Abstract
The contribution of osteoclasts to hematopoietic stem/progenitor cell (HSPC) retention in the bone marrow is controversial. Studies of HSPC trafficking in osteoclast-deficient mice are limited by osteopetrosis. Here, we employed two non-osteopetrotic mouse models to assess the contribution of osteoclasts to basal and granulocyte colony-stimulating factor (G-CSF) induced HSPC mobilization. We generated Rank−/− fetal liver chimeras using Csf3r−/− recipients to produce mice lacking G-CSF receptor expression in osteoclasts. Basal and G-CSF-induced HSPC mobilization was normal in these chimeras. We next acutely depleted osteoclasts in wild-type mice using the RANK ligand inhibitor osteoprotegerin (OPG). Marked suppression of osteoclasts was observed after a single injection of OPG-Fc. Basal and G-CSF-induced HSPC mobilization in OPG-Fc treated mice were comparable to control mice. Together, these data show that osteoclasts are not required for the efficient retention of HSPCs in the bone marrow and are dispensable for HSPC mobilization by G-CSF.
INTRODUCTION
Granulocyte-colony stimulating factor (G-CSF) is the most commonly used agent to mobilize hematopoietic stem/progenitor cells (HSPCs) from the bone marrow to blood. G-CSF induces HSPC mobilization, in large part, by suppressing CXCL12 production from bone marrow stromal cells.1–4 We recently reported that expression of the G-CSFR only in CD68+ monocytic-lineage cells is sufficient to induce HSPC mobilization by G-CSF.5 Within the monocytic lineage, the G-CSFR is expressed on monocytes, macrophage, myeloid dendritic cells, and osteoclasts. There is evidence linking macrophages with HSPC trafficking. Specifically, pharmacologic or genetic approaches that delete macrophages result in suppression of CXCL12 production from stromal cells and HSPC mobilization.6,7 However, the contribution of osteoclasts to HSPC mobilization is less clear. Kollet and colleagues reported that activation of osteoclasts by injection of RANK ligand (RANKL) is associated with moderate HSPC mobilization.8 Likewise, Cho et al showed that activation of osteoclasts with RANKL enhanced mobilization in response to inflammatory stress.9 These data suggest that osteoclasts promote HSPC egress from the bone marrow. Conversely, there is data suggesting that osteoclasts may contribute to HSPC retention in the bone marrow. Inhibition of osteoclast activity with bisphosphonate treatment is associated with a modest increase in HSPC mobilization by G-CSF.7,10 Moreover, Miyamoto and colleagues showed that in several transgenic mouse lines with impaired osteoclast activity (specifically, Csf1, Fos, or Rankl deficient mice) G-CSF-induced HSPC mobilization is increased.10 Each of these studies has certain limitations. Most notably, osteoclast deficiency is associated with osteopetrosis, extramedullary hematopoiesis, and constitutive HSPC mobilization, potentially confounding studies of HSPC trafficking. Here, we employed two non-osteopetrotic mouse models to assess the contribution of osteoclasts to basal and G-CSF induced HSPC mobilization.
METHODS
Mice
Sex- and age-matched wild-type, Csf3r−/− mice11 and Rank+/− mice12 on a C57BL/6 background were maintained under standard pathogen free conditions according to methods approved by the Washington University Animal Studies Committee.
Rank−/− fetal liver chimeras
Fetal liver cells were harvested from a Rank+/− mouse intercross at 13.5–14.5 days post-conception. Genotyping was performed on fetal tissue to identify Rank−/− fetal livers.12 Two million cells were injected retro-orbitally into lethally irradiated (1,100 cGy) wild-type or Csf3r−/− 6–12 week old mice.
G-CSF and OPG-Fc
6–8 week old male wild-type mice were used in these experiments. Recombinant human G-CSF (Amgen) was administered at a dose of 250 μg/kg/day subcutaneously for 7 days. Mice were analyzed 3 to 4 hours after the final cytokine dose. Recombinant OPG-Fc was a generous gift from Amgen. Mice were given a single subcutaneous injection of 100 μg of OPG-Fc or saline alone.
