Abstract
Background
Tumor cell infiltration is a major mechanism of treatment escape in glioblastoma. Src is an intracellular tyrosine kinase that mediates tumor cell motility and invasiveness. We evaluated the efficacy and safety of bosutinib, a tyrosine kinase inhibitor that potently inhibits Src and Abl, in patients with recurrent glioblastoma.
Methods
In this two-arm study, patients with histologically confirmed recurrent glioblastoma and ≤ 2 relapses, not previously treated with anti-vascular endothelial growth factor therapy, were administered oral bosutinib 400 mg daily. Arm A planned for 6 patients who were candidates for surgical resection to be given bosutinib for 7-9 days prior to resection. Arm B was a two-stage design phase 2 trial targeting 30 patients. The primary endpoint was progression-free survival at 6 months (PFS6) in Arm B.
Results
After 9 patients enrolled onto stage 1 of Arm B, 9 (100%) patients progressed within 6 months. Therefore, the study met the pre-specified criteria for early closure and both Arms were closed. In Arm B, Median PFS was 7.71 weeks and median OS was 50 weeks. Best objective response was stable disease in one patient (11.1%). Seven patients (77.8%) had treatment-related AEs of any grade and 2 (22.2%) were grade ≥3. Arm A was closed after 2 patients enrolled. Src activation was evident in all archival tumor samples.
Conclusion
Bosutinib monotherapy does not appear to be effective in recurrent glioblastoma. However, Src remains a potential target based on its upregulation in tumor samples and role in glioma invasion.
Keywords: glioblastoma, bosutinib, Src inhibitor, invasion
Background
Patients with glioblastoma (GBM) have a poor prognosis. Despite treatment including maximal surgical resection, concurrent radiation and temozolomide and adjuvant temozolomide [1], median progression-free survival (PFS) and overall survival (OS) remain 6.9 months and 14.6 months, respectively, and 5-year survival is approximately 10% [2]. Prognosis at recurrence is dismal and treatment options are limited [3, 4]. There is an urgent need to identify novel therapeutic targets for drug development.
GBMs are highly infiltrative tumors, making pathways involved in tumor cell motility and invasion rational targets. Src is an intracellular tyrosine kinase that coordinates multiple extracellular factors involved in cell-cell and cell-matrix adhesions. Upregulation of Src reduces these adhesions, promoting increased cell motility, invasion and metastatic potential [5, 6]. Activated Src is increased in several solid tumors, including several GBM cell lines and 61% of GBM tumor specimens from first resection [7]. In pre-clinical GBM models, Src inhibition reduced cell proliferation and viability, and increased glioma cell apoptosis [7, 8]. Src might also play a role in glioma angiogenesis and inhibition in pre-clinical models reduced vessel density and VEGF-induced vascular permeability [9, 10]. Therefore, Src is a potentially attractive therapeutic target in GBM.
Several clinical trials in GBM have investigated dasatinib – a potent Src inhibitor that also inhibits c-Kit, c-Fms, and platelet-derived growth factor receptor-β (PDGFR- β) [11]. However, phase 1 studies of dasatinib in combination with the nitrosourea lomustine (CCNU) [12] and epidermal growth factor receptor (EGFR) inhibitor erlotinib [13], as well as a phase 2 trial of dasatinib monotherapy [14, 15] did not demonstrate efficacy. A retrospective study of dasatinib and bevacizumab also did not reveal a significant improvement in PFS or OS [16].
Bosutinib is a potent third generation tyrosine kinase inhibitor (TKI) that dually targets Src and the oncogene Abl with IC50 values for enzyme inhibition of 3.5 and 1 nM, respectively. It is approved for use in Philadelphia chromosome-positive chronic myelogenous leukemias (CML) with resistance or intolerance to first or second-generation TKIs [17, 18]. It differs from dasatinib in its specificity for Src and Bcr-Abl, while minimizing activity against c-KIT or PDGFR [19, 20]. Bosutinib is orally administered and well tolerated in CML patients with low-grade gastrointestinal toxicity reported most commonly [21]. Although bosutinib lacks brain penetration in animal models, a CSF penetrance of only 1% (ratio of CSF to plasma concentrations) would achieve concentrations in the CSF that completely inhibit Src enzyme activity in vitro. The objective of this phase 2 study was to evaluate the efficacy and safety of bosutinib in patients with recurrent GBM.
