Abstract
Investigations from multiple laboratories support the existence of melanoma initiating cells (MICs) that potentially contribute to melanoma's drug resistance. ABT-737, a small molecule BCL-2/BCL-XL/BCL-W inhibitor, is promising in cancer treatments, but not very effective against melanoma, with the anti-apoptotic protein MCL-1 as the main contributor to resistance. The synthetic retinoid fenretinide (4-HPR) has shown promise for treating breast cancers. Here, we tested whether the combination of ABT-737 with 4-HPR is effective in killing both the bulk of melanoma cells and MICs. The combination synergistically decreased cell viability and caused cell death in multiple melanoma cells lines (carrying either BRAF or NRAS mutations), but not in normal melanocytes. The combination increased the NOXA expression and caspase-dependent MCL-1 degradation. Knocking-down NOXA protected cells from combination-induced apoptosis, implicating the role of NOXA in the drug synergy. The combination treatment also disrupted primary spheres (a functional assay for MICs) and decreased the percentage of ALDHhigh cells (a marker of MICs) in melanoma cell lines. Moreover, the combination inhibited the self-renewal capacity of MICs, measured by secondary sphere forming assays. In vivo, the combination inhibited tumor growth. Thus, this combination is a promising treatment strategy for melanoma, regardless of mutation status of BRAF or NRAS.
Introduction
Metastatic melanoma is a devastating disease resistant to conventional therapies. Despite several recent FDA approved drugs for melanomas, there is still a pressing need for therapies using novel approaches (Arbiser et al., 2012; Chapman et al., 2011; Finn et al., 2012; Garbe et al., 2011; Wong et al., 2013). It is proposed that cancer stem cells, or cancer initiating cells (CICs), are responsible for initiation, progression, and resistance to treatment (Lee et al., 2014). Recent studies provide evidence that a subpopulation of melanoma cells possess characteristics similar to CICs, termed melanoma initiating cells (hereafter, MICs) (Civenni et al., 2011; Fang et al., 2005; Monzani et al., 2007; Roesch et al., 2010; Santini et al., 2012). We previously demonstrated the existence of human melanoma cells that fulfill the criteria for MICs (self-renewal and differentiation), and these cells are more resistant to chemotherapy (Luo et al., 2012). As a strategy in preventing relapse, it is crucial to look for therapies that de-bulk melanoma tumors and also target MIC populations.
The BCL2 (BCL-2) family is important in regulating the intrinsic apoptotic pathway, and includes three groups: 1) Anti-apoptotic proteins BCL-2, BCL2L (BCL-XL), BCLW (BCL-W), MCL1 (MCL-1), A1 and BCL-B; 2) Multi-domain pro-apoptotic proteins BAX, BAK and BOK, which are effectors of apoptosis; 3) BH3-only pro-apoptotic proteins BIM, PUMA, BAD, NOXA, BIK, BMF and tBID, which are initiators of apoptosis (see review (Czabotar et al., 2014)). Interactions between different members are not mutually exclusive or equal. Some BH3-only proteins only bind to one group of anti-apoptotic proteins, e.g., NOXA only binds to MCL-1/A1, and BAD only to BCL-2/BCL-XL/BCL-W. Conversely, BIM, PUMA, and tBID bind to multiple anti-apoptotic proteins. These various combinations of interactions control the initiation of apoptosis.
The anti-apoptotic BCL-2 protein family contributes to melanoma's resistance to apoptosis (Eberle and Hossini, 2008; Haass and Schumacher, 2014; Mohana-Kumaran et al., 2014; Placzek et al., 2010; Soengas and Lowe, 2003), and clinical trials targeting anti-apoptotic Bcl-2 family members are currently underway (Thomas et al., 2013). ABT-737 and its oral bioactive form, ABT-263, are small molecule BCL-2/BCL-XL/BCL-W inhibitors that show promising results in cancer therapy, either alone or in combination with other chemotherapeutics (Oltersdorf et al., 2005; Thomas et al., 2013). However, many labs, including ours, found that ABT-737 by itself is not very effective for de-bulking melanoma, and that the anti-apoptotic protein MCL-1 is the primary contributor to resistance (Miller et al., 2009; Reuland et al., 2011; Reuland et al., 2012). Therefore, compounds targeting MCL-1 are good potential partners to be combined with ABT-737.
To combine with ABT-737, we chose fenretinide (4-HPR), for its ability to inhibit MCL-1 indirectly and for its killing action in CICs of other cancers (Kang et al., 2008; Torrisi and Decensi, 2000; Zhang et al., 2013). This combination has synergistic cytotoxicity in other cancers (Bruno et al., 2012; Fang et al., 2011; Kang et al., 2008), however, it has never been tested on melanoma cell lines or any cancer initiating cell populations.
One of the common ways of isolating or enriching CICs is by the use of surface markers (Shakhova and Sommer, 2013; Stecca et al., 2013). However, the use of surface markers like CD271 or CD133 to enrich MICs has produced contradictory results (Shakhova and Sommer, 2013). To avoid such issues, we employed three surface-marker-independent assays to examine the effects of drug treatments on the MIC populations: primary sphere forming, secondary sphere forming, and the Aldefluor assays. Melanoma-spheres display “stemness,” exhibit self-renewal capacity and tumorigenicity, and sustain tumor growth in vivo, allowing for the generation of human melanoma xenografts (Santini et al., 2012; Stecca et al., 2013). We used a primary sphere forming functional assay to test drug potency on melanoma cells harboring stem-like features, the MICs. Post-drug treatment, and using the cells from the primary sphere assay, a secondary sphere assay described by Stecca et al. (Stecca et al., 2013) quantified the self-renewal capacity of the enriched, resistant MICs. Furthermore, we and others established that cells with higher aldehyde dehydrogenase (ALDH) activity are enriched in MICs (Luo et al., 2012; Santini et al., 2012). Therefore, we used the Aldefluor assay to further examine the effects of drug treatments on MICs.
The present study tested the effects of a combination melanoma treatment with ABT-737 (a small molecule BCL-2/BCL-XL/BCL-W inhibitor) and 4-HPR (an indirect MCL-1 inhibitor), focusing on de-bulking the melanoma and killing MICs and/or inhibiting their self-renewal capacity.
