Abstract
Oxidation of polyunsaturated fatty acids (PUFA) through enzymatic or non-enzymatic free radical-mediated reactions can yield an array of lipid metabolites including eicosanoids, octadecanoids, docosanoids and related species. In mammals, these oxygenated PUFA mediators play prominent roles in the physiological and pathological regulation of many key biological processes in the cardiovascular, renal, reproductive and other systems including their pivotal contribution to inflammation. Mass spectrometry-based technology platforms have revolutionized our ability to analyze the complex mixture of lipid mediators found in biological samples, with increased numbers of metabolites that can be simultaneously quantified from a single sample in few analytical steps. The recent development of high-sensitivity and high-throughput analytical tools for lipid mediators affords a broader view of these oxygenated PUFA species, and facilitates research into their role in health and disease. In this review, we illustrate current analytical approaches for a high-throughput lipidomic analysis of eicosanoids and related mediators in biological samples.
1. INTRODUCTION
Polyunsaturated fatty acids (PUFA) can be oxygenated by means of enzymatic or free radical-mediated reactions into numerous bioactive oxygenated lipid mediators. One of the best-studied classes of these oxygenated mediators are the eicosanoids (from the Greek "eikosa," meaning "twenty"), derivatives of the 20-carbon PUFA arachidonic acid (AA; 20:4n-6), eicosapentaenoic acid (EPA; 20:5n-3) and dihomo-γ-linolenic acid (DGLA; 20:3n-6). For the purpose of this review, the use of the 'eicosanoid' terminology has been extended to include the oxygenated products of other fatty acids including the octadecanoid derivatives of 18-carbon PUFA, such as linoleic acid (LA; 18:2n-6) and alpha-linolenic acid (ALA; 18:3n-3) and the docosanoids produced from 22-carbon PUFA, such as docosapentaenoic acid (DPA; 22:5) and docosahexaenoic acid (DHA; 22:6n-3).
In humans, eicosanoids and related mediators mediate a wide array of critical biological processes such as ovulation, initiation of labor, bone metabolism, nerve growth and development, wound healing, kidney function, blood-vessel tone, blood coagulation, immune responses, and, most notably, inflammation [1–3]. Some of the most successful drug classes, such as the nonsteroidal anti-inflammatory drugs (NSAID) (e.g., acetylsalicylic acid, or aspirin, ibuprofen, and celecoxib, or Celebrex®), target prostanoid formation [4].
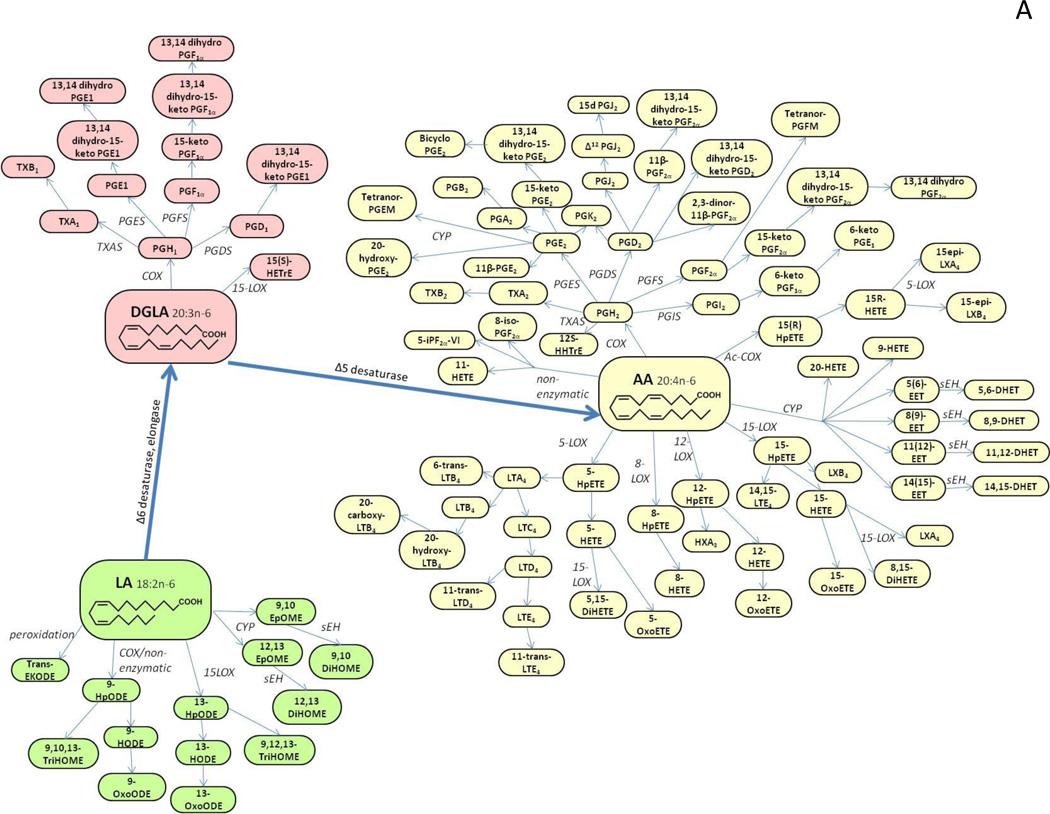
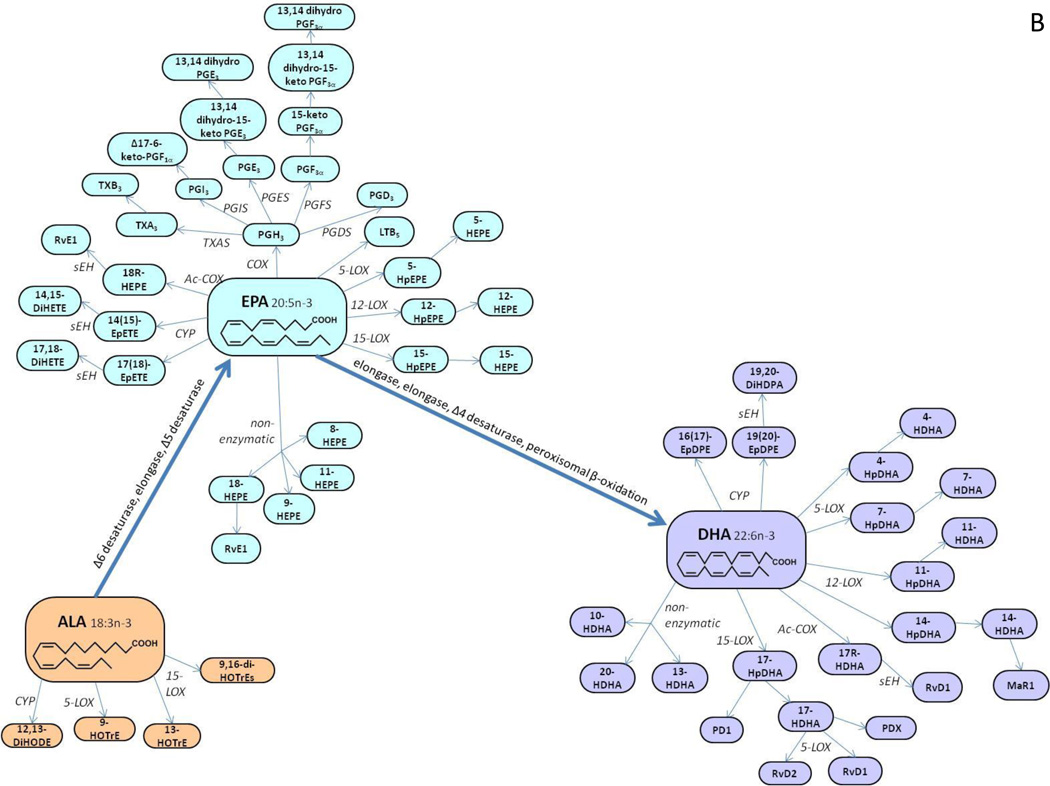
The enzymatic or non-enzymatic oxygenation of PUFA can result in hundreds of oxygenated species, including prostanoids and iso-prostanes (isoP), leukotrienes, regio- and stereo-isomers of mono- and poly- hydroxyl-, hydroperoxy-, epoxy- and keto-fatty acids (Figure 1A,B) [1, 5–7]. Because such mediators might have opposite and redundant properties, it would be difficult to explain the molecular mechanisms of biological processes by studying only a limited number of eicosanoid species. Indeed, it is the overall balance between various oxygenated PUFA species that seems to modulate many biological processes. Thus, the ability to simultaneously measure a wide range of metabolites could further our understanding of their roles in health and disease, as well as provide a set of biomarkers for disease diagnosis or prognosis.
Figure 1.
A. Schematic outline of oxygenated species derivatives of the omega-6 fatty acids linoleic acid C18:2 (LA), dihomo-γ-linolenic acid C20:3 (DGLA) and arachidonic acid C20:4 (AA), via the cyclooxygenase (COX), lipoxygenase (LOX), CYP-450 (CYP) or free radical catalyzed pathways.
B. Schematic outline of oxygenated species derivatives of the omega-3 fatty acids α-linolenic acid C18:3 (ALA), eicosapentaenoic acid C20:5 (EPA) and docosahexaenoic acid C22:6 (DHA), via the COX, LOX, CYP or free radical catalyzed pathways.
Abbreviations: dihydroxy-eicosatrienoic acid (DHET), dihydroxy-eicosatetraenoic acid (DiHETE), dihydroxy-octadecadienoic acid (DiHODE), dihydroxy-octadecenoic acid (DiHOME), epoxy-keto-octadecenoic acid (EKODE), epoxy-eicosatrienoic acid (EET), epoxy-docosapentaenoic acid (EpDPE), epoxy-eicosatetraenoic acid (EpETE), epoxy-octadecenoic acid (EpOME), hydroxy-docosahexaenoic acid (HDHA), hydroxy-eicosapentaenoic acid (HEPE), hydroxy-eicosatrienoic acid (HETrE), hydroxy-eicosatetraenoic acid (HETE), hydroxy-heptadecatrienoic acid (HHTrE), hydroxy-octadecadienoic acid (HODE), hydroxy-octadecatrienoic acid (HOTrE), hydroperoxy-docosahexaenoic acid (HpDHA), hydroperoxy-eicosapentaenoic acid (HpEPE), hydroperoxy-eicosatetraenoic acid (HpETE), hydroperoxy-octadecadienoic acid (HpODE), hepoxilin (HX), leukotriene (LT), lipoxin (LX), oxo-eicosatetraenoic acid (OxoETE), oxo-octadecadienoic acid (OxoODE), prostaglandin (PG), prostaglandin E metabolite (PGEM), prostaglandin F metabolite (PGFM), resolvin (Rv), soluble epoxide hydrolase (sEH), trihydroxy-octadecenoic acid (TriHOME), thromboxane (TX).
The analysis of oxygenated PUFA metabolites faces three major challenges: First, these lipid mediators are present at extremely low concentration in biological tissues. Second, these species are not stored in tissues, but are transiently formed on demand and have limited stability. Third, the same PUFA substrate can be oxidized in different positions of its acyl chain, leading to many isomeric species, each with specific metabolic actions. As a consequence, their measurements require rapid, highly-sensitive, accurate and specific analytical methodologies.
Here we review current analytical approaches for a high-throughput analysis of eicosanoids, octadecanoids, docosanoids and related oxygenated PUFA species in biological samples.
