Highlights
-
•
Reward-modulated cognitive control is supported by amygdalar incentive processing.
-
•
NAcc activation supports inhibitory control in youths.
-
•
NAcc activation hinders inhibitory control in adults.
Keywords: Adolescent, Reward, Motivation, Development, Inhibitory control, Antisaccade
Abstract
We investigated changes in brain function supporting inhibitory control under age-controlled incentivized conditions, separating age- and performance-related activation in an accelerated longitudinal design including 10- to 22-year-olds. Better inhibitory control correlated with striatal activation during neutral trials, while Age X Behavior interactions in the striatum indicated that in the absence of extrinsic incentives, younger subjects with greater reward circuitry activation successfully engage in greater inhibitory control. Age was negatively correlated with ventral amygdala activation during Loss trials, suggesting that amygdala function more strongly mediates bottom-up processing earlier in development when controlling the negative aspects of incentives to support inhibitory control. Together, these results indicate that with development, reward-modulated cognitive control may be supported by incentive processing transitions in the amygdala, and from facilitative to obstructive striatal function during inhibitory control.
1. Introduction
Adolescence is recognized as a period of increased behavioral risk associated with greater mortality (Eaton et al., 2012). Although direct links between real-world risk-taking and brain maturation have yet to be established, research to date suggests that neural systems supporting cognitive control and incentive processing follow different developmental trajectories, which may lead to increased impulsivity in the face of rewarding situations (Casey et al., 2008, Galvan et al., 2006, Luna et al., 2014, Steinberg, 2005). Although initial neurodevelopmental studies have been influential in guiding research toward the interaction of reward processing and cognitive control, there are three limitations in the existing literature. First, in tasks where performance increases with age (e.g., the antisaccade task; Luna et al., 2001), many prior studies have not compared neural activation patterns due to both task performance and age. That is to say, while developmental studies often control performance differences by using tasks that generate equal performance or though analytic models, in the present study we placed both behavior and age into the same model to account for shared vs. unique variance explained by each, allowing for the examination of their interaction. Second, most developmental studies have been cross-sectional in design, limiting implications toward developmental change (Singer and Willett, 2003). We address these limitations by focusing on how incentives, age, and performance, modulate brain activity during inhibitory control throughout middle childhood to young adulthood using an accelerated longitudinal design.
Behavioral studies indicate peak sensitivity to reward during adolescence (Cauffman et al., 2010), yet neuroimaging results have been inconsistent. Functional magnetic resonance imaging (fMRI) studies have shown developmental peaks in striatal activation when processing rewards (Ernst et al., 2005, Galvan et al., 2006, Van Leijenhorst et al., 2010), as well as developmental troughs (Bjork et al., 2004, Bjork et al., 2010, Lamm et al., 2014).
Relatively less is known about the development processes underlying loss compared to what is known of these processes for reward (Spear, 2011). In adults, behavioral economics studies indicate that losses are valued two-fold compared to gains (Kahneman and Tversky, 1979, Tversky and Kahneman, 1992) suggesting a psychological difference between rewards and losses. Behaviorally, adolescents and adults tend to exhibit similar levels of loss-aversion, while neuronally adolescents recruit striatal and frontal regions to a greater degree than adults when making decisions involving losses (Barkley-Levenson et al., 2012, Weller et al., 2010). While the circuitry underlying the processing of losses and gains similarly include anterior cingulate, nucleus accumbens (NAcc), and amygdala, it is differentially engaged during these two types of tasks (Levin et al., 2012, Tom et al., 2007).
In concert with motivation, inhibitory control, which is a core component of executive function, continues to mature through adolescence (Fischer et al., 1997, Luna et al., 2004, Munoz et al., 1998) supported by age-related changes in frontoparietal activation (Bunge et al., 2002, Ordaz et al., 2013). The antisaccade (AS) task probes the integrity of cortico-subcortical inhibitory control (Hallett, 1978) and elicits decreases in dorsolateral PFC activation from childhood to adolescence, when it reaches adult-like levels (Ordaz et al., 2013). The AS task elicits increases in dACC activation from childhood into adulthood, and correlates with performance (Ordaz et al., 2013). These results suggest that inhibitory control is largely available by adolescence but with continued specialization that may undermine cognitive control and influence decision-making.
The effect of incentives on cognitive control have shown that incentives enhance activation in task-relevant neural regions (Krawczyk and D’Esposito, 2011, Krawczyk et al., 2007, Locke and Braver, 2008, Yamamoto et al., 2013). In a rewarded AS task, behavioral performance was greater for reward than for non-reward trials, and rewards activated oculomotor circuitry supporting inhibitory control (Geier et al., 2010). Alternatively, others have found that when reward is contingent on suppressing an small immediate reward in favor of a larger delayed reward, regions supporting inhibitory control show relatively decreased activation (O’Connor et al., 2012). The authors suggest that successful inhibitory control over an immediate reward requires attentional disengagement. This would be similar to behavioral studies that have found success in delay of gratification to be facilitated by strategies that involve diverting attention from the immediate reward by engaging in other activities, such as making up unrelated games (Mischel et al., 1989).
To examine the developmental effects of potential rewards and losses on cognitive control, we performed an incentivized AS fMRI study using an accelerated longitudinal design. The study sample consisted of individuals ranging from 10- to 20-years of age, with each being sampled two or three times at approximately 15-month intervals. We selected 22 regions typically associated with reward processing and inhibitory control and thought to underlie incentive and cognitive processing, including those that have been found to change through development (e.g. striatum, orbitofrontal cortex, ventromedial prefrontal cortex). Based on past results (Ernst et al., 2005, Galvan et al., 2006, Van Leijenhorst et al., 2010) including our own (Geier et al., 2010, Padmanabhan et al., 2011), we make the following hypotheses. Activation in reward and cognitive control regions will show distinct age related effects across different incentives. During incentive trials, activity in ventral striatum will peak during adolescence while it will not change in neutral trials. Performance will improve with age, and with incentives, especially in younger subjects. As a second aim, we also sought to characterize the shape (linear vs. curvilinear) of developmental trajectories afforded by a longitudinal design.