Results
4-HPR and ABT-737 synergistically reduced cell viability and increased apoptosis in melanoma cells but not in melanocytes
To explore whether treatment with 4-HPR and ABT-737 affected the cell viability and apoptosis of the melanoma cells, we used the MTS assay, the Annexin V assay, and bright-field morphological analysis (Figure 1). The data from the MTS assays were analyzed with Calcusyn to obtain the combination index (CI) value for all of the combinations (Supplemental Table S1). The combination treatment was strongly synergistic over a wide range of drug concentrations, with CI values <0.3 for all melanoma cell lines tested. Cells treated with various doses of 4-HPR and ABT-737 showed synergy occurring from 5-10 μM 4-HPR and at or above 1.1 μM ABT-737 for all cell lines except A375, which showed synergy at much lower 4-HPR doses (Figure 1a and Supplemental Table 1). The Annexin V assay demonstrated that a combination of 4-HPR and 3.3 μM ABT-737 caused dramatic apoptosis in every melanoma cell line we tested, ranging from ∼40-70%, even though the single agents had little effect (Figure 1b). The combination induced similar effects in the melanoma cell lines carrying mutated BRAF or NRAS, and significantly increased the percentage of Annexin V+ cells for all melanoma cell lines tested (p < 0.01). Melanoma cells treated with the combination showed signs of cell death, such as detachment from the substrate, misshape morphology, and blebbing, while melanocytes remained largely unaffected (Figure 1c). Moreover, the primary melanocyte line HEMNLP2 was resistant to the drug combination (Figure 1a and 1b). The immortalized melanocyte line PIG1 showed only modest effects at the highest doses (Figure 1a). Overall, the above assays indicated that the combination treatment synergistically reduced cell viability and caused cell death in multiple melanoma cell lines, but not in melanocytes.
Figure 1. The effects of 4-HPR combined with ABT-737 in melanomas and melanocytes.
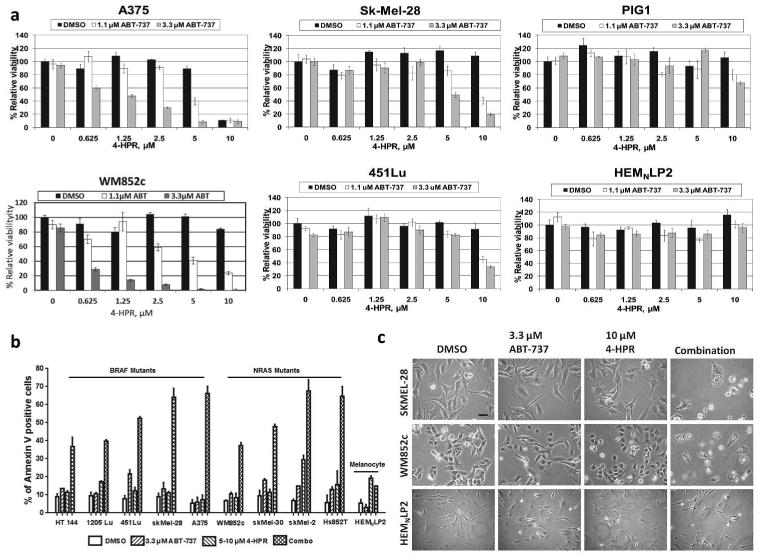
(a) The MTS assay shows that 4-HPR combined with ABT-737 synergistically killed melanomas (A375, SK-MEL-28, WM852c, and 451Lu), but had little effect on melanocytes (HEMNLP2 and PIG1). Cells were treated with the indicated drugs for 48 h before the MTS assay. (b) The Annexin V assay shows massive apoptosis in melanoma cells induced by 48 h treatment of 4-HPR and ABT-737. In all melanoma cells (either mutant BRAF or NRAS), the combination treatment significantly induced apoptosis compared to all other treatments (DMSO or single drug, p < 0.01). (c) Visual appearance of cells showing cell death in combination treatments for melanoma cells, but not for melanocytes. Scale bar = 100μm.
The combination induced caspase-dependent MCL-1 degradation and increased NOXA expression
We and others have shown that MCL-1 is the primary protein that allows melanoma's resistance to ABT-737, but an increase of NOXA expression or a decrease of MCL-1 expression overcomes this resistance (Lucas et al., 2012; Miller et al., 2009; Reuland et al., 2011; Reuland et al., 2012). Only the combination treatment notably increased NOXA expression and cleaved PARP (Figure 2a). In addition, only the combination treatment decreased the levels of MCL-1 (Figure 2a). Quantification indicated that the combination treatment dramatically increased the NOXA/MCL-1 ratio by at least 5-fold in all the melanoma cell lines tested (Figure 2a).
Figure 2. The combination of 4-HPR with ABT-737 induced PARP cleavage, increased the NOXA/MCL-1 ratio and caused Caspase-mediated MCL-1 degradation.
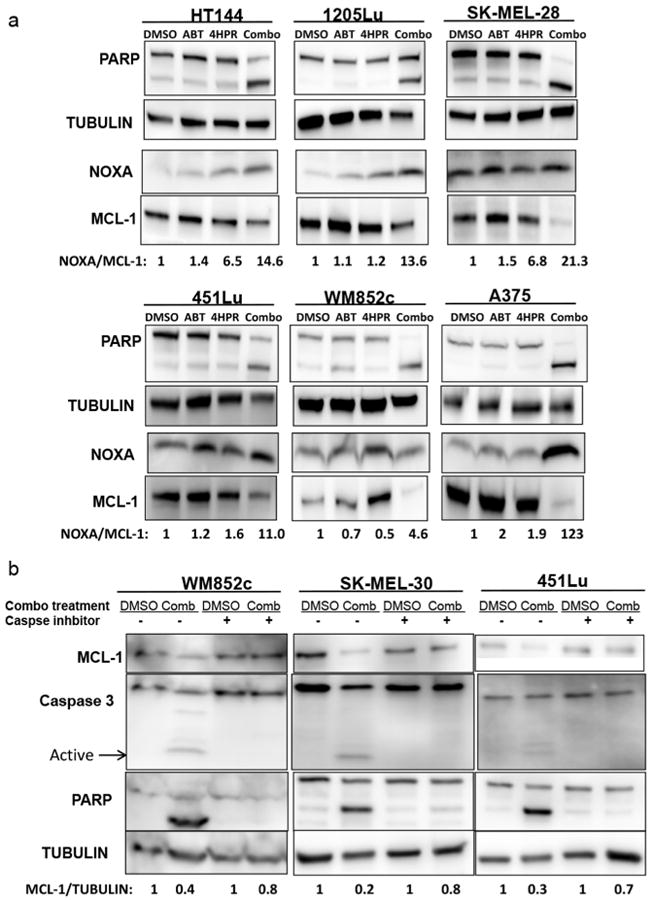
(a) Immunoblot of cell lysates treated with either vehicle control (DMSO), 3.3μM ABT-737 (ABT), 5-10μM 4-HPR (4HPR), or the combination of the two drugs (Combo). The ratio of NOXA/MCL-1 in the control was set as “1” for each cell line. (b) Immunoblot of cell lysates treated with either vehicle control (DMSO) or the combination with or without co-treatment of 30μM pan caspase inhibitor Z-VAD-FMK. WM852c and SK-MEL-30 are NRAS mutants, and all other cell lines shown here are BRAF mutants. The normalized MCL-1 levels in the Combo with or without the Caspase inhibitor were compared to the respective DMSO conditions.