Patients and Methods
Patients
Eligible patients (aged ≥ 18 years) had histologically confirmed GBM (World Health Organization grade IV astrocytoma); had received temozolomide and radiation as first-line therapy; had ≤ 2 prior systemic treatments; had Karnofsky performance status (KPS) ≥ 60%, and had archived tumor material available. In addition, recurrent GBM patients enrolled on Arm A were candidates for resection
Key exclusion criteria included concomitant use of CYP3A4/5 inhibitors or potent inducers; clinically relevant gastrointestinal abnormalities; uncontrolled or significant cardiovascular disease within 6 months of registration; history of a different malignancy (unless disease-free for at least 3 years and deemed to be at low risk for recurrence of that malignancy). Individuals with cervical cancer in situ, and basal cell or squamous cell carcinoma of the skin were eligible if diagnosed and treated within the past 3 years. Patients were not eligible if they had evidence of other malignancy requiring therapy other than surgery within the last 3 years. Patients previously treated with anti-vascular endothelial growth factor (VEGF) directed therapy or alternating electric field therapy were excluded since the data regarding the efficacy of any therapy after progression on these modalities were lacking at the time of study initiation. Patients enrolled on Arm A were removed from study if their diagnostic pathology demonstrating only gliosis or necrosis without active tumor.
The Dana-Farber/Harvard Cancer Center institutional review board approved the study protocol. All procedures were performed in accordance with the ethical standards of the Helsinki Declaration. All patients provided written informed consent.
Study design
This open-labeled, dual-arm, single-agent, study of bosutinib (SKI-606) in patients with recurrent GBM was conducted at Massachusetts General Hospital Cancer Center and Dana-Farber Cancer Institute (ClinicalTrials.gov: NCT01331291). There were two treatment arms. Patients enrolled on Arm A were candidates for resection with the exploratory outcome of measuring intratumoral Src phosphorylation. The target enrollment for Arm A was 6 patients. Arm B was designed as a Simon two stage, phase 2 trial with a target enrollment of 30 patients (see Statistical Analysis). The primary endpoint was progression-free survival at six months (PFS6). Secondary analyses included adverse events (AEs), overall survival (OS), median progression-free survival (PFS), overall response rate (ORR) according to the Macdonald criteria, [22] and response duration. Exploratory endpoints included pharmacodynamic tumor response assessed by phosphorylated Src (pSrc) in Arm A.
Patients on Arm A received bosutinib 400mg by mouth daily for 7-9 days prior to surgery. After at least 10 days had elapsed post-operatively, these subjects resumed bosutinib at 400mg daily in 28-day cycles until disease progression, intolerability, or withdrawal of consent. Patients on Arm B received bosutinib 400mg by mouth daily in 28-day cycles until disease progression, intolerability, or withdrawal of consent. Bosutinib 400mg daily was the recommended phase 2 dose based on phase 1 data in patients with advanced solid tumors [23].
Tumor Assessments
For the secondary outcome of overall response rate, tumor response was assessed by Macdonald criteria [22]. Patients in both arms had a contrast-enhanced baseline MRI within 21 days of starting bosutinib. In Arm A, an MRI was conducted within 24-48 hours post-surgery and again prior to reinitiating bosutinib. In both arms, tumors were assessed serially with MRI on every other cycle.
Safety
All AEs from the time of enrollment until the end of study were graded using the Cancer Therapy Evaluation Program (CTEP) Active Version of the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE). For both arms, clinical and basic laboratory assessments (hematology, blood chemistry, urinalysis, and coagulation studies) were performed at baseline, cycle 1/day 1, cycle 1/day 14, and day 1 of each subsequent cycle. In addition, patients on Arm A had clinical and laboratory assessments on day 1 pre-surgery and the day of surgery.