2. Biosynthesis of oxygenated lipid mediators
2.1. Polyunsaturated fatty acid substrates
PUFA are the immediate metabolic precursors for the biosynthesis of oxygenated species. They belong to two main families: omega-3 (n-3) PUFA and omega-6 (n-6) PUFA, so named according to the position of the first double bond in their fatty acyl chain, starting from the methyl end (Figure 1). Mammals cannot insert a double bond at either the n-3 or n-6 position of a fatty acyl chain and must rely entirely on dietary intake for PUFA (hence the characterization of their precursors LA and ALA as essential macronutrients) [8, 9]. Once absorbed in the gut and transported through the lymph in chylomicrons, PUFA can undergo further elongation and desaturation in the liver, and then circulate through the blood to all tissues, usually via lipoproteins and albumin. Tissue and cellular PUFA can be found as free fatty acids, esterified to complex lipids (e.g., glycerophospholipids, glycerolipids, sphingolipids and cholesteryl esters) or conjugated to amino acids and ethanolamine [10].
Some of the health effects associated with PUFA consumption are mediated through the formation of various oxygenated species [11, 12]. In particular, current nutritional research shows that a diet enriched in n-3 PUFA offers health benefits and anti-inflammatory properties, whereas an excess of n-6 PUFA can contribute to the pathogenesis of many chronic inflammatory diseases, including cardiovascular and autoimmune diseases [13]. Such evidence suggests that, in addition to pharmacological treatments, nutritional interventions can also be used to modulate eicosanoid and related species composition and their biological activities [14–18].
2.2. Reactions mediating the formation and deactivation of eicosanoids and related mediators
Polyunsaturated fatty acids can be oxygenated via enzymatic and/or non-enzymatic reactions. The three major enzymatic pathways involved in the generation of oxygenated species are catalyzed by cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP) isoforms.
Cyclooxygenase Metabolites
COX-1 and COX-2 possess dual cyclooxygenase and peroxidase activities, catalyzing the oxidative cyclization of the central 5 carbons within 20-carbon PUFA to prostaglandin (PG) H2. PGH2 is subsequently converted to a variety of prostanoids including PG E, D, and F, prostacyclin (PGI2) and thromboxanes (TX), by cell-specific terminal synthases and isomerases (Figure 1A,B). 20-Carbon PUFA DGLA, AA and EPA give rise to series-1,-2 and -3 prostanoids, respectively. 18-Carbon PUFA, such as LA, and 22-carbon PUFA, such as DHA, can be partially metabolized to hydroxy-fatty acids such as HODE and HDHA, respectively [19–21]. After acetylation of the active site of COX by aspirin, the enzyme generates (R)-hydroxy-derivatives such as 15(R)-HETE, 18(R)-HEPE and 17(R)-HDHA [22, 23].
Lipoxygenase Metabolites
LOX activities catalyze the stereoselective insertion of molecular oxygen into PUFA with the formation of hydroperoxy fatty acids (e.g., HpETE and HpEPE) that are subsequently reduced to the corresponding hydroxy- fatty acids (e.g. HETE and HEPE). LOX isoforms are conventionally named after the stereospecific carbon at which they oxygenate AA, e.g. 5-, 12-15-LOX [24]. In the 5-LOX active site, 5-hydroperoxy-PUFA can also undergo a catalytic rearrangement to form leukotrienes A-series (LTA), which can then be hydrolyzed by the LTA hydrolase to generate LT B-series (LTB), or, alternatively undergo conjugation to glutathione by the LTC synthase to form cysteinyl-LT such as LTC4 [1]. Monohydroxy fatty acids such as HETE, HEPE and HDHA can participate in transcellular metabolism and, subject to further lipoxygenations, can form polyhydroxy-PUFA such as the AA-derived lipoxins and EPA- and DHA-derived resolvins and protectins [25].
Cytochrome P450 Metabolites
The superfamily of CYP monooxygenases catalyzes the hydroxylation and/or epoxygenation of PUFA [26]. In addition to producing LOX-like monohydroxy fatty acids, CYP can add a hydroxyl moiety to the omega-carbons of PUFA to form a unique class of omega-hydroxylated HETE (e.g. 20-HETE) [27]. The epoxidation of PUFA by CYP isoforms results in the formation of epoxyeicosatrienoic acids (EETs) that can be hydrolyzed to the corresponding dihydroxyeicosatrienoic acids (DHETs) by the soluble-enzyme epoxide hydrolase (sEH) [26, 28–30].
Non-Enzymatically Produced Metabolites
Oxidative stress and reactive oxygen species can lead to free-radical catalyzed peroxidation of PUFA, generating a range of isoP and a multitude of hydroxy fatty acids [31–35]. In contrast to enzymatic oxygenation of PUFA, which leads to the formation of pure oxygenated enantiomers and regioisomers, lack of enzymatic control in the free-radical-mediated chemical reactions results in a racemic mixtures of stereo- and regio-isomers. Non-enzymatically derived species have been shown to serve as useful biomarkers of oxidative stress and tissue damage [36–39].
Catabolism of eicosanoids and related species
The biological activities of eicosanoids and other oxygenated lipids are regulated by efficient mechanisms for deactivation and excretion. Human metabolism of eicosanoids involves mainly dehydrogenation of hydroxyl groups and reduction of double bonds [40]. Further catabolism of PUFA oxygenated species might involve hydroxylation (e.g., CYP-mediated omega-hydroxylation), beta-oxidation and glucuronidation, which increase their water solubility and thus urinary excretion from the body [1, 41, 42]. The overall catabolism further contributes to the complexity of the PUFA oxygenated metabolites.
3. Analysis of oxygenated fatty acid metabolites
Historically, measurements of eicosanoids and related species have been performed using radiometric and enzymatic immunoassays, which often lacked specificity and targeted only a few compounds [43–46]. Liquid chromatography with UV or fluorescence detection [47, 48], and gas chromatography coupled to mass spectrometry (GC-MS) have also been used [49]. However, these methodologies require multi-step procedures involving derivatization reactions, increasing the analysis time and the risk of thermal decomposition. Recent advances in biological mass spectrometry of lipids [50] have paved the way to a growing number of liquid chromatography-mass spectrometry (LC-MS) methodologies with much enhanced sensitivity and specificity, and the ability to simultaneously analyze large numbers of oxygenated PUFA species [51–58].
The important biological role of these lipid mediators, and their potential value as biomarkers, has created a growing demand for a comprehensive, high-throughput screening methodology driving the use of the most innovative technologies. A typical analytical platform for eicosanoids and related species includes the optimization of five major steps: 1) sample collection and storage; 2) sample preparation and extraction; 3) liquid chromatography; 4) ionization; and 5) mass spectrometric detection.
3.1. Sample collection and storage
Sample collection is a key step in the workflow for the analysis of PUFA oxygenated metabolites. Tissue degradation and free-radical oxidation might occur within seconds at room temperature, leading to alterations in the composition of eicosanoids and other species. Therefore, to avoid artifacts in the analysis, biological samples should be rapidly collected and processed: tissue samples are quickly snap frozen in liquid nitrogen; biofluids, such as blood, are drawn, stored on ice and rapidly processed for serum or plasma preparation; cells are harvested in cold solvents to immediately quench their metabolism [59–62]. All biological samples should be stored at −80°C until further processed and freeze/thaw cycles should be avoided.
It is worth noting that during the preparation of plasma samples, platelets may become activated and produce large quantities of eicosanoids. This problem can be minimized if blood is collected directly in containers containing anticoagulant, such as EDTA, which can sequester calcium ions and then inhibit phospholipase A2’s (e.g., sPLA2, cPLA2. Similarly, serum samples derived from coagulating blood have much higher concentrations of lipid mediators, compared to plasma [63].
3.2. Sample preparation and extraction
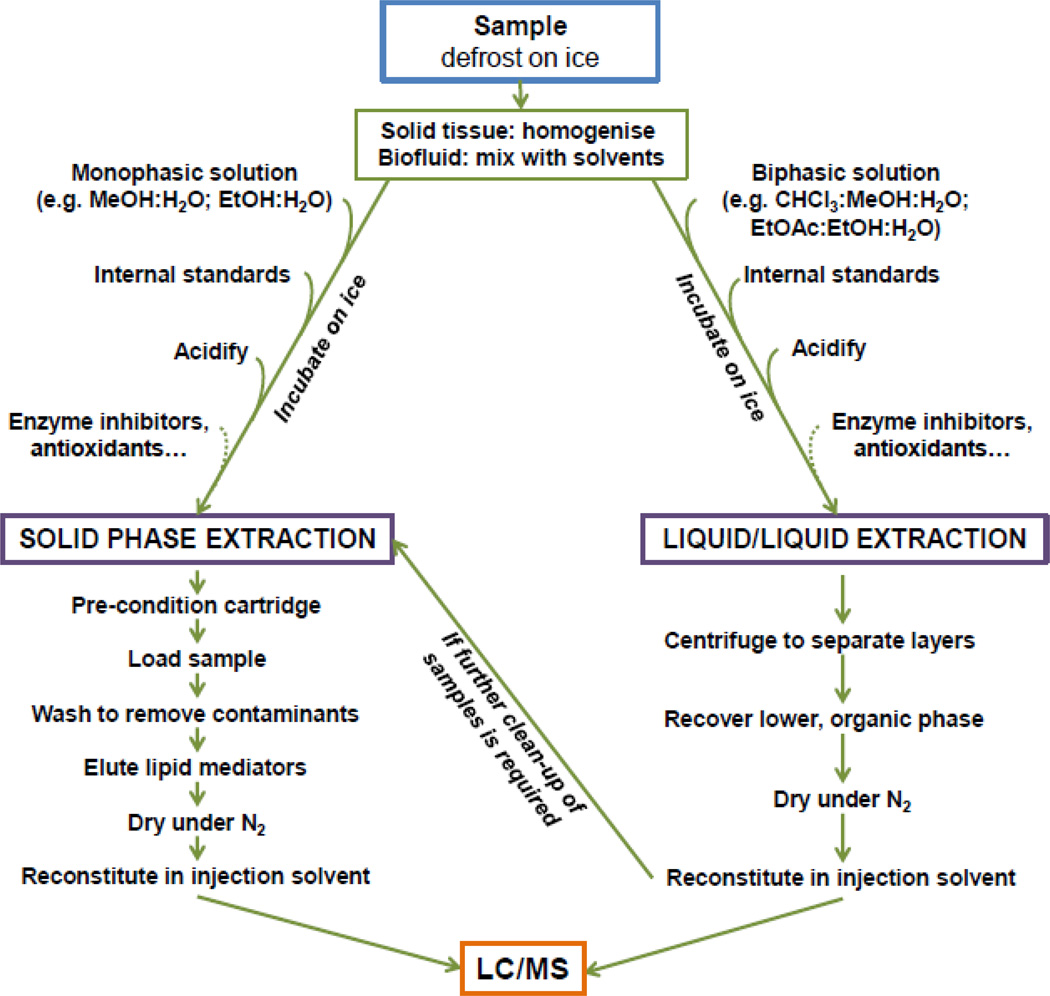
Eicosanoids and similar species are present at very low abundance in both tissues and biofluids. The quality of sample preparation is, therefore, an important factor for a successful analysis. Many PUFA oxygenated species are unstable at room temperature, and the whole sample preparation procedure should be conducted in cold conditions whenever possible and, antioxidants could be used to minimize non-enzymatic oxidation (e.g., butylated hydroxytoluene, BHT) [64]. Normalization of the extraction efficiency and quantitation is achieved by adding stable, isotope-labeled compounds (internal standards) prior to extraction, as described below. Liquid-liquid extraction and/or solid-phase extraction (SPE) procedures are usually used prior to MS analysis, to eliminate non-lipid contaminants and highly abundant species like phospholipids, which may create dynamic-range and ion-suppression problems (Figure 2).
