Abstract
Background
We reported that DNA-PK is critical for the expression of NF-κB-dependent genes in TNF-α-treated glioblastoma cells, suggesting an involvement in inflammatory diseases.
Objective
To investigate the role of DNA-PK in asthma.
Methods
Cell culture and ovalbumin or house dust mite (HDM)-based murine asthma models were used in this study.
Results
DNA-PK was essential for monocyte adhesion to TNF-α–treated-endothelial cells. Administration of the DNA-PK inhibitor, NU7441, reduced airway eosinophilia, mucus hypersecretion, airway hyperresponsiveness (AHR), and OVA-specific IgE production in mice that were pre-challenged with ovalbumin. Such effects correlated with a marked reduction in lung VCAM-1 expression and production of several cytokines, including IL-4/IL-5/IL-13/eotaxin/IL-2/IL-12 and the chemokines MCP-1/KC with negligible effect on IL-10/IFN-γ production. DNA-PK inhibition, by gene heterozygosity, also prevented manifestation of asthma-like traits. These results were confirmed in a chronic model of asthma using HDM, a human allergen. Remarkably, such protection occurred without causing SCID. Adoptive transfer of Th2-skewed OT-II-WT CD4+ T cells reversed IgE and Th2 cytokine production but not AHR in ovalbumin-challenged DNA-PKcs+/− mice. DNA-PK inhibition reduced IL-4/IL-5/IL-13/eotaxin/IL-8/MCP-1 production without affecting IL-2/IL-12/IFN-γ/IP-10 production in CD3/CD28-stimulated human CD4+T cells potentially by blocking expression of gata-3. These effects occurred without significant reductions in T-cell proliferation. In mouse CD4+T cells, DNA-PK inhibition, in vitro, severely blocked C3/CD28-induced gata-3 and t-bet expression in CD4+T cells and prevented differentiation of Th1 and Th2 cells under respective Th1- and Th2-skewing conditions.
Conclusion
Our results suggest DNA-PK as a novel determinant of asthma and a potential target for the treatment of the disease.
Keywords: DNA-PK, asthma, GATA-3, Th2 cytokines, adhesion molecules, eosinophilia, SCID, T cell receptor
INTRODUCTION
DNA-dependent protein kinase (DNA-PK) is important for repairing double-stranded DNA breaks (DSBs) that are caused by DNA-damaging agents through non-homologous end joining (NHEJ) 1, 2. DNA-PK is composed of a 450-kDa catalytic subunit (DNA-PKcs) and two DNA-binding subunits (Ku70 and Ku80). DNA-PKcs phosphorylates a number of proteins, including p53, transcription factors, RNA polymerase, histones, and Ku70/Ku80 2. An important question in the research on DNA-PK is why do humans have high levels of the kinase although only less than 1% is needed to repair DNA damage 1, 3? Recently, our laboratory reported in a pioneering study that DNA-PK is required for the expression of vascular cell adhesion molecule (VCAM)-1, as well as many other nuclear factor (NF)-κB-dependent genes that are downstream of TNF-α 4. We provided evidence that the mechanism by which DNA-PK regulates NF-κB-dependent genes in response to TNF-α or IL-1β may be dependent upon its ability to phosphorylate the p50 subunit of NF-κB at serine residue-20. The phosphorylation of this site appears to greatly influence the interaction between the p50 and p65 subunits of NF-κB, and it subsequently exerts a dominant role on the ability of the transcription factor to bind its κB sequence to the VCAM-1 gene promoter 4. Collectively, our reported results elucidated a novel mechanism by which DNA-PK positively regulates the expression of NF-κB-dependent genes by regulating p50 NF-κB, which is a subunit that has largely been regarded as a p65-aiding or gene-suppressing subunit. More importantly, these findings suggested a possible role for DNA-PK in inflammatory diseases, such as asthma.
The pathophysiology of asthma involves many cellular and subcellular events 5. Exposure to allergens and other environmental factors triggers various responses that ultimately lead to bronchial constriction and impaired airflow in the lungs 5. Airway inflammation appears to be central to the pathogenesis of allergic asthma, and this inflammation is thought to partly result from an overproduction of inflammatory factors, such as adhesion molecules, cytokines, and chemokines, as well as a number of enzymes that are involved in altering tissue homeostasis, such as nitric oxide synthases 5. Many of the aforementioned inflammatory factors are highly dependent on NF-κB-associated signal transduction 6. The role of NF-κB in asthma is demonstrated by the observation that p50 NF-κB-deficient mice do not mount the typical eosinophilic airway inflammation in an ovalbumin-induced asthma model 7. This defect is associated with the requirement of p50 NF-κB for the expression of gata-3, whose product is essential for the expression of Th2 cytokines, including IL-4, IL-5, and IL-13 8. Consequently, factors that regulate NF-κB including its p50 subunit such as DNA-PK may influence the onset of the disease.
In the current study, we tested the hypothesis that DNA-PK plays a critical role in the onset of asthma through the regulation of inflammatory factors. An in vitro system of inflammation, acute and chronic animal models of asthma, adoptive transfers, and anti-CD3- and anti-CD28-treated CD4+T cells including those isolated from human subjects were used to conduct the study. The results suggested that DNA-PK is an important participant in the pathogenesis of asthma independent of its role in T and B cells development. In addition, DNA-PK may be a viable therapeutic target for the treatment of the disease.
METHODS
Animals
Male C57BL/6J mice (6–8 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME, USA). C57BL/6 DNA-PKcs+/− mice were generated by backcrossing the mice under the C57BL/6xSV129 mixed background with C57BL/6 wild-type (WT) mice for at least nine generations. The last generation was interbred to generate the C57BL/6 DNA-PKcs−/− mice. Mice were kept in a specific pathogen-free facility at LSUHSC and allowed unlimited access to sterilized chow and water. Experimental protocols were approved by the LSUHSC Animal Care and Use Committee.