Pharmacodynamics
Src immunohistochemistry (IHC) of archival tumor samples was performed on paraffin slides. Slides were deparaffinized through xylene and dehydrated through ethanol. Heated EDTA buffer was used for antigen retrieval and slides were blocked with peroxidase for 5 minutes and 10% normal goat serum/5% milk/1% bovine serum in tris-buffered saline with tween for one hour at room temperature. Slides were incubated in Src primary antibody [Src (36D10) #2109, Cell Signaling] overnight at 4°C and SignalStain® Boost IHC Detection Reagent (HRP, rabbit, 8114, Cell Signal) secondary for 30 minutes at room temperature and developed with DAB. Immunohistochemistry staining was scored as follows: 1+, < 10% of the cells stained; 2+, 25 – 50% of the cells stained; 3+, 50 – 75% of cells stained; 4+, > 75% of cells stained.
Immunohistochemistry for phosphorylated Src was performed on archival tumor samples and post-treatment resection tumor specimens from the two patients who were enrolled on Arm A. Archival and post-treatment resection samples were collected as per standard procedure, fixed in formalin and embedded in paraffin. All H&E slides were reviewed with a neuropathologist (A.S.R.) and included viable tumor tissue. All archival and post-surgical slides were prepared and stained at the same time to eliminate batch effect. Slides were scored as noted above using pSrc primary antibody [pSrc (Tyr416) #2101, Cell Signaling]. Livers from human hepatocellular carcinoma xenografts were used as positive controls.
Statistical Analysis
The study utilized a Simon two stage optimal trial design with PFS6 in Arm B as the primary outcome [24]. PFS6 was defined as the proportion of patients alive and progression-free at six months. PFS was calculated as the time in weeks from the first dose of bosutinib to disease progression (per Macdonald criteria) or death from any cause. In a pooled database of patients with recurrent GBM, the 6-month progression-free survival rate was determined to be 9% [16]. If the true PFS6 in Arm B were 30% or more, there would be interest in further investigation of bosutinib. Arm B tested the null hypothesis (H0) with 5% significance level that the PFS6 was ≤9% versus the alternative hypothesis (Ha) that the PFS6 was ≥30%. The optimal Simon two-stage design that minimized expected sample size with 80% power required that 10 subjects enroll in Stage I and that this arm of the study would terminate if 9 or 10 subjects experienced progression or death within 6 months. If 2 or more subjects were alive and progression free at 6 months, an additional 20 subjects were planned to be enrolled onto Stage II for a total of 30 subjects. If at the end of the trial, 25 or more total had experienced progression or death within 6 months, Ha would be rejected. The expected sample size was 14.5 and the probability of early termination was 0.775. If PFS6 is < 9%, there is a type I error rate of 0.034). If PFS6 > 30%, there is a 0.185 probability of concluding that the drug is not effective (81.5% power).
The primary and secondary endpoints and safety analyses were conducted for all patients who received ≥ 1 dose of bosutinib in each cohort. Kaplan-Meier was used to graphically depict the distributions of OS and PFS among patients with disease progression or death. Best ORR (complete, response, partial response, stable disease) was calculated as a proportion with 2-sided 95% confidence interval (CI). Cox proportional hazards models were used to assess the relationship between time-to-event end points and baseline variables. GraphPad Prism software (GraphPad Software Inc, La Jolla, CA) was used for statistical analysis.
Results
Patients
Between August 2, 2011 and February 6, 2013, 11 patients were enrolled in this study – 2 in Arm A and 9 in Arm B (Table 1). In the entire study, median age at enrollment was 52 (range 36 – 62), median KPS 90 (range 90 – 70). Seven (63.6%) patients were male, and 9 (81.8%) white. Seven (63.6%) had gross total resections at diagnosis and 3 (27.3%) subtotal resections. All patients received standard, initial therapy with temozolomide and radiation at diagnosis, and 1 (9.1%) was also treated with an investigational intravenous radiation sensitizer PPX (CT-2103) concurrently with radiation. Median time from diagnosis to trial enrollment was 10 months (range 8.58 – 46.03).