2. Methods
2.1. Participants
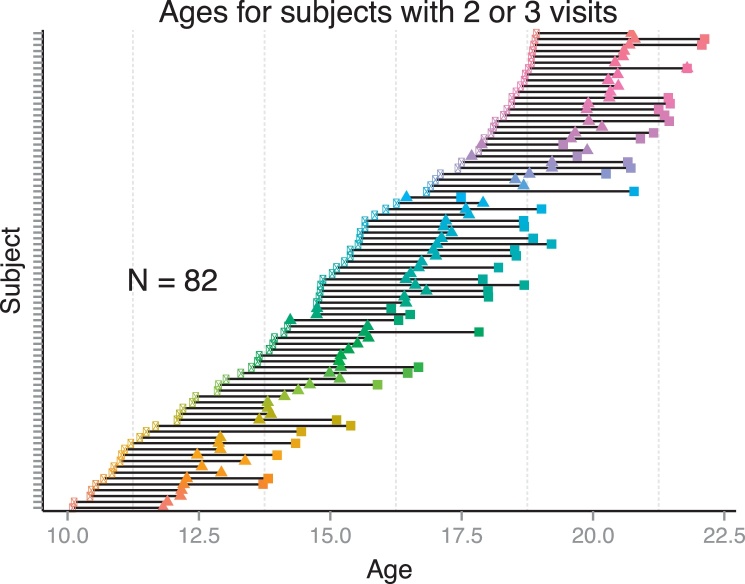
The data for these analyses include 187 initial participants ranging in age from 10- to 20-years. Data was collected as part of an ongoing study and participants were enrolled from Pittsburgh and surrounding areas for behavioral testing and neuroimaging approximately every 15 months for two-and-a-half years. After accounting for motion, whole-brain coverage, behavioral measures, number of trials, and number of visits, the resulting data set included eighty-two subjects (41 females; Fig. 1) providing data across two (N = 49) or three (N = 33) visits. Participants were compensated $75, plus up to an additional $25 based on accumulation of points. Immediately prior to scanning, subjects were asked to rate how ‘valuable’ (7-point Likert scale) they considered their chosen reward. In addition, each participant was asked to write down at least one item they might purchase with this compensation as a means to increase the salience of the reward. Subjects were instructed that they could win (rewarded trials) or lose (potential loss trials) points on each trial depending on their performance and that these points would be tallied at the end of the session. Subjects were remunerated based on the proportion of points earned out of a total of 280 using the following scale: 0–70 points (US $10), 71–140 (US $15), 141–210 (US $20), 211–280 (US $25.00 or the chosen gift card). This point-based approach allowed a separation between trial outcomes and dollar amounts, which was intended better adjust for potential differences in the subjective value of dollar amounts across age. IRB approved consent and assent forms were signed and collected from all participants and from the parents of minors.
Fig. 1.
Distribution of ages for subjects included in the current data set.
2.2. Design
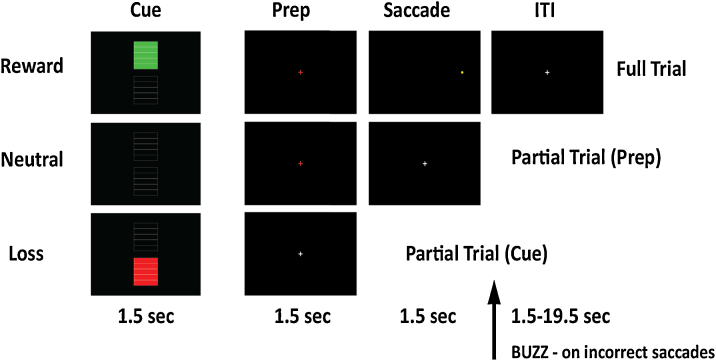
The design for this incentivized antisaccade task was based on a similar task used by Geier and Luna (2012). Subjects were informed that they would see a cue indicating whether correct performance would result in a gain of points (Reward trials), incorrect performance would incur a loss of points (Loss trials), or neither correct nor incorrect performance would affect accrual of points (neutral trials; Fig. 2). Reward and Loss trials were worth plus or minus 5 points, respectively, which was indicated by the number of green or red bars appearing in the Cue display. Following each 1.5 s cue was a 1.5 s preparatory epoch, followed by a 1.5 s saccade event. The display of the saccade event contained a small yellow dot at one of six pseudorandomly selected peripheral locations; subjects were required to saccade away from the dot upon presentation. After the saccade event, correct responses were followed by a cash register sound, while incorrect responses were followed by a buzzer sound at the beginning of the intertrial interval. Intertrial intervals varied from 1.5 to 19.5 s following an exponential distribution. A total of 56 trials for each Reward, Neutral, and Loss condition were presented across 4 runs. An additional 72 partial trials with either a cue alone or cue and preparatory epoch without a saccade event were also presented to estimate better the hemodynamic response to each event type in other analyses (Ollinger et al., 2001a, Ollinger et al., 2001b). Here, we collapsed across Cue, Delay, and Response epochs to gain more power in identifying our effects of interest.
Fig. 2.
Experimental design Reward, Neutral, and Loss Cues were displayed for 1.5 s and followed by either a Prep and Saccade stimulus each lasting 1.5 s (Full Trial), a 1.5 s Prep stimulus (Partial Trial), or an intertrial interval (ITI; Partial Trial).
2.3. Data acquisition
Eye-tracking data in the MR scanner were collected using a long-range optics eye-tracking system from Applied Science Laboratories (Model 504LRO; Bedford, MA). Eye-position was obtained via pupil-corneal reflection observed in the reflection of a head coil-mounted mirror with 0.5° of visual angle. Video monitoring was also used to ensure compliance. A 9-point calibration was performed prior to the experimental session and between runs when necessary. Stimuli were presented using E-prime software (Psychology Software Tools, Inc., Pittsburgh, PA) and projected onto a flat screen behind the scanner, visible to the subject through the coil-mounted mirror. Eye data were scored off-line using ILAB (Gitelman, 2002) and MATLAB software (MathWorks, Inc.).
