Abstract
Rationale and Objectives
To compare the effectiveness of MAVRIC SL with conventional 2D-FSE MR techniques at 3T in imaging patients with a variety of metallic implants.
Materials and Methods
Twenty-one 3T MR studies were obtained in 19 patients with different types of metal implants. Paired MAVRIC SL and 2D-FSE sequences were reviewed by 2 radiologists, and compared for in-plane and through-plane metal artifact, visualization of the bone implant interface and surrounding soft tissues, blurring, and overall image quality using a 2-tailed Wilcoxon signed rank test. The area of artifact on paired images was measured and compared using a paired Wilcoxon signed rank test. Changes in patient management resulting from MAVRIC SL imaging were documented.
Results
Significantly less in-plane and through-plane artifact was seen with MAVRIC SL, with improved visualization of the bone-implant interface and surrounding soft tissues, and superior overall image quality (p = 0.0001). Increased blurring was seen with MAVRIC SL (p=0.0016). MAVRIC SL significantly decreased the image artifact compared to 2D-FSE (p=0.0001). Inclusion of MAVRIC SL to the imaging protocol determined the need for surgery or type of surgery in 5 patients, and ruled out the need for surgery in 13 patients. In 3 patients the area of interest was well seen on both MAVRIC SL and 2D-FSE images, so the addition of MAVRIC had no effect on patient management.
Conclusion
Imaging around metal implants with MAVRIC SL at 3T significantly improved image quality and decreased image artifact compared to conventional 2D-FSE imaging techniques, and directly impacted patient management.
Keywords: MAVRIC SL, magnetic resonance imaging, image quality, metallic susceptibility artifact
INTRODUCTION
Metallic orthopedic implants are now commonplace in modern joint and spine surgery. However, subsequent magnetic resonance (MR) imaging of symptomatic patients with orthopedic hardware is often limited by metallic susceptibility artifacts, resulting in signal loss, pile-up artifact and geometric distortion (1, 2, 3). Two MR imaging methods have recently been introduced that significantly reduce susceptibility artifact near metal implants: Slice Encoding for Metal Artifact Correction (SEMAC) (4, 5) and Multi-Acquisition Variable Resonance Image Combination (MAVRIC) (6, 7). Both techniques use multiple excitations to excite the overall volume being imaged, but SEMAC excites standard slices (limited spatial bands), whereas MAVRIC excites limited frequency bands. As the extent of susceptibility artifact is directly proportional to field strength, these metal suppression sequences are typically applied at field strengths no higher than 1.5 Tesla (1.5T) (4, 5, 6, 7, 8, 9). However, the increasing use of 3 tesla (3T) scanners makes it important to develop and evaluate metal suppression techniques at higher field strengths.
The SEMAC technique uses additional phase encoding in the slice direction to correct for through plane distortion, combined with view-angle tilting and a spin-echo acquisition to avoid in-plane distortion and T2* signal loss. The 3-dimensional (3D) spin-echo acquisition is used to resolve the profile of each excited slice in the region of interest. MAVRIC excites a series of limited spectral bandwidths, and uses the same 3D spin-echo readout to resolve distortion, exploiting the limited excited frequency range to avoid in-plane distortion. Overall, MAVRIC's spectral overlap strategy to smoothly combine separately encoded spectral bins tends to yield composite images that have higher signal-to-noise ratios (SNR) than images constructed with SEMAC's non-overlapping slices (10). Unfortunately, in cases where surface coils fail to provide adequate volume selectivity around large joints, such as the hip and shoulder, MAVRIC's lack of z-selectivity can result in significant through-plane aliasing.
A hybrid acquisition sequence has recently been developed that mitigates this type of aliasing by combining the slice-selectivity of SEMAC with the overlapped excitation and combination properties of MAVRIC (10) (Figure 1). The hybrid technique was initially known as volume-selective three-dimensional multi-spectral imaging (VS-3D-MSI), but has more recently been renamed Multi-Acquisition with Variable Resonance Image Combination SeLective (MAVRIC SL). MAVRIC SL has been shown to reduce metal artifact around small metal implants even at the higher field strength of 3T (10). However, few attempts have been made to image larger metal implants at 3T.
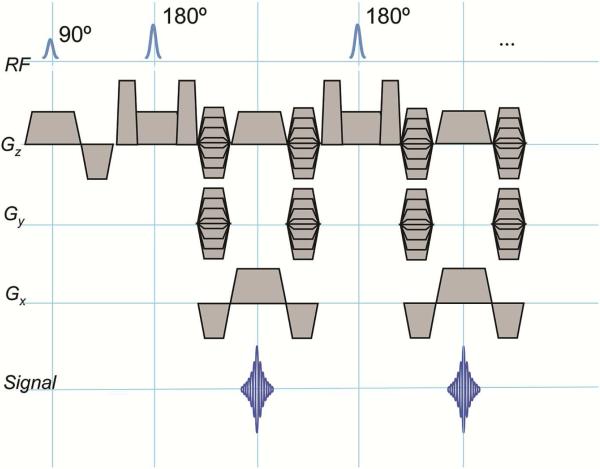
Figure 1.
Pulse sequence diagram for MAVRIC SL, where RF is radiofrequency, Gz is the slice-select gradient, Gy is the phase-encoding gradient and Gx is the frequency-encoding gradient. The MAVRIC-SL sequence is based on a standard spin-echo-train imaging sequence (such as 3D FSE, Cube, Space, VISTA). The excitation and refocusing RF pulses are Gaussian shaped, with overlap between adjacent excited regions, which are typically about 8 - 12mm thick. Typically a reduced refocusing flip angle (135 or 120 degrees) is used instead of 180 degrees, to reduce SAR (specific absorption rate). Phase encoding in both slice and phase directions is used, allowing the sequence to resolve through-slice distortions. A view-angle-tilting (VAT) gradient is applied on the slice axis during the readout gradient to correct in-plane distortions.
Symptomatic patients with metal implants requiring detailed radiologic evaluation are routinely evaluated with MR imaging at our institution using a 3T scanner. We describe our initial clinical experience with MAVRIC SL at 3T, and compare it with conventional imaging sequences.
MATERIALS AND METHODS
Subjects
This study is in compliance with the Health Insurance Portability and Accounting Act (HIPAA) and was approved by our Institutional Review Board. Between September 2010 and November 2011, and following informed consent, a total of 39 patients underwent MR imaging on a 3T scanner using MAVRIC SL. Twenty of these were excluded because conventional 2D-FSE images were not acquired in the same anatomic plane as the MAVRIC SL acquisitions, precluding the possibility of appropriate comparison studies. The remaining 19 patients who were included in the study had a total of 21 MRI studies with MAVRIC SL and 2D-FSE acquisitions acquired in the same anatomic plane. The study population comprised of 8 women (age range 22-74 years; mean age 47.5 years) and 11 men (age range 18-86 years; mean age 53.4 years). The implants included 7 total hip arthroplasties, 4 dynamic hip screws, 1 intramedullary femoral nail, 1 femoral neck pinning, 2 spinal fusions, 1 anterior cruciate ligament reconstruction, 1 plated proximal tibial osteotomy, 1 navicular screw, and 3 total knee arthroplasties. Of these, one patient had bilateral painful total knee replacements and another had bilateral symptomatic total hip arthroplasties.