Figure 2.
Workflow for the sample preparation options for the analysis of oxygenated PUFA species found in various biological tissue samples. Samples (liquid and/or solid tissues) can be prepared using solid phase extraction (SPE) or liquid/liquid extraction, or a combination of the two, before analysis by liquid chromatography/mass spectrometry (LC/MS). Acid is used to protonate the analytes prior to reversed phase SPE or liquid-liquid extractions; enzyme inhibitors can include protease or cyclooxygenase inhibitors, depending on the experimental design.
Internal standards
A mixture of deuterated eicosanoids and related species is often used to normalize endogenous metabolite levels for both extraction efficiency and instrument response. With hundreds of oxygenated PUFA species routinely analyzed, using an appropriate internal standard, such as a stable isotope-labeled molecule (e.g., deuterated), for each species is impractical. Many of the deuterated standards are indeed expensive to synthesize, labile, and may contain impurities. We must therefore compromise, normalizing the individual, molecular–ion peak intensity with a limited number of internal standards that would match groups of endogenous species for chemistry, retention time, and ionization efficiencies. Table 1 includes typical deuterated internal standards and the PUFA mediators with which they were paired [40, 52, 54, 56, 65–68].
Table 1.
List of MRM transitions (M1 = precursor ion; M2 = production) for most common natural oxygenated PUFA species, and non-natural compounds used as internal standards (ISTD). The precursor fatty acids, metabolite class, and predominant metabolic pathway are also reported for each compound. Compounds can be additionally identified by retention time, which are not listed because they will differ depending on the specific chromatographic methods and experimental conditions employed. An example of selected compounds and their identification by retention time, as well as unique fragmentation pattern, is shown in Figure 3 [52, 54, 56, 65–68].
| Compound Name | M1 | M2 | ISTD | Precursor | Pathway | Class | |
|---|---|---|---|---|---|---|---|
| 1 | 12-HHTrE | 279.2 279.2 |
179.2 163.2 |
(d8) 12(S)-HETE | AA | COX | Alcohol |
| 2 | (d4) 15-deoxy-Δ12,14-PGJ2 | 319.2 | 275.3 | ISTD | AA | COX | Prostanoid |
| 3 | 2,3-dinor-11b PGF2α | 325.2 | 145.1 | (d4) PGF2α | AA | COX | Prostanoid |
| 4 | Tetranor-PGEM | 327.1 327.1 |
309.2 291.1 |
(d4) PGE2 | AA | COX | Prostanoid |
| 5 | Tetranor-PGFM | 329.2 329.2 |
311.2 293.2 |
(d4) PGF2α | AA | COX | Prostanoid |
| 6 | PGJ2 | 333.2 333.2 |
233.1 271.2 |
(d4) PGD2 | AA | COX | Prostanoid |
| 7 | Δ12-PGJ2 | 333.2 333.2 |
233.1 271.2 |
(d4) 15-deoxy-Δ12,14-PGJ2 | AA | COX | Prostanoid |
| 8 | 15-deoxy-Δ12,14-PGJ2 | 315.2 | 271.2 | (d4) 15-deoxy-Δ12-PGJ2 | AA | COX | Prostanoid |
| 9 | PGA2 | 333.2 | 271.2 | (d4) PGE2 | AA | COX | Prostanoid |
| 10 | PGK2 | 349.2 | 205.1 | (d4) PGE2 | AA | COX | Prostanoid |
| 11 | 13,14-dihydro-15-keto PGD2 | 351.2 | 175.2 | (d4) PGD2 | AA | COX | Prostanoid |
| 12 | 13,14-dihydro-15-keto PGE2 | 351.2 351.2 351.2 |
175.2 333.2 207.2 |
(d4) PGE2 | AA | COX | Prostanoid |
| 13 | 15-keto PGF2α | 351.2 351.2 |
219.1 113.1 |
(d4) PGF2α | AA | COX | Prostanoid |
| 14 | PGD2 | 351.2 | 271.2 | (d4) PGD2 | AA | COX | Prostanoid |
| 15 | PGE2 | 351.2 | 271.2 | (d4) PGE2 | AA | COX | Prostanoid |
| 16 | 11β-PGE2 | 351.2 | 271.2 | (d4) PGE2 | AA | COX | Prostanoid |
| 17 | 13,14-dihydro-15-keto PGF2α | 353.2 353.2 353.2 |
113.1 183.1 291.1 |
(d4) PGF2α | AA | COX | Prostanoid |
| 18 | 11β-13,14-dihydro-15-keto PGF2α | 353.2 | 113.2 | (d4) PGF2α | AA | COX | Prostanoid |
| 19 | PGF2α | 353.2 | 193.2 | (d4) PGF2α | AA | COX | Prostanoid |
| 20 | 11β-PGF2α | 353.2 | 193.2 | (d4) PGF2α | AA | COX | Prostanoid |
| 21 | (d4) PGD2 | 355.2 | 275.2 | ISTD | AA | COX | Prostanoid |
| 22 | (d4) PGE2 | 355.2 | 275.2 | ISTD | AA | COX | Prostanoid |
| 23 | 13,14-dihydro PGF2α | 355.2 355.2 |
275.2 311.2 |
(d4) PGF2α | AA | COX | Prostanoid |
| 24 | (d4) PGF2α | 357.3 | 197.2 | ISTD | AA | COX | Prostanoid |
| 25 | 6-keto PGE1 | 367.2 | 143.1 | (d4) PGE2 | AA | COX | Prostanoid |
| 26 | 20-hydroxy PGE2 | 367.2 | 287.2 | (d4) PGE2 | AA | COX | Prostanoid |
| 27 | 6-keto PGF1α | 369.2 | 163.1 | (d4) 6-keto PGF1α | AA | COX | Prostanoid |
| 28 | TXB2 | 369.2 | 169.1 | (d4) TXB2 | AA | COX | Thromboxane |
| 29 | (d4) 6-keto PGF1α | 373.2 | 167.2 | ISTD | AA | COX | Thromboxane |
| 30 | (d4) TXB2 | 373.2 | 173.1 | ISTD | AA | COX | Thromboxane |
| 31 | 15-KETE (15-OxoETE) |
317.2 | 113.2 | (d8) 5(S)-HETE | AA | LOX | Ketone |
| 32 | 5-KETE (5-OxoETE) |
317.2 | 203.2 | (d8) 5(S)-HETE | AA | LOX | Ketone |
| 33 | 12-KETE (12-OxoETE) |
317.2 | 273.3 | (d8) 12(S)-HETE | AA | LOX | Alcohol |
| 34 | 5-HETE | 319.2 | 115.1 | (d8) 5(S)-HETE | AA | LOX | Alcohol |
| 35 | 8-HETE | 319.2 | 155.1 | (d8) 5(S)-HETE | AA | LOX | Alcohol |
| 36 | 11-HETE | 319.2 | 167.1 | (d8) 12(S)-HETE | AA | LOX | Alcohol |
| 37 | 15-HETE | 319.2 319.2 |
175.1 219.2 |
(d8) 5(S)-HETE | AA | LOX | Alcohol |
| 38 | 12-HETE | 319.2 | 179.2 | (d8) 12(S)-HETE | AA | LOX | Alcohol |
| 39 | (d8) 5(S)-HETE | 327.3 | 116.1 | ISTD | AA | LOX | Alcohol |
| 40 | (d8) 12(S)-HETE | 327.3 | 184.2 | ISTD | AA | LOX | Alcohol |
| 41 | 15-HpETE | 335.2 317.2 |
113.1 113.1 |
(d8) 5(S)-HETE | AA | LOX | Hydroxyperoxide |
| 42 | LTB4 | 335.2 | 195.1 | (d4) LTB4 | AA | LOX | Leukotriene |
| 43 | 6-trans-LTB4 | 335.2 | 195.1 | (d4) LTB4 | AA | LOX | Leukotriene |
| 44 | 5-HpETE | 335.2 317.2 |
203.2 203.2 |
(d8) 5(S)-HETE | AA | LOX | Hydroxyperoxide |
| 45 | Hepoxilin A3 | 335.2 335.2 |
273.2 127.2 |
(d8) 12(S)-HETE | AA | LOX | Hepoxilin |
| 46 | 12-HpETE | 335.2 317.2 |
273.3 153.3 |
(d8) 12(S)-HETE | AA | LOX | Hydroxyperoxide |
| 47 | (d4) LTB4 | 339.2 | 197.1 | ISTD | AA | LOX | Leukotriene |
| 48 | 5(S),6(R)-Lipoxin A4 | 351.2 | 115.1 | (d4) LTB4 | AA | LOX | Lipoxin |
| 49 | 5(S),6(S)-Lipoxin A4 | 351.2 | 115.1 | (d4) LTB4 | AA | LOX | Lipoxin |
| 50 | 20-hydroxy LTB4 | 351.2 | 195.1 | (d4) LTB4 | AA | LOX | Leukotriene |
| 51 | 5(S),14(R)-Lipoxin B4 | 351.2 | 221.2 | (d4) LTB4 | AA | LOX | Lipoxin |
| 52 | 20-carboxy LTB4 | 365.2 | 347.2 | (d4) LTB4 | AA | LOX | Leukotriene |
| 53 | LTE4 | 438.2 | 333.2 | (d3) LTE4 | AA | LOX | Leukotriene |
| 54 | 11-trans LTE4 | 438.2 | 333.2 | (d3) LTE4 | AA | LOX | Leukotriene |
| 55 | 14,15-LTE4 | 438.2 | 333.2 | (d3) LTE4 | AA | LOX | Leukotriene |
| 56 | (d3) LTE4 | 441.2 | 336.2 | ISTD | AA | LOX | Leukotriene |
| 57 | LTD4 | 495.2 | 177.1 | (d3) LTE4 | AA | LOX | Leukotriene |
| 58 | 11-trans LTD4 | 495.2 | 177.1 | (d3) LTE4 | AA | LOX | Leukotriene |
| 59 | 20-HETE | 319.2 319.2 |
275.2 245.2 |
d6-20-HETE | AA | CYP | Alcohol |
| 60 | 8(9)-EpETrE (8(9)-EET) |
319.2 | 155.1 | (d11) 14,15-DiHETrE | AA | CYP | Epoxide |
| 61 | 11(12)-EpETrE (11(12)-EET) |
319.2 | 167.1 | (d11) 14,15-DiHETrE | AA | CYP | Epoxide |
| 62 | 14(15)-EpETrE (14(15)-EET) |
319.2 319.2 |
175.1 219.2 |
(d11) 14,15-DiHETrE | AA | CYP | Epoxide |
| 63 | 5(6)-EpETrE (5(6)-EET) |
319.2 | 191.2 | (d11) 14,15-DiHETrE | AA | CYP | Epoxide |
| 64 | (d6) 20-HETE | 325.3 | 279.2 | ISTD | AA | CYP | Alcohol |
| 65 | bicyclo-PGE2 | 333.2 | 113.2 | (d4) PGE2 | AA | CYP | Prostanoid |
| 66 | 5,6-DiHETE | 335.2 | 115.1 | (d4) LTB4 | AA | CYP | Diol |
| 67 | 5,15-DiHETE | 335.2 | 115.2 | (d4) LTB4 | AA | CYP | Diol |
| 68 | 8,15-DiHETE | 335.2 335.3 353.2 |
127.2 155.1 235.2 |
(d4) LTB4 | AA | CYP | Diol |
| 69 | 8,9-DiHETrE (8,9-DHET) |
337.2 | 127.1 | (d11) 14,15-DiHETrE | AA | CYP | Diol |
| 70 | 5,6-DiHETrE (5,6-DHET) |
337.2 | 145.1 | (d11) 14,15-DiHETrE | AA | CYP | Diol |
| 71 | 11,12-DiHETrE (11,12-DHET) |
337.2 | 167.2 | (d11) 14,15-DiHETrE | AA | CYP | Diol |
| 72 | 14,15-DiHETrE (14,15-DHET) |
337.2 | 207.2 | (d11) 14,15-DiHETrE | AA | CYP | Diol |
| 73 | (d11) 14,15-DiHETrE | 348.3 | 207.1 | ISTD | AA | CYP | Diol |