Various triggers induce caspase-mediated MCL-1 degradation, contributing to accelerated apoptosis (Miller et al., 2009; Ramirez-Labrada et al., 2014). We examined this potential mechanism using a pan-caspase inhibitor as described (Miller et al., 2009; Ramirez-Labrada et al., 2014). Co-treatment of the pan-caspase inhibitor Z-VAD-FMK with the combination blocked caspase 3 activity and PARP cleavage, as expected. MCL-1 expression also increased in the combination-treated cells to a level close to the control cells, suggesting the MCL-1 degradation is mediated by caspases (Figure 2b). Thus, these data suggest that the combination induced expression of pro-apoptotic NOXA and caspase–dependent degradation of anti-apoptotic protein MCL-1. Results were similar in both BRAF mutated (451Lu) and NRAS mutated cells (WM852c and SK-MEL-30).
Inhibition of NOXA lessened the effects of the drug combination
We examined the killing potency of the combination of 4-HPR and ABT-737 on cells after knocking down NOXA, BIM, BID, PUMA, or TP53 (p53) (Figure 3). Of these, only knock-down of NOXA resulted in a significant reduction in cell death compared to the control in all the cell lines tested (p < 0.05), although the protections were not complete (Figure 3a-d, and supplementary Figure S1). In addition, knock-down of BIM significantly protected SK-MEL-30 (p < 0.01) but not A375 cells, even though the knockdown efficiency seemed to be compatible. Interestingly, BIM expression was higher in SK-MEL-30 than in A375 cells (Supplementary Figure S2). These data suggest the combination-induced killing is NOXA-dependent, and BIM may play a role in a cell-line dependent manner.
Figure 3. Knockdown of NOXA decreased drug-induced cell death.
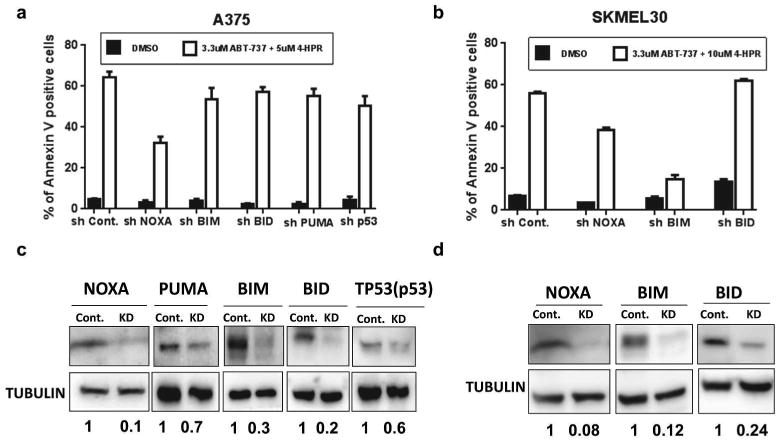
Annexin V assays of the indicated knockdown cell lines derived from A375 (a) or SK-MEL-30 (b). Immuoblots (c and d) of lysates from the cell lines used in (a and b respectively) show efficient knockdown of indicated proteins. In A375, knockdown of NOXA by shRNA (shNoxa) significantly reduced combination-induced apoptosis as compared to control (shCont) (p <0.05), but knockdown of other pro-apoptotic proteins did not. In SK-MEL-30, both knockdown of NOXA and BIM significantly reduced combination-induced apoptosis as compared to control (p<0.01).
The combination of 4-HPR with ABT-737 caused cytotoxicity in MICs and also increased the NOXA/MCL-1 ratio in sphere cultures of multiple melanoma cell lines
We examined whether this combination is also effective against MICs using sphere forming and the Aldeflour assays (Figure 4). In all of nine melanoma cell lines, the combination severely disrupted the primary spheres compared to the control (p< 0.001, Figure 4a and b). The combination also significantly decreased the number of spheres as compared to ABT-737 alone in eight of the cell lines (p < 0.001), and as compared to 4-HPR in two of them (p< 0.05 or lesser, Figure 4b). 4-HPR significantly decreased the sphere numbers in all the cell lines (p< 0.01 or lesser) and ABT-737 significantly decreased the number in 1205Lu, WM852c and Hs852T compared to DMSO (p <0.01).
Figure 4. The combination of 4-HPR with ABT-737 caused cytotoxicity in MICs.
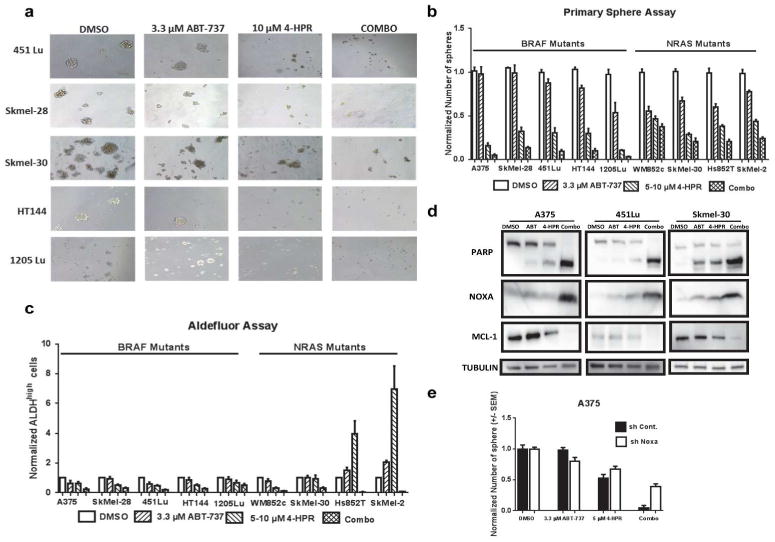
(a) Brightfield analysis showing complete disruption of primary spheres after 48 h of the combination treatment. Scale bar = 100 μm. (b) Quantification of the number of spheres from melanoma cell lines with either mutant BRAF or NRAS. The combination significantly reduced the number of spheres compared to the vehicle (DMSO) or ABT-737 in multiple melanoma cell lines (p < 0.001). (c) Quantification of the ALDH assay. The combination treatment significantly reduced the relative percentage of ALDHhigh cells compared to the control or ABT-737 in multiple melanoma cell lines. (d) Immunoblots of lysates from the cells used in ‘b’ showing that the combination induced PARP cleavage, increased NOXA, and reduced MCL-1. (e) Primary sphere assays with NOXA knockdown (shNoxa) or control (shCont.) of A375 after being treated with indicated drugs. Knockdown of NOXA significantly protected cells from the combination-induced disruption of spheres compared to the control (p <0.01).