Image Acquisition
All images were acquired using General Electric (GE) 3T systems (GE Healthcare, Waukesha, WI). Hips were imaged with an 8-channel cardiac array coil, knees were imaged with an 8-channel phased array transmit-receive knee coil, lumbar spines were imaged with an 8-channel phased array receive only coil, and the foot was imaged with an 8-channel receive-only coil. Conventional 2D-FSE and MAVRIC SL sequences were acquired in the coronal, axial or sagittal planes, using similar scan parameters, and a readout bandwidth of BW±125kHz. The acquisition matrix varied by body part.
2D-FSE sequences were obtained with proton density, T2, T2 or inversion recovery weighting using the following parameters. 2D FSE PD and T2 IDEAL were acquired with TR/TE 2400 – 4000ms/ 35 – 105ms, number of excitation (NEX) 1, slice thickness 3 – 5 mm, echo train length (ETL 8). 2D FSE STIR was acquired with TR/TE 4000 – 8800/32 – 50, TI 150 – 170 ms, NEX 0.5 – 1.5, slice thickness 3 – 5 mm. The scan times for all 2D FSE sequences varied between 3 – 5 minutes. MAVRIC SL sequences were obtained with either proton density (PD) or short tau inversion recovery (STIR) weighting. MAVRIC SL PD was acquired using the following parameters: TR/TE(ms) 3850 - 4000/ 32 - 38, NEX 0.5, slice thickness 3 – 5 mm, echo train length (ETL) 20, acquisition time 8 – 10 minutes. MAVRIC SL IR was acquired with a TR/TE 4000 – 8000/32 - 37, inversion time (TI) 150 – 170 ms, NEX 0.5, slice thickness 3 – 5 mm, acquisition time 12 minutes. MAVRIC SL images were acquired using 22 spectral bins with bandwidth 2.25kHz, separated by 1 kHz and were reconstructed using bin overlap deblurring followed by sum-of-squares combination of the spectral bins (10). The reconstruction was done using a prototype reconstruction on the scanner, which is now built-in on scanners that support MAVRIC SL.
Image Analysis
The MR images were retrospectively reviewed independently by two fellowship trained musculoskeletal radiologists (16 and 3 years of experience in musculoskeletal radiology respectively) and the paired 2D-FSE and MAVRIC SL images were compared in matching anatomical planes for both qualitative and quantitative parameters as follows.
Qualitative Analysis
The paired MAVRIC SL and 2D-FSE images were qualitatively and independently compared side-by-side by the reviewers for six imaging parameters, namely the extent of in-plane and through plane artifact, visualization of bone-implant interface, visualization of surrounding soft tissues, image blurring, and overall image quality. Images were graded on a 5 point scale from −2 to +2: −2 = image A much better than B, −1 = image A somewhat better than B, 0 = no difference between images, +1 = image B somewhat better than A. and +2 = image B much better than image A. Readers were blinded as to the type of acquisition that they were grading.
Quantitative Analysis
Comparison of the extent of metal artifact was difficult given the different shapes of metallic implant used in each patient, and the differing patterns of metal artifact generated on 2D-FSE and MAVRIC SL. Paired images were reviewed to identify the slice where the metal artifact was maximal, and a region of interest encompassing the implant and surrounding artifact was drawn using OsiriX by a single observer (Figure 2). Artifact was defined as areas of signal void, bright signal pile-up, or geometric distortion.
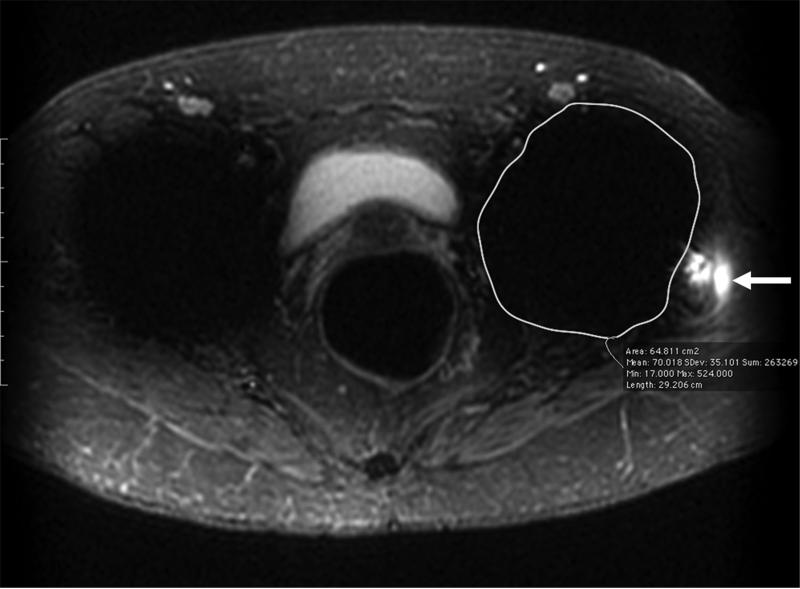
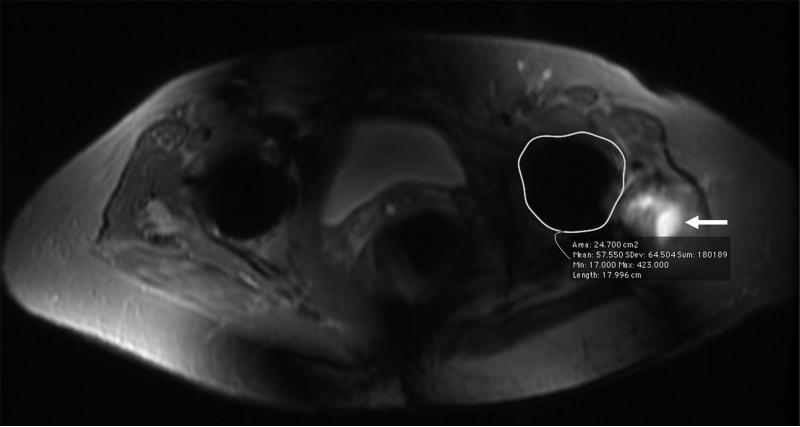
Figure 2.
