US were also included as controls. As shown in Fig 2a (left panel), pre-FS and post-FS individuals exhibit higher levels of IgE anti-Dsg1 than the control groups. The right panel of Fig 2a shows that these pre-FS and post-FS individuals also have significantly higher levels of IgE anti-LJM11 as compared with HC-LV and HC-US. These results suggest that the IgE antibodies against Dsg1 and LJM11 develop before the onset of FS among susceptible individuals from this endemic area. In addition, similar to IgG4 antibodies in FS, IgE anti-LJM11 and anti-Dsg1 antibodies from both pre-FS and post-FS are also cross-reactive, as their IgE binding to LJM11 can be inhibited by Dsg1 autoantigen (Fig 2b). Notably, pre-FS individuals have significantly lower levels of IgE anti-Dsg1 than that from post-FS patients (Fig 2a, left panel), suggesting that anti-LJM11 IgE develops prior to that of IgE autoantibodies.
Figure 2. IgE antibodies among Individuals before or after their onset of FS.
a. Anti-Dsg1 and anti-LJM11 IgE levels among pre-FS and post-FS individuals were determined by ELISA and compared with IgE and IgG4 anti-Dsg1 and LJM11 of normal controls (HC) from both FS endemic region (LV) and non-FS endemic region (US). b. The binding of serum IgE antibodies from pre-FS and post-FS individuals to LJM11 is inhibited by recombinant human Dsg1. IgE anti-LJM11 ELISA is conducted in the presence and absence of Dsg1. The percentage of inhibition is determined by the reduction of RLUs in the presence of Dsg1 (50[.proportional]g/ml) divided by RLUs without Dsg1 for each serum sample. Statistical significance between any two groups is assessed by t-test. Significant difference is marked by * (p<0.05) or ** (p<0.01), and p values are indicated.
Considering that IgG4 and IgE antibody responses to allergens are closely linked, it is likely that the generation of IgE anti-LJM11, introduced by sand fly bites, result in the development of IgE and IgG4 to this antigen that cross-reacts with Dsg1 and subsequently lead to clinical FS. The definitive evolution and association of IgG4 and IgE antibody responses in FS requires further analysis of the CDR3 sequences of these two classes of antibody populations. Our current findings suggest that LJM11 might be the initial target of the IgE response in FS susceptible individuals, hence those individuals with higher levels of anti-LJM11 IgE may have a higher risk to develop FS. It may provide a basis for the possible employment of IgE as an early predictor of FS.
ACKNOWLEDGEMENTS
Funding support is provided by NIH grant RO1-AR32599 (LAD), and K01-AR056378 (YQ).
Abbreviations
- Dsg1
desmoglein 1
- FS
Fogo selvagem
- PF
pemphigus foliaceus
- PV
pemphigus vulgaris
- SGLL
salivary gland antigens from sand fly (Lutzomyia longipalpis)
- LV
Limao Verde in Brazil
- HC
healthy control
- RLUs
Relative Light Units
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Amagai M, Komai A, Hashimoto T, et al. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–7. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- Aoki V, Millikan RC, Rivitti EA, et al. Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem). J Investig Dermatol Symp Proc. 2004;9:34–40. doi: 10.1111/j.1087-0024.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- Diaz LA, Sampaio SA, Rivitti EA, et al. Endemic pemphigus foliaceus (Fogo Selvagem): II. Current and historic epidemiologic studies. J Invest Dermatol. 1989;92:4–12. doi: 10.1111/1523-1747.ep13070394. [DOI] [PubMed] [Google Scholar]
- Hamilton RG, Morrison SL. Epitope mapping of human immunoglobulin-specific murine monoclonal antibodies with domain-switched, deleted and point-mutated chimeric antibodies. Journal of immunological methods. 1993;158:107–22. doi: 10.1016/0022-1759(93)90263-7. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L, Holtzman N, Burnett L. A Quantitative in Vitro Study of the Chromatographic Distribution and Immunoglobulin Characteristics of Human Blocking Antibody. J Immunol. 1968;101:317. [PubMed] [Google Scholar]
- Moraes ME, Fernandez-Vina M, Lazaro A, et al. An epitope in the third hypervariable region of the DRB1 gene is involved in the susceptibility to endemic pemphigus foliaceus (fogo selvagem) in three different Brazilian populations. Tissue Antigens. 1997;49:35–40. doi: 10.1111/j.1399-0039.1997.tb02707.x. [DOI] [PubMed] [Google Scholar]
- Muller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343–7. doi: 10.1097/01.all.0000173783.42906.95. [DOI] [PubMed] [Google Scholar]
- Nagel A, Lang A, Engel D, et al. Clinical activity of pemphigus vulgaris relates to IgE autoantibodies against desmoglein 3. Clin Immunol. 2009;134:320–30. doi: 10.1016/j.clim.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Qaqish BF, Prisayanh P, Qian Y, et al. Development of an IgG4-based predictor of endemic pemphigus foliaceus (fogo selvagem). J Invest Dermatol. 2009;129:110–8. doi: 10.1038/jid.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Jeong JS, Maldonado M, et al. Cutting Edge: Brazilian Pemphigus Foliaceus Anti-Desmoglein 1 Autoantibodies Cross-React with Sand Fly Salivary LJM11 Antigen. J Immunol. 2012;189:1535–9. doi: 10.4049/jimmunol.1200842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Prisayanh P, Andraca E, et al. IgE, IgM, and IgG4 anti-desmoglein 1 autoantibody profile in endemic pemphigus foliaceus (fogo selvagem). J Invest Dermatol. 2011;131:985–7. doi: 10.1038/jid.2010.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock B, Martins CR, Theofilopoulos AN, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem). N Engl J Med. 1989;320:1463–9. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]