Ovalbumin (OVA) sensitization and challenge, house dust mite extracts (HDM) challenge, and AHR measurement
Mice were sensitized to 100 μg of Grade V chicken OVA (Sigma-Aldrich, St. Louis MO) mixed with 2 mg aluminum hydroxide in saline by i.p. injection twice, once a week as previously described 9. Mice were then challenged with aerosolized 3% OVA for 30 min once for the single challenge protocol or once daily for three days for the multiple challenge protocol. Isoflurane anesthetized mice were challenged intranasally with 25 μl of saline or 1 mg/ml whole HDM (Dermatophagoides pteronyssinus) extract (Greer Labs, Lenoir, NC), 3 times per week for 5 weeks. Intraperitoneal administration of the DNA-PK inhibitor, NU7441 (Tocris Bioscience, Bristol, UK), or vehicle (saline) was conducted 30 min after OVA or HDM challenge. The dosage and route of NU7441 administration were determined according to the study by Zhao et al.10, which examined the plasma pharmacokinetics of the drug. Mice were sacrificed 48 h later for bronchoalveolar lavage (BAL) or lung fixation and processing. Some mice were subjected to AHR measurements 24 h after OVA challenge as described 11.
Organ recovery, tissue processing, cell staining, Th2 cytokine and OVA-specific IgE assessments, FACS analysis, and immunohistochemistry (IHC)
Lungs from sacrificed animals were fixed with formalin for histological analysis or subjected to BAL for cell counting and cytokine assessments as described 11. Tissue sections were subjected to hematoxylin and eosin (H&E) or Periodic Acid-Schiff (PAS) staining using standard protocols. Some sections were subjected to IHC, as previously described 11, with antibodies to VCAM-1 (Biocare Medical, Concord, CA). BAL fluids were subjected to cyto-spin, and the cells were stained with Diff-Quik (IMEB Inc, San Marcos, CA) for inflammatory cell assessments. Cytokine assessments were conducted using the Bio-Rad Bioplex or the Millipore MILLIPLEX MAP Human Cytokine/Chemokine system for mouse or human, according to the manufacturer’s instructions. OVA-specific IgE levels were quantified by sandwich ELISA (Serotec, Raleigh, NC). Spleen weights from different groups were recorded and total splenic cell count was assessed using a cell counter (Nexelcom cellometer, Lawrence, MA). For the populations of CD4+ and CD8+T cells, splenocytes were stained with anti-mouse CD3e allophycocyanin (145-2c11-APC), anti-mouse CD8a (Ly-2-53-6.7-FITC), and anti-mouse CD4 phycoerythrin (GK1.5-PE) followed by FACS analysis. B cell populations were determined by staining with anti-mouse CD19 (1D3-FITC) and anti-Hu/Mo CD45R (B220) (RA3-6B2). Also single cell suspensions from spleen and lung draining lymph nodes were generated and stained with anti-mouse CD19 (1D3-APC) and anti-mouse CD138 (281–2- PE) for plasma B-cells. All antibodies that were used for FACS analysis were from BD Biosciences (San Jose, CA). The multiplex assay and FACS were conducted at the LSUHSC Comprehensive Alcohol Research Center Core. Two de-identified lung specimens from individuals who died of severe asthma and a normal lung from an individual who died from an asthma-unrelated condition were acquired from the LSUHSC Pathology Department. Sections from these samples and from the different experimental groups were subjected to IHC, as previously described 11, with antibodies to VCAM-1 (Biocare Medical, Concord, CA).
Cell culture, treatments, immunoblot analysis, and cell adhesion assay
Human umbilical vein endothelial cells (HUVECs) and the monocytic cell line, U937, were purchased from Life Technologies Inc. (Portland, OR) and ATCC (Manassas, VA), respectively. Lung smooth muscle cells (LSMCs) were isolated from WT or DNA-PKcs+/− mice, as previously described 11. Knockdown of DNA-PKcs in HUVECs was achieved using a lentiviral vector (sc-35200-v; Santa Cruz Biotechnology, Santa Cruz, CA) encoding a DNA-PKcs-targeting shRNA. The treatments with TNF-α, preparation of protein extracts, and immunoblot analysis with antibodies to VCAM, actin, or GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) were conducted, as described 4. For the cell adhesion assay, HUVECs were stimulated with TNF-α for 18 h before the addition of calcein-AM (Life Technologies)-labeled U937 cells. Non-adherent monocytes were removed, and fluorescence was measured.