Table 1.
Baseline Patient Demographics and Characteristics in all Arms
| Median age (range) | 52 (range 36 – 62) |
| Median KPS (range) | 90 (70 – 90) |
| Male, n (%) | 7 (63.6) |
| White, n (%) | 9 (81.8) |
| Surgery | |
| Gross total resection, n (%) | 7 (63.6) |
| Subtotal resection, n (%) | 3 (27.3) |
| Prior chemoradiation, n (%) | 11 (100) |
For the entire study, median duration of treatment with bosutinib was 7.71 weeks (range 1.86 – 23.86) with median number of cycles of 2 (range 1 – 6). Median follow-up time was 23.57 weeks (range 2.57 – 63.71). The reason for discontinuing treatment was progression in 10 (90.9%) patients and death due to disease progression in 1 (9.1%) patient.
Efficacy
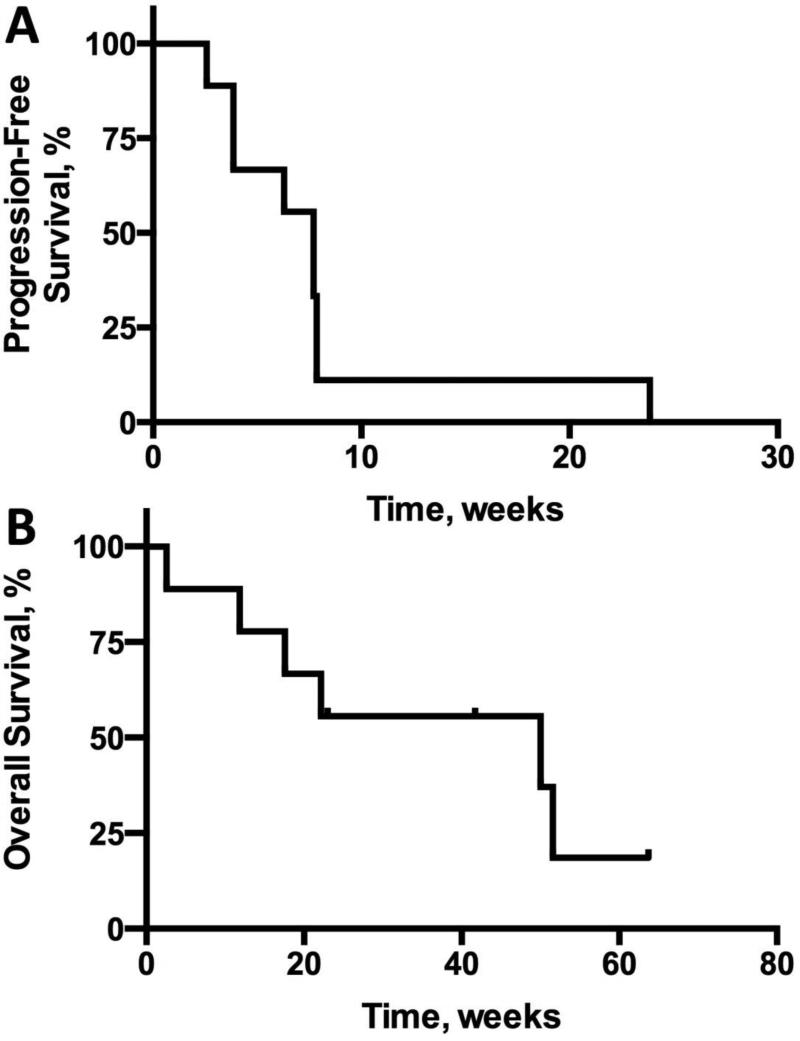
The primary endpoint of Arm B, a phase II trial, was progression-free survival at 6 months. After 9 patients were enrolled on Arm B, the interim analysis revealed that 9 (100%) patients had disease progression including death in 1 (11.1%) patient. Based on the Simon two stage design, this met the pre-specified criteria for study termination. Median PFS in Arm B from initiation of study treatment to date of progression was 7.71 weeks (95% CI 2.6 – 7.9). Median OS from initiation of study treatment for patients on Arm B was 50 weeks (95% CI 2.9 – NA) (Figure 1). The best objective response in patients on Arm B was stable disease in one patient (11.1%) and progressive disease in 8 (88.9%). Specific treatment regimens after study discontinuation were not recorded.