| 74 | 9-HETE | 319.2 319.2 |
123.1 167.1 |
(d8) 12(S)-HETE | AA | non-enzymatic | Alcohol |
| 75 | (+/−) 5-iPF2α-VI | 353.2 | 115.1 | (d4) PGF2α | AA | non-enzymatic | IsoP |
| 76 | 8-iso PGF2α | 353.2 | 193.2 | (d4) PGF2α | AA | non-enzymatic | IsoP |
| 77 | 1α,1β-dihomo PGF2α | 381.3 | 337.2 | (d4) PGF2α | ADA | COX | Prostanoid |
| 78 | 9-HOTrE | 293.2 | 171.1 | (d4) 9(S)-HODE | ALA | LOX | Alcohol |
| 79 | 12,13-DiHODE | 311.2 | 293.2 | (d4) 9,10-DiHOME | ALA | CYP | Diol |
| 80 | 15-keto PGF1α | 353.2 353.2 |
113.1 221.1 |
(d4) 6-keto PGF1α | DGLA | COX | Prostanoid |
| 81 | 13,14-dihydro-15-keto PGD1 | 353.2 | 209.1 | (d4) PGD2 | DGLA | COX | Prostanoid |
| 82 | PGD1 | 353.2 353.2 |
273.2 317.2 |
(d4) PGD2 | DGLA | COX | Prostanoid |
| 83 | PGE1 | 353.2 353.2 |
273.2 317.2 |
(d4) PGE2 | DGLA | COX | Prostanoid |
| 84 | 13,14-dihydro-15-keto PGF1α | 355.2 | 193.2 | (d4) PGF2α | DGLA | COX | Prostanoid |
| 85 | PGF1α | 355.2 355.2 |
293.2 311.2 |
(d4) PGF2α | DGLA | COX | Prostanoid |
| 86 | TXB1 | 371.2 | 171.1 | (d4) TXB2 | DGLA | COX | Thromboxane |
| 87 | 15-HETrE | 321.2 321.1 |
221.2 303.2 |
(d8) 5(S)-HETE | DGLA | LOX | Alcohol |
| 88 | 17-HDoHE (17-HDHA) |
343.2 343.2 |
201.2 281.3 |
(d8) 5(S)-HETE | DHA | LOX | Alcohol |
| 89 | Neuroprotectin D1 (PD1) |
359.2 359.2 |
153.1 206.1 |
(d8) 12(S)-HETE | DHA | LOX | Protectin |
| 90 | 10,17-DiHDoHE (PDX) |
359.2 359.2 |
153.2 206.1 |
(d8) 12(S)-HETE | DHA | LOX | Protectin |
| 91 | Resolvin D1 | 375.2 | 141.0 | (d11) 14,15-DiHETrE | DHA | LOX | Resolving |
| 92 | 16(17)-EpDPE | 343.2 | 233.2 | (d11) 14,15-DiHETrE | DHA | CYP | Epoxide |
| 93 | 19(20)-EpDPE | 343.2 343.3 |
281.3 285.2 |
(d11) 14,15-DiHETrE | DHA | CYP | Epoxide |
| 94 | 19,20-DiHDPA | 361.2 349.2 |
229.3 269.2 |
(d11) 14,15-DiHETrE | DHA | CYP | Diol |
| 95 | PGD3 | 349.2 | 269.2 | (d4) PGD2 | EPA | COX | Prostanoid |
| 96 | PGE3 | 349.2 | 269.2 | (d4) PGE2 | EPA | COX | Prostanoid |
| 97 | PGF3α | 351.2 | 193.2 | (d4) PGF2α | EPA | COX | Prostanoid |
| 98 | Δ17-6-keto PGF1α | 367.2 | 163.1 | (d4) 6-keto PGF1α | EPA | COX | Prostanoid |
| 99 | TXB3 | 367.2 | 169.1 | (d4) TXB2 | EPA | COX | Thromboxane |
| 100 | 5-HEPE | 317.2 | 115.1 | (d8) 5(S)-HETE | EPA | LOX | Alcohol |
| 101 | 12-HEPE | 317.2 | 179.1 | (d8) 12(S)-HETE | EPA | LOX | Alcohol |
| 102 | 15-HEPE | 317.2 317.2 |
175.1 219.2 |
(d8) 5(S)-HETE | EPA | LOX | Alcohol |
| 103 | LTB5 | 333.2 | 195.1 | (d4) LTB4 | EPA | LOX | Leukotriene |
| 104 | Resolvin E1 | 349.2 | 195.1 | (d11) 14,15-DiHETrE | EPA | LOX | Resolving |
| 105 | 14(15)-EpETE | 317.2 | 207.1 | (d11) 14,15-DiHETrE | EPA | CYP | Epoxide |
| 106 | 17(18)-EpETE | 317.2 | 259.2 | (d11) 14,15-DiHETrE | EPA | CYP | Epoxide |
| 107 | 14,15-DiHETE | 335.2 | 207.1 | (d11) 14,15-DiHETrE | EPA | CYP | Diol |
| 108 | 17,18-DiHETE | 335.2 | 247.2 | (d11) 14,15-DiHETrE | EPA | CYP | Diol |
| 109 | 13-KODE (13-OxoODE) |
293.2 | 113.1 | (d4) 9(S)-HODE | LA | LOX | Ketone |
| 110 | 9-KODE (9-OxoODE) |
293.2 | 185.2 | (d4) 9(S)-HODE | LA | LOX | Ketone |
| 111 | 9-HODE | 295.2 | 171.1 | (d4) 9(S)-HODE | LA | LOX | Alcohol |
| 112 | 13-HODE | 295.2 | 195.2 | (d4) 9(S)-HODE | LA | LOX | Alcohol |
| 113 | (d4) 9(S)-HODE | 299.2 | 172.1 | ISTD | LA | LOX | Alcohol |
| 114 | 13-HpODE | 311.2 293.2 |
113.2 113.2 |
(d4) 9(S)-HODE | LA | LOX | Hydroxyperoxide |
| 115 | 9-HpODE | 311.2 293.2 |
185.2 185.2 |
(d4) 9(S)-HODE | LA | LOX | Hydroxyperoxide |
| 116 | 9,10,13-TriHOME | 329.2 | 171.1 | (d4) 9(S)-HODE | LA | LOX | Triol |
| 117 | 9,12,13-TriHOME | 329.2 | 211.2 | (d4) 9(S)-HODE | LA | LOX | Triol |
| 118 | 9(10)-EpOME | 295.2 | 171.2 | (d4) 9,10-DiHOME | LA | CYP | Epoxide |
| 119 | 12(13)-EpOME | 295.2 | 195.2 | (d4) 12,13-DiHOME | LA | CYP | Epoxide |
| 120 | 12,13-DiHOME | 313.2 | 183.2 | (d4) 12,13-DiHOME | LA | CYP | Diol |
| 121 | 9,10-DiHOME | 313.2 | 201.1 | (d4) 9,10-DiHOME | LA | CYP | Diol |
| 122 | (d4) 12,13-DiHOME | 317.3 | 185.2 | ISTD | LA | CYP | Diol |
| 123 | (d4) 9,10-DiHOME | 317.3 | 203.2 | ISTD | LA | CYP | Diol |
Solid Phase Extraction (SPE)
In SPE, oxygenated PUFA species are extracted from biological matrix by loading, washing and eluting them through a column using appropriate solvent conditions (Figure 2). SPE involves using commercial columns pre-packed with various sorbents, which include the reversed-phase C18 (e.g., Sep-Pak, Waters Corp, Milford, MA [64, 69], polymer-based reversed phase materials (e.g., Oasis HLB, Waters Corp, Milford, MA [51, 52, 65, 70] and Strata-X, Phenomenex, Torrance, CA [54, 56]) and anion-exchange polymer-based resins [45, 53, 71, 72] cartridges. A reversed-phase C18 cartridge will allow specificity of retention based on the eicosanoids overall hydrophobicity. Polymeric materials that contain both lipophilic and hydrophilic functional groups will provide a broader retention of more lipid metabolites. Anion-exchange materials with a polymeric backbone in addition to a positively charged functional group will selectively retain eicosanoids based on both hydrophobic interactions with the acyl chains and anion-exchange interactions with the carboxylic groups of the PUFA-derivatives.
Notably, no SPE method gives 100% recovery for all lipid species and the efficiency for each identified lipid must be quantified; hence the importance of including proper internal standards in all studies. Leukotrienes are particularly challenging for certain extraction systems and must be carefully checked. Although SPE cartridges can be expensive in terms of both time and supplies, SPE protocols appear to be indispensable to analyze low abundance of mediators in complex matrices, and can be easily standardized for optimal intra- and inter-lab reproducibility.
Liquid-liquid Extraction (LLE)
In LLE, eicosanoids and related species are extracted from biological samples with mixtures of water and organic solvent, leading to phase partitioning of lipids into the organic layer (Figure 2). One of the most commonly used LLE protocols involve the use of chloroform-methanol mixtures according to Bligh and Dyer [73]. Alternative extraction procedures using ether [74], acetone/chloroform [64], hexane–isopropanol [75], and ethyl acetate/ethanol mixtures have been proposed for a wide range of tissues. LLE has proven to be a valid high-throughput alternative to SPE for the extraction of lipid metabolites from biological matrices such as urine samples [53, 76] and brain samples [64, 77]. Additionally, LLE can be used in combination with SPE to maximize the purification of oxygenated PUFA species prior to further MS analysis [66, 78–80].
3.3. Liquid Chromatography
High Performance Liquid Chromatography (HPLC)
Chromatographic separation is necessary for analyzing very low-abundance and isomeric metabolites. Liquid chromatography (LC) has become the chromatographic solution of choice for separating the complex mixture of oxygenated species prior to detection by mass spectrometry. In contrast to GC, in LC no derivatization of the analytes is required, which facilitates the analysis. The high level of chemical similarity and the high number of isomeric species, however, sometimes makes separating the oxygenated PUFA species by conventional high-performance LC (HPLC) quite challenging.
Ultra High Performance Liquid Chromatography (UHPLC)
One approach to increasing chromatographic resolution uses a column packed with reduced particle sizes (<2 µm). The high pressure (10,000–15,000 psi) needed to operate these columns led to the development of ultra-high performance liquid chromatography (UHPLC) [81–83]. Sub-2-µm particles provide narrow chromatographic peaks (often <3 s), which results not only in better resolution but also in lower detection limits and shorter times for the chromatographic run (<5–10 min), compared with conventional HPLC [81–84]. Thus, in conjunction with mass spectrometry, UHPLC can reduce ion suppression caused by co-eluting lipids, and isobaric and isomeric interferences [53, 58].