Correct responses in the antisaccade task were defined as those in which the first eye movement during the saccade epoch with velocity greater than or equal to 30°/s (Gitelman, 2002) was made toward the mirror location of the peripheral cue and extended beyond a 2.5°/visual angle from central fixation. Incorrect responses occurred when the first saccade during the saccade epoch was directed toward the peripheral stimulus and exceeded the 2.5°/visual angle central fixation zone but were subsequently directed to the correct location, indicating that the instructions were being followed. Trials in which no eye movements were generated, or in which the tracker lost fixation, were excluded from analyses. The overall proportion of trials excluded was 10% (SD = 9%). However, this proportion was greater for participants in the 10- to 14-year age range (M = 13.9%, SD = 12%) than those in the older 14- to 18-year (M = 8%, SD = 7%) and 18- to 23-year (M = 9.3%, SD = 7.7%) age ranges.
Imaging data were collected using a 3.0-T Siemens Allegra scanner at the Brain Imaging Research Center, University of Pittsburgh, Pittsburgh, PA. High-resolution anatomical data were collected using a magnetization prepared rapid acquisition gradient-echo (MP-RAGE) pulse sequence with 192 slices (1-mm slice thickness) in the sagittal plane. Functional data were collected using a gradient-echo echo-planar imaging (EPI) sequence sensitive to BOLD contrast (T2*) with the following parameters: TR = 1.5 s, TE = 29 ms, flip angle = 70°, and a 64 × 64 matrix with a field of view of 20 × 20 cm. Twenty-nine slices with a height of 4 mm were collected, for an anisotropic voxel size of 3.125 mm × 3.125 mm × 4 mm.
Preprocessing of the functional data followed standard techniques: despiking using AFNI's 3dDespike, slice timing correction, motion correction using mcflirt (Jenkinson et al., 2002), brain extraction, registration of functional to non-linearly registered anatomical data, spatial smoothing using SUSAN (Smith and Brady, 1997) with FWHM of 5 mm, high pass filtering of 0.008 Hz, and normalization.
2.4. Analyses
Analyses were refined by only including data meeting the following inclusion criteria: runs with fewer than 15% volumes having greater than 3 mm motion between volumes; visits sharing 90% of whole-brain coverage with all other subjects; subject visits with greater than 50% accuracy in antisaccade performance per condition (excluded 1 visit); visits with 20 or more correct antisaccade trials per condition (excluded 5 visits); and participants with 2 or 3 yearly visits.
Fixed-effects analyses were run using FSL to generate parameter estimates (PE) at the visit-level for each subject for Reward (Rew), Neutral (Neu), and Loss (Loss) conditions, as well as Rew > Neu and Loss > Neu contrasts, using the jittered intertrial interval as baseline. Nuisance regressors included the time-courses of two voxels from the right and left lateral ventricles to account for physiological noise, the six motion regressors used in motion correction, and the convolved hemodynamic response from trials that resulted in an incorrect response and trials that could not be rated due to missing eye-tracking data or those without a saccade (i.e. partial trials). This is to say, only correct trials were used in the analyses, with an event duration of 4.5 s to model the Cue, Preparatory, and Saccade epochs. We then used mixed-effects regression on the PEs obtained from the visit-level regression analysis using the nlme package for R on a voxel-by-voxel basis. Spurious effects of outliers were controlled for by resampling individual PEs using the R function boot with 500 iterations. Bootstrapped parameter estimates and standard errors were used to calculate t values, p values, and z values, in order to generate statistical brain maps.
Our analyses focused on a set of a priori ROIs (Table 1) known to be involved in antisaccade performance (i.e., frontal and supplementary eye fields, pre SMA, caudate, and putamen) (Geier et al., 2010, Luna et al., 2001, Velanova et al., 2008, Velanova et al., 2009) and in reward and loss processing (i.e. amygdala, orbitofrontal cortex, ventral medial prefrontal cortex, and striatum). Antisaccade-related ROIs were drawn with a 10 mm or 7 mm (pre-SMA, SEF) sphere surrounding the peak voxel of the associated cluster identified by neurosynth (www.Neurosynth.org) using the name of each ROI as a keyword. One exception to this was the ROI for posterior parietal cortex, which used the term “preparatory”, as this term provided a closer fit to activations from prior antisaccade studies. The resulting z-statistic images for these ROIs were then corrected for multiple comparisons using false discovery rate correction with a q-value of 0.05.
Table 1.
ROIs.
| Region | Basis | x | y | z | Radius (mm) | n_vox |
|---|---|---|---|---|---|---|
| Amy_R | H-O Anat Atlas | 23 | −3 | −18 | – | 434 |
| Amy_L | H-O Anat Atlas | −23 | −5 | −18 | – | 390 |
| caudate_R | H-O Anat Atlas | 13 | 10 | 11 | – | 675 |
| caudate_L | H-O Anat Atlas | −13 | 9 | 10 | – | 632 |
| NAcc_R | H-O Anat Atlas | 9 | 12 | −7 | – | 110 |
| NAcc_L | H-O Anat Atlas | −10 | 12 | −7 | – | 119 |
| OFC_R | H-O Anat Atlas | 29 | 24 | −16 | – | 1444 |
| OFC_L | H-O Anat Atlas | −30 | 24 | −17 | – | 1650 |
| putamen_R | H-O Anat Atlas | 26 | 2 | 0 | – | 1011 |
| putamen_L | H-O Anat Atlas | −25 | 0 | 0 | – | 979 |
| vmPFC | H-O Anat Atlas | 0 | 44 | −18 | – | 1011 |
| dACC_sphere | Coordinate | 0 | 22 | 30 | 10 | 515 |
| dlPFC_L_sphere | Coordinate | −42 | 38 | 28 | 10 | 515 |
| dlPFC_R_sphere | Coordinate | 40 | 40 | 28 | 10 | 515 |
| FEF_L_sphere | Coordinate | −26 | −6 | 52 | 10 | 515 |
| FEF_R_sphere | Coordinate | 26 | −6 | 52 | 10 | 515 |
| PPC_L_sphere | Coordinate | −28 | −64 | 48 | 10 | 515 |
| PPC_R_sphere | Coordinate | 30 | −62 | 46 | 10 | 515 |
| preSMA_sphere | Coordinate | 0 | 6 | 58 | 7 | 179 |
| SEF_sphere | Coordinate | 0 | 0 | 68 | 7 | 179 |
| vlPFC_L_sphere | Coordinate | −48 | 36 | −4 | 10 | 515 |
| vlPFC_R_sphere | Coordinate | 48 | 36 | −6 | 10 | 515 |
Note: H-O Anat Atlas – Harvard-Oxford anatomical atlas; x, y, z coordinates listed in MNI space; coordinates for H-O ROIs are centroid.