Figure 1.
Kaplan-Meier analysis of (A) progression-free survival and (B) overall survival for patients enrolled on Arm B.
Toxicity
In the entire study, all patients received bosutinib at a dose of 400mg daily. Including both arms, 3 (27.3%) patients experienced dose holds for a median of 2.5 days (range 2 – 5). One (9.1%) patient was held for seizures, one (9.1%) for grade 1/2 elevated transaminases, and one (9.1%) for external ear pain. There were no dose reductions. The most common AE of any grade was lymphopenia in 9 (81.8%), fatigue in 6 (54.6%), and nausea in 6 (54.6%) (Table 2). Seven patients (63.6%) had AEs of any grade considered related to treatment with bosutinib although only 2 (18.2%) were grade ≥3.
Table 2.
Adverse Events in all Arms
| Adverse Event | All enrolled, n (%) | Possibly, probably, or definitely related to bosutinib, n (%) | ||
|---|---|---|---|---|
| Any grade | Grade 3 / 4 | Any grade | Grade 3 / 4 | |
| Lymphopenia | 9 (81.8) | 3 (27.3) | 2 (18.2) | 1 (9.1) |
| Fatigue | 6 (54.6) | 1 (9.09) | 0 (0) | 0 (0) |
| Nausea | 6 (54.6) | 0 (0) | 5 (45.5) | 0 (0) |
| Seizure | 5 (45.5) | 1 (9.09) | 0 (0) | 0 (0) |
| Elevated ALT | 5 (45.5) | 0 (0) | 4 (36.4) | 0 (0) |
| Pain | 5 (45.5) | 0 (0) | 2 (18.2) | 0 (0) |
| Diarrhea | 4 (36.4) | 0 (0) | 3 (27.3) | 0 (0) |
| Hypophosphatemia | 4 (36.4) | 0 (0) | 2 (18.2) | 1 (9.1) |
| Elevated ALT | 5 (45.5) | 0 (0) | 4 (36.4) | 0 (0) |
| Rash | 3 (27.3) | 0 (0) | 2 (18.2) | 0 (0) |
| Lung infection | 3 (27.3) | 2 (18.2) | 0 (0) | 0 (0) |
| Elevated AST | 2 (18.2) | 0 (0) | 2 (18.2) | 0 (0) |
| Cerebral edema | 1 (9.09) | 1 (9.09) | 0 (0) | 0 (0) |
Correlative analysis of tumor samples
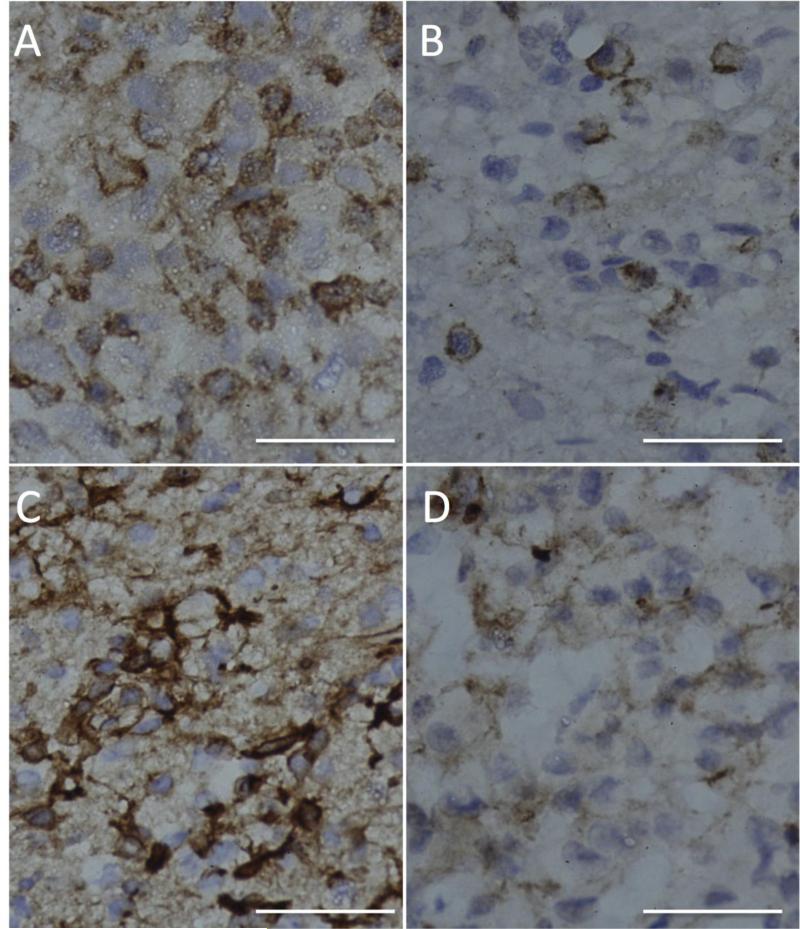
Immunohistochemistry for Src and pSrc was performed on all diagnostic (archival) tumor samples and post-bosutinib resection specimen samples from 2 patients on Arm A and were scored independently by two observers (J.W.T. and A.S.R.). Eleven out of 11 (100%) archival tumors had evidence of Src upregulation and activation, as Src staining was 3+ or greater in all specimens and all specimens had at least 1+ pSrc (Table 3, Figure 2). At the time of study closure, only 2 patients had enrolled onto Arm A. For the two patients on Arm A, pSrc levels scored lower in the post-treatment tumor specimen in comparison to the paired archival specimen (Figure 2). In addition, the pSrc:total Src ratios decreased in the post-treatment recurrent specimens for both patients; patient X scored 3+:3+: in the diagnostic specimen and 1+:3+ in the resection specimen and patient Y scored 3+:4+ in the diagnostic specimen and 2+:4+ in the resection specimen.
Table 3.