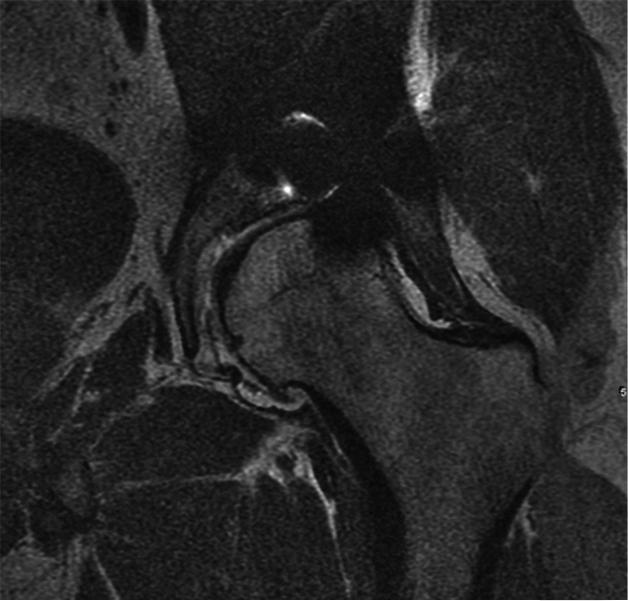
(A) Axial inversion recovery and (B) axial MAVRIC SL inversion recovery images of the left hip in a 74-year-old woman with bilateral total hip arthroplasties and a painful left hip due to disruption of the gluteus minimus and medius tendons. An ovoid area of signal void is seen around the acetabular component, and the maximum area of artifact is measured in the same place on both images to allow quantitative comparison On the axial inversion recovery image the area of artifact measured 64.8 cm2, compared to 24.2 cm2 on the axial MAVRIC SL inversion recovery image. A small amount of fluid is seen lateral to the left hip, corresponding to fluid in the greater trochanteric bursa (arrow).
Clinical History
Subsequent changes in patient management resulting from the addition of MAVRIC SL imaging were recorded for each patient as detailed in their electronic medical records.
Statistical Analysis
Statistical analysis was performed using Excel 12.0 (Microsoft). Statistical significance was set at p < 0.05 for all tests of significance.
The two-tailed Wilcoxon signed rank test was performed to detect a difference between the MAVRIC SL and 2D-FSE image quality grades given for each of the six image characteristics compared. The maximal area of artifact for each implant was also compared using a 2-tailed Wilcoxon signed rank test.
A weighted Cohen's kappa coefficient (κ) was calculated to measure inter-observer agreement in the qualitative analyses. The degree of agreement was categorized using previously defined guidelines as follows: a κ value of less than 0 indicated poor agreement; a κ value of 0—0.20, slight agreement; a κ value of 0.21—0.40, fair agreement; a κ value of 0.41—0.60, moderate agreement; a κ value of 0.61—0.80, substantial agreement; and a κ value of 0.81—1.0, near perfect agreement (11).
RESULTS
Qualitative Analysis
The image quality on MAVRIC SL and 2D FSE was compared and graded independently by the 2 readers, for each of the 6 parameters, and the mean of the 2 scores are shown in Table 1. Both in-plane (mean grade: −2) and through-plane (mean grade: −1.9) artifacts were significantly decreased with MAVRIC SL compared to 2D-FSE acquisitions (Figure 3) (p = 0.0001, κ = 1; p = 0.0001, κ = 1). Visualization of the bone-implant interface (mean grade: −1.95) as well as visualization of the surrounding soft tissues (mean grade: −1.86) was also significantly improved in MAVRIC SL compared to 2D-FSE (Figure 3) (p = 0.0001, κ = 1; p = 0.0001, κ = 1). Blurring (mean grade: +0.71) was significantly worse on the MAVRIC SL acquisitions (Figure 4) (p = 0.0016, κ = 1), (mean grade: −1.83). However, despite the increased blurring the overall image quality was still significantly better on MAVRIC SL (p = 0.0001, κ = 0.83).
Table 1.
Comparison of image quality between MAVRIC SL and 2D-FSE images
| Study Characteristics | Mean grades for Readers 1 and 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study # | Structure Imaged | Plane | MAVRIC SL image contrast | 2D-FSE image contrast | Extent of in-plane metal artifact | Extent of through-plane metal artifact | Visualization of bone-implant interface | Visualization of surrounding soft tissues | Image blurring | Overall image quality |
| 1 | Spine | Sagittal | PD | T2 | −2 | −2 | −1 | −2 | 1 | −1 |
| 2 | Spine | Axial | PD | T2 | −2 | −2 | −2 | −2 | 1 | −2 |
| 3 | Hip | Axial | PD | T2 IDEAL | −2 | −2 | −2 | −2 | 1 | −2 |
| 4 | Hip | Coronal | PD | T1 | −2 | −2 | −2 | −2 | 0 | −2 |
| 5 | Hip | Axial | IR | T1 | −2 | −2 | −2 | −2 | 1 | −1 |
| 6 | Hip | Axial | PD | T2 IDEAL | −2 | −2 | −2 | −2 | 0 | −2 |
| 7 | Hip | Axial | PD | T2 IDEAL | −2 | −2 | −2 | −2 | 0 | −1.5 |
| 8 | Hip | Coronal | PD | PD | −2 | −2 | −2 | −2 | 1 | −2 |
| 9 | Hip | Coronal | PD | PD | −2 | −2 | −2 | −2 | 0 | −2 |
| 10 | Hip | Coronal | PD | T1 | −2 | −2 | −2 | −2 | 0 | −2 |
| 11 | Hip | Coronal | IR | T1 | −2 | −1 | −2 | 0 | 1 | −1 |
| 12 | Hip | Axial | IR | T1 | −2 | −2 | −2 | −2 | 2 | −2 |
| 13 | Hip | Axial | PD | T1 | −2 | −2 | −2 | −2 | 2 | −2 |
| 14 | Hip | Coronal | PD | T1 | −2 | −2 | −2 | −2 | 0 | −2 |
| 15 | Hip | Coronal | PD | PD | −2 | −2 | −2 | −2 | 1 | −2 |
| 16 | Knee | Sagittal | PD | PD | −2 | −1 | −2 | −2 | 1 | −2 |
| 17 | Knee | Axial | IR | T1 | −2 | −2 | −2 | −2 | 0 | −2 |
| 18 | Knee | Coronal | PD | T1 | −2 | −2 | −2 | −2 | 1 | −2 |
| 19 | Knee | Sagittal | PD | PD | −2 | −2 | −2 | −1 | 0 | −2 |
| 20 | Knee | Coronal | IR | T1 | −2 | −2 | −2 | −2 | 1 | −2 |
| 21 | Foot | Sagittal | IR | T1 | −2 | −2 | −2 | −2 | 1 | −2 |
| Mean grade for all patients | −2 | 1.9 | −1.95 | −1.86 | 0.71 | −1.83 | ||||
MAVRIC SL = multi-acquisition variable resonance image combination slice selective, 2D-FSE = two-dimensional fast spin-echo, PD = Proton-density, IR = Inversion-recovery, IDEAL = Iterative decomposition of water and fat with echo asymmetry and least-squares estimation
Grading scale: −2 = MAVRIC SL much better than 2D-FSE, −1 = MAVRIC SL somewhat better than 2D-FSE, 0 = no difference between images, +1 = 2D-FSE somewhat better than MAVRIC SL. and +2 = 2D-FSE much better than MAVRIC SL
Figure 3.