Because a primary question of interest to many developmentalists is whether age-related change is linear or quadratic, and because many general patterns can be approximated through the use of polynomials, we tested linear and polynomial models of development against our primary model of interest containing an Age X Behavior interaction for each ROI and incentive condition or contrast:
-
•
Linear: PEij = Intercept + β1(Age) + u1 + eij
-
•
Quadratic: PEij = Intercept + β1(Age) + β2(Age2) + u1 + eij
-
•
Age X Behavior: PEij = Intercept + β1(Age) + β2(Accuracy) + β3(Age × Accuracy) + u1 + eij
where subscript ij represents individual i at visit j. We did not explore a fourth possible model containing both Age and Age2 interacting with Accuracy, as our initial analyses demonstrated the superior fit of the model that did not include an Age2 term, and because performance in the antisaccade task has primarily been associated with age in a linear, or curvilinear (i.e. inverse) function with an extended age-range (Luna et al., 2004), rather than U- or inverted U-shaped trajectories. Age in years was mean-centered for the linear and quadratic models. For the Age X Behavior model, Reward condition accuracy was used as the behavioral measure for Reward trials, Loss condition accuracy was used for Loss trials, and accuracy in neutral trials was used as behavior for neutral trials. Age was represented in years for the Age X Behavior model to allow a sensible interpretation of the Age X Behavior interaction. Here, u1 represents a random intercept effect nested within subjects, while eij represents the normally distributed residual error.
To compare model fits, we collected the mean Akaike information criterion (AIC) across voxels from every ROI for each model and from an unconditional model that included only the intercept. AIC is a goodness of fit measure that seeks to balance model fit and complexity by penalizing the addition of parameters: a lower AIC value indicates a better fit to the data relative to an alternative model. A rule of thumb for comparing AIC values is that a decrease of 2 or less is weak evidence, 4–7 moderate, and 10+ strong for preferring one model over another (Burnham and Anderson, 2004). Generally, Model X is a better fit to the data than Model Y if the AIC for Model X decreases AIC by more than 1 + k, where k is number of additional parameters.
3. Results
3.1. Value ratings
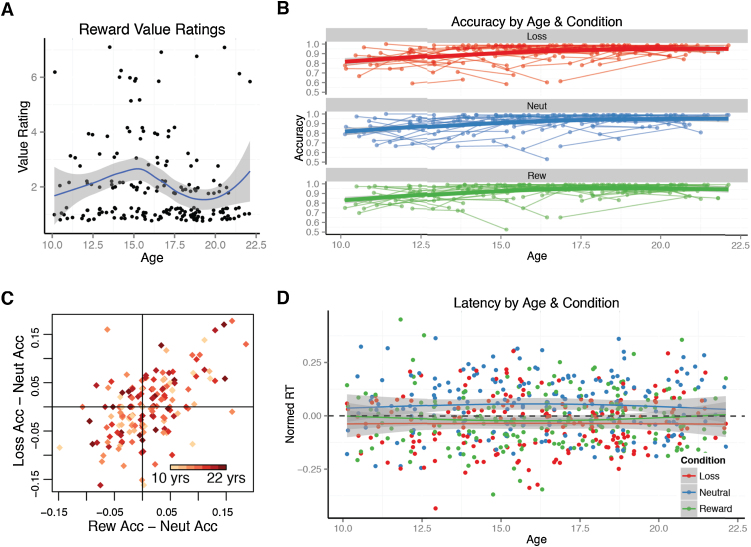
The type of reward selected – visa debit cards (46%), cash (39%), iTunes (4%) and Barnes & Noble (4%) gift cards – did not differ by age. All but four participants included in these analyses (ages 11.5, 13.9, 21.4, and 21.8 years) rated the subjective value of their chosen reward using a reversed Likert scale, 1 indicating the most value and 7 indicating the least value. As can be seen in Fig. 3A, although adolescents may appear to devalue the performance-based incentive (higher scores indicate less reward value), general linear models including Age or Age and Age2 as predictors of value ratings showed no associations with age, suggesting that, if anything, adolescents show greater variability in their ratings than younger or older participants.
Fig. 3.
Reward value and antisaccade performance. (A) The self-reported value (1 = high value, 7 = low value) of gift cards appeared to be less for adolescents and a few older participants. Loss curve with standard error. (B) Accuracy improved with age most strongly between 10- and 14-years of age, and did not differ by condition. (C) The effect of positive and negative incentives were correlated with another, either improving or worsening performance. (D) Latencies were unaffected by Reward, Loss, or Neutral condition. Rew – Reward, Neut – Neutral, Acc – accuracy.
3.2. Behavior
Correct response rate (‘accuracy’) in each condition was good overall and improved with age (Fig. 3B). Linear mixed-model regression with Age and Condition as variables followed by MCMC sampling showed that Age was a significant predictor (p < 0.0001). Performance did not differ by incentive condition although there was a trend for greater accuracy in the Reward compared to Neutral condition (p < 0.1).
To examine the relative effect of incentive on accuracy at the individual level for each visit, we ran a correlation on difference scores created by subtracting accuracy in the Neutral condition from accuracy in the Reward and Loss conditions. This correlation showed a strong relationship between incentive conditions, r = 0.545, t(195) = 9.083, p < 0.001, such that for some individuals, reward and loss cues tended to improve performance, whereas for others, incentives tended to degrade performance (Fig. 3C). This effect did not interact with age (p > 0.19). To examine the consistency with which incentives affected performance across individuals, we collected intraclass correlation coefficients (ICC) on these difference scores. The results from these tests show that Reward–Neutral difference scores were significantly correlated within individuals across visits, ICC = 0.226, p < 0.05, 95% CI [0.056, 0.395], but that Loss–Neutral difference scores were not, ICC = 0.017, 95% CI [-0.143, 0.192].