In eight out of nine melanoma cell lines, the combination significantly decreased the percentage of ALDHhigh cells compared to the DMSO control (p < 0.01 or less) (Figure 4c). In seven out of nine melanoma cell lines, the combination significantly decreased the percentage of ALDHhigh cells compared to ABT-737 (p < 0.05) (Figure 4c). In Hs852T and SK-MEL-2, the combination significantly decreased the percentage of ALDHhigh cells compared to DMSO, ABT-737 as well as 4-HPR (p < 0.05 or lesser) (Figure 4c). Consistent with these results, the combination also reduced JARID1B expression (Supplemental Figure S3), another potential marker for MICs (Roesch et al., 2013). Interestingly, single treatment of ABT-737 increased the JARID1B expression compared to control in one of the cell lines.
With the sphere lysates, we then performed immunoblot (Figure 4d). The data were similar to our findings in monolayer conditions; the combination treatment increased the expression of NOXA and cleaved PARP, and decreased the expression of MCL-1 (Figure 4d vs. Figure 2). Similarly, knock-down of NOXA also reduced the effects of the combination treatment in the sphere-forming ability of multiple cell lines (Figure 4e and Supplemental Figure S4). Taken together, results indicate that the combination treatment induced NOXA-dependent cytotoxicity in the MIC population.
4-HPR combined with ABT-737 inhibited the self-renewal capacity of MICs in multiple melanoma cell lines in vitro
One cause of cancer relapse is the self-renewal capacity of CICs (Beck and Blanpain, 2013); the secondary sphere formation assay is a means of measuring this capacity in vitro. Instead of just enriching stem-like cells, as in the primary sphere-forming assay, this assay measures the cell population's ability to regenerate after drug treatment. Primary spheres formed after indicated drug treatments were dissociated and replated at the same viable cell density. However, no drugs were added during the secondary sphere-forming assay. This assay specifically assesses if any of the remaining cells—those that escaped chemotherapeutics—are capable of self-renewing and regenerating into a mass of tumor cells.
The most striking and consistent result across all cell lines was that the combination treatment almost eliminated all secondary sphere formation. Interestingly, unlike in the primary sphere assay, 4-HPR treatment alone did not significantly inhibit the formation of the secondary spheres in most of the cell lines (Figure 5a), indicating that 4-HPR did not kill a subpopulation of MICs capable of proliferation post drug treatment. Statistical analyses indicated that the combination treatment significantly decreased the number of secondary spheres formed compared to DMSO (p < 0.01), ABT-737 (p < 0.01), or 4-HPR treatment (p < 0.05 or less) in all seven cell lines (Figure 5b). Furthermore, single drug treatments showed an increased number of secondary spheres, compared to the control in some of the BRAF mutated cell lines (Figure 5c), though the p-value reached significance only in the 451Lu (p < 0.05).
Figure 5. The Combination of 4-HPR and ABT-737 inhibited the self-renewal capacity of MICs in vitro.
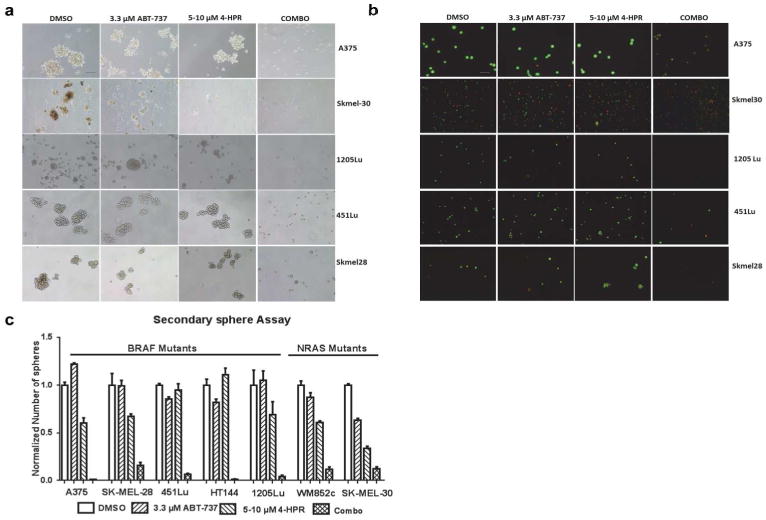
(a) Brightfield analysis of the secondary sphere assay showing that only the combination inhibited the formation of secondary spheres. Scale bar = 100 μm. (b) Visualization of the cells with ethidium bromide/acridine orange (EtBr/AO) staining indicated that the majority of cells in the control or single drug treatment were alive (green), but the majority of cells in the combination were dead (orange). Scale bar = 100 μm. (c) Quantification of the number of secondary spheres from melanoma cell lines with either mutated BRAF or NRAS. The combination significantly reduced the number of spheres compared to all other treatments in multiple melanoma cell lines (p < 0.05 or less).
Visualization of these cells with EtBr/AO staining indicated the majority of cells in the control or single drug treatments were alive, but the majority of cells in the combination treatments were dead (Figure 5b). Thus, the results show that the combination prevented the formation of secondary spheres in multiple cell lines, demonstrating that the combination decreased MIC's self-renewal capability.
In patient-derived samples, 4-HPR combined with ABT-737 significantly disrupted spheres
To determine whether the combination treatment of ABT-737 with 4-HPR had a similar effect on samples more clinically relevant than cell lines, we examined the effects of the combination in patient tumor samples maintained in a tumor xenograft mouse model (PDX). Drug responses more closely match clinical outcomes in the PDX model, and the PDX model provides more material than fresh tumor tissues (Tentler et al., 2012). Drug treatment was tested in four patient samples that we established in a PDX model for melanoma (Luo et al., 2012). Spheres took longer to form in most of patient derived cells, delaying the start of drug treatment, but otherwise cells were handled in the same way. Interestingly, the effects of the combination treatment on primary sphere formation were more similar to the effects of secondary sphere formation of the melanoma cell lines. In the PDX model cells, primary spheres were severely disrupted (Figure 6a), with significantly reduced sphere numbers compared to all other treatment conditions: DMSO (p < 0.001), ABT-737 (p < 0.01 or less), and 4-HPR (p < 0.01 or less) (Figure 6b).