Reversed-Phase Liquid Chromatography
Reversed-phase LC columns are often used to separate eicosanoids and related mediators. For underivatized metabolites, protonation of the carboxylic acid using a weak organic acid, such as formic or acetic acid, in the mobile phase allows better retention on the reversed-phase column, ensuring chromatographic separation. Oxygenated PUFA metabolites containing longer acyl chains elute from the reversed phase LC column later than shorter-chain lipids (e.g., C20:3 > C18:3), while the retention time decreases as the number of double bonds increases (e.g., C18:2 > C18:3). N-3 PUFA tend to elute earlier than n-6 PUFA (e.g., docosapentaenoic acid (DPA) n-3 earlier than DPA n-6).
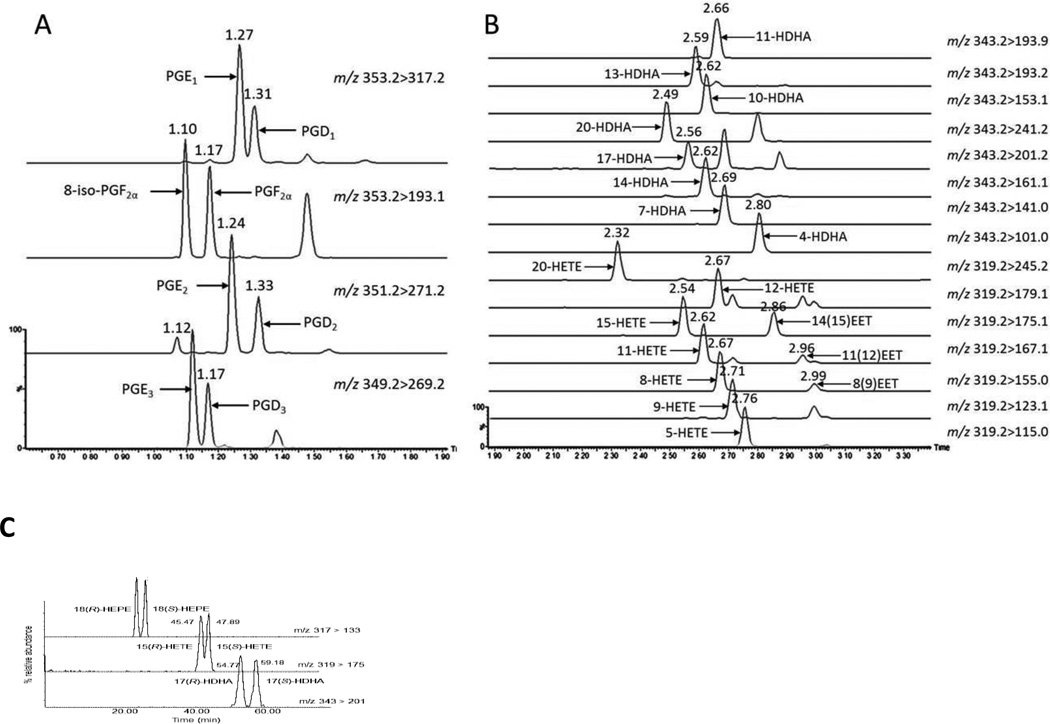
Using an UHPLC with a reversed-phase C18 column, both mediators and their free fatty-acid precursors can be separated in less than 10 minutes in a single chromatographic run, providing key information to help us better understand the intricate regulation of oxygenated PUFA metabolism (Figure 3A,B). Although reversed-phase HPLC and UHPLC applications allow for good separation of most species, the peaks detected might correspond to racemic mixtures; with the exception of diastereo-isomers (e.g., xS,yS and xR,yS), separation of the R and S enantiomers usually requires the use of chiral chromatography (Figure 3C), as discussed below.
Figure 3.
Representative UHPLC-MS/MS chromatograms showing separation of pairs of isobaric species with identical fragmentation patterns by chromatography (A), and of co-eluting metabolites by compound-specific precursor and fragment ions (B). Using a combination of UHPLC with a C18, reversed-phase column, a mixture of commercially available oxygenated PUFA species elutes according to their polarity, number of double bonds and acyl-chain length, allowing the separation of most isomeric and isobaric species (e.g., PGE2 and PGD2) in less than 10 minutes according to retention time and identifying fragmentation patterns (Table 1). A representative UHPLC-MS chromatogram for the chiral separation of enantiomers is shown in panel (C) (adapted from [57]).
Chiral Liquid Chromatography
Most of the high-throughput methods currently used to analyze eicosanoids and related species are based on reversed-phase chromatography, and this approach cannot distinguish between enantiomers. Whilst enzymatic formation of eicosanoids and other PUFA mediators is highly stereoselective, non-enzymatic reactions proceed without stereoselectivity, generating equal amount of enantiomeric species. Thus, the use of either normal phase or reversed-phase chiral chromatography is necessary when attempting to distinguish between enzymatic and non-enzymatic pathways of oxygenated PUFA formation [85–88] (Figure 3C). Chiral chromatography has a number of drawbacks, including the need for long equilibration and elution times (up to 10–60 min) and limited sensitivity. These limitations do not facilitate use in high throughput applications, and chiral chromatography tends to be used for targeted questions aiming to explore specific oxygenated species and not screening assays [89, 90].
3.4. Ionization
Electrospray ionization (ESI) has emerged as the most applied ionization technique for the analysis of all classes of eicosanoids, octadecanoids and docosanoids by LC-MS. ESI generates an abundance of both positive and negative molecular ion species ([M+H]+ and [M-H]−), but the majority of the applications using ESI for non-derivatized metabolites have been in the negative-ion mode [55]. Although mostly detected as deprotonated molecular ion species ([M-H]−), depending on the particular operating conditions of the MS instrument, oxygenated derivatives may be also detected as dehydrated ions ([M–H–H2O]−) [52].
The presence of the weak acid in most of the mobile phases used for the analysis of oxygenated PUFA species by LC-MS (e.g., 0.1% formic acid and acetic acid), offsets the formation of carboxylate anions in the ESI source. To improve detection limits and method sensitivity, alternative derivatization methodologies have been developed. In charge-reversal derivatization, carboxylic acids are converted into cationic derivatives with quaternary amines, improving the detection by ESI [91–93]. In electron capture atmospheric pressure chemical ionization (APCI), eicosanoids and related species are tagged before analysis with an electron-capturing group such as the pentafluorobenzyl moiety, which enhances detection by APCI [94].
3.5. Mass Spectrometry
Tandem Mass Spectrometry
To maximize the sensitivity and specificity of analysis, tandem mass spectrometry (MS/MS) performed on triple quadrupole instruments is often the preferred technology for the analysis of oxygenated PUFA mediators, offering a very selective and sensitive system appropriate for targeted quantitative analyses. Fragmentation information is required to define chemical structures and distinguish between the numerous isobaric species; an acquisition mode named multiple-reaction monitoring (MRM) is typically used to selectively monitor the transition of a selected precursor ion (M1) to a specific product ion (M2); this can be assisted by LC-separation, as shown in the case of the isobaric species PGE2 and PGD2 that have identical fragmentation patterns (Figure 3A) [66]. Furthermore, in the case of co-eluting metabolites, compound-specific precursor ions and their corresponding fragment ions allows selective detection and quantitation of those compounds, a tool important in distinguishing e.g. HETE or HDHA species (Figure 3B; Table 1) [57].
Recent reports have shown that the combination of UHPLC chromatographic separation and MRM transitions performed on triple-quadrupole instruments enables the analysis of over one hundred oxygenated PUFA species in a single acquisition [9–13] including a recent report of 184 metabolites including 26 internal standards separated and accurately quantified including some in picogram/fentomole amounts in a 5 min UHPLC run [68]. To enhance the sensitivity of detection, these MRM transitions are monitored in defined retention-time windows, maximizing dwell times by reducing overlapping transitions. A list of characteristic MRM transitions (compound-specific and selective precursor-to-product ion transitions) for more than 100 common metabolites detected using negative electrospray ionization appears in Table 1.
Quantitation
Quantitation of oxygenated PUFA species is achieved by generating calibration curves using commercially-available standards and class-appropriate deuterated internal standards (see also Internal Standards paragraph under Sample Preparation and Extraction section). Although, at an early discovery phase it is often impractical, the use of stable isotope standards for each eicosanoid of interest is ultimately required for the absolute quantitation and validation of the initial discoveries. Usually, eicosanoid concentrations in biological samples are normalized by volume, tissue weight, cell number, or protein or DNA concentration. Despite potential limitations in their measurements (see also the Sample Preparation and Extraction Section), the standardization of the operating procedures for sample collection and preparation can lead to sufficiently accurate results to reveal answers to biochemical questions. For instance, when comparing samples from parallel measurements in which the precision is very high, the lower accuracy in absolute quantity does not impact upon the study outcome.
4. FUTURE DIRECTIONS
Discovery of Novel PUFA-Derived Species
The discovery of novel oxygenated metabolites derivatives of various PUFA present in biological samples could lead to the identification of unexplored biochemical pathways conferring novel biological activities. With the implementation of modern analytical technologies, we can expect that many more novel structures will be discovered and added to current targeted-lipidomics approaches. Three examples are provided below.
During the last decade, a growing number of oxygenated PUFA species, including the anti-inflammatory mediators lipoxins, resolvins, protectins and maresins, have been discovered, while their complete biochemical characterization is still underway [95–99]. Furthermore, the existence of very long chain PUFA in humans (C24–C36) [100–103] might suggest the existence of their respective oxygenated metabolites, albeit they have not yet been characterized.
Besides existing in their unbound or free form, oxygenated PUFA moieties are found esterified to complex lipids (e.g., glycerophospholipids, glycerolipids and cholesteryl esters) [34, 70, 77, 104–116] or amide-conjugated to ethanolamine (e.g., prostamides) [115, 117] and various amino acids [10, 118], whose mechanism of formation and action has not yet been fully elucidated.
Unusual eicosanoids and related molecules derived from gut microbiome metabolism [119, 120] and dietary sources such as plants (e.g., jasmonic acid) [118], animal meat, and dairy (conjugated-linoleic acid) [121], further contribute to the overall complexity of oxygenated PUFA species found in human tissues and biological fluids, possibly mediating yet-to-be explored biological activities.
Biomarkers
Many enzymatically and non-enzymatically formed eicosanoids have been proposed as biomarkers in clinical research [37–39, 122, 123]. As the verification and validation of these markers of health and disease progresses, there will be a need to develop more reproducible and cost-effective means for their measurement; LC-MS is already used in clinical laboratories, and robust and validated assays for oxygenated PUFA mediators could become part of the routine clinical tests.
Systems Biology
Recent evidence indicates that genetic polymorphisms contribute to the absorption, transport, biosynthesis, metabolism and biological effects of PUFA and their derivatives [124–130]. For example, the efficacy of n-3 PUFA supplementation seems to be related to the ApoE polymorphism [125, 127, 128]. Thus, the integration of oxygenated PUFA measurements with various "omics" techniques, including metabolomics, proteomics and genomics, will allow a more accurate understanding of the role of this system in health and disease, eventually supporting precision and personalized medicine [131, 132].
Novel MS-based approaches
The recent advances in high resolution mass spectrometry and hybrid instruments offer novel analytical capabilities for the analysis of PUFAs and their precursors and metabolites [76, 133, 134]. Although such instruments might suffer from limited dynamic range and sensitivity compared with triple quadrupoles, they can perform additional MS/MS modes of acquisition, such as data-dependent acquisition [133, 135, 136] and data-independent acquisition [76, 134, 137]. Such acquisition modes allow us to acquire extensive fragmentation data from a wider range of lipids in a single analytical run, obviating the need to manually select specific MRM transitions.