The analysis of saccade latencies for correct responses used a linear mixed-model with age and incentive condition as factors with correct trials only. Initial analysis of RTs found a large effect of age (p < 10−9), and because RT is known to decrease with age, we normalized (mean divided by standard deviation) each subject's RT with respect to their RTs on correct trials at each visit. Although the incentive conditions generally showed a decrease in RT compared to the neutral condition (Fig. 3D), these changes in RT were not significant for the Loss (p = 0.110) or Reward (p = 0.091) conditions. Interactions between incentive conditions and age were not significant.
Finally, we examined whether reward value ratings predicted accuracy in Reward, Neutral, and Loss conditions. They did not. Correlation r values were between −0.05 and 0.05 for all conditions, and p values > 0.53.
3.3. fMRI
Model comparisons showed that the addition of Age2 to the linear model did not improve AIC, on average increasing AIC by 2.53, 3.23, and 1.40 points for Reward, Neutral, and Loss trials, respectively, across ROIs. Rather, an increase in AIC is clearly demonstrative of poorer fit. In contrast, adding Behavior and the Age X Behavior interaction to the linear model improved (decreased) AIC by 7.10, 8.27, and 7.12 points for Reward, Neutral, and Loss trials, respectively, demonstrating a superior model fit to the data even after penalizing for additional parameters. Thus, all reported results are obtained from an Age X Behavior model unless otherwise specified.
3.4. Mean activation (intercepts)
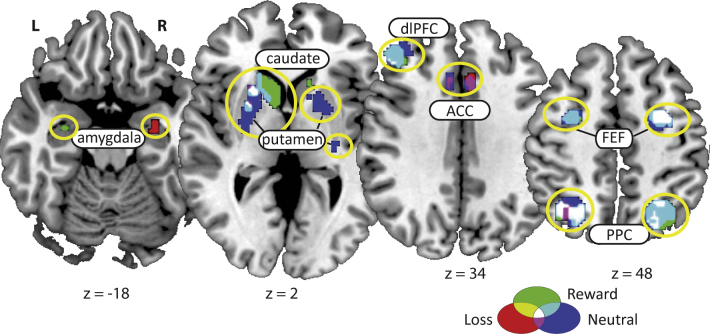
The main effects during Reward, Loss, and Neutral trials are illustrated in Fig. 4 and Table 2.
Fig. 4.
Main effects during Reward, Loss, and neutral trials activation was found in several occulomotor regions involved in the antisaccade task (FEF, putamen, PPC, dlPFC). Activation was also found in ACC during Neutral and Loss trials, caudate during Neutral and Reward trials, and in amygdala during all three trial types. ACC – anterior cingulate cortex, dlPFC – dorsolateral prefrontal cortex, PPC – posterior parietal cortex, FEF – frontal eye fields.
Table 2.
ROIs by condition with significant main effects (intercepts).
| Condition | ROI | Hemi | Sign | N vox | z-value | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Reward | Amygdala | R | + | 27 | 3.62 | 16 | −4 | −14 |
| Reward | Caudate | L | + | 234 | 3.87 | −8 | 4 | 10 |
| Reward | Caudate | R | + | 524 | 5.02 | 14 | 6 | 12 |
| Reward | dlPFC | R | + | 304 | 3.81 | 46 | 40 | 32 |
| Reward | FEF | L | + | 200 | 4.86 | −30 | 2 | 48 |
| Reward | FEF | R | + | 88 | 3.46 | 32 | 0 | 48 |
| Reward | nAcc | R | + | 31 | 3.16 | 6 | 14 | −2 |
| Reward | PPC | L | + | 459 | 4.1 | −32 | −60 | 54 |
| Reward | PPC | R | + | 395 | 4.86 | 26 | −58 | 38 |
| Reward | preSMA | – | + | 59 | 3.63 | −4 | 10 | 54 |
| Reward | Putamen | R | + | 105 | 4.11 | 22 | 20 | −6 |
| Neutral | Caudate | L | + | 108 | 4.39 | −14 | 4 | 12 |
| Neutral | Caudate | R | + | 65 | 4.16 | 18 | 20 | −2 |
| Neutral | dACC | – | + | 120 | 3.84 | 8 | 22 | 36 |
| Neutral | dlPFC | R | + | 341 | 4.99 | 40 | 34 | 22 |
| Neutral | FEF | L | + | 207 | 4.63 | −28 | 2 | 52 |
| Neutral | FEF | R | + | 95 | 4.12 | 30 | 0 | 48 |
| Neutral | PPC | L | + | 381 | 4.23 | −20 | −66 | 42 |
| Neutral | PPC | R | + | 448 | 5.63 | 26 | −60 | 40 |
| Neutral | Putamen | L | + | 262 | 4.5 | −22 | 16 | −8 |
| Neutral | Putamen | R | + | 444 | 5.83 | 22 | 20 | −4 |
| Neutral | vmPFC | − | 268 | 4.36 | 0 | 50 | −22 | |
| Loss | Amygdala | L | + | 57 | 3.8 | −30 | −6 | −22 |
| Loss | dACC | – | + | 155 | 4.43 | −8 | 22 | 34 |
| Loss | dlPFC | R | + | 142 | 3.34 | 36 | 34 | 24 |
| Loss | FEF | L | + | 115 | 5.24 | −30 | 2 | 48 |
| Loss | PPC | L | + | 186 | 3.81 | −24 | −70 | 46 |
| Loss | PPC | R | + | 376 | 4.41 | 30 | −54 | 42 |
| Loss | preSMA | – | + | 23 | 3.33 | −4 | 10 | 54 |
| Loss | Putamen | R | + | 88 | 4.48 | 22 | 20 | −4 |
Note: dlPFC – dorsolateral prefrontal cortex, nAcc – nucleus accumbens, dACC – dorsal anterior cingulate cortex, FEF – frontal eye fields, PPC – posterior parietal cortex, vmPFC – ventral medial prefrontal cortex, preSMA – pre-supplementary motor area.