Human blood collection, mouse splenocyte preparation, CD4+T cell purification, TCR stimulation, and RT-PCR
CD4+T cell isolation, stimulation, measurement of proliferation, and RT-PCR were as described 11, 12
T-helper cells skewing, Th1 and Th2 cytokines assessment, and adoptive transfer
CD4+ T cells were purified from a single cell suspension procured from spleens of OVA-sensitized wild-type or DNA-PKcs+/− mice by negative selection with the Easy Sep kit from Stem Cell Technologies (Vancouver, Canada). All CD4+ T cells were stimulated on coated plates (1 μg/ml anti-CD3 and 0.5ug/ml anti-CD28). CD4+ subsets were generated by culture under the following conditions: Th1, IL-12 (10 ng/ml; R&D Systems), and anti–IL-4 (10 μg/ml; clone 11B11); Th2, IL-4 (100 U/ml; R&D Systems) and anti–IL-12 (10 μg/ml; clone C17.8). Some groups of wild-type CD4+ subsets were treated with NU7441. On day 3 post-stimulation, cells were re-plated with IL-2 alone for an additional 2 days. The extracted total RNA from the cells was used for the generation of cDNA using reverse transcriptase III (Invitrogen) and analyzed by real-time PCR using primers specific for mouse gata-3 11, Il-4 (5′-CCCCAGCTAGTTGTCATCCTG-3′ and 5′-CGCATCCGT GGATATGGCTC-3′), Il-5 (5′-GGGCTTCCTGCTCCTATCTA-3′ and 5′-CAGTCATGGCA CAGTCTGAT-3′), Il-13 (5′-GGTCCACACAGGGCAACT-3′ and 5′-AATAAGATCAAGAAGAAATGTGCTCAA-3′); Ifn-γ (5′-TCAAGTGGCATAGATGTGGA-AGAA-3′ and 5′-TGGCTCTGCAGGATTTTCATG-3′) and t-bet (5′-GCCAGGGAA-CCGCTTATATG-3′and 5′-GACGATCATCTGGGTCACATTGT-3′). In addition, OT-II mice on a C57BL/6 background (Jackson Laboratory, Bar Harbor, ME) were sacrificed and splenic CD4+ T cells were skewed towards a Th2 population in the presence of ovalbumin peptide (Fremont, CA). The produced Th2-like cells were injected i.v into the tail vein of recipient mice (1×106 cells/ mouse). All mice were subjected to aerosolized ovalbumin challenge daily for 4 days. AHR was assessed 24 h later and all mice were sacrificed at 48 h after the last challenge.
Data Analysis
All data are expressed as mean ± standard deviation (S.D.) of values from at least six mice per group, unless stated otherwise, or triplicate conditions when cells were used. The Prism software (GraphPad, San Diego, CA) was used to analyze the differences between experimental groups by one-way analysis of variance, followed by Tukey’s multiple comparison tests. For some results, analysis was conducted using unpaired Student’s t-test.
RESULTS
DNA-PK protein level and function are critical for VCAM-1 expression and are required for the adhesion of inflammatory cells to endothelial cells upon TNF-α treatment
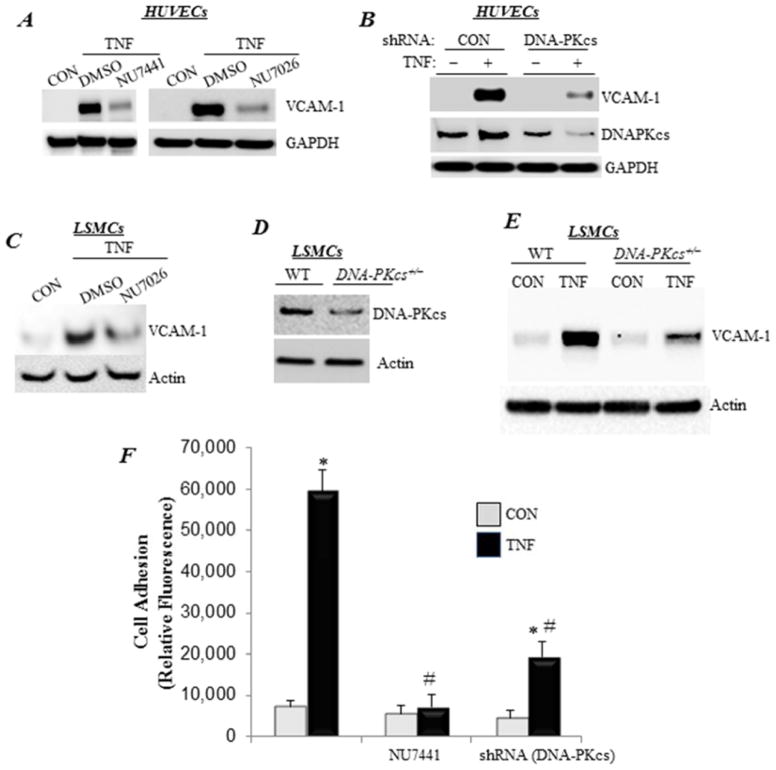
Treatment of HUVECs with TNF-α induced a robust expression of VCAM-1 (Fig. 1A), which was significantly reduced in the presence of DNA-PK inhibitors, such as NU7441 or NU7026. In addition, partial knockdown of the catalytic subunit of DNA-PK (DNA-PKcs) achieved a similar effect as that by the drugs (Fig. 1B), thus demonstrating specificity of the effects. The connection between DNA-PK and VCAM-1 expression was not limited to endothelial cells. In mouse LSMCs, pharmacological inhibition of DNA-PK markedly reduced VCAM-1 expression upon TNF-α exposure (Fig. 1C). DNA-PKcs heterozygosity, which significantly reduced the expression of the kinase (Fig. 1D)13, decreased the expression of TNF-α-induced VCAM-1 in LSMCs (Fig. 1E). These results suggest that DNA-PK may play an important role in inflammatory cell recruitment. We next utilized an in vitro cell adhesion assay using U937, a human monocytic cell line, to test this hypothesis. TNF-α induced a high level of monocyte adhesion (Fig. 1F), whereas DNA-PKcs knockdown or inhibition with NU7441 dramatically reduced such adhesion. These results provide preliminary evidence on the involvement of DNA-PK in inflammatory diseases.
Figure 1. DNA-PK protein level and function are required for VCAM-1 expression and adhesion of inflammatory cells to endothelial cells upon TNF-α treatment.