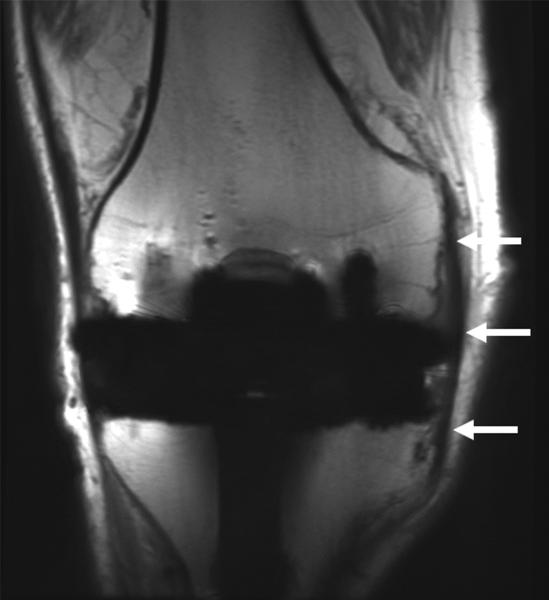
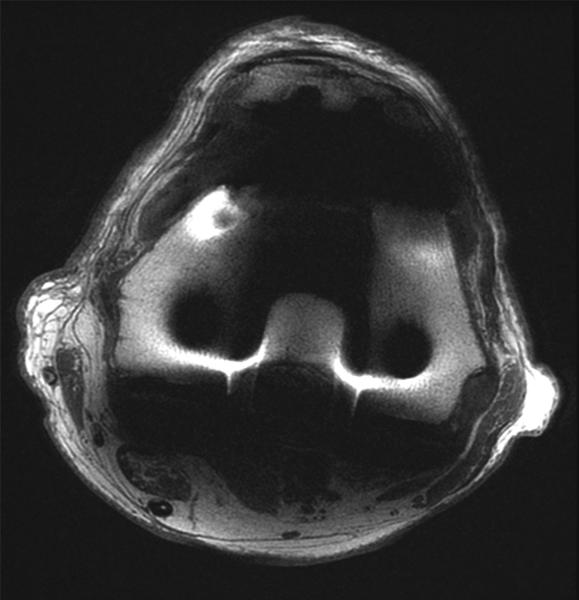
(A) Coronal 2D FSE and (B) MAVRIC SL proton density-weighted images of the right knee following a total knee arthroplasty demonstrate significantly less metal artifact on MAVRIC SL images. This allows visualization of bone immediately adjacent to the prosthesis, and demonstrates an intact medial collateral ligament (arrows). (C) Axial 2D FSE and (D) MAVRIC SL proton density-weighted images in the same patient shows decreased in-plane artifact in the patella (P), trochlear (T) and posterior femur (F) on MAVRIC SL, and there is an absence of through-plane artifact (*) in the central femur on MAVRIC SL. A small joint effusion is clearly seen on MAVRIC SL (arrow).
Figure 4.
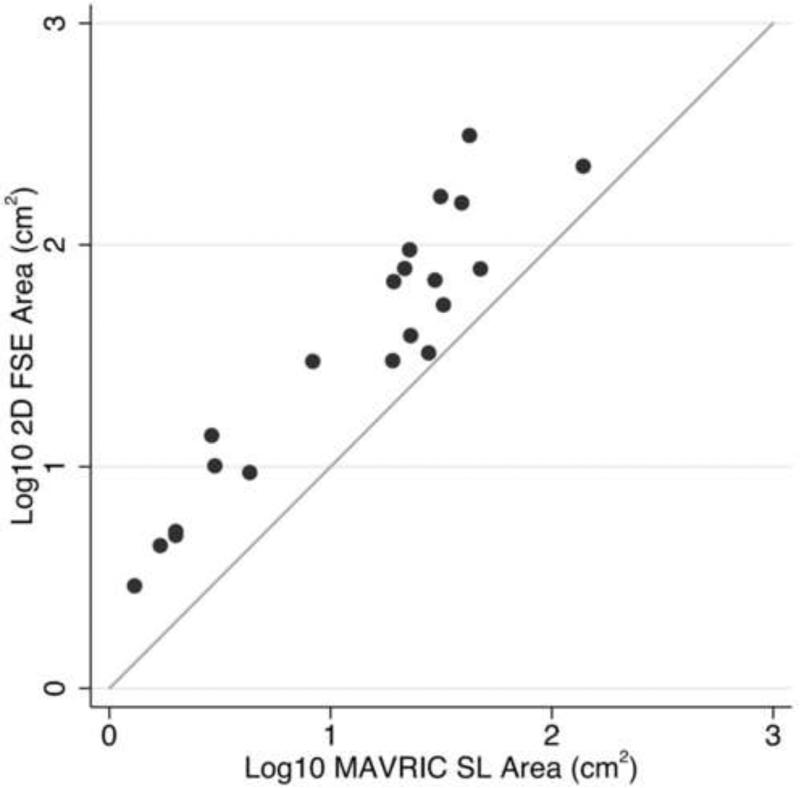
Scatter plot comparing the area of artifact around implants on paired 2D FSE and MAVRIC SL images. A log scale was used for clarity. The diagonal line indicates identical X and Y values.
Quantitative Analysis
The area of metal artifact measured on MAVRIC SL images was significantly less than that on 2D FSE images (p < 0.0001) (Figure 4). The median artifact area on 2D-FSE images was 39.0cm2 compared to 21.6cm2 on MAVRIC SL, and the median difference between artifact areas on MAVRIC SL and 2D FSE was 21.3 cm2. The measured artifact area was much greater for the total hip and total knee prostheses. However, the majority of our arthroplasty patients were referred from outside institutions, and the type of implant and metal composition was not available.
Clinical History
The addition of MAVRIC SL imaging made a significant difference to patient management in 18 out of the 21 studies (86%). In 5 of the 21 studies, findings that were only adequately seen on MAVRIC SL images either resulted in surgical intervention (Figures 5 and 6) or determined what type of surgery the patient subsequently received (Table 2). In 13 of the 21 studies, the imaging findings on MAVRIC SL images ruled-out the need for surgical intervention and led to conservative medical management (Figure 7) (Table 3). The 2 patients who had bilateral symptomatic arthroplasties were both in the group that was managed conservatively. In 3 cases the addition of MAVRIC SL made no significant difference to the patient's subsequent management, as the metal artifact did not interfere with visualization of the region of interest. One was a patient with diffuse cystic angiomatosis and ischial tuberosity pain, who had a femoral rod placed for prior bisphosphonate-induced insufficiency fracture. No abnormality was demonstrated in the region of the ischial tuberosity; another was a knee post ACL reconstruction for assessment of the ACL graft and a cyclops lesion was well seen on both MAVRIC SL and 2D-FSE; the final case was a knee with prior proximal tibial osteotomy for assessment of an osteochondral graft, which was well seen on both MAVRIC SL and 2D-FSE images.
Figure 5.