3.5. Age effects
Linear effects of Age were found in four ROIs: PPC, vlPFC, FEF, and amygdala (Table 3). Activation in ventral basolateral amygdala was negatively correlated with age during Loss trials. Activation in vlPFC was negatively correlated with age during neutral trials, while FEF activation was positively correlated with age during Loss Trials. In addition, the difference in FEF activation between Loss and neutral trials also correlated positively with age.
Table 3.
ROIs showing effects of age, behavior, and Age X Behavior interactions.
| Condition | ROI | Hemi | var | Sign | n vox | z-val | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| Neutral | Caudate | L | beh | + | 108 | 3.69 | -8 | 0 | 12 |
| Neutral | Caudate | R | ageXbeh | − | 316 | 4.38 | 12 | 0 | 12 |
| Neutral | Caudate | R | beh | + | 319 | 4.35 | 12 | 0 | 12 |
| Neutral | dlPFC | R | age | − | 22 | 4.25 | 40 | 34 | 22 |
| Neutral | nAcc | R | ageXbeh | − | 18 | 3.34 | 6 | 14 | −2 |
| Neutral | nAcc | R | beh | + | 23 | 3.49 | 6 | 14 | −2 |
| Neutral | Putamen | L | ageXbeh | − | 576 | 4.12 | −30 | −18 | 2 |
| Neutral | Putamen | L | beh | + | 622 | 4.17 | −28 | 8 | −6 |
| Neutral | Putamen | R | ageXbeh | − | 280 | 3.87 | 24 | 6 | 12 |
| Neutral | Putamen | R | beh | + | 358 | 4.07 | 24 | 6 | 12 |
| Neutral | vlPFC | L | age | − | 20 | 3.58 | −56 | 32 | −2 |
| Loss | Amy | L | age | − | 45 | 3.69 | −28 | −2 | −28 |
| Loss | FEF | L | age | + | 271 | 4.12 | −22 | −12 | 50 |
| Loss | FEF | R | age | + | 43 | 3.41 | 30 | −10 | 50 |
| Loss | vlPFC | L | ageXbeh | − | 42 | 3.66 | −52 | 42 | 2 |
| Loss | vlPFC | L | beh | + | 35 | 3.69 | −52 | 42 | 4 |
| Loss > Neutral | FEF | L | age | + | 160 | 3.7 | −24 | −8 | 46 |
Note: x, y, z coordinates in MNI space.
The comparison of AIC values for each model (mean AIC of ROI) found that the quadratic model was a better fit to the data for PPC in the Loss condition (Table 4). In both right and left PPC, U-shaped patterns of activation were found, with a trough during adolescence.
Table 4.
ROIs with significant Age2 terms for quadratic model.
| Contrast | ROI | Hemi | Shape | n vox | z-value | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Loss | PPC | L | ∪ | 304 | 3.29 | −24 | −64 | 42 |
| Loss | PPC | R | ∪ | 291 | 4.25 | 32 | −64 | 40 |
Note: x, y, z coordinates in MNI space.
3.6. Age and behavior interaction
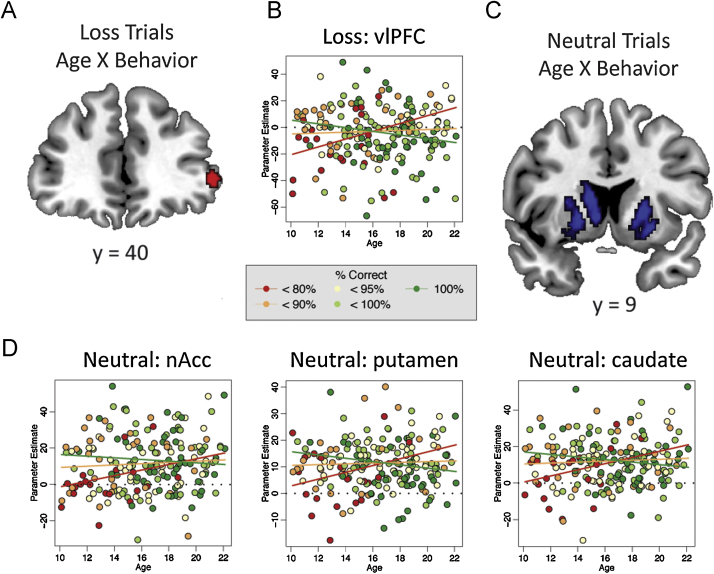
Significant effects of behavior were found in NAcc, caudate, and putamen, for neutral trials, and in vlPFC for Loss trials (Table 3). Positive betas for behavior show that as activation in these regions increased, there was a general improvement in AS performance in their respective condition. In all four of these same ROIs, interactions between age and behavior were also found. These results demonstrated that for younger participants, increased activation in NAcc, caudate, putamen, and vlPFC was associated with improved AS performance, but among older participants, increased activation in these regions was associated with worse AS performance (Fig. 5). The point of inflection (i.e., the age at which activation changed from beneficial to deleterious) was 16.9 years for caudate (Neutral), 19.92 years for NAcc (Neutral), 17.11 years for left putamen (Neutral), 16.8 years for right putamen (Neutral), and 16.02 years for vlPFC (Loss).
Fig. 5.
Age X Behavior interactions in Loss and neutral trials. During Loss trials, an Age X Behavior interaction was found in ventral lateral PFC, while during neutral trials, Age X Behavior interactions were found in nucleus accumbens, putamen, and caudate. Interactions show that for younger participants (left side of scatterplots), greater activation was associated with greater performance (green line higher than red line), while for older participants (right side of scatterplots), greater activation was associated with worse performance (green line lower than red line). Individual parameter estimates are color coded by percent correct. vlPFC – ventrolateral prefrontal cortex, nAcc – nucleus accumbens. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This longitudinal study investigated maturation of the neural substrates supporting inhibitory control under incentivized conditions. Overall, results supported some of our hypotheses but not others. In the present design, we did not find evidence for the hypothesized peak in striatal activity during incentives. While similar circuitries were engaged across incentives, associations with age showed predominantly linear associations that engaged different regions for each incentive condition. Activation increased with age in FEF during loss, and in frontoparietal regions during neutral trials. Additionally, during loss, activation in basolateral amygdala decreased and showed a trough during adolescence in PPC. These results suggest that developmental changes in incentive processing is not peaking in adolescence but may continue to show increased or decreased engagement through young adulthood depending on the region involved. We also hypothesized that incentives would improve performance in younger subjects. Results indicated that in childhood, increased engagement of frontostriatal regions was associated with better performance, but through adolescence this relationship inverted and greater activation was associated with a decline in performance. These results suggest that optimal mature performance is supported by concise activation of frontostriatal systems, and evidence of increased engagement in adulthood may reflect continued immaturities.