(A) HUVECs were pretreated with 1 μM NU7026 or NU7441 or 0.1% DMSO for 30 min before treatment with TNF-α (TNF) for 24 h in the continued presence of the drugs. Protein extracts were subjected to immunoblot analysis with antibodies to VCAM-1 or GAPDH. (B) HUVECs were transduced with a lentiviral vector encoding control or shRNA targeting DNA-PKcs. Cells were then treated with TNF-α for 24 h, after which protein extracts were subjected to immunoblot analysis with antibodies to VCAM-1, DNA-PKcs, or GAPDH. (C) LSMCs were pretreated with NU7026 or 0.1% DMSO for 30 min before treatment with TNF-α for 24 h in the continued presence of the drugs. Protein extracts were subjected to immunoblot analysis with antibodies to VCAM-1 or actin. (D) Immunoblot analysis of protein extracts from WT or DNA-PKcs+/− LSMCs with antibodies to DNA-PKcs or actin. (E) WT or DNA-PKcs+/− LSMCs were treated with TNF-α for 24 h. VCAM-1 protein expression was assessed by immunoblot analysis. (F) HUVECs, subjected to DNA-PKcs knockdown or treated with NU7441, were stimulated with TNF-α for 18 h before the addition of calcein-AM-labeled U937 cells. Non-adherent monocytes were removed, and fluorescence was measured. Fluorescence is expressed in arbitrary units. *, difference from untreated cells; #, difference from TNF-α-treated cells; p<0.01.
DNA-PK is involved in allergic lung inflammation, and its inhibition reduces eosinophilia, mucus hypersecretion, AHR, and IgE production in a mouse model of asthma
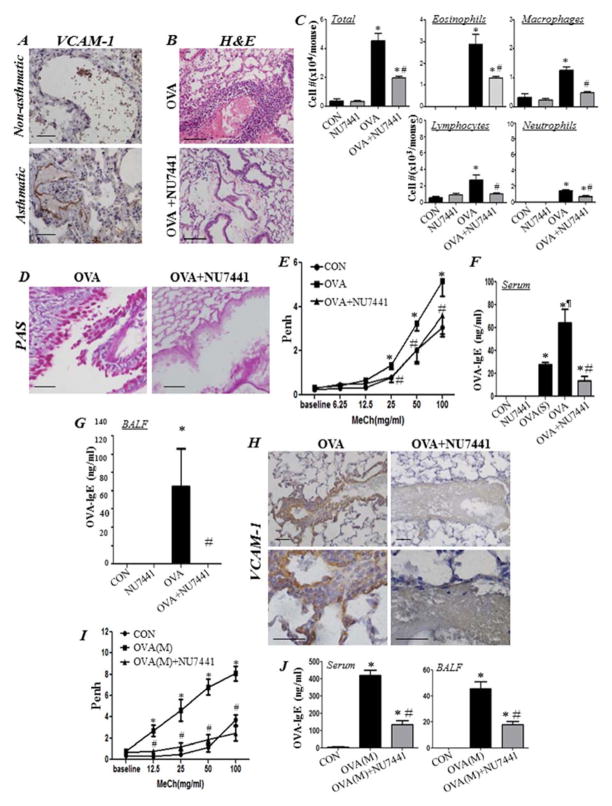
Figure 2A shows robust expression of VCAM-1 primarily in endothelial cells of lung tissue from an individual who died from asthma. Challenge of OVA-sensitized C57BL/6 mice to aerosolized OVA induced the substantial infiltration of eosinophils into the lungs (Fig. 2B–C). Moreover, this extent of eosinophilia was significantly reduced in mice that received a single i.p. injection of NU7441 (5 mg/kg) 30 minutes after OVA challenge (Fig. 2B–C). NU7441 administration also reduced the total number of inflammatory cells, including lymphocytes, monocytes, macrophages, and neutrophils that were recruited to the lungs of OVA-challenged mice (Fig. 2C). Such effects of the drug were also mirrored by a significant reduction in mucus hypersecretion in the lungs of OVA-challenged animals (Fig. 2D). Additionally, NU7441 administration completely blocked AHR to inhaled methacholine 24 h after OVA challenge (Fig. 2E). We next assessed whether the protective effects of NU7441 against eosinophilia and mucus production coincided with a blockade of IgE production, a major hallmark of human asthma. OVA sensitization was sufficient to induce substantial levels of IgE in sera of mice, which significantly increased upon OVA challenge (Fig. 2F). DNA-PK inhibition severely reduced the production of OVA-specific IgE in sera of treated animals (Fig. 2F). Remarkably, treatment with the drug caused a complete inhibition of BALF OVA-specific IgE (Fig. 2G). OVA challenge induced a robust increase in VCAM-1 expression, primarily in endothelial cells and, to a lesser extent, in inflammatory and smooth muscle cells (Fig. 2H). This expression was markedly reduced in the lungs of mice that were treated with NU7441 after OVA challenge. We next examined whether DNA-PK inhibition blocks AHR and IgE production upon repeated challenges with OVA. Fig. 2I shows that a single administration of NU7441 was sufficient to block AHR manifestation after multiple challenges with OVA. NU7441 treatment also caused a significant reduction in OVA-specific IgE levels both in sera and BALF of treated animals (Fig. 2J).
Figure 2. DNA-PK is involved in allergic lung inflammation, and its inhibition reduces eosinophilia, mucus hypersecretion, AHR, and IgE production in a mouse model of asthma.