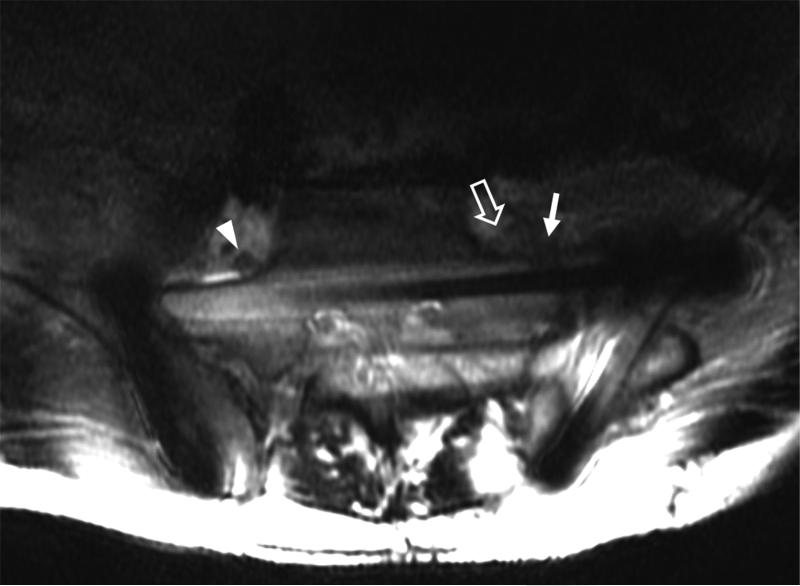
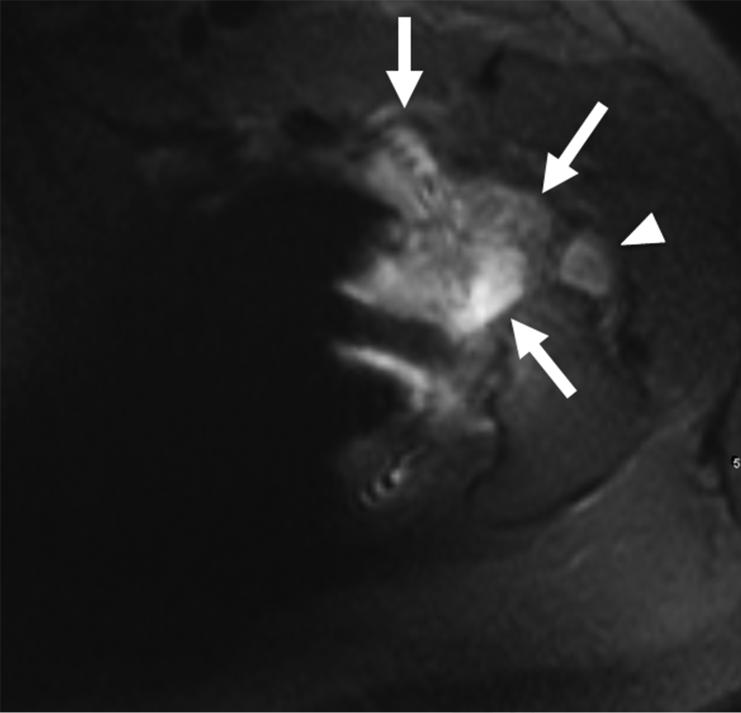
(A) Axial 2D FSE T2 and (B) Axial MAVRIC SL inversion recovery images of the sacrum in a 63-year-old male with acute onset of foot drop on the left after open reduction and internal fixation of complex pelvic and sacral fractures. The right L5 nerve root can be seen clearly in both images (arrowhead). The location of the left L5 nerve root is largely obscured by metal artifact on axial T2 (arrow) and visualization of the sacrum is limited. On the axial MAVRIC SL image the left L5 nerve root appears thickened (open arrow), and is in close proximity to the underlying sacral fracture (arrow).
Figure 6.
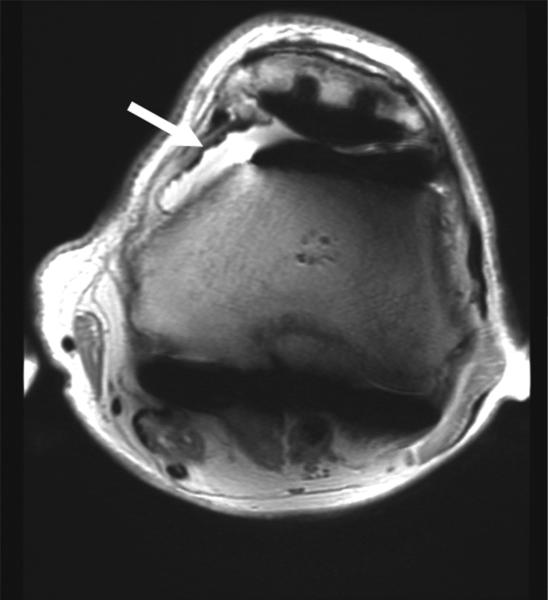
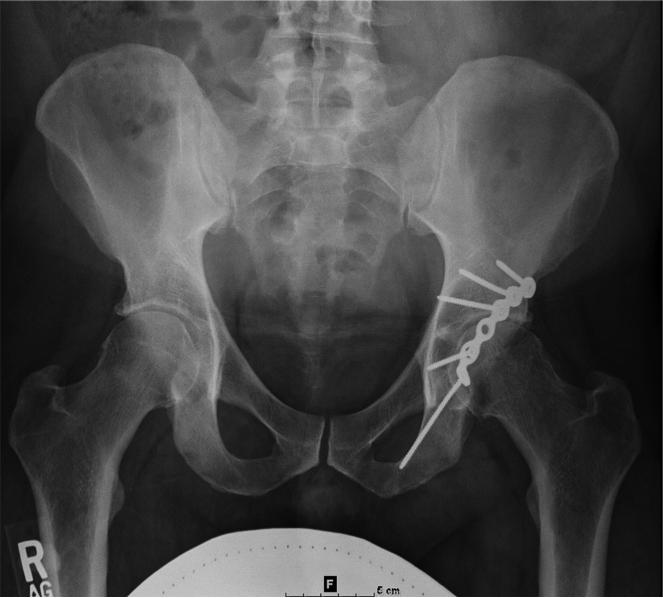
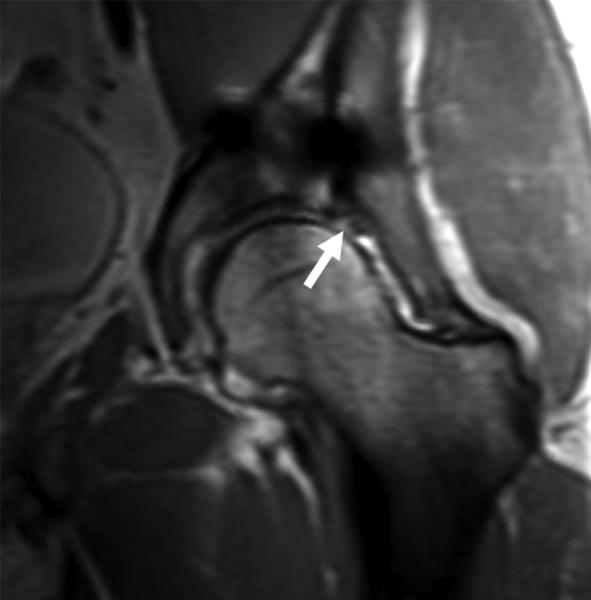
(A) AP radiograph of the pelvis in a 38 year-old male with a plated posterior acetabular wall fracture. (B) Coronal 2D FSE T1 and (C) coronal MAVRIC SL proton density-weighted images of the left hip. Significantly decreased artifact is seen on the MAVRIC SL, allowing visualization of a superior labral tear (arrow), and adjacent joint space narrowing.