Colony-forming cells (CFU-C) assays
We plated 10 μL blood, 5 × 104 nucleated spleen cells, or 2.0 × 104 nucleated bone marrow cells in 1.25 mL methylcellulose media supplemented with a cocktail of recombinant cytokines (MethoCult 3434; StemCell Technologies). Colonies were counted after 7 days of culture in a humidified chamber with 5% carbon dioxide (CO2) at 37°C.
Real-time quantitative RT-PCR
Femurs were flushed directly with TRIzol reagent (Invitrogen), and RNA was isolated according to the manufacturer’s instructions. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed using the TaqMan One-step RT-PCR Master Mix Reagents Kit on a GeneAmp 5700 Sequence Detection System (Applied Biosystems). TaqMan primer/probe mixes for cathepsin K (Mm00484039_m1), tartrate-resistant acid phosphatase (Mm00475698_m1), Csf3r (Mm00432735_m1), and beta-actin (Mm00607939_s1) were obtained from Applied Biosystems.
Flow Cytometry
The following antibodies were used (all from eBiosciences): Gr-1 (RB6-8C5), B220 (RA3-6B2), CD3e (145-2C11), and Ter-119 (TER-119); Sca-1 (D7); and c-kit (2B8). Cells were analyzed either on a FACScan or Gallios flow cytometer.
TRAP staining
Paraffin-embedded bone marrow sections were stained for TRAP and quantified by histomorphometry, as described previously.13
Statistics
Statistical significance of differences was calculated using 2-tailed Student’s t tests.
RESULTS & DISCUSSION
To generate adult mice with defective osteoclasts but without severe osteopetrosis, we first generated Rank−/− fetal liver chimeras (Figure 1). RANK signaling is required for osteoclast development;12 thus, we predicted that Rank−/− fetal liver chimeras would lack osteoclasts. However, osteoclasts number was only modestly reduced in these mice (Figure 1A–D). This is likely secondary to radio-resistant recipient osteoclasts and is consistent with a recent report showing recipient macrophage expansion following transplantation, if donor macrophage development is compromised.14
Figure 1. G-CSF signaling in osteoclasts is dispensable for HSPC mobilization by G-CSF.
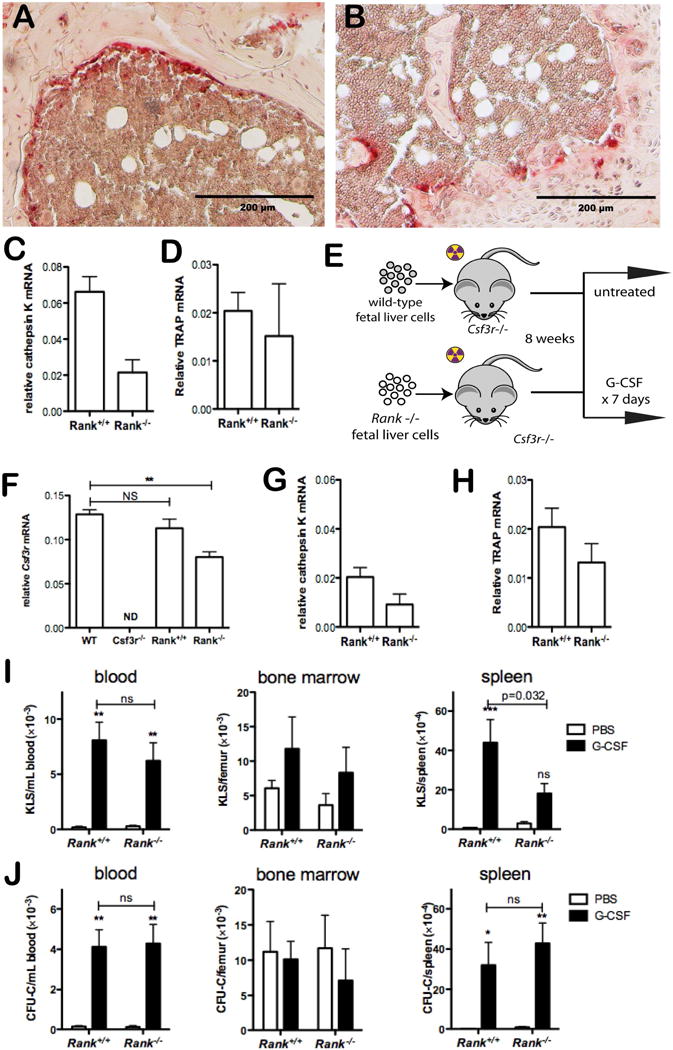