In data-dependent acquisition (DDA) mode of operation, the MS/MS experiment performs one scan based on the data acquired in the previous scan. For example, a DDA experiment switches from full-scan MS mode to MS/MS mode when the most intense ions from an inclusion list are detected or after other specific criteria are met. When performing DDA during fast UHPLC analysis, however, the occurrence of narrow, rapidly eluting peaks might result in a loss of data in the MS mode while MS/MS data are being acquired [133, 135, 136].
In data-independent acquisition mode of operation, alternating low and elevated collision energy are utilized independently to the previous scan, without need of inclusion lists and pre-selection of precursor ions by the quadrupole mass analyzer. Low collision-energy acquisition generates information about the precursor ions, and elevated collision energy provides information about product ions. Data-independent acquisitions collect both precursor and product ion information, for virtually all detectable ions, which ultimately constitutes an important repository of knowledge for both qualitative and quantitative applications [76, 134, 137].
Fluxomic approaches
A complete fluxomics analysis of the eicosanoid biosynthetic pathways has recently been developed that allows one to predict and quantitatively determine with LC/MS the temporal changes of each eicosanoid metabolite [135, 138, 139] without using labeled precursors. This is possible because when cells are at rest, levels of free AA and various eicosanoids are minimal, but when cells are activated by various receptor mediated triggers, the cytosolic phospholipase A2 is activated to release AA instigating changes in AA metabolite levels over time [23].
5. CONCLUSIONS
Oxygenated metabolites of PUFA play an active role in health and disease, conferring a range of beneficial as well as toxic effects. Their production through multiple enzymatic and non-enzymatic pathways results in hundreds of bioactive lipids. The application of MS-based lipidomic methodologies has revealed the challenges presented by increasingly complex lipid profiles, and has precipitated the realization that bioactivities mediated by eicosanoids and related oxygenated PUFA mediators may largely depend upon their overall balance.
The application of state-of-the-art MS-based analytical platforms permits the rapid identification and quantification of numerous species. These data are creating novel opportunities to enhance our knowledge of oxygenated PUFA functions in the context of health and disease. High-throughput methodology for the measurement of eicosanoids, octadecanoids, docosanoids and other species could lead to the development of panels of biomarkers to assess inflammatory status, disease diagnosis and/or prognosis, assisting pharmacological and/or nutritional interventions.
Highlights.
Oxidation of polyunsaturated fatty acids can yield an array of eicosanoids
These lipid mediators play key pathophysiological roles in humans
Mass spectrometry-based technology allows us to analyze such lipid mediators
Development of high-throughput approaches facilitates the analysis of eicosanoids
ACKNOWLEDGEMENTS
We wish to thank National Institutes of Health Grants RO1 GM20501 and U54 GM069338 (EAD), and The Wellcome Trust Grant WT094028 (AN) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolaou A, Mauro C, Urquhart P, Marelli-Berg F. Polyunsaturated Fatty Acid-derived lipid mediators and T cell function. Front Immunol. 2014;5:75. doi: 10.3389/fimmu.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolaou A. Eicosanoids in skin inflammation. Prostaglandins Leukot Essent Fatty Acids. 2013;88:131–138. doi: 10.1016/j.plefa.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Hwang SH, Wecksler AT, Wagner K, Hammock BD. Rationally designed multitarget agents against inflammation and pain. Curr Med Chem. 2013;20:1783–1799. doi: 10.2174/0929867311320130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011;365:1812–1823. doi: 10.1056/NEJMra1104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le HD, Fallon EM, Kalish BT, de Meijer VE, Meisel JA, Gura KM, Nose V, Pan AH, Bistrian BR, Puder M. The effect of varying ratios of docosahexaenoic acid and arachidonic acid in the prevention and reversal of biochemical essential fatty acid deficiency in a murine model. Metabolism. 2013;62:499–508. doi: 10.1016/j.metabol.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu W-C, Cook-Johnson RJ, James MJ, Mühlhäusler B, Gibson RA. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2010;83:61–68. doi: 10.1016/j.plefa.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Nilsson A. Sources of eicosanoid precursor fatty acid pools in tissues. J Lipid Res. 2001;42:1521–1542. [PubMed] [Google Scholar]
- 11.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? British journal of clinical pharmacology. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2014 doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Experimental Biology and Medicine. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 14.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51:2074–2081. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman JW, Stok JE, Vidal JD, Corbin CJ, Huang Q, Hammock BD, Conley AJ. Cytochrome p450-dependent lipid metabolism in preovulatory follicles. Endocrinology. 2004;145:5097–5105. doi: 10.1210/en.2004-0710. [DOI] [PubMed] [Google Scholar]
- 16.Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J Lipid Res. 2012;53:1662–1669. doi: 10.1194/jlr.P025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruins MJ, Dane AD, Strassburg K, Vreeken RJ, Newman JW, Salem N, Jr, Tyburczy C, Brenna JT. Plasma oxylipin profiling identifies polyunsaturated vicinal diols as responsive to arachidonic acid and docosahexaenoic acid intake in growing piglets. J Lipid Res. 2013;54:1598–1607. doi: 10.1194/jlr.M034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nording ML, Yang J, Georgi K, Hegedus Karbowski C, German JB, Weiss RH, Hogg RJ, Trygg J, Hammock BD, Zivkovic AM. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 Fatty acids. PloS one. 2013;8:e76575. doi: 10.1371/journal.pone.0076575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engels F, Willems H, Nijkamp F. Cyclooxygenase-catalyzed formation of 9-hydroxylinoleic acid by guinea pig alveolar macrophages under non-stimulated conditions. FEBS letters. 1986;209:249–253. doi: 10.1016/0014-5793(86)81121-8. [DOI] [PubMed] [Google Scholar]
- 20.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. Resolvins A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment that Counter Proinflammation Signals. The Journal of experimental medicine. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagarde M, Liu M, Véricel E, Calzada C, Chen P, Driss F, Guichardant M. Docosahexaenoic acid, protectin synthesis: relevance against atherothrombogenesis. Proc Nutr Soc. 2014;73:186–189. doi: 10.1017/S0029665113003704. [DOI] [PubMed] [Google Scholar]
- 22.Sharma NP, Dong L, Yuan C, Noon KR, Smith WL. Asymmetric acetylation of the cyclooxygenase-2 homodimer by aspirin and its effects on the oxygenation of arachidonic, eicosapentaenoic, and docosahexaenoic acids. Molecular pharmacology. 2010;77:979–986. doi: 10.1124/mol.109.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norris PC, Gosselin D, Reichart D, Glass CK, Dennis EA. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12746–12751. doi: 10.1073/pnas.1404372111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brash AR, Schneider C, Hamberg M. Applications of stereospecifically-labeled fatty acids in oxygenase and desaturase biochemistry. Lipids. 2012;47:101–116. doi: 10.1007/s11745-011-3612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and Maresins: New Pro-Resolving Families of Mediators in Acute Inflammation and Resolution Bioactive Metabolome. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. doi: 10.1016/j.bbalip.2014.08.006. pii: S1388-1981 (2014) 00161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annual review of pharmacology and toxicology. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harder D, Campbell W, Roman R. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. Journal of vascular research. 1995;32:79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- 28.Capdevila JH, Falck JR. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins & other lipid mediators. 2002;68–69:325–344. doi: 10.1016/s0090-6980(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 29.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. The Journal of biological chemistry. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 31.Morrow JD, Harris TM, Roberts LJ., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 32.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith WL, Murphy RC. Oxidized lipids formed non-enzymatically by reactive oxygen species. The Journal of biological chemistry. 2008;283:15513–15514. doi: 10.1074/jbc.R800006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zemski Berry KA, Murphy RC. Free radical oxidation of plasmalogen glycerophosphocholine containing esterified docosahexaenoic acid: structure determination by mass spectrometry. Antioxid Redox Signal. 2005;7:157–169. doi: 10.1089/ars.2005.7.157. [DOI] [PubMed] [Google Scholar]
- 35.de La Torre A, Lee YY, Oger C, Sangild PT, Durand T, Lee JC, Galano JM. Synthesis, discovery, and quantitation of dihomo-isofurans: biomarkers for in vivo adrenic acid peroxidation. Angew Chem Int Ed Engl. 2014;53:6249–6252. doi: 10.1002/anie.201402440. [DOI] [PubMed] [Google Scholar]
- 36.Roberts LJ, II, Morrow JD. Measurement of F<sub>2</sub>-isoprostanes as an index of oxidative stress in vivo. Free Radical Biology and Medicine. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 37.Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. The FASEB Journal. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 38.Sbardella E, Greco A, Stromillo ML, Prosperini L, Puopolo M, Cefaro LA, Pantano P, De Stefano N, Minghetti L, Pozzilli C. Isoprostanes in clinically isolated syndrome and early multiple sclerosis as biomarkers of tissue damage and predictors of clinical course. Mult Scler. 2013;19:411–417. doi: 10.1177/1352458512457721. [DOI] [PubMed] [Google Scholar]
- 39.Cracowski J-L, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends in pharmacological sciences. 2002;23:360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 40.Balgoma D, Checa A, Sar DG, Snowden S, Wheelock CE. Quantitative metabolic profiling of lipid mediators. Molecular nutrition & food research. 2013;57:1359–1377. doi: 10.1002/mnfr.201200840. [DOI] [PubMed] [Google Scholar]
- 41.Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. Journal of lipid research. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- 42.Reilly MP, Lawson JA, FitzGerald GA. Eicosanoids and isoeicosanoids: indices of cellular function and oxidant stress. The Journal of nutrition. 1998;128:434S–438S. doi: 10.1093/jn/128.2.434S. [DOI] [PubMed] [Google Scholar]
- 43.Reinke M. Monitoring thromboxane in body fluids: a specific ELISA for 11-dehydrothromboxane B2 using a monoclonal antibody. Am J Physiol. 1992;262:E658–E662. doi: 10.1152/ajpendo.1992.262.5.E658. [DOI] [PubMed] [Google Scholar]
- 44.Shono F, Yokota K, Horie K, Yamamoto S, Yamashita K, Watanabe K, Miyazaki H. A heterologous enzyme immunoassay of prostaglandin E2 using a stable enzyme-labeled hapten mimic. Anal Biochem. 1988;168:284–291. doi: 10.1016/0003-2697(88)90320-x. [DOI] [PubMed] [Google Scholar]