(A) Lung tissue sections from an asthmatic and a non-asthmatic individual were subjected to IHC staining with antibodies to human VCAM-1. WT mice were subjected to OVA sensitization followed by challenge or left untreated. Mice were administered i.p. 5 mg/kg NU7441 or saline thirty minutes after OVA challenge. Control mice received either 5 mg/kg of NU7441 or saline. Mice were sacrificed 48 h later. (B) H&E stain of lung sections. (C) Assessment of differentially stained cells in BALF. Data are expressed as total number of cells per mouse. Data are means ± SD of values from at least six mice per group. *, difference from unchallenged mice; #, difference from OVA-challenged mice; p < 0.01. (D) PAS staining of lung sections. (E) Assessment of Penh recorded 24 h after a single OVA challenge using whole body plethysmography upon exposure to the indicated concentrations of aerosolized methacholine (MeCh). Results are plotted as maximal fold increase of Penh relative to baseline and expressed as mean ± SEM where n=6 mice per group. *, difference from control mice; #, difference from OVA-challenged mice, p < 0.01. Assessment of sera (F) or BALF (G) collected from the different experimental groups for OVA-specific IgE. *, difference from control mice; #, difference from OVA-challenged mice; ¶, difference from OVA-sensitized but not challenged (OVA(S)) mice, p < 0.01. (H) IHC staining of lung sections with antibodies to mouse VCAM-1. Bars: 5 μm. (I) Assessment of Penh after multiple OVA challenges (M) and a single NU7441 administration thirty minutes after the last challenge; measurements were conducted 24 h after the last OVA challenge. Results are plotted and analyzed as in (E). (J) Assessment of OVA-specific IgE in sera or BALF collected from the experimental groups described in (I). *, difference from control mice, p < 0.01; #, difference from OVA-challenged mice; ¶, difference from OVA-sensitized but not challenged mice (OVA(S)), p < 0.01.
DNA-PK inhibition blocks OVA-induced asthma-like traits by reducing the production of Th2 cytokines in the mouse model of asthma
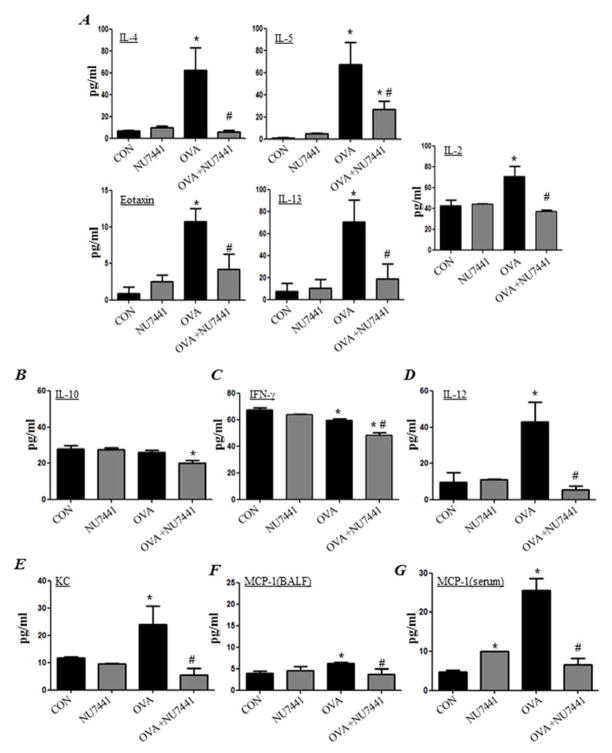
OVA exposure induced the production of IL-2, IL-4, IL-5, IL-13, and eotaxin (Fig. 3A). Treatment of mice with NU7441 after OVA challenge significantly reduced or blocked the production of the aforementioned cytokines and chemokines with little to no effect on IL-10 (Fig. 3B) and IFN-γ levels (Fig. 3C). However, the OVA-induced increase in IL-12 was completely abrogated by treatment with NU7441 (Fig. 3D). Treatment with NU7441 also blocked the production of keratinocyte-derived chemokine (KC) (Fig. 3E) in the lungs of OVA-challenged mice. The levels of MCP-1 barely increased above baseline in the lungs of OVA-challenged mice. Any increase that occurred was completely reduced by NU7441 treatment (Fig. 3F). In sera of OVA-challenged mice, however, MCP-1 levels were more pronounced and treatment with NU7441 completely blocked such increased production (Fig. 3G).
Figure 3. Effect of DNA-PK inhibition on OVA-induced production of Th1 and Th2 cytokines.
Assessment of BALF for IL-4, IL-5, eotaxin, IL-13, or IL-2 (A); IL-10 (B); IFN-γ (C); IL-12 (D); KC (E); or MCP-1 (F). Assessment of sera for MCP-1 (G). Data are means ± SD of values from at least six mice per group. *, difference from unchallenged mice; #, difference from OVA-challenged mice; p < 0.01.
DNA-PKcs gene heterozygosity prevents eosinophilia, mucus hypersecretion, production of Th2 cytokine, increase in OVA-specific IgE levels, and AHR in an animal model of asthma
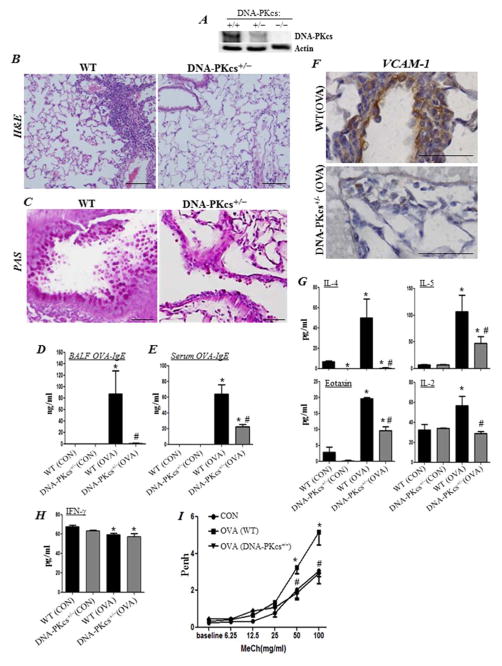
Similarly to cells in culture, DNA-PKcs gene heterozygosity reduces the expression of the kinase in whole tissue (Fig. 4A). Fig. 4B shows that DNA-PKcs heterozygosity markedly blocked OVA-challenge-mediated eosinophilia. Concomitantly, mucus production was reduced in the DNA-PKcs+/− mice (Fig. 4C). The blockade of airway eosinophilia and mucus production in OVA-challenged DNA-PKcs+/− mice was associated with a complete blockade in OVA-specific IgE production in the lungs (Fig. 4D), as well as its significant reduction in the sera of treated animals (Fig. 4E). VCAM-1 expression was also reduced in the lungs of OVA-challenged DNA-PKcs+/− mice (Fig. 4F). This was associated with a marked reduction in the production of IL-4, IL-5, IL-2, and eotaxin (Fig. 4G). However, DNA-PKcs heterozygosity exerted no effect on the levels of IFN-γ in control or OVA-challenged mice (Fig. 4H). Additionally, DNA-PKcs heterozygosity completely blocked AHR to increasing doses of methacholine in OVA-challenged mice (Fig 4I). These results confirm those that were attained using the kinase inhibitor and provide support to the notion that the effect of DNA-PK inhibition on asthma is specific.