Src and pSrc Immunohistochemistry results from study patients' archival brain tumor samples
| Score n (%) | 1 + | 2+ | 3+ | 4+ |
| Src, n= 11 | 0 (0) | 0 (0) | 1 (9.1) | 10 (90.9) |
| pSrc, n = 11 | 1 (9.1) | 4 (36.4) | 3 (27.3) | 3 (27.3) |
Figure 2. Representative histologic images for tumor specimens from patients enrolled on Arm A.
Immunohistochemical sections stained with pSrc antibody (brown) (A) Patient X diagnostic tumor specimen. (B) Patient X post-treatment resection tumor specimen (C) Patient Y diagnostic tumor specimen. (D) Patient Y post-treatment resection tumor specimen. Bar 50 μm.
Discussion
Although analysis of archival tumor specimens demonstrated upregulation and activation of Src in all tumors, bosutinib did not appear to have single-agent efficacy in patients with recurrent GBM. In arm B, there were no objective radiographic responses and no patient met the study's primary efficacy endpoint of PFS6 [25] at the interim analysis and, consequently, the study was terminated early after 9 patients were enrolled in Arm B and 2 patients enrolled on Arm A. Including both arms, the best radiographic response was stable disease, and the longest duration on study was 23 weeks. Bosutnib was well tolerated with only 2 (18.2%) grade ≥3 AEs (lymphopenia and hypophosphatemia).
Although our study did not identify efficacy for bosutinib monotherapy in recurrent GBM, Src may remain a rational therapeutic target. Our analysis of Src in diagnostic tumor specimens is consistent with previous clinical and preclinical reports suggesting Src is upregulated in GBM [7]. Additionally, Src is a known mediator of motility and invasion in GBM [8, 26]. Preclinical evidence also suggests that activation of Src family kinases may promote GBM invasion after anti-angiogenic therapy [27]. In mice bearing GBM orthotopic xenografts, dasatinib, a Src inhibitor, effectively blocked the increased invasion induced by bevacizumab [27]. It is unclear why bosutinib did not demonstrate significant efficacy in our trial. One possibility may be because in animal models there is no CNS penetration [28]. Because of the small sample size of post-treatment specimens, which limited our ability to generate statistical conclusions, intratumoral concentrations of bosutinib were not measured. We observed some evidence of on-target inhibition in the two post-treatment specimens, as the pSrc:total Src ratios were decreased compared to the paired diagnostic specimens. However, the activation status of Src in the recurrent GBM population is unknown, and it's possible that Src activation is decreased in GBM tumors after standard chemoradiation. Analysis of Src activation in recurrent tumors warrants further investigation.