- 45.Dahl JH, van Breemen RB. Rapid quantitative analysis of 8-iso-prostaglandin-F(2alpha) using liquid chromatography-tandem mass spectrometry and comparison with an enzyme immunoassay method. Anal Biochem. 2010;404:211–216. doi: 10.1016/j.ab.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bessard J, Cracowski JL, Stanke-Labesque F, Bessard G. Determination of isoprostaglandin F2alpha type III in human urine by gas chromatography-electronic impact mass spectrometry. Comparison with enzyme immunoassay. J Chromatogr B Biomed Sci Appl. 2001;754:333–343. doi: 10.1016/s0378-4347(00)00621-6. [DOI] [PubMed] [Google Scholar]
- 47.Weichert H, Stenzel I, Berndt E, Wasternack C, Feussner I. Metabolic profiling of oxylipins upon salicylate treatment in barley leaves—preferential induction of the reductase pathway by salicylate. FEBS letters. 1999;464:133–137. doi: 10.1016/s0014-5793(99)01697-x. [DOI] [PubMed] [Google Scholar]
- 48.Toyo'oka T, Ishibashi M, Terao T, Imai K. Sensitive fluorometric detection of prostaglandins by high performance liquid chromatography after precolumn labelling with 4-(N,N-dimethylaminosulphonyl)-7-(1-piperazinyl)-2, 1, 3-benzoxadiazole (DBD-PZ) Biomedical Chromatography. 1992;6:143–148. doi: 10.1002/bmc.1130060310. [DOI] [PubMed] [Google Scholar]
- 49.Baranowski R, Pacha K. Gas chromatographic determination of prostaglandins. Mini Rev Med Chem. 2002;2:135–144. doi: 10.2174/1389557024605465. [DOI] [PubMed] [Google Scholar]
- 50.Harkewicz R, Dennis EA. Applications of mass spectrometry to lipids and membranes. Annual review of biochemistry. 2011;80:301–325. doi: 10.1146/annurev-biochem-060409-092612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundstrom SL, Saluja R, Adner M, Haeggstrom JZ, Nilsson G, Wheelock CE. Lipid mediator metabolic profiling demonstrates differences in eicosanoid patterns in two phenotypically distinct mast cell populations. J Lipid Res. 2013;54:116–126. doi: 10.1194/jlr.M030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strassburg K, Huijbrechts AM, Kortekaas KA, Lindeman JH, Pedersen TL, Dane A, Berger R, Brenkman A, Hankemeier T, van Duynhoven J, Kalkhoven E, Newman JW, Vreeken RJ. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal Bioanal Chem. 2012;404:1413–1426. doi: 10.1007/s00216-012-6226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sterz K, Scherer G, Ecker J. A simple and robust UPLC-SRM/MS method to quantify urinary eicosanoids. J Lipid Res. 2012;53:1026–1036. doi: 10.1194/jlr.D023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolaou A, Masoodi M, Mir A. Lipidomic analysis of prostanoids by liquid chromatography-electrospray tandem mass spectrometry. Methods Mol Biol. 2009;579:271–286. doi: 10.1007/978-1-60761-322-0_14. [DOI] [PubMed] [Google Scholar]
- 55.Murphy RC, Barkley RM, Zemski Berry K, Hankin J, Harrison K, Johnson C, Krank J, McAnoy A, Uhlson C, Zarini S. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346:1–42. doi: 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 56.Dumlao DS, Buczynski MW, Norris PC, Harkewicz R, Dennis EA. High-throughput lipidomic analysis of fatty acid derived eicosanoids and N-acylethanolamines. Biochim Biophys Acta. 2011;1811:724–736. doi: 10.1016/j.bbalip.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massey KA, Nicolaou A. Lipidomics of oxidized polyunsaturated fatty acids. Free Radic Biol Med. 2013;59:45–55. doi: 10.1016/j.freeradbiomed.2012.08.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaik JSB, Miller TM, Graham SH, Manole MD, Poloyac SM. Rapid and simultaneous quantitation of prostanoids by UPLC–MS/MS in rat brain. Journal of Chromatography B. 2014;945:207–216. doi: 10.1016/j.jchromb.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dennis EA, Deems RA, Harkewicz R, Quehenberger O, Brown HA, Milne SB, Myers DS, Glass CK, Hardiman G, Reichart D, Merrill AH, Jr, Sullards MC, Wang E, Murphy RC, Raetz CR, Garrett TA, Guan Z, Ryan AC, Russell DW, McDonald JG, Thompson BM, Shaw WA, Sud M, Zhao Y, Gupta S, Maurya MR, Fahy E, Subramaniam S. A mouse macrophage lipidome. The Journal of biological chemistry. 2010;285:39976–39985. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pastor A, Farré M, Fitó M, Fernandez-Aranda F, de la Torre R. Analysis of ECs and related compounds in plasma: artifactual isomerization and ex vivo enzymatic generation of 2-MGs. Journal of lipid research. 2014;55:966–977. doi: 10.1194/jlr.D043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamlage B, Maldonado SG, Bethan B, Peter E, Schmitz O, Liebenberg V, Schatz P. Quality markers addressing preanalytical variations of blood and plasma processing identified by broad and targeted metabolite profiling. Clinical chemistry. 2014;60:399–412. doi: 10.1373/clinchem.2013.211979. [DOI] [PubMed] [Google Scholar]
- 62.Metherel AH, Aristizabal Henao JJ, Stark KD. EPA and DHA levels in whole blood decrease more rapidly when stored at −20 degrees C as compared with room temperature, 4 and −75 degrees C. Lipids. 2013;48:1079–1091. doi: 10.1007/s11745-013-3827-x. [DOI] [PubMed] [Google Scholar]
- 63.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B. The human serum metabolome. PloS one. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Golovko MY, Murphy EJ. An improved LC-MS/MS procedure for brain prostanoid analysis using brain fixation with head-focused microwave irradiation and liquid-liquid extraction. J Lipid Res. 2008;49:893–902. doi: 10.1194/jlr.D700030-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Analytical chemistry. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masoodi M, Nicolaou A. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid communications in mass spectrometry. 2006;20:3023–3029. doi: 10.1002/rcm.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong A, Sagar DR, Ortori CA, Kendall DA, Chapman V, Barrett DA. Simultaneous tissue profiling of eicosanoid and endocannabinoid lipid families in a rat model of osteoarthritis. Journal of lipid research. 2014 doi: 10.1194/jlr.M048694. jlr. M048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Armando AM, Quehenberger O, Yan C, Dennis EA. Comprehensive Ultra Performance Liquid Chromatographic Separation and Mass Spectrometric Analysis of Eicosanoid Metabolites in Human Samples. Journal of Chromatography A. 2014;1359:60–69. doi: 10.1016/j.chroma.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. The Journal of biological chemistry. 2012;287:10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shearer GC, Newman JW. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Current atherosclerosis reports. 2009;11:403–410. doi: 10.1007/s11883-009-0061-3. [DOI] [PubMed] [Google Scholar]
- 71.Lee CY, Jenner AM, Halliwell B. Rapid preparation of human urine and plasma samples for analysis of F2-isoprostanes by gas chromatography-mass spectrometry. Biochem Biophys Res Commun. 2004;320:696–702. doi: 10.1016/j.bbrc.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Vigor C, Bertrand-Michel J, Pinot E, Oger C, Vercauteren J, Le Faouder P, Galano JM, Lee JC, Durand T. Non-enzymatic lipid oxidation products in biological systems: assessment of the metabolites from polyunsaturated fatty acids. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;964:65–78. doi: 10.1016/j.jchromb.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 73.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 74.Lee SH, Williams MV, DuBois RN, Blair IA. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2003;17:2168–2176. doi: 10.1002/rcm.1170. [DOI] [PubMed] [Google Scholar]
- 75.Saunders RD, Horrocks LA. Simultaneous extraction and preparation for high-performance liquid chromatography of prostaglandins and phospholipids. Analytical biochemistry. 1984;143:71–75. doi: 10.1016/0003-2697(84)90559-1. [DOI] [PubMed] [Google Scholar]
- 76.Brose SA, Baker AG, Golovko MY. A fast one-step extraction and UPLC-MS/MS analysis for E2/D 2 series prostaglandins and isoprostanes. Lipids. 2013;48:411–419. doi: 10.1007/s11745-013-3767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brose SA, Thuen BT, Golovko MY. LC/MS/MS method for analysis of E(2) series prostaglandins and isoprostanes. J Lipid Res. 2011;52:850–859. doi: 10.1194/jlr.D013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Powell WS. Precolumn extraction and reversed-phase high-pressure liquid chromatography of prostaglandins and leukotrienes. Analytical biochemistry. 1987;164:117–131. doi: 10.1016/0003-2697(87)90375-7. [DOI] [PubMed] [Google Scholar]
- 79.Takabatake M, Hishinuma T, Suzuki N, Chiba S, Tsukamoto H, Nakamura H, Saga T, Tomioka Y, Kurose A, Sawai T. Simultaneous quantification of prostaglandins in human synovial cell-cultured medium using liquid chromatography/tandem mass spectrometry. Prostaglandins, leukotrienes and essential fatty acids. 2002;67:51–56. doi: 10.1054/plef.2002.0381. [DOI] [PubMed] [Google Scholar]
- 80.Rago B, Fu C. Development of a high-throughput ultra performance liquid chromatography–mass spectrometry assay to profile 18 eicosanoids as exploratory biomarkers for atherosclerotic diseases. Journal of Chromatography B. 2013;936:25–32. doi: 10.1016/j.jchromb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Churchwell MI, Twaddle NC, Meeker LR, Doerge DR. Improving LC–MS sensitivity through increases in chromatographic performance: Comparisons of UPLC–ES/MS/MS to HPLC–ES/MS/MS. Journal of Chromatography B. 2005;825:134–143. doi: 10.1016/j.jchromb.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 82.Plumb RS, Johnson KA, Rainville P, Smith BW, Wilson ID, Castro-Perez JM, Nicholson JK. UPLC/MSE; a new approach for generating molecular fragment information for biomarker structure elucidation. Rapid Communications in Mass Spectrometry. 2006;20:1989–1994. doi: 10.1002/rcm.2550. [DOI] [PubMed] [Google Scholar]
- 83.Swartz ME. UPLC™: an introduction and review. Journal of Liquid Chromatography & Related Technologies. 2005;28:1253–1263. [Google Scholar]
- 84.Martín-Venegas R, Casillas R, Jáuregui O, Moreno JJ. Rapid simultaneous analysis of cyclooxygenase, lipoxygenase and cytochrome P-450 metabolites of arachidonic and linoleic acids using high performance liquid chromatography/mass spectrometry in tandem mode. Journal of pharmaceutical and biomedical analysis. 2011;56:976–982. doi: 10.1016/j.jpba.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 85.Oh SF, Vickery TW, Serhan CN. Chiral lipidomics of E-series resolvins: aspirin and the biosynthesis of novel mediators. Biochim Biophys Acta. 2011;1811:737–747. doi: 10.1016/j.bbalip.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schneider C, Yu Z, Boeglin WE, Zheng Y, Brash AR. Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. Methods Enzymol. 2007;433:145–157. doi: 10.1016/S0076-6879(07)33008-5. [DOI] [PubMed] [Google Scholar]
- 87.Lee SH, Blair IA. Targeted chiral lipidomics analysis of bioactive eicosanoid lipids in cellular systems. BMB Rep. 2009;42:401–410. doi: 10.5483/bmbrep.2009.42.7.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mesaros C, Blair IA. Targeted chiral analysis of bioactive arachidonic Acid metabolites using liquid-chromatography-mass spectrometry. Metabolites. 2012;2:337–365. doi: 10.3390/metabo2020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bayer M, Mosandl A, Thaçi D. Improved enantioselective analysis of polyunsaturated hydroxy fatty acids in psoriatic skin scales using high-performance liquid chromatography. Journal of Chromatography B. 2005;819:323–328. doi: 10.1016/j.jchromb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki N, Hishinuma T, Saga T, Sato J, Toyota T, Goto J, Mizugaki M. Determination of urinary 12 (<i>S</i>)-hydroxyeicosatetraenoic acid by liquid chromatography–tandem mass spectrometry with column-switching technique: sex difference in healthy volunteers and patients with diabetes mellitus. Journal of Chromatography B. 2003;783:383–389. doi: 10.1016/s1570-0232(02)00666-9. [DOI] [PubMed] [Google Scholar]