Figure 4. DNA-PKcs heterozygosity is sufficient to reduce asthma-associated traits.
WT and DNA-PKcs+/− mice were subjected to OVA sensitization followed by challenge or left untreated as described above. (A) Immunoblot analysis of lung tissue with antibodies to DNA-PKcs or actin. H&E (B) and PAS (C) staining of lung sections. Assessment of BALF (D) or sera (E) collected from the different experimental groups for OVA-specific IgE; *, difference from unchallenged mice; #, difference from OVA-challenged WT mice; p < 0.01. (F) IHC staining of lung sections with antibodies to mouse VCAM-1; bars: 5 μm. BALF from the different experimental groups were assessed for IL-4, IL-5, eotaxin, IL-2 (G) or IFN-γ (H). (I) Assessment of Penh using whole body plethysmography. Results are plotted as maximal fold increase of Penh relative to baseline and expressed as mean ± SEM where n=6 mice per group. *, difference from control WT mice; #, difference from OVA-challenged WT mice, p < 0.01.
DNA-PK inhibition pharmacologically or by gene heterozygosity blocks asthma-like manifestation in a HDM asthma model
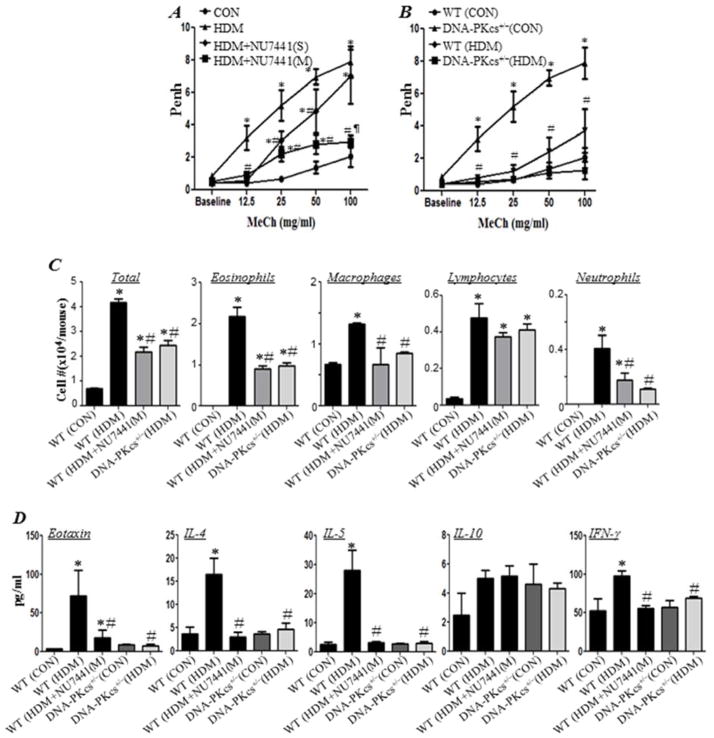
Despite the success of the OVA asthma model in mechanistic studies, it still has many limitations including the development of tolerance during chronic exposure and the requirement for systemic priming with adjuvant. A better model that more closely resembles human asthma is inhaled delivery of HDM. This model involves mucosal sensitization within the lungs with a true allergen to induce Th2-driven inflammation 14. Fig. 5A shows that NU7441 administration concomitantly with HDM exposure markedly reduced AHR to inhaled methacholine. Interestingly, a single administration of the DNA-PK inhibitor at the end of the HDM exposure protocol provided a significant protection against AHR. Similarly, DNA-PKcs heterozygous provided protection against AHR in HDM-exposed mice suggesting specificity of the effect (Fig. 5B). Protection against AHR by NU7441 administration, either concomitantly with HDM exposure or at the end of allergen exposure, or by gene heterozygosity was accompanied by a substantial reduction in eosinophilia and overall lung cellularity (Fig. 5C). These effects were also mirrored by a major decrease in the Th2 cytokines eotaxin, IL-4, and IL-5 but not IL-10 (Fig. 5D). The moderate HDM-mediated increase in IFN-γ was also blocked by DNA-PK inhibition.
Figure 5. DNA-PK inhibition pharmacologically or by gene heterozygosity blocks asthma-like manifestation in a chronic HDM asthma model.