Table 2.
Imaging findings seen only on MAVRIC SL that either resulted in surgical intervention or determined what type of surgery was necessary
| Presenting symptom | Imaging findings on MAVRIC SL | Surgical findings and intervention | Outcome |
|---|---|---|---|
| 63 year old male with acute onset of foot drop after open reduction and internal fixation of complex pelvic and sacral fractures | Impingement of L5 nerve root over the fracture site | L5 nerve root tethered and compressed along the superior aspect of the sacrum at the site of fracture. Nerve decompressed and dissected free from scar tissue, with resection of superior margin of anterior sacrum | Significant improvement in pain at 2 week follow up, but persistent foot drop. Foot drop persists at 2 year follow up. |
| 74 year old female with recurrent hip pain after total hip replacement | Complete tear of the gluteus medius and minimus tendons | Extensive tear of gluteus medius and minimus tendons, with severe fibrofatty atrophy of the gluteus minimus muscle. Abductor tendon reconstruction rerouting the tensor fascia lata through the greater trochanter | Markedly improved abductor strength at 2 week follow up (>4/5 muscle strength). Continued to do well at 6 month follow up. |
| 57 year old female with a total hip replacement and 2 prior dislocations of the prosthetic hip. Presenting with hip stiffness and weakness, primarily of abductor strength. Radiographs demonstrated a steep acetabular cup, with evidence of polyethylene wear but no obvious periprosthetic osteolysis. | Hip joint effusion but no evidence of particle disease. Intact gluteal tendons, but marked atrophy and fatty infiltration of the gluteal muscles | Acetabular component replaced for abnormal cup position and marked polyethylene wear, with no need for revision of femoral component or gluteal tendon reconstruction. | No further instability of hip at 1 year follow up after intense physical therapy |
| 22 year old synchronized swimmer with hip pain 6 months after placement of 3 percutaneous screws for femoral neck stress fracture | Absence of osteonecrosis in the femoral head | Removal of symptomatic hardware, stress fracture well healed | Asymptomatic at 2 week follow up and returned to swimming for the season. No further issues at 6 month follow up. |
| 38 year old male with prior plate and screw fixation of a left acetabular fracture with continued hip pain | Tear of the anterosuperior acetabular labrum, with bony prominence of femoral head-neck junction | Acetabular labrum torn from acetabular margin from 11 - 1 o'clock position, with large chondral flap. Underwent labral repair, chondral debridement and microfracture, plus osteochondroplasty of femoral head-neck junction. | Residual pain and clicking at 3 month follow up, but significant improvement at 4 month follow up. However, 2 year follow up showed progression of osteoarthritis and is being considered for hip arthroplasty |
NB. MAVRIC SL = multi-acquisition variable resonance image combination slice selective
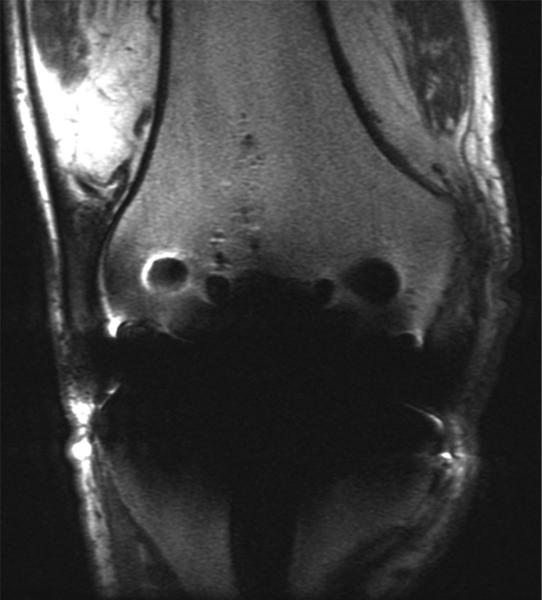
Figure 7.
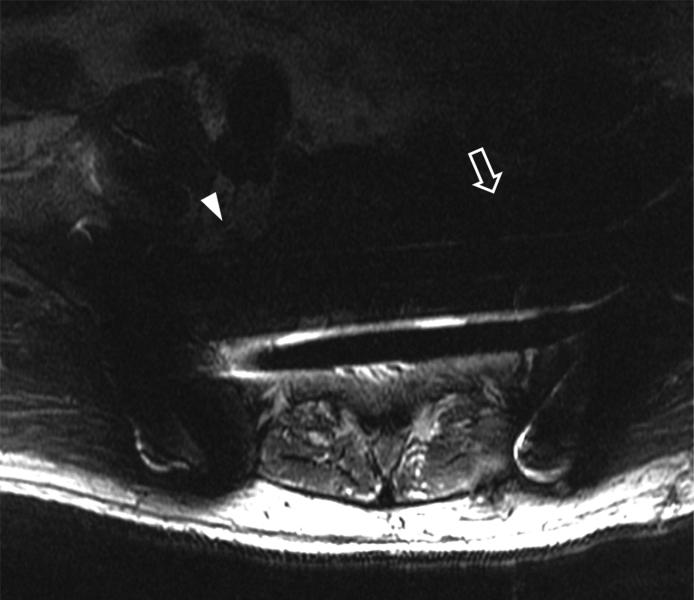
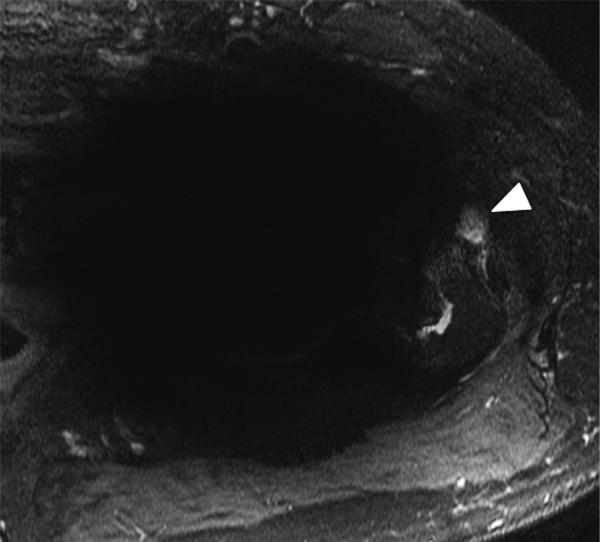
(A) Axial inversion recovery and (B) axial MAVRIC SL inversion recovery images of the left hip in a 64-year-old male with left hip pain after a resurfacing hip arthroplasty. Significantly decreased metal artifact is seen on MAVRIC SL images, although the images appear more blurry. A small focal fluid collection is seen on both sequences (arrowhead). However, the large joint effusion and synovitis (arrows) is only seen on MAVRIC SL. The effusion was aspirated, and subsequent steroid injection lead to symptomatic improvement.