4.1. Behavioral results
Consistent with prior work, we found that performance in the AS task improved with age (Geier and Luna, 2012, Luna et al., 2004, Velanova et al., 2008). Results showed that incentives affect performance with variability in those who showed improvement and those who showed hampering of performance. This may reflect how incentives contribute to heightened performance in some, while for others it can result in “choking under pressure” (Mobbs et al., 2009). The ICCs for behavioral data showed that performance on Reward trials was more stable within individuals than performance on Loss trials, suggesting that antisaccade performance may involve circuitry that is more susceptible to change over development or to day-to-day fluctuations, and this circuitry is more heavily engaged during loss than during reward conditions.
4.2. Age-related activation
After separating activation that was related to behavior, we found several regions where activation was correlated with age, including cortical control regions and subcortical regions supporting loss and reward.
Age related changes were evident across cortical control regions including VLPFC, PPC, and FEF. Activation in VLPFC, a key region supporting cognitive control (Ridderinkhof et al., 2004), was found to benefit younger subjects and hamper performance in older subjects. VLPFC activation has been found to normatively decrease in magnitude with age during the AS task (Ordaz et al., 2013). Greater activation during adulthood may reflect a pattern of processing closer to immaturity that could lead to poorer performance. In contrast to this linear effect in VLPFC, PPC showed a U-shaped curve with a nadir during adolescence. PPC in childhood may support attentional modulation (Asplund et al., 2010) as has been found to be predominant at this age during AS performance (Hwang et al., 2010). During adulthood, PPC may provide more direct support to antisaccade performance in contrast to prosaccade performance as has been found elsewhere (Brown et al., 2007). FEF showed a positive correlation with Age, but during Loss trials only. FEF is one of the core regions supporting correct AS performance (Everling et al., 1998). Loss trials may have been more difficult and adults may have supported correct performance by engaging this crucial region. Taken together, these results suggest that with development there is specialization in recruiting regions specific to AS performance, rather than relying on circuitry supporting general processes of cognitive control.
Age was also negatively correlated with activity in the ventral aspect of the amygdala during Loss trials. The BL amygdala, with its innervation from sensory regions, as well as from cingulate, insula, and PFC, has been associated with the throughput of bottom-up processing in mediating consummatory conditioning, information updating, value-encoding, as well as participating in attentional function (Parkes and Balleine, 2013, Pessoa, 2010, Pickens et al., 2003, Seymour and Dolan, 2008). Loss trials may contain an emotional component above Neutral and Reward trials that is more effectively curtailed with maturation, and reflected in attenuated ventral amygdala activity. Thus, our findings may reflect a decrease in amygdala-mediated bottom-up processing through adolescence that would be consistent with the maturation of cognitive control and response inhibition during adolescence. Future work will be important in substantiating this initial finding.
Increased striatal activation was associated with better performance under non-incentivized neutral trials in younger participants, while for older participants greater striatal activation was predictive of worse overall AS performance. Similar to the developmental trajectory of findings in VLPFC, increased activity in NAcc may be a marker for earlier development supporting better performance, while its continued dependence in adulthood may hamper performance. The nature of striatal activation during neutral trials, which are absent of extrinsic incentives, may be related to the ability to generate intrinsic motivation to support performance. Reward circuitry can be activated in the absence of extrinsic incentives when simply making a choice (Leotti and Delgado, 2011) and when difficulty is greater (Schouppe et al., 2014). Thus, under non-incentivized conditions, activation of motivation circuitry in children may enhance activity of cognitive control circuitry, thus enhancing performance. On the other hand, in adults, a disengagement of reward-related circuitry supports better performance (Mobbs et al., 2009, O’Connor et al., 2012). Hence, while striatal activity may support cognitive control in childhood when the task is difficult, in adulthood striatal engagement may limit the efficacy of cognitive control circuitry.
It is interesting that an Age X Behavior interaction was not found in the striatum during Reward trials, when we would expect to find increased striatal activation. One possibility for the absence of this finding could be that striatal activation was robust across all levels of performance during reward trials (see Table 2) that it reached a ceiling effect undermining the ability to find interactions. Another possibility is that Reward and Loss conditions evoke activation in slightly distinct aspects of motivation circuitry. This possibility is not exclusive from the first in that multiple converging afferents on the striatum may activate it more strongly during one condition than another. On the other hand, afferents from regions differentially involved with reward and loss could also combine differentially, leading to the observation of an Age X Behavior interaction in one condition but not the other, as found here. Each of these suggestions is speculative, and more work is necessary to confirm their generalizability across different contexts.
5. Conclusion
Results indicated that while greater striatal activity supports cognitive control early in development, it was found to hamper performance in adulthood where engagement of specialized control regions may support optimal control. Results showed a decrease in ventral amygdala activity through development suggesting a transition in the mediating role of the amygdala in bottom-up processing during inhibitory control. Thus, adolescence may mark the transition in the balance between facilitative and obstructive striatal function and cognitively-driven amygdala function during inhibitory control, which may further interact with the relative balance between externally and internally motivating processes.
Conflicts of interest
The authors declare that they have no competing financial or personal conflicts of interest.
Acknowledgements
We would like to thank members of the Laboratory for Neuroscognitive Development at the University of Pittsburgh for their help in collecting data and discussions, and the anonymous reviewers for thier helpful and thoughtful comments.