Fetal liver cells from either Rank+/+ or Rank−/− mice were transplanted into lethally-irradiated mice. (A & B) Shown are representative bone sections from Rank+/+ (A) and Rank−/− (B) chimeras stained for tartrate-resistant acid phosphatase (TRAP), a marker of osteoclasts. (C & D) The degree of osteoclast loss in the mice was quantified by measuring bone marrow cathepsin K mRNA (C) or TRAP mRNA (D) expression relative to β-actin mRNA (n = 2–4 mice per genotype). (E) Fetal liver cells from either Rank+/+ or Rank−/− mice were transplanted into lethally-irradiated Csf3r−/− mice. Eight weeks later, mice were treated with either PBS or 250 μg/kg/day G-CSF for 7 days. Shown is the expression of Csf3r (F) Cathepsin K (G) or TRAP (H) mRNA in the bone marrow of wild type mice (WT), Csf3r−/− mice, or untreated Rank+/+ or Rank−/− Csf3r−/− chimeras relative to β-actin mRNA (n = 4–6 per cohort). (I & J) The number of KLS cells (I) or CFU-Cs (J) in the peripheral blood, bone marrow, and spleen (n = 4–6 mice per donor genotype per treatment group) is shown. *P < 0.05, **P < 0.01, ***P < 0.001, ns: not significant, nd: not detected.
To circumvent this problem, we transplanted Rank−/− (or Rank+/+) fetal liver cells into Csf3r−/− recipients (Figure 1E). Since donor osteoclast development is blocked in the absence of RANK signaling, any remaining osteoclasts in these mice should lack the G-CSF receptor. Donor engraftment with Csf3r sufficient cells was confirmed by measuring Csf3r mRNA expression in bone marrow cells 6–8 weeks after transplantation (Figure 1F). Of note, the number of osteoclasts (as measured by bone marrow cathepsin K and TRAP expression) was comparable in Csf3r−/− recipients reconstituted with wild-type or Rank−/− bone marrow (Figure 1G & H). As expected, these mice are not osteopetrotic, as evidence by bone marrow histology (Suppl. Figure 1) and normal bone marrow and spleen cellularity (Suppl. Figure 2). At baseline, the number of bone marrow, peripheral blood, and spleen Kit+ Sca+ lineage− (KLS) cells and colony forming cells (CFU-C) were similar in Rank−/− Csf3r−/− and Rank+/+ Csf3r−/− chimeras (Figure 1I & J). Moreover, a similar robust mobilization response to G-CSF with both types of chimeras was observed.