Table 3.
Imaging findings on MAVRIC SL resulting in a decision not to operate
| Subject Population | Imaging findings | Subsequent management | Outcome |
|---|---|---|---|
| 73 year old male with painful back years after spinal fusion with transfacet screws and iliac crest bone graft | Post-operative changes, with no significant neural compressive lesion | Physical therapy and trigger point injections for hypertonic muscles | Interval improvement at 5 week follow up |
| 57 year old female with hip pain, status-post total hip replacement | Partial-thickness gluteus medius tendon tear | Physical therapy | Interval improvement at 4 month follow up |
| 64 year old male with hip pain, status-post total hip replacement | Large hip joint effusion | Physical therapy | No follow up recorded in patient's medical records |
| 55 year old female with hip pain, status-post total hip replacement (THR) | Pseudotumor ruled out | Physical therapy | No improvement – felt to be over-loading THR due to severe osteoarthritis in contralateral hip, which was subsequently replaced with excellent clinical outcome in both hips |
| 45 year old male with hip pain, status-post total hip replacement | Pseudotumor ruled out | Bone scan, hip aspiration, and steroid injection iliopsoas tendon sheath | Negative bone scan and hip aspirate. No improvement after steroid injection. No additional follow up recorded. |
| 65 year old male with metastatic chondrosarcoma and hip pain, status-post total hip replacement for ununited femoral fracture in irradiated femur | No tumor recurrence | Steroid injection | Excellent result at 5 month follow up |
| 27 year old male with hip pain, status-post placement of bilateral femoral neck screws for slipped capital femoral epiphyses (2 studies, 1 patient) | Degenerative change | Bilateral steroid injection | No significant relief and was subsequently referred to spine clinic |
| 52 year old male status post chondrosarcoma resection, with extensive reconstruction of proximal femur | No definite tumor recurrence | Physical therapy | Fractured femoral plate 3 months later and treated surgically |
| 86 year old male with bilateral knee pain, status post total knee arthroplasties (2 studies, 1 patient) | No significant pathology identified | Physical therapy | No follow up recorded in patient's medical records |
| 47 year old female with knee pain status post total knee arthroplasty, with instability on flexion | No significant pathology identified | Physical therapy | 3 month course of physical therapy, but knee remained unstable and was subsequently revised with liner exchange. Excellent result at follow up |
| 18 year old male with painful foot, status-post open reduction and internal fixation of prior navicular stress fracture | Healed stress fracture | Physical therapy, with taping and icing for 2 weeks | No pain at 6 month follow up |
NB. MAVRIC SL = multi-acquisition variable resonance image combination slice selective
DISCUSSION
Metal implants are commonplace, and post-operative evaluation with MRI has previously been limited by metal artifact, particularly at higher field strengths. The metal induces inhomogeneity of the local magnetic field around the implants, resulting in areas of signal void, geometric distortion and signal pile-up. Modifications can be applied to conventional fast-spin-echo pulse sequences in an attempt to minimize metal artifact and improve image quality, including the use of a wide receiver bandwidth, thinner slice thickness, orienting the frequency encoding direction along the long axis of the implant, and decreasing voxel size. However, even with optimized parameters, the metal artifact can still obscure pathology in the vicinity of the metal, particularly at higher field strengths. A variety of metal suppression techniques have been developed to improve imaging near metal. Slice encoding for metal artifact correction (SEMAC) and multi-acquisition variable-resonance imaging combination (MAVRIC) both employ a 3D spin echo acquisition, and reduce susceptibility artifacts while maintaining clinically viable scan-times using standard clinical hardware (4, 5, 6, 7). SEMAC selectively excites multiple slices, and uses view angle tilting (VAT) technique to remove in-plane distortion (12). A 3D spin echo acquisition is then used to resolve the profiles of each excited slice, which minimizes through plane distortion (4,5). SEMAC has been investigated on GE MRI scanners as well as Siemens and Phillips, and with its slice selectivity can be used to image the spine in patients with metallic spinal implants (13). MAVRIC utilizes a series of limited excitation bandwidth to reduce in-plane distortions, but is therefore not slice selective, limiting its use in certain areas such as in imaging of the spine. Although the overlap of selective excitations in MAVRIC produces high SNR, it can also be combined with the selectivity used in the SEMAC approach to form a widely available hybrid MAVRIC SL technique. Similar methodologies are also being developed by other MRI vendors to decrease metal artifact. Siemens has also combined Multiple Slab Acquisition with view angle tilting (MSVAT) with the 3D turbo spin echo sequence SPACE to produce a hybrid technique MSVAT-SPACE, which uses multiple thin slabs and slice-oversampling to significantly decrease susceptibility artifact associated with metallic implants (14, 15).
Koch and colleagues carried out a preliminary evaluation of MAVRIC SL by comparing it with MAVRIC and conventional 2D-FSE imaging (10). Their study showed that although the through-plane aliasing in MAVRIC may not compromise diagnostic accuracy around small metal implants, such as in the ankle, the severity of through-plane aliasing could be extremely detrimental to MAVRIC's diagnostic capability around larger metal implants, such as those used in the shoulder, hip, and spine. MAVRIC SL imaging, on the other hand, successfully diminished susceptibility artifacts and through-plane aliasing not only around large joints at 1.5T, but also around a small stainless steel ankle implant at 3T. Our study has shown that imaging of patients with a variety of metal implants using MAVRIC SL lead to a dramatic improvement in image quality compared to conventional 2D-FSE imaging techniques. Imaging with MAVRIC SL resulted in less in-plane and through-plane artifact, improving visualization of the bone-implant interface and surrounding soft tissues. The imaging findings seen on MAVRIC SL images had a direct impact on patient management, resulting in surgical intervention in 5 patients, while ruling out the need for surgery in 13 others. Lastly, MAVRIC SL imaging was successful at 3T, not only around a small metallic implant in the foot, but also around larger metal implants in the hip, knee, and spine.
Although our results support the use of MAVRIC SL as a viable metal artifact suppression technique at 3T, imaging with MAVRIC SL does have some disadvantages. Like MAVRIC and SEMAC, MAVRIC SL uses a 3D-FSE acquisition, requiring an increased number of excitations to acquire the data for each excited slice, and thus extending scan times compared to 2D-FSE techniques (16). The longer scan time may result in motion artifact, as patients find it increasingly hard to remain still for the duration of the scan, although motion artifact was not a limiting factor in our study. The scan duration also makes it impractical to acquire multiple MAVRIC SL sequences in all 3 orthogonal imaging planes. However, the addition of MAVRIC SL in one or two orthogonal imaging planes may be sufficient to answer the clinical question being posed, making it unnecessary to acquire multiple other sequences in an effort to make the correct diagnosis. The acquisition time for MAVRIC SL will also continue to decrease with the use of parallel imaging and better coils.