Footnotes
Available online 19 September 2014
References
- Asplund C.L., Todd J.J., Snyder A.P., Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat. Neurosci. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson E.E., van Leijenhorst L., Galvan A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Dev. Cogn. Neurosci. 2012;3:72–83. doi: 10.1016/j.dcn.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G., Caggiano D., Bennett S., Hommer D. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS ONE. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.R., Vilis T., Everling S. Frontoparietal activation with preparation for antisaccades. J. Neurophysiol. 2007;98(3):1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Bunge S., Dudukovic N., Thomason M., Vaidya C.J., Gabrieli J. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham K.P., Anderson D.R. Multimodel inference understanding AIC and BIC in model selection. Sociol. Methods Res. 2004;33(2):261–304. [Google Scholar]
- Casey B., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E., Shulman E., Steinberg L., Claus E., Banich M., Graham S., Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Dev. Psychol. 2010;46(1):193–207. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Eaton D.K., Kann L., Kinchen S., Shanklin S., Flint K.H., Hawkins J., Wechsler H. Youth risk behavior surveillance – United States, 2011. MMWR Surveill Summ. 2012;61(4):1–162. [PubMed] [Google Scholar]
- Ernst M., Nelson E., Jazbec S., McClure E., Monk C., Leibenluft E., Pine D. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Everling S., Dorris M.C., Munoz D.P. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J. Neurophysiol. 1998;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Fischer B., Biscaldi M., Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Res. 1997;754(1):285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T., Parra C., Penn J., Voss H.U., Glover G., Casey B. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26(25):6885. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Luna B. Developmental effects of incentives on response inhibition. Child Dev. 2012;83(4):1262–1274. doi: 10.1111/j.1467-8624.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman D.R. ILAB: a program for postexperimental eye movement analysis. Behav. Res. Methods Instrum. Comput.: J. Psychon. Soc. Inc. 2002;34(4):605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Hallett P.E. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hwang K., Velanova K., Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J. Neurosci.: Off. J. Soc. Neurosci. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kahneman D., Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Krawczyk D.C., D’Esposito M. Modulation of working memory function by motivation through loss-aversion. Hum. Brain Mapp. 2011;34(4):762–774. doi: 10.1002/hbm.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk D.C., Gazzaley A., D’Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Lamm C., Benson B.E., Guyer A.E., Perez-Edgar K., Fox N.A., Pine D.S., Ernst M. Longitudinal study of striatal activation to reward and loss anticipation from mid-adolescence into late adolescence/early adulthood. Brain Cogn. 2014:1–10. doi: 10.1016/j.bandc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti L.A., Delgado M.R. The inherent reward of choice. Psychol. Sci. 2011;22(10):1310–1318. doi: 10.1177/0956797611417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin I., Xue G., Weller J.A., Reimann M., Lauriola M., Bechara A. A neuropsychological approach to understanding risk-taking for potential gains and losses. Front. Neurosci. 2012;6:1–11. doi: 10.3389/fnins.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke H., Braver T.S. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn. Affect. Behav. Neurosci. 2008;8(1):99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., Geier C. The adolescent sensation seeking period: development of reward processing and its effects on cognitive control. In: Reyna V.F., Zayas V., editors. The Neuroscience of Risky Decision Making. Bronfenbrenner Series on the Ecology of Human Development. American Psychological Association; Washington, DC: 2014. pp. 93–121. [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J., Sweeney J.A. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Mischel W., Shoda Y., Rodriguez M.L. Delay of gratification in children. Sci. New Ser. 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Hassabis D., Seymour B., Marchant J.L., Weiskopf N., Dolan R.J., Frith C.D. Choking on the money: reward-based performance decrements are associated with midbrain activity. Psychol. Sci. 2009;20(8):955–962. doi: 10.1111/j.1467-9280.2009.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz D.P., Broughton J.R., Goldring J.E., Armstrong I.T. Age-related performance of human subjects on saccadic eye movement tasks. Exp. Brain Res. 1998;121(4):391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- O’Connor D.A., Rossiter S., Yücel M., Lubman D.I., Hester R. Successful inhibitory control over an immediate reward is associated with attentional disengagement in visual processing areas. Neuroimage. 2012;62(3):1841–1847. doi: 10.1016/j.neuroimage.2012.05.040. [DOI] [PubMed] [Google Scholar]
- Ollinger J.M., Corbetta M., Shulman G.L. Separating processes within a trial in event-related functional MRI: II analysis. Neuroimage. 2001;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger J.M., Shulman G.L., Corbetta M. Separating processes within a trial in event-related functional MRI: I the method. Neuroimage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 2013;33(46):18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A., Geier C.F., Ordaz S.J., Teslovich T., Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev. Cogn. Neurosci. 2011;1(4):517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes S.L., Balleine B.W. Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J. Neurosci. 2013;33(20):8753–8763. doi: 10.1523/JNEUROSCI.5071-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Emotion and cognition and the amygdala: from what is it? To what's to be done? Neuropsychologia. 2010;48(12):3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens C.L., Saddoris M.P., Setlow B., Gallagher M., Holland P.C., Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J. Neurosci. 2003;23(35):11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., van den Wildenberg W.P.M., Segalowitz S.J., Carter C.S. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Schouppe N., Demanet J., Boehler C.N., Ridderinkhof K.R., Notebaert W. The role of the striatum in effort-based decision-making in the absence of reward. J. Neurosci. 2014;34(6):2148–2154. doi: 10.1523/JNEUROSCI.1214-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B., Dolan R.J. Emotion, decision making, and the amygdala. Neuron. 2008;58(5):662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Singer J.D., Willett J.B. Oxford University Press; New York: 2003. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. [Google Scholar]
- Smith S., Brady J. SUSAN – a new approach to low level image processing. Int. J. Comput. Vis. 1997;23(1):45–78. [Google Scholar]
- Spear L.P. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev. Cogn. Neurosci. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn. Sci. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Tom S., Fox C., Trepel C., Poldrack R. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. J. Risk Uncertain. 1992;5(4):297–323. [Google Scholar]
- Van Leijenhorst L., Gunther Moor B., Op de Macks Z., Rombouts S., Westenberg P.M., Crone E. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J. Neurosci. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller J.A., Levin I., Denburg N. Trajectory of risky decision making for potential gains and losses from ages 5 to 85. J. Behav. Decis. Mak. 2010;24:331–344. [Google Scholar]
- Yamamoto S., Kim H.F., Hikosaka O. Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. J. Neurosci. 2013;33(27):11227–11238. doi: 10.1523/JNEUROSCI.0318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]