In summary, our results suggest that bosutinib monotherapy is not associated with antitumor activity in recurrent GBM patients. We confirmed Src upregulation and activation in pre-treatment GBM tumors. Our correlative findings, along with the role of Src in glioma cell invasion, suggest Src warrants continued investigation as a target for therapy. Therapeutic efficacy may be realized in combination with other therapies, such as anti-angiogenic agents.
Acknowledgments
J.W.T. is supported by U.S. National Cancer Institute of the National Institutes of Health under Award Number K12CA090354. A.S.C. is supported by a Richard B. Simches Scholars Award and an Early Career Research Award from The Ben and Catherine Ivy Foundation. We thank Gino Ferraro and Carolyn Smith for assistance with immunohistochemistry.
Footnotes
Conflict of Interest
J. Taylor, J. Dietrich, E. Gerstner, A. Norden, M. Rinne, D. Cahill, A. Stemmer-Rachamimov, P. Wen, R. Betensky, and A. Chi have no conflict of interest.
T. Batchelor has received research funding from Pfizer, Incorporated
D. Giorgio, K. Snodgrass, and A. Randall are employed by Pfizer, Incorporated
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiation Oncology G, National Cancer Institute of Canada Clinical Trials G Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The lancet oncology. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. doi:10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 4.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. doi:10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nature cell biology. 2002;4:632–638. doi: 10.1038/ncb829. doi:10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 6.Irby RB, Yeatman TJ. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer research. 2002;62:2669–2674. [PubMed] [Google Scholar]
- 7.Du J, Bernasconi P, Clauser KR, Mani DR, Finn SP, Beroukhim R, Burns M, Julian B, Peng XP, Hieronymus H, Maglathlin RL, Lewis TA, Liau LM, Nghiemphu P, Mellinghoff IK, Louis DN, Loda M, Carr SA, Kung AL, Golub TR. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nature biotechnology. 2009;27:77–83. doi: 10.1038/nbt.1513. doi:10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angers-Loustau A, Hering R, Werbowetski TE, Kaplan DR, Del Maestro RF. SRC regulates actin dynamics and invasion of malignant glial cells in three dimensions. Molecular cancer research : MCR. 2004;2:595–605. [PubMed] [Google Scholar]
- 9.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Molecular cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 10.Weissenberger J, Steinbach JP, Malin G, Spada S, Rulicke T, Aguzzi A. Development and malignant progression of astrocytomas in GFAP-v-src transgenic mice. Oncogene. 1997;14:2005–2013. doi: 10.1038/sj.onc.1201168. doi:10.1038/sj.onc.1201168. [DOI] [PubMed] [Google Scholar]
- 11.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. Journal of medicinal chemistry. 2004;47:6658–6661. doi: 10.1021/jm049486a. doi:10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi E, Stupp R, van den Bent MJ, van Herpen C, Laigle Donadey F, Gorlia T, Hegi M, Lhermitte B, Strauss LC, Allgeier A, Lacombe D, Brandes AA. EORTC 26083 phase I/II trial of dasatinib in combination with CCNU in patients with recurrent glioblastoma. Neuro-oncology. 2012;14:1503–1510. doi: 10.1093/neuonc/nos256. doi:10.1093/neuonc/nos256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reardon DA, Vredenburgh JJ, Desjardins A, Peters KB, Sathornsumetee S, Threatt S, Sampson JH, Herndon JE, 2nd, Coan A, McSherry F, Rich JN, McLendon RE, Zhang S, Friedman HS. Phase 1 trial of dasatinib plus erlotinib in adults with recurrent malignant glioma. Journal of neuro-oncology. 