WT or DNA-PKcs+/− mice were subjected to a chronic i.n. challenge with HDM. WT mice were administered NU7441 (5mg/kg) either 30 minutes after each HDM exposure (M) or once 30 minutes after the last HDM exposure (S). Twenty four hours after the last HDM exposure, mice were subjected to Penh measurements then sacrificed 24 h later. (A) Penh of HDM-challenged WT mice that received NU7441 with HDM exposure. (B) Penh of HDM-challenged WT or DNA-PKcs+/− mice; note the measurements for HDM-exposed and control mice are the same as those depicted in (A). Results are plotted as maximal fold increase of Penh relative to baseline and expressed as mean ± SEM where n=6 mice per group. (C) Cells of BAL fluids were differentially stained, and total, eosinophils, macrophages, lymphocytes, and neutrophils were counted. Data are expressed as total number of cells per mouse. Data are means ± SD of values from at least six mice per group. (D) BAL fluids from the different experimental groups were assessed for eotaxin, IL-4, IL-5, IL-10 and IFN-γ. *, difference from WT control mice; #, difference from WT HDM-challenged mice; ¶, difference from WT HDM-challenged mice treated with single administration of NU7441 (HDM+NU7441(S)); p <0.01.
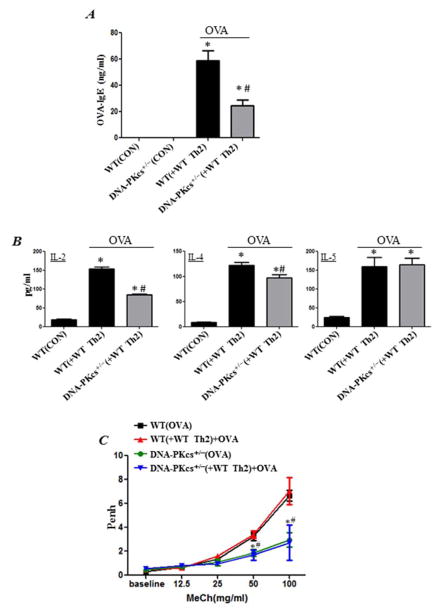
Adoptive transfer of in vitro Th2-skewed CD4+ ++ T cells partially reverses IgE and Th2 cytokine production but not AHR in OVA-exposed DNA-PKcs+/− mice
To begin understanding the role of DNA-PK in immune vs. structural cells during an allergic response, we examined whether adoptive transfer of Th2-skewed CD4+ T cells isolated from OT-II mice reverses asthma-like traits in naïve DNA-PKcs+/− mice upon exposure to the OVA. The results shows that the adoptive transfer was sufficient to partially reverse IgE production in DNA-PKcs+/− mice (Fig. 6A) and to induce production of Th2 cytokines such as IL-2, IL-4, and IL-5 (Fig. 6B) upon exposure to aerosolized OVA to levels equivalent or close to those observed in the WT counterparts. Interestingly, however, although the cell transfer induced AHR in OVA-treated WT mice, it was not sufficient to reverse such response in DNA-PKcs+/− mice (Fig. 6C). The number of eosinophils was minimal in lungs of WT mice upon OVA exposure and as such it was difficult to determine whether the adoptive transfer was sufficient to restore eosinophilia in DNA-PKcs+/− mice. Nevertheless, these results suggest an important role for DNA-PK in the T cell function as well as the function of non-immune cells such as smooth muscle cells that are necessary for AHR manifestation.
Figure 6. Adoptive transfer of in vitro Th2-skewed CD4+ T cells partially reverses IgE and Th2 cytokine production but not AHR in OVA-exposed DNA-PKcs+/− mice.
Splenic CD4+ T cells from sacrificed OT-II mice were skewed towards Th2 population in presence of OT-II peptide. 1×106 cells/ mouse were injected i.v into the tail vein of each recipient mouse. All mice were subjected to aerosolized OVA challenge daily for 4 days. Twenty four hour after the last OVA exposure, mice were subjected to Penh measurements then sacrificed 24 h later. (A) Assessment of sera for OVA-specific IgE. (B) Assessment of sera for IL-2, IL-4, or IL-5. *, difference from control WT mice; #, difference from WT mice receiving adoptive transfer (WT(+WT Th2)), p < 0.01. (C) Assessment of Penh in the different experimental groups 24 h after the last OVA challenge. Results are plotted as maximal fold increase of Penh relative to baseline and expressed as mean ± SEM where n=6 mice per group. *, difference from OVA-sensitized and challenged WT mice (WT(OVA)); #, difference from OVA-challenged WT-Th2 recipient mice (WT(+WT Th2)+OVA)); p < 0.01.