The Rank−/− Csf3r−/− chimera data show that G-CSF signaling in osteoclasts is not required for HSPC mobilization by G-CSF. However, it is possible that osteoclasts may regulate HSPC trafficking independent of the G-CSF receptor. To address this possibility, we acutely depleted osteoclasts by treating wild-type mice with OPG-Fc. Osteoprotegrin (OPG) is a decoy receptor for RANKL.15 Recombinant OPG-Fc is a chimeric molecule fusing the RANKL binding domain of OPG with the Fc portion of human immunoglobulin. OPG-Fc has a long circulating half-life, and treatment with OPG-Fc reversibly depletes osteoclasts in rats.16 As expected, we observed a marked suppression of osteoclasts in wild-type mice after a single injection of OPG-Fc that persisted for at least 9 days. Cathepsin K and tartrate resistant acid phosphatase (TRAP) mRNA expression in the bone marrow decreased 50-fold and 8.4-fold, respectively (Figure 2A & B), and TRAP+ cells were nearly absent [Figure 2C &D and Suppl. Figure 3; number of osteoclasts per mm bone surface: 3.5 ± 0.11 (saline treated) versus 0.19 ± .02 (day 7 after OPG-Fc treatment), P < 0.001]. Of note, OPG-Fc had no effect on monocytes, macrophages, and myeloid dendritic cell number (Suppl. Figure 4). Osteoclast depletion by itself did not result in mobilization of KLS cells to blood or spleen (Figure 2E). We next treated mice with OPG-Fc followed by G-CSF (Figure 2F). Again, severe osteoclast suppression was observed in OPG-Fc treated mice, whether or not G-CSF also was given (Figure 2G). Importantly, G-CSF induced KLS cell and CFU-C mobilization to blood and spleen was similar in OPG-Fc treated and control mice (Figure 2H & I).
Figure 2. Osteoclast ablation does not perturb basal or G-CSF-induced HSPC mobilization.
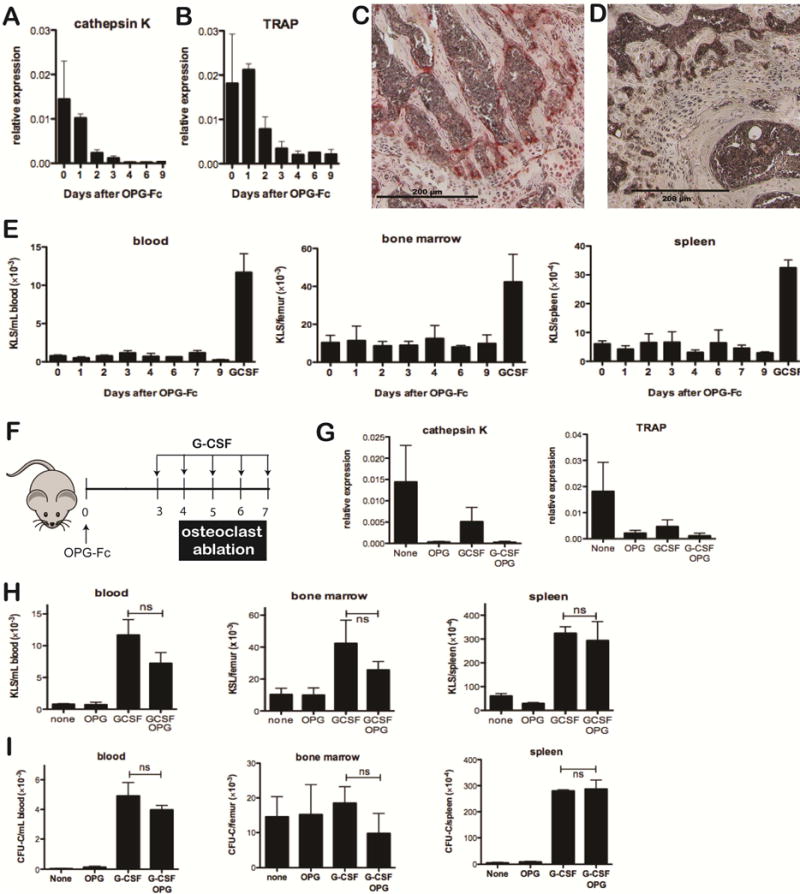
(A & B) Mice were treated with a single dose of OPG-Fc at day 0. Osteoclast number was assessed by measuring bone marrow cathepsin K (A) or TRAP (B) mRNA expression relative to β-actin mRNA (n = 2–3 per time point). (C & D) Representative sections of trabecular bone stained for TRAP from untreated (C) or OPG-Fc treated (D) mice 7 days after treatment with a single dose of OPG-Fc. (E) Wild-type mice were given a single dose of OPG-Fc at day 0; the frequency of KLS cells in the peripheral blood, bone marrow, and spleen was assayed at the indicated time points. The last bar on each graph is based on mice treated with G-CSF alone for 5 days (n = 2–5 per time point). (F) Mice were treated with either a single dose of OPG-Fc, a five-day course of G-CSF beginning 3 days after the OPG-Fc treatment, or a 5-day course of G-CSF alone. (G) Cathepsin K or TRAP mRNA levels in the bone marrow was measured on day 7. (H & I) The number KLS cells (H) or CFU-C (I) in the peripheral blood, bone marrow, and spleen was measured at the conclusion of the G-CSF course (n = 2–5 per time point). ns: not significant.