2012;108:499–506. doi: 10.1007/s11060-012-0848-x. doi:10.1007/s11060-012-0848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lassman ABWM, Gilbert M, Aldape K, Wright J, Wagner H, Brachman D, Malkin M, Mehta M. Phase 2 trial of dasatinib in patients with recurrent glioblastoma (RTOG 0627) Neuro-oncology. 2008;10 doi: 10.1093/neuonc/nov011. abstract MA-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lassman ABWM, GIlbert MR, Aldape KD, Beumer JJ, Wright J, Takebe N, Puduvalli VK, Hormigo A, Gaur R, Werner-Wask M, Mehta MP. Phase II trial of dasatinib in target selected patients with recurrent glioblastoma (RTOG 0627). Neuro-oncology. 2011;13:iii64. doi: 10.1093/neuonc/nov011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu-Emerson C, Norden AD, Drappatz J, Quant EC, Beroukhim R, Ciampa AS, Doherty LM, Lafrankie DC, Ruland S, Wen PY. Retrospective study of dasatinib for recurrent glioblastoma after bevacizumab failure. Journal of neuro-oncology. 2011;104:287–291. doi: 10.1007/s11060-010-0489-x. doi:10.1007/s11060-010-0489-x. [DOI] [PubMed] [Google Scholar]
- 17.Cortes JE, Kantarjian HM, Brummendorf TH, Kim DW, Turkina AG, Shen ZX, Pasquini R, Khoury HJ, Arkin S, Volkert A, Besson N, Abbas R, Wang J, Leip E, Gambacorti-Passerini C. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–4576. doi: 10.1182/blood-2011-05-355594. doi:10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes JE, Kim DW, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicius L, Malhotra H, Powell C, Gogat K, Countouriotis AM, Gambacorti-Passerini C. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486–3492. doi: 10.1200/JCO.2011.38.7522. doi:10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, Frost P, Ye F, Boschelli DH, Boschelli F. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer research. 2003;63:375–381. [PubMed] [Google Scholar]
- 20.Puttini M, Coluccia AM, Boschelli F, Cleris L, Marchesi E, Donella-Deana A, Ahmed S, Redaelli S, Piazza R, Magistroni V, Andreoni F, Scapozza L, Formelli F, Gambacorti-Passerini C. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer research. 2006;66:11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. doi:10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 21.Amsberg GK, Schafhausen P. Bosutinib in the management of chronic myelogenous leukemia. Biologics : targets & therapy. 2013;7:115–122. doi: 10.2147/BTT.S30182. doi:10.2147/BTT.S30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 23.Daud AI, Krishnamurthi SS, Saleh MN, Gitlitz BJ, Borad MJ, Gold PJ, Chiorean EG, Springett GM, Abbas R, Agarwal S, Bardy-Bouxin N, Hsyu PH, Leip E, Turnbull K, Zacharchuk C, Messersmith WA. Phase I study of bosutinib, a src/abl tyrosine kinase inhibitor, administered to patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:1092–1100. doi: 10.1158/1078-0432.CCR-11-2378. doi:10.1158/1078-0432.CCR-11-2378. [DOI] [PubMed] [Google Scholar]
- 24.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled clinical trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 26.Lund CV, Nguyen MT, Owens GC, Pakchoian AJ, Shaterian A, Kruse CA, Eliceiri BP. Reduced glioma infiltration in Src-deficient mice. Journal of neuro-oncology. 2006;78:19–29. doi: 10.1007/s11060-005-9068-y. doi:10.1007/s11060-005-9068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huveldt D, Lewis-Tuffin LJ, Carlson BL, Schroeder MA, Rodriguez F, Giannini C, Galanis E, Sarkaria JN, Anastasiadis PZ. Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PloS one. 2013;8:e56505. doi: 10.1371/journal.pone.0056505. doi:10.1371/journal.pone.0056505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(2008) Bosutinib (SK-606) Investigator's Brochure.