- 91.Bollinger JG, Thompson W, Lai Y, Oslund RC, Hallstrand TS, Sadilek M, Turecek F, Gelb MH. Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal Chem. 2010;82:6790–6796. doi: 10.1021/ac100720p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lamos SM, Shortreed MR, Frey BL, Belshaw PJ, Smith LM. Relative quantification of carboxylic acid metabolites by liquid chromatography-mass spectrometry using isotopic variants of cholamine. Anal Chem. 2007;79:5143–5149. doi: 10.1021/ac062416m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pettinella C, Lee SH, Cipollone F, Blair IA. Targeted quantitative analysis of fatty acids in atherosclerotic plaques by high sensitivity liquid chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:168–176. doi: 10.1016/j.jchromb.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 94.Singh G, Gutierrez A, Xu K, Blair IA. Liquid chromatography/electron capture atmospheric pressure chemical ionization/mass spectrometry: analysis of pentafluorobenzyl derivatives of biomolecules and drugs in the attomole range. Analytical chemistry. 2000;72:3007–3013. doi: 10.1021/ac000374a. [DOI] [PubMed] [Google Scholar]
- 95.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, Serhan CN. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One. 2014;9:e102362. doi: 10.1371/journal.pone.0102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013;3:1940. doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balas L, Guichardant M, Durand T, Lagarde M. Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date. Biochimie. 2014;99:1–7. doi: 10.1016/j.biochi.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Chen P, Fenet B, Michaud S, Tomczyk N, Vericel E, Lagarde M, Guichardant M. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009;583:3478–3484. doi: 10.1016/j.febslet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 100.Aveldano MI, Sprecher H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. The Journal of biological chemistry. 1987;262:1180–1186. [PubMed] [Google Scholar]
- 101.Aveldano MI. A novel group of very long chain polyenoic fatty acids in dipolyunsaturated phosphatidylcholines from vertebrate retina. The Journal of biological chemistry. 1987;262:1172–1179. [PubMed] [Google Scholar]
- 102.Aveldano MI. Long and very long polyunsaturated fatty acids of retina and spermatozoa: the whole complement of polyenoic fatty acid series. Advances in experimental medicine and biology. 1992;318:231–242. doi: 10.1007/978-1-4615-3426-6_19. [DOI] [PubMed] [Google Scholar]
- 103.Harkewicz R, Du H, Tong Z, Alkuraya H, Bedell M, Sun W, Wang X, Hsu YH, Esteve-Rudd J, Hughes G, Su Z, Zhang M, Lopes VS, Molday RS, Williams DS, Dennis EA, Zhang K. Essential role of ELOVL4 protein in very long chain fatty acid synthesis and retinal function. The Journal of biological chemistry. 2012;287:11469–11480. doi: 10.1074/jbc.M111.256073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ravandi A, Leibundgut G, Hung MY, Patel M, Hutchins PM, Murphy RC, Prasad A, Mahmud E, Miller YI, Dennis EA, Witztum JL, Tsimikas S. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J Am Coll Cardiol. 2014;63:1961–1971. doi: 10.1016/j.jacc.2014.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aldrovandi M, Hammond VJ, Podmore H, Hornshaw M, Clark SR, Marnett LJ, Slatter DA, Murphy RC, Collins PW, O'Donnell VB. Human platelets generate phospholipid-esterified prostaglandins via cyclooxygenase-1 that are inhibited by low dose aspirin supplementation. J Lipid Res. 2013;54:3085–3097. doi: 10.1194/jlr.M041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O'Donnell VB, Murphy RC. New families of bioactive oxidized phospholipids generated by immune cells: identification and signaling actions. Blood. 2012;120:1985–1992. doi: 10.1182/blood-2012-04-402826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hutchins PM, Moore EE, Murphy RC. Electrospray MS/MS reveals extensive and nonspecific oxidation of cholesterol esters in human peripheral vascular lesions. J Lipid Res. 2011;52:2070–2083. doi: 10.1194/jlr.M019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kuhn H, Hazen SL, Goodall AH, Hamali HA, Collins PW, O'Donnell VB. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. The Journal of biological chemistry. 2010;285:6891–6903. doi: 10.1074/jbc.M109.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakamura T, Henson PM, Murphy RC. Occurrence of oxidized metabolites of arachidonic acid esterified to phospholipids in murine lung tissue. Anal Biochem. 1998;262:23–32. doi: 10.1006/abio.1998.2749. [DOI] [PubMed] [Google Scholar]
- 110.Morgan AH, Hammond VJ, Morgan L, Thomas CP, Tallman KA, Garcia-Diaz YR, McGuigan C, Serpi M, Porter NA, Murphy RC, O'Donnell VB. Quantitative assays for esterified oxylipins generated by immune cells. Nat Protoc. 2010;5:1919–1931. doi: 10.1038/nprot.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, Witztum JL, Tsimikas S, Miller YI. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet: macrophage binding and activation. The Journal of biological chemistry. 2010;285:32343–32351. doi: 10.1074/jbc.M110.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized low density lipoprotein. The Journal of biological chemistry. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choi SH, Yin H, Ravandi A, Armando A, Dumlao D, Kim J, Almazan F, Taylor AM, McNamara CA, Tsimikas S, Dennis EA, Witztum JL, Miller YI. polyoxygenated cholesterol ester hydroperoxide activates TLR4 and SYK dependent signaling in macrophages. PLoS One. 2013;8:e83145. doi: 10.1371/journal.pone.0083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. Journal of Biological Chemistry. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 115.Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci. 2014;35:284–292. doi: 10.1016/j.tips.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, Bonventre JV, Prescott SM, Roberts LJ., 2nd Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. The Journal of biological chemistry. 2006;281:4616–4623. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 117.Matias I, Chen J, De Petrocellis L, Bisogno T, Ligresti A, Fezza F, Krauss A-P, Shi L, Protzman CE, Li C. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. Journal of Pharmacology and Experimental Therapeutics. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- 118.Mosblech A, Feussner I, Heilmann I. Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem. 2009;47:511–517. doi: 10.1016/j.plaphy.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 119.Prost I, Dhondt S, Rothe G, Vicente J, Rodriguez MJ, Kift N, Carbonne F, Griffiths G, Esquerré-Tugayé M-T, Rosahl S. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiology. 2005;139:1902–1913. doi: 10.1104/pp.105.066274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noverr MC, Erb-Downward JR, Huffnagle GB. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clinical microbiology reviews. 2003;16:517–533. doi: 10.1128/CMR.16.3.517-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Banni S. Conjugated linoleic acid metabolism. Curr Opin Lipidol. 2002;13:261–266. doi: 10.1097/00041433-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 122.Fernie AR, Trethewey RN, Krotzky AJ, Willmitzer L. Metabolite profiling: from diagnostics to systems biology. Nature Reviews Molecular Cell Biology. 2004;5:763–769. doi: 10.1038/nrm1451. [DOI] [PubMed] [Google Scholar]
- 123.Laiakis EC, Strassburg K, Bogumil R, Lai S, Vreeken RJ, Hankemeier T, Langridge J, Plumb RS, Fornace AJ, Jr, Astarita G. Metabolic Phenotyping Reveals a Lipid Mediator Response to Ionizing Radiation. Journal of proteome research. 2014 doi: 10.1021/pr5005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fontaine-Bisson B, Wolever TM, Chiasson JL, Rabasa-Lhoret R, Maheux P, Josse RG, Leiter LA, Rodger NW, Ryan EA, Connelly PW, Corey PN, El-Sohemy A. Genetic polymorphisms of tumor necrosis factor-alpha modify the association between dietary polyunsaturated fatty acids and fasting HDL-cholesterol and apo A-I concentrations. The American journal of clinical nutrition. 2007;86:768–774. doi: 10.1093/ajcn/86.3.768. [DOI] [PubMed] [Google Scholar]
- 125.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Jr, Weiner M, Shinto L, Aisen PS. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA : the journal of the American Medical Association. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, Kennedy D. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. The American journal of clinical nutrition. 2013;97:1134–1143. doi: 10.3945/ajcn.112.053371. [DOI] [PubMed] [Google Scholar]
- 127.Thifault E, Cormier H, Bouchard-Mercier A, Rudkowska I, Paradis AM, Garneau V, Ouellette C, Lemieux S, Couture P, Vohl MC. Effects of Age, Sex, Body Mass Index and APOE Genotype on Cardiovascular Biomarker Response to an n-3 Polyunsaturated Fatty Acid Supplementation. Journal of nutrigenetics and nutrigenomics. 2013;6:73–82. doi: 10.1159/000350744. [DOI] [PubMed] [Google Scholar]
- 128.Minihane AM, Khan S, Leigh-Firbank EC, Talmud P, Wright JW, Murphy MC, Griffin BA, Williams CM. ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:1990–1997. doi: 10.1161/01.atv.20.8.1990. [DOI] [PubMed] [Google Scholar]
- 129.Morris PG, Zhou XK, Milne GL, Goldstein D, Hawks LC, Dang CT, Modi S, Fornier MN, Hudis CA, Dannenberg AJ. Increased levels of urinary PGE-M, a biomarker of inflammation, occur in association with obesity, aging, and lung metastases in patients with breast cancer. Cancer prevention research. 2013;6:428–436. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 131.Jacobs DM, Gaudier E, van Duynhoven J, Vaughan EE. Non-digestible food ingredients, colonic microbiota and the impact on gut health and immunity: a role for metabolomics. Current drug metabolism. 2009;10:41–54. doi: 10.2174/138920009787048383. [DOI] [PubMed] [Google Scholar]
- 132.Hyotylainen T, Oresic M. Systems biology strategies to study lipidomes in health and disease. Prog Lipid Res. 2014;55:43–60. doi: 10.1016/j.plipres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 133.Norskov NP, Hedemann MS, Laerke HN, Knudsen KE. Multicompartmental nontargeted LC-MS metabolomics: explorative study on the metabolic responses of rye fiber versus refined wheat fiber intake in plasma and urine of hypercholesterolemic pigs. Journal of proteome research. 2013;12:2818–2832. doi: 10.1021/pr400164b. [DOI] [PubMed] [Google Scholar]
- 134.Goudarzi M, Weber WM, Mak TD, Chung J, Doyle-Eisele M, Melo DR, Brenenr DJ, Guilmette RA, Fornace AJ. Metabolomic and lipidomic analysis of serum from mice exposed to an internal emitter, Cesium-137, using a shotgun LC-MS approach. Journal of proteome research. 2014 doi: 10.1021/pr500913n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu Y, Hong S, Tjonahen E, Serhan CN. Mediator-lipidomics: databases and search algorithms for PUFA-derived mediators. J Lipid Res. 2005;46:790–802. doi: 10.1194/jlr.D400020-JLR200. [DOI] [PubMed] [Google Scholar]
- 137.Masoodi M, Eiden M, Koulman A, Spaner D, Volmer DA. Comprehensive lipidomics analysis of bioactive lipids in complex regulatory networks. Anal Chem. 2010;82:8176–8185. doi: 10.1021/ac1015563. [DOI] [PubMed] [Google Scholar]
- 138.Gupta S, Maurya MR, Stephens DL, Dennis EA, Subramaniam S. An integrated model of eicosanoid metabolism and signaling based on lipidomics flux analysis. Biophys J. 2009;96:4542–4551. doi: 10.1016/j.bpj.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kihara Y, Gupta S, Maurya MR, Armando A, Shah I, Quehenberger O, Glass CK, Dennis EA, Subramaniam S. Modeling of eicosanoid fluxes reveals functional coupling between cyclooxygenases and terminal synthases. Biophys J. 2014;106:966–975. doi: 10.1016/j.bpj.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]