Collectively, our data show that osteoclasts are not required for the efficient retention of HSPCs in the bone marrow and are dispensable for HSPC mobilization by G-CSF. Consequently, the increase in basal and G-CSF induced HSPC mobilization observed in constitutive models of osteoclast deficiency are likely secondary to osteopetrosis. Of note, treatment with bisphosphonates augments HSPC mobilization by G-CSF in mice,7,10 suggesting that off-target effects of these agents, rather than osteoclast suppression, is responsible for the enhanced mobilization response. Collectively, these data suggest that pharmacologic strategies to augment HSPC mobilization by disrupting osteoclast function are unlikely to be effective.
Supplementary Material
KEYPOINTS.
Osteoclasts are dispensable for HSPC retention in the bone marrow
Osteoclasts are not required for G-CSF induced HSPC mobilization
Acknowledgments
We thank Amgen for their generous gift of OPG-Fc. This work was supported by a grant from the National Institutes of Health: RO1 HL60772 (DCL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution: MR, TS, and APS participated in the experimental design, performed the experiments, analyzed the data, and participated in writing the manuscript. DCL participated in the experimental design, data interpretation, and assisted with the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. [erratum appears in Nat Immunol 2002 Aug;3(8):787] Nature Immunology. 2002;3(7):687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 2.Levesque JP, Hendy J, Takamatsu Y, et al. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111(2):187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christopher MJ, Liu F, Hilton MJ, et al. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114(7):1331–9. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christopher MJ, Rao M, Liu F, et al. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251–60. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–71. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23):4815–28. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 8.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 9.Cho KA, Joo SY, Han HS, et al. Osteoclast activation by receptor activator of NFkappaB ligand enhances the mobilization of hematopoietic progenitor cells from the bone marrow in acute injury. Int J Mol Med. 2010;26(4):557–63. doi: 10.3892/ijmm_00000499. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto K, Yoshida S, Kawasumi M, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208(11):2175–81. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Wu HY, Wesselschmidt R, et al. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity. 1996;5(5):491–501. doi: 10.1016/s1074-7613(00)80504-x. [DOI] [PubMed] [Google Scholar]
- 12.Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CA, Pettit AR, Toulson S, et al. Responses in vivo to purified poly(3-hydroxybutyrate-co-3-hydroxyvalerate) implanted in a murine tibial defect model. J Biomed Mater Res A. 2009;91(3):845–54. doi: 10.1002/jbm.a.32238. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 16.Ominsky MS, Li X, Asuncion FJ, et al. RANKL inhibition with osteoprotegerin increases bone strength by improving cortical and trabecular bone architecture in ovariectomized rats. J Bone Miner Res. 2008;23(5):672–82. doi: 10.1359/jbmr.080109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


