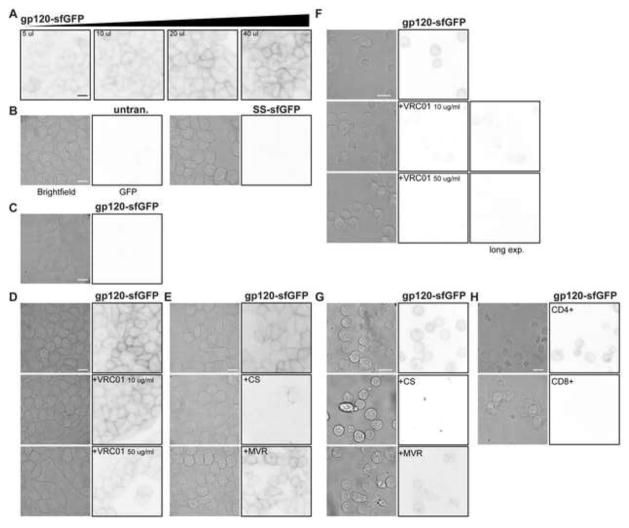
Fig. 2.
gp120-sfGFP binds CD4/CCR5 expressing TZM-bl cells. TZM-bl or HeLa cells were plated in chamber slides and incubated overnight. TZM-bl cells were then exposed to (A) a range of concentrations of gp120-sfGFP, 5–40 μL (~0.2–0.45 μg/mL) and (B) SS-sfGFP and control media. (C) Parental HeLa cells were exposed to 40 μl gp120-sfGFP and no binding was apparent. TZM-bl cells were co-incubated with 40 μL (~0.45 μg/mL) gp120-sfGFP and (D) 10 or 50 μg/ml VRC01 antibody, (E) 100 μg/ml cellulose sulfate (CS) or 10 μM Maraviroc (MVR). Brightfield and inverted fluorescence images were used to determine the fluorescence binding pattern; images are representative of cells from at least two independent experiments, scale bar = 10 μm. (E–G) gp120-sfGFP binds primary lymphocytes. After three days of stimulation with PHA, PBMCs were incubated with 40 μL (~0.45 μg/mL) gp120-sfGFP or controls in the presence or absence of the inhibitors (E) VRC01 antibody, (F) cellulose sulfate (CS) or Maraviroc (MVR). After incubation, PBMCs were transferred to lysine-coated coverglass chamber slides to promote PBMC attachment to the glass for fluorescence microscopy. (G) After 3 days of PHA-stimulation, CD8-negative (labeled as CD4-positive) and CD8-positive (labeled as CD8-positive) PBMC were incubated with gp120-sfGFP for 1 h. Brightfield paired with inverted fluorescence images were used to determine the fluorescence binding pattern. Incubation of PBMCs with SS-sfGFP or control media did not result in fluorescence (data not shown). Images are representative of cells from at least two different donors, scale bar = 10 μm.