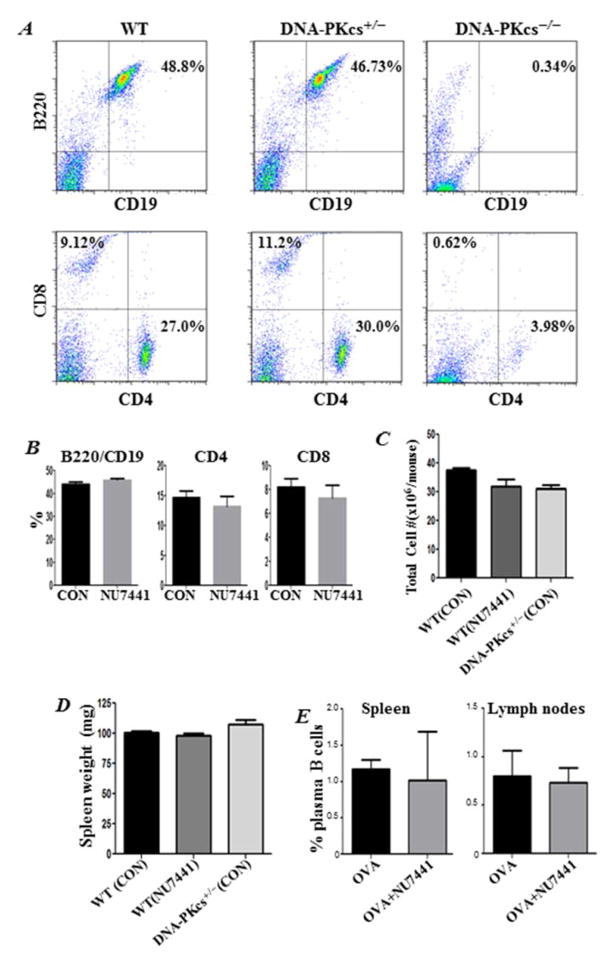
DNA-PK inhibition by gene heterozygosity or pharmacological drugs does not cause a SCID phenotype, alter the fate of plasma B cells, or change overall spleen weight
DNA-PK deficiency or mutations have been associated with a defective NHEJ and, thus, the development of a SCID phenotype in mice 3, 15. Figure 7A confirms this phenotype and shows that the spleens of DNA-PKcs−/− mice were almost completely devoid of B (B220+CD19+) and T (CD4+ and CD8+) cells. Interestingly, DNA-PKcs heterozygosity did not cause this defect, as DNA-PKcs+/− mice exhibited B and T cell populations comparable to those in WT (Fig. 7A), which is consistent with published reports 15. Similarly, inhibition of DNA-PK by NU7441 did not affect the levels of T and B cells (Fig. 7B). These minimal to no effects on T and B cells by DNA-PK inhibition were mirrored by a lack of an effect on the total number of spleen cells (Fig. 7D) and of the overall weight of spleens (Fig. 7E) in treated animals. DNA-PK inhibition by NU7441 or gene heterozygosity did not affect the fate of CD4+ T cells in spleens of OVA-challenged mice (data not shown). NU7441 treatment caused no effect on mouse CD19/CD138 plasma B cells in spleens or lung-draining lymph nodes of OVA-challenged animals (Fig. 7E). These results suggest that the role of DNA-PK in inflammation may be different from that in the generation of B and T cells, and that the basis for the protective effects conferred by its inhibition is not associated with a deficiency in B and T cells or the development of a SCID phenotype.
Figure 7. DNA-PK inhibition pharmacologically or by gene heterozygosity does not cause a SCID phenotype, alter the fate of plasma B cells, or change spleen weight.
(A) Single-cell suspensions from spleens of WT, DNA-PKcs+/−, or DNA-PKcs−/− mice were stained with fluorescently labeled antibodies to CD19 or B220. Cells were also stained with antibodies to CD4 or CD8 followed by FACS analysis. (B) WT mice received either saline or 5mg/kg NU7441 and sacrificed 48 h later. Single cell suspensions from spleens were subjected to FACS analysis with antibodies to B220, CD19, CD4 or CD8. (C) Comparison of total cell counts in spleens of DNA-PKcs+/− mice and WT mice that received saline or NU7441. (D) Comparison of spleen weight of the different groups. (E) Assessment of CD19/CD138-positive plasma B cells in single-cell suspensions from spleens or lung draining-lymph nodes from OVA-challenged mice that received saline or NU7441.
Differential roles of DNA-PK in the production of Th2 and Th1 cytokines upon TCR stimulation in human CD4+T cells without a prominent effect on their proliferation
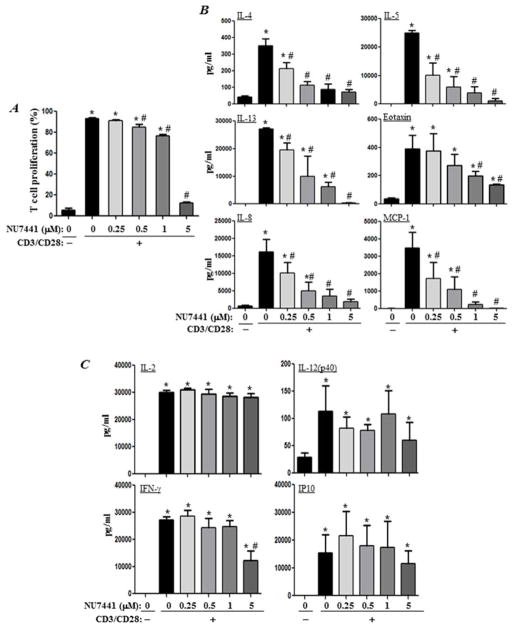
We next examined the effect of DNA-PK inhibition on the proliferation of CD4+T cells that were isolated from the peripheral blood of healthy human subjects. As shown in Figure 8A, DNA-PK inhibition with a low concentration (0.25 μM) of NU7441 exerted no effect on T-cell proliferation, whereas medium concentrations of the drug exerted little to moderate effects. At 0.5 and 1 μM, NU7441 treatment reduced cell proliferation by less than 15 and 25%, respectively. However, at 5 μM, the drug almost completely blocked the proliferation of CD4+T cells.
Figure 8. Effects of pharmacological inhibition of DNA-PK on CD4+T cell proliferation and production of Th1 and Th2 cytokines upon TCR stimulation.
(A) Negatively selected human CD4+T cells from healthy donors were stimulated in triplicates with antibodies to CD3/CD28 in the absence or presence of different concentrations of NU7441. Cell proliferation was assessed by CFSE staining. (B–C) CD4+T cells were stimulated as in (A) and cytokines were assessed in culture media 72 hours later. Data are given as means ± SD of values obtained from duplicates of the triplicates. *, difference from non-stimulated cells; #, difference from CD3/CD28- stimulated cells; p <0.01.
Next, we determined whether the reduction in cytokine production observed upon DNA-PK inhibition via drugs or gene heterozygosity in the animal model was strictly associated with an effect on T-cell function. NU7441 treatment caused a severe reduction in the production of the Th2 cytokines (IL-4, IL-5, IL-13, and eotaxin) and chemokines (IL