Figure 6. The combination of 4-HPR with ABT-737 had cytotoxic effects in PDX tumor samples and in a mouse xenograft model.
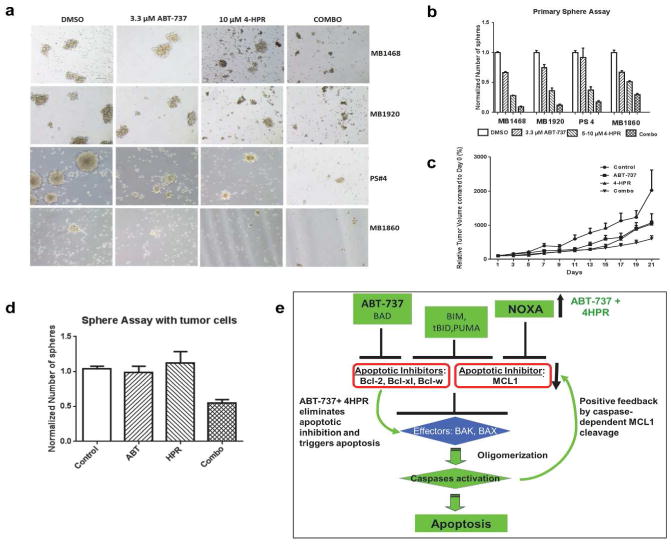
(a) Brightfield analysis of PDX tumor samples showing complete disruption of spheres after 48 h of combination treatment. Scale bar = 100 μm. (b) Quantification of the number of spheres for the PDX samples. The combination treatment significantly reduced the number of spheres compared to vehicle (DMSO, p < 0.001)), ABT-737 (p < 0.01 or less), and 4-HPR (p < 0.01 or less) in multiple PDX samples. (c) Relative tumor volumes in a mouse xenograft model. The tumor volume at day 0 was set as 100%. The combination significantly inhibited the tumor growth compared to control and 4-HPR. (d) Sphere assays with the tumor cells collected at the end of the xenograft experiment from ‘c’. The combination significantly reduced the number of spheres compared to vehicle or individual treatments (p < 0.05). The graph represents the mean of three different sets of tumors collected. (e) A simplified model to illustrate how the combination of ABT-737 plus 4HPR triggers apoptosis by altering Bcl-2 family members. Initially, the combination eliminates the functions of multiple apoptotic inhibitors and activates apoptosis by two ways: ABT-737 alone blocks BCL-2/BCL-XL/BCL-W, and the combination induces NOXA expression, which inhibits MCL-1. Consequently, the combination treatments synergistic mechanism is achieved by MCL-1 degradation by a small percentage of activated caspases, which produces a positive feedback signal for further apoptosis activation.
4-HPR and ABT-737 reduce tumor growth in an in vivo mouse xenograft model
In a mouse xenograft model, the rate of tumor growth in the combination group was significantly slower compared to the control group (p = 0.002), with no significant difference in the tumor growth rate among the control, ABT-737 alone, or 4-HPR alone groups. At the end of the treatment period on days 19 and 21, the relative tumor volume of the combination group was significantly reduced compared to both the control group (p < 0.001) and 4-HPR alone group (p < 0.05) (Figure 6d). Single drug treatments, ABT-737 or 4-HPR alone, were not significantly different from the control. These results show that the combination of ABT-737 and 4-HPR significantly reduced the growth of melanoma tumors in vivo compared to vehicle or individual drugs (Figure 6c).
To determine whether treatments in vivo also affect tumor cells' capacity to form spheres, we performed sphere-forming assays with the single cell suspensions isolated from the surviving tumors of the above experiment. No drugs were added to the cells during the sphere assay. These mouse-xenograft derived tumor cells took longer than the cell lines to form spheres, and the combination significantly reduced the number of spheres compared to vehicle or individual treatments (p < 0.05) (Figure 6d). Immunoblots show the combination induced PARP cleavage and increased the NOXA/MCL-1 ratio (Supplemental Figure 5), similar to the in vitro results.
Discussion
This study examined the effects of combining ABT-737 with 4-HPR on melanoma, looking at the efficacy of killing both the bulk of tumor cells and the MICs. Regarding de-bulking the tumor cells, we confirmed by MTS assays, Annexin V assays, and the detection of PARP cleavage by immunoblot that the combination treatment synergistically decreased cell viability and induced apoptosis in multiple cells lines (Figures 1 and 2). Moreover, BRAF or NRAS status did not affect the sensitivity to the drug combination. Given the lack of treatment options for NRAS mutated melanomas, it is exciting that this combination may lead to better patient outcomes.
To examine the effect on MIC populations, we utilized primary and secondary sphere formation assays and an ALDH activity assay. In multiple melanoma cell lines, the combination and 4-HPR alone significantly disrupted the primary spheres and decreased the percentage of ALDHhigh cells, compared to vehicle (DMSO) and ABT-737. Strikingly, only the combination significantly inhibited the formation of secondary spheres in these cells. The primary spheres and ALDHhigh cell populations are enriched in MICs, but the secondary sphere assay measures the capacity of self-renewal. Only the combination treatment significantly decreased self-renewal capacity, preventing proliferation post-treatment, essentially inhibiting the re-growth of tumor cells. Thus, the combination was more potent than the control or either drug alone in eliminating MICs, and has the potential to prevent relapse in melanoma patients.
Overall, in melanoma cell lines and PDX patient samples, the combination treatment, but not individual treatments, is cytotoxic to the bulk of melanoma cells, and more importantly, to the MICs. This treatment would potentially hinder relapse by blocking tumor regeneration. Collectively, results of in vitro monolayer, sphere and ALDH assays, and mouse in vivo, all show the combination treatment vigorously kills melanoma cells and MICs compared to vehicle or the single drug.
We investigated several potential mechanisms for the synergistic effects between ABT-737 and 4-HPR in melanomas. Although 4-HPR is known to cause cell death by inducing reactive oxygen species (ROS) and ceramide synthesis (Maurer et al., 2000), our results indicated that ROS or ceramide were not involved in melanoma death (Supplemental Figures 6 & 7). Instead, the combination induced caspase-dependent MCL-1 degradation and increased NOXA expression (Figure 2). Changes in the expression of NOXA and MCL-1 were similarly observed in both mutated BRAF and NRAS melanomas, and may serve as biomarkers of sensitivity to the combination treatment. Moreover, blocking NOXA expression partially protected multiple melanoma cell lines from the combination (Figure 3 and Supplemental Figure 1). Thus, our data suggest that this drug combination induces cell death in melanoma by antagonizing multiple anti-apoptotic BCL-2 family members, including BCL-2, BCLXL, and BCL-W (by ABT-737) and MCL-1 (by induction of NOXA and caspase-dependent MCL-1 degradation) (Figure 6e). Consequently, a small amount of activated caspases led to further degradation of MCL-1, producing a positive feedback signal for further activation of apoptosis, as seen in the combination-induced synergy (Figure 6e).
Currently two leading activation models, direct and indirect, address how interactions between members of the BCL-2 family regulate the activation of apoptosis (Czabotar et al., 2014). The consensus is that these two models are not mutually exclusive, and the predominant pathway depends on the biological context (Czabotar et al., 2014). In the direct activation model, certain BH3-only proteins directly activate the effectors BAX and BAK, and anti-apoptotic BCL-2 members primarily sequester BH3-only proteins. In the indirect model, BAX and BAK become activated only if all of the anti-apoptotic proteins are neutralized by BH3-only proteins. Our results are consistent with the indirect activation model.
We have tested a few combinations with ABT-737 previously, and found that combination with proteasome inhibitor Velcade was very toxic (Miller et al., 2009; Reuland et al., 2011; Reuland et al., 2012), and the MIC populations were quite resistant to TMZ (Luo et al., 2012). In contrast, 4-HPR is considered a chemoprevention agent due to its low toxicity (Malone et al., 2003). Moreover, current targeted melanoma treatments, with PLX4032 for instance, only kill tumor cells harboring BRAF mutations. Of BRAF carriers, response rate is 50% and relapse is all too common. Consequently, melanoma oncology is in dire need of multiple therapies using novel mechanistic approaches that target non-BRAF mutated tumors, or heterogeneous populations within the same melanoma. The combination of 4-HPR and ABT-737 may provide a potent treatment option, regardless of mutation status.
Materials and Methods
Primary and secondary Sphere forming Assays
All sphere assays were performed with poly-hema (Sigma, St. Louis, MO) coated plates or dishes (Reginato et al., 2003), in stem cell media as described previously (Dontu et al., 2003; Iwanaga et al., 2012). Specifically, the media contained DME/F12 (Hyclone) supplemented with B27 (Invitrogen), 20 ng/mL EGF and 20 ng/mL bFGF (BD Biosciences), and 4 μg/mL heparin (Sigma).
Primary Sphere Assay
Cells were plated at a density of 1-5 viable cell/μl for melanoma cell lines and 20 viable cell/μl for the patient samples. Fresh media was added every 2-3 days. The criteria described in (Stecca et al., 2013) was used to define a sphere. Briefly, a sphere is a compact spherical-roundish mass of cells with an approximate diameter greater than 50 μm. The spheres were treated with indicated drugs on day 5. After 48 h, the numbers of spheres were counted.
Secondary Sphere Assay
Primary spheres, formed as mentioned above for indicated drug treatments, were dissociated into single cells and replated as described in (Stecca et al., 2013). The procedures were the same as for the primary sphere assay, except that no drugs were added during the secondary sphere assays. At least three repeats of both the primary and secondary sphere assays were done for each cell line. The data were normalized as the relative fold compared to the vehicle (DMSO) control, and the number of spheres in the DMSO control was set at “1”. The ethidium bromide/acridine orange staining assay, as described previously (Ribble et al., 2005; Smith et al., 2012), was used to estimate live, dead, or apoptotic cells of the secondary spheres dissociated with PBS-EDTA (Stecca et al., 2013).
Other methods
Further information about other materials and methods are provided in the Supplementary Material online.
Supplementary Material
Supplemental Figure S1. Knockdown of NOXA decreased drug-induced cell death. Annexin V assays with NOXA knockdown (shNoxa) or control (shCont.) derived from A375 (a), SK-MEL-28 (b), or WM852c(c) after being treated with indicated drugs for 48 h. The insets show immunoblots of lysates from the respective cell lines used in that graph indicating efficient knockdown of NOXA. shNoxa was significantly different from shCont upon the combination treatment for all three cell lines (p < 0.05). There was no statistically significant differences between the shCont and shNoxa for the vehicle (DMS0) or the single drug treatments for all the cell lines tested.
Supplemental Figure S2. Higher overall expressions of BIM in SK-MEL-30 than in A375 cells. Immunoblot of cell lysates treated with either vehicle control (DMSO), 3.3μM ABT-737 (ABT), 10μM 4-HPR (4-HPR), or the combination of the two drugs (Combo). SK-MEL-30 exhibits higher level of BIM than A375 in vehicle (DMSO) treated cells. Combination treatment did not significantly affect BIM expression in either cell lines.
Supplemental Figure S3. The combination treatment of ABT-737 plus 4-HPR decreased JARID1B expression. Immunoblot of cell lysates treated with indicated treatment: vehicle control (DMSO), 3.3μM ABT-737 (ABT), 10μM 4-HPR (4-HPR), or the combination of the two drugs (Combo). The Combination treatment reduced the expression of JARED 1B compared to the control in all the cell lines tested.
Supplemental Figure S4. Knock-down of NOXA lessens the effects of combining 4-HPR and ABT-737 on sphere-forming capacity of melanoma cells. Primary sphere assays with NOXA knockdown (shNoxa) or control (shCont.) derived from SK-MEL-30 (a), SK-MEL-28 (b), or WM852c (c) after being treated with indicated drugs. Knockdown of NOXA significantly protected cells from the combination-induced disruption of spheres compared to the control in all the cell lines tested (p <0.01). There were no statistical differences between the shCont. and shNoxa for control or the single drug treatments in any of the cell lines, except 4-HPR treatment in SK-MEL-30 (p <0.01).
Supplemental Figure S5. The effects of combination treatment of ABT-737 plus 4-HPR on the tumor samples harvested from in vivo experiments of Figure 6c. Immunoblot of cell lysates from the tumor samples harvested at the end of the in vivo xenograft experiment of Figure 6c post treatments of with indicated drugs: vehicle control (DMSO), ABT-737 (ABT), 4-HPR or the combination of the two drugs (Combo).
Supplemental Figure S6. Pretreatment with antioxidants does not abrogate the effects of combining 4-HPR and ABT-737. (a) MTS assays with A375 cells pretreated with vehicle, 100 μM vitamin C, or 1 mM vitamin E for 2 h prior to the addition of varying concentrations of 4-HPR (0.625-10 μM) with or without 3.3 μM ABT-737 show no difference in sensitivity to the drug combination after 48 h. (b) Annexin V assays of A375 cells pretreated with 10 mM NAC or vehicle for 2 h prior to the addition of 5 μM 4-HPR and 3.3 μM ABT-737 show no difference in sensitivity between control and NAC treatment. (c) WM852c cells pretreated with varying concentrations of NAC (1.25-10 mM) for 2 h prior to the addition of 10 μM 4-HPR and 3.3 μM ABT-737 show no difference in sensitivity to the drug combination, but there is a tendency for higher NAC concentrations to promote more cell death.
Supplemental Figure S7. Pretreatment with inhibitors of ceramide biosynthesis does not abrogate the effects of combining 4-HPR and ABT-737. MTS Assays of 1205Lu (a) and A375 (b) melanoma cell lines, pretreated with various inhibitors of ceramide biosynthesis for 2 h prior to the administration of indicated doses of 4-HPR and ABT-737 for 48 h. MTS Assays of 1205Lu (c) and A375 (d) melanoma cell lines, pretreated with an inhibitor of glucosylceramide synthase, PDMP prior to the administration of indicated doses of 4-HPR and ABT-737 for 48 h. L-cycloserine, myriocin and Fumonisin B1 are inhibitors of the de novo ceramide synthesis pathway. Desipramine and GW4869 are inhibitors of sphingomyelinase pathway. MTS assays show that inhibitors of the de novo and salvage ceramide synthesis pathways did not affect sensitivity to the drug combination compared to vehicle, but an inhibitor of glucosylceramide synthase, PDMP, sensitized melanoma cells to 4-HPR.
Supplemental Table 1: Combination Index (CI) of the data in Fig.1. The results from Figure 1a were used with Calcusyn to obtain CI of the drug combination. CI values < 0.9 indicate synergism. Smaller CI values indicate stronger synergy. CI values between 0.9-1.1 indicate additive. CI values > 1.1 indicate antagonism.
Acknowledgments
This work was supported in part by a Veterans Administration merit grant from the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development) to DAN; by a Southwestern Skin Cancer SPORE Pilot project to YGS; and by NIH training grant 5T32AR007411-29 to SNR (PI: DAN). We thank the University of Colorado Skin Cancer Biorepository for providing human melanoma samples and melanoma cell lines. We are grateful to Karen Helm, Christine Childs, and Lester Acosta of the CU Cancer Center Flow Cytometry Core facility (supported by Cancer Center Support Grant P30CA046934 and NIAMS Skin Disease Research Core Center grant P30 AR 057212) for their expert technical assistance. We thank Abbott Laboratories (Saul Rosenberg, Steven Elmore, and colleagues) for providing the ABT-737 compound.
Abbreviations
- 4-HPR
N-(4-Hydroxyphenyl)retinamide
- MIC
Melanoma Initiating Cells
- CSC
Cancer Stem Cells
Footnotes
Conflict of Interest: The authors declared no conflicts of interest.
References
- Arbiser JL, Bips M, Seidler A, et al. Combination therapy of imiquimod and gentian violet for cutaneous melanoma metastases. Journal of the American Academy of Dermatology. 2012;67:e81–3. doi: 10.1016/j.jaad.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727–38. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- Bruno S, Ghiotto F, Tenca C, et al. N-(4-hydroxyphenyl)retinamide promotes apoptosis of resting and proliferating B-cell chronic lymphocytic leukemia cells and potentiates fludarabine and ABT-737 cytotoxicity. Leukemia. 2012;26:2260–8. doi: 10.1038/leu.2012.98. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civenni G, Walter A, Kobert N, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews Molecular cell biology. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle J, Hossini AM. Expression and function of bcl-2 proteins in melanoma. Current genomics. 2008;9:409–19. doi: 10.2174/138920208785699571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Fang H, Harned TM, Kalous O, et al. Synergistic activity of fenretinide and the Bcl-2 family protein inhibitor ABT-737 against human neuroblastoma. Clin Cancer Res. 2011;17:7093–104. doi: 10.1158/1078-0432.CCR-11-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn L, Markovic SN, Joseph RW. Therapy for metastatic melanoma: the past, present, and future. BMC Med. 2012;10:23. doi: 10.1186/1741-7015-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: current status and future prospects. The oncologist. 2011;16:5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass NK, Schumacher U. Melanoma never says die. Experimental dermatology. 2014;23:471–2. doi: 10.1111/exd.12400. [DOI] [PubMed] [Google Scholar]
- Iwanaga R, Wang CA, Micalizzi DS, et al. Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways. Breast Cancer Res. 2012;14:R100. doi: 10.1186/bcr3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Wan Z, Kang YH, et al. Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst. 2008;100:580–95. doi: 10.1093/jnci/djn076. [DOI] [PubMed] [Google Scholar]
- Lee N, Barthel SR, Schatton T. Melanoma stem cells and metastasis: mimicking hematopoietic cell trafficking? Lab Invest. 2014;94:13–30. doi: 10.1038/labinvest.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas KM, Mohana-Kumaran N, Lau D, et al. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res. 2012;18:783–95. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- Luo Y, Dallaglio K, Chen Y, et al. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30:2100–13. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone W, Perloff M, Crowell J, et al. Fenretinide: a prototype cancer prevention drug. Expert opinion on investigational drugs. 2003;12:1829–42. doi: 10.1517/13543784.12.11.1829. [DOI] [PubMed] [Google Scholar]
- Maurer BJ, Melton L, Billups C, et al. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92:1897–909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- Miller LA, Goldstein NB, Johannes WU, et al. BH3 mimetic ABT-737 and a proteasome inhibitor synergistically kill melanomas through Noxa-dependent apoptosis. J Invest Dermatol. 2009;129:964–71. doi: 10.1038/jid.2008.327. [DOI] [PubMed] [Google Scholar]
- Mohana-Kumaran N, Hill DS, Allen JD, et al. Targeting the intrinsic apoptosis pathway as a strategy for melanoma therapy. Pigment Cell Melanoma Res. 2014;27:525–39. doi: 10.1111/pcmr.12242. [DOI] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–46. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Placzek WJ, Wei J, Kitada S, et al. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell death & disease. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Labrada A, Lopez-Royuela N, Jarauta V, et al. Two death pathways induced by sorafenib in myeloma cells: Puma-mediated apoptosis and necroptosis. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2014 doi: 10.1007/s12094-014-1201-y. [DOI] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Paulus JK, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–40. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Reuland SN, Goldstein NB, Partyka KA, et al. The combination of BH3-mimetic ABT-737 with the alkylating agent temozolomide induces strong synergistic killing of melanoma cells independent of p53. PLoS One. 2011;6:e24294. doi: 10.1371/journal.pone.0024294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuland SN, Goldstein NB, Partyka KA, et al. ABT-737 synergizes with Bortezomib to kill melanoma cells. Biol Open. 2012;1:92–100. doi: 10.1242/bio.2011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble D, Goldstein NB, Norris DA, et al. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–94. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Vultur A, Bogeski I, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer cell. 2013;23:811–25. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini R, Vinci MC, Pandolfi S, et al. Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells. 2012;30:1808–18. doi: 10.1002/stem.1160. [DOI] [PubMed] [Google Scholar]
- Shakhova O, Sommer L. Testing the cancer stem cell hypothesis in melanoma: the clinics will tell. Cancer Lett. 2013;338:74–81. doi: 10.1016/j.canlet.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Smith SM, Ribble D, Goldstein NB, et al. Laboratory Methods in Cell Biology. Elsevier Inc.; 2012. A Simple Technique for Quantifying Apoptosis in 96-Well Plates; pp. 361–8. (Ed.) PMC, ed. [Google Scholar]
- Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–51. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- Stecca B, Santini R, Pandolfi S, et al. Culture and isolation of melanoma-initiating cells. Curr Protoc Stem Cell Biol. 2013;Chapter 3 doi: 10.1002/9780470151808.sc0306s24. Unit 3 6. [DOI] [PubMed] [Google Scholar]
- Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–50. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Quinn BA, Das SK, et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther Targets. 2013;17:61–75. doi: 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi R, Decensi A. Fenretinide and cancer prevention. Curr Oncol Rep. 2000;2:263–70. doi: 10.1007/s11912-000-0077-x. [DOI] [PubMed] [Google Scholar]
- Wong CY, Helm MA, Kalb RE, et al. The Presentation, Pathology, and Current Management Strategies of Cutaneous Metastasis. N Am J Med Sci. 2013;5:499–504. doi: 10.4103/1947-2714.118918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mi JQ, Fang H, et al. Preferential eradication of acute myelogenous leukemia stem cells by fenretinide. Proc Natl Acad Sci U S A. 2013;110:5606–11. doi: 10.1073/pnas.1302352110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Knockdown of NOXA decreased drug-induced cell death. Annexin V assays with NOXA knockdown (shNoxa) or control (shCont.) derived from A375 (a), SK-MEL-28 (b), or WM852c(c) after being treated with indicated drugs for 48 h. The insets show immunoblots of lysates from the respective cell lines used in that graph indicating efficient knockdown of NOXA. shNoxa was significantly different from shCont upon the combination treatment for all three cell lines (p < 0.05). There was no statistically significant differences between the shCont and shNoxa for the vehicle (DMS0) or the single drug treatments for all the cell lines tested.
Supplemental Figure S2. Higher overall expressions of BIM in SK-MEL-30 than in A375 cells. Immunoblot of cell lysates treated with either vehicle control (DMSO), 3.3μM ABT-737 (ABT), 10μM 4-HPR (4-HPR), or the combination of the two drugs (Combo). SK-MEL-30 exhibits higher level of BIM than A375 in vehicle (DMSO) treated cells. Combination treatment did not significantly affect BIM expression in either cell lines.
Supplemental Figure S3. The combination treatment of ABT-737 plus 4-HPR decreased JARID1B expression. Immunoblot of cell lysates treated with indicated treatment: vehicle control (DMSO), 3.3μM ABT-737 (ABT), 10μM 4-HPR (4-HPR), or the combination of the two drugs (Combo). The Combination treatment reduced the expression of JARED 1B compared to the control in all the cell lines tested.
Supplemental Figure S4. Knock-down of NOXA lessens the effects of combining 4-HPR and ABT-737 on sphere-forming capacity of melanoma cells. Primary sphere assays with NOXA knockdown (shNoxa) or control (shCont.) derived from SK-MEL-30 (a), SK-MEL-28 (b), or WM852c (c) after being treated with indicated drugs. Knockdown of NOXA significantly protected cells from the combination-induced disruption of spheres compared to the control in all the cell lines tested (p <0.01). There were no statistical differences between the shCont. and shNoxa for control or the single drug treatments in any of the cell lines, except 4-HPR treatment in SK-MEL-30 (p <0.01).
Supplemental Figure S5. The effects of combination treatment of ABT-737 plus 4-HPR on the tumor samples harvested from in vivo experiments of Figure 6c. Immunoblot of cell lysates from the tumor samples harvested at the end of the in vivo xenograft experiment of Figure 6c post treatments of with indicated drugs: vehicle control (DMSO), ABT-737 (ABT), 4-HPR or the combination of the two drugs (Combo).
Supplemental Figure S6. Pretreatment with antioxidants does not abrogate the effects of combining 4-HPR and ABT-737. (a) MTS assays with A375 cells pretreated with vehicle, 100 μM vitamin C, or 1 mM vitamin E for 2 h prior to the addition of varying concentrations of 4-HPR (0.625-10 μM) with or without 3.3 μM ABT-737 show no difference in sensitivity to the drug combination after 48 h. (b) Annexin V assays of A375 cells pretreated with 10 mM NAC or vehicle for 2 h prior to the addition of 5 μM 4-HPR and 3.3 μM ABT-737 show no difference in sensitivity between control and NAC treatment. (c) WM852c cells pretreated with varying concentrations of NAC (1.25-10 mM) for 2 h prior to the addition of 10 μM 4-HPR and 3.3 μM ABT-737 show no difference in sensitivity to the drug combination, but there is a tendency for higher NAC concentrations to promote more cell death.
Supplemental Figure S7. Pretreatment with inhibitors of ceramide biosynthesis does not abrogate the effects of combining 4-HPR and ABT-737. MTS Assays of 1205Lu (a) and A375 (b) melanoma cell lines, pretreated with various inhibitors of ceramide biosynthesis for 2 h prior to the administration of indicated doses of 4-HPR and ABT-737 for 48 h. MTS Assays of 1205Lu (c) and A375 (d) melanoma cell lines, pretreated with an inhibitor of glucosylceramide synthase, PDMP prior to the administration of indicated doses of 4-HPR and ABT-737 for 48 h. L-cycloserine, myriocin and Fumonisin B1 are inhibitors of the de novo ceramide synthesis pathway. Desipramine and GW4869 are inhibitors of sphingomyelinase pathway. MTS assays show that inhibitors of the de novo and salvage ceramide synthesis pathways did not affect sensitivity to the drug combination compared to vehicle, but an inhibitor of glucosylceramide synthase, PDMP, sensitized melanoma cells to 4-HPR.
Supplemental Table 1: Combination Index (CI) of the data in Fig.1. The results from Figure 1a were used with Calcusyn to obtain CI of the drug combination. CI values < 0.9 indicate synergism. Smaller CI values indicate stronger synergy. CI values between 0.9-1.1 indicate additive. CI values > 1.1 indicate antagonism.