MAVRIC SL also utilizes a longer echo train length (ETL=20) that can result in increased blurring compared to 2D-FSE (ETL=8). In addition, the echo train view-ordering schemes of the 2D-FSE and 3D-FSE implementations are substantially different. This can introduce additional blurring in the 3D-FSE acquisition utilized in MAVRIC SL. MAVRIC SL imaging is currently not available with phase oversampling or “no phase wrap,” and therefore a wider field of view was used to avoid phase wrapping, thus compromising in-plane resolution. The lower in-plane resolution of MAVRIC SL may also be perceived as blurring.
As described in the introduction, the MAVRIC SL approach (like SEMAC and MAVRIC) is designed to address the susceptibility-induced artifacts that cause static magnetic field variations, resulting in signal loss, pile-up and geometric distortion artifacts (2). The radiofrequency (RF) magnetic field also suffers distortion near metals, primarily due to RF currents induced in the implant, which are worse at 3T than at 1.5T (17). The result of this distortion is some shading near the metal, which is generally quite benign compared with the substantial susceptibility artifacts near metal. Most vendors have simple, but proprietary algorithms to correct for coil shading, and although these typically do not affect diagnosis, reviewing the images is easier as the radiologist does not have to adjust brightness/contrast as much to compensate for shading in the adjacent soft tissues. The use of parallel imaging methods may also mitigate shading, since the coil combination methods typically correct in part for shading.
Limitations of our study included the small number of patients, weakening statistical power. A wide variety of metal implants were included in the study, making direct comparisons between individual implants difficult. However, the overall aim of our study was to demonstrate the utility of MAVRIC SL in imaging patients with differing sizes and shapes metal implants, rather than a focused study on a single type of implant in one joint. Although the reviewers were blinded to the imaging sequence being evaluated, a dramatic difference in image quality was seen between the 2 sequences, which inevitably introduced observer bias.
The area of metal artifact was measured by a single observer in the quantitative study. Due to the differing shapes and sizes of the metal implants it was difficult to determine the most appropriate place to measure the artifact around each implant. Many of the implants included in our study consisted of multiple components such as plate and screws, in a variety of different orientations, making it challenging to accurately compare area of artifact measurements between 2D-FSE and MAVRIC SL. This also precluded attempting volume measurements of artifact. The area measurements were performed on images where the artifact around the implant was maximal. Other studies have used phantoms with a unique grid design to assess the magnitude of in-plane and through-plane image distortion (9). However, accurately quantifying the degree of in-plane and through-plane distortion in vivo is challenging. Lastly, as most patients were referred from outside institutions, we did not know the manufacturer or the type of metal used in each implant, and therefore could not formally evaluate whether the composition of the implant affected imaging findings for each patient.
In conclusion, imaging patients with metal implants using MAVRIC SL at 3T significantly improved image quality and decreased the extent of metal artifact compared to 2D-FSE imaging, allowing increased diagnostic confidence and thereby directly impacting patient management. We expect that the addition of MAVRIC SL to conventional imaging sequences at 3T will enable increased diagnostic accuracy in patients presenting with complications relating to their implants, or pathology in structures adjacent to the implant. However, larger prospective randomized studies are needed to establish the utility of MAVRIC SL as an appropriate diagnostic tool in symptomatic patients with metal implants.
Acknowledgement
Grant support: NIH 5K24 AR062068, NIH 1R01 EB017739, GE Healthcare
The authors would like to thank Jarrett Rosenberg PhD for his assistance with the statistical analysis in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys. 1996 Jun;23(6):815–850. doi: 10.1118/1.597854. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves BA, Worters PW, Pauly KB, et al. Metal-induced artifacts in MRI. AJR Am J Roentgenol. 2011 Sep;197(3):547–555. doi: 10.2214/AJR.11.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris CA, White LM. Metal artifact reduction in musculoskeletal magnetic resonance imaging. Orthop Clin North Am. 2006 Jul;37(3):349–359. doi: 10.1016/j.ocl.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Lu W, Pauly KB, Gold GE, et al. SEMAC: Slice Encoding for Metal Artifact Correction in MRI. Magn Reson Med. 2009 Jul;62(1):66–76. doi: 10.1002/mrm.21967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hargreaves BA, Chen W, Lu W, et al. Accelerated slice encoding for metal artifact correction. J Magn Reson Imaging. 2010 Apr;31(4):987–996. doi: 10.1002/jmri.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch KM, Lorbiecki JE, Hinks RS, et al. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med. 2009 Feb;61(2):381–390. doi: 10.1002/mrm.21856. [DOI] [PubMed] [Google Scholar]
- 7.Hayter CL, Koff MF, Shah P, et al. MRI after arthroplasty: comparison of MAVRIC and conventional fast spin-echo techniques. Am J Roentgenol. 2011 Sep;197(3):W405–411. doi: 10.2214/AJR.11.6659. [DOI] [PubMed] [Google Scholar]
- 8.Chen CA, Chen W, Goodman SB, et al. New MR imaging methods for metallic implants in the knee: artifact correction and clinical impact. J Magn Reson Imaging. 2011 May;33(5):1121–1127. doi: 10.1002/jmri.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koff MF, Shah P, Koch KM, et al. Quantifying image distortion of orthopedic materials in magnetic resonance imaging. J Magn Reson Imaging. 2013 Sep;38(3):610–618. doi: 10.1002/jmri.23991. [DOI] [PubMed] [Google Scholar]
- 10.Koch KM, Brau AC, Chen W, et al. Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med. 2011 Jan;65(1):71–82. doi: 10.1002/mrm.22523. [DOI] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- 12.Cho ZH, Kim DJ, Kim YK. Total inhomogeneity correction including chemical shifts and susceptibility by view angle tilting. Med Phys. 1988;15:7–11. doi: 10.1118/1.596162. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Lim D, Kim E, et al. Feasibility of fat-saturated T2-weighted magnetic resonance imaging with slice encoding for metal artifact correction (SEMAC) at 3T. Magnetic Resonance Imaging. 2014;32(8):1001–1005. doi: 10.1016/j.mri.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Tao A, Padua A, Goerner F, Nittka M, et al. SEMAC-VAT and MSVAT-SPACE sequence strategies for metal; artifact reduction in 1.5T magnetic resonance imaging. Investigative Radiology. 2012;47(5):267–276. doi: 10.1097/RLI.0b013e318240a919. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Nittka M, Paul D, Lauer L. MSVAT-SPACE for fast metal implants imaging.. Proceedings of the 18th ISMRM Annual Meeting; Stockholm Sweden. 2010.p. 3171. [Google Scholar]
- 16.Irarrazabal P, Nishimura DG. Fast three dimensional magnetic resonance imaging. Magn Reson Med. 1995 May;33(5):656–662. doi: 10.1002/mrm.1910330510. [DOI] [PubMed] [Google Scholar]
- 17.Koch KM, King KF, McKinnon GC. B1 Effects When Imaging Near Metal Implants at 3T.. Proceedings of the 18th ISMRM Annual Meeting; Stockholm Sweden. 2010.p. 3082. [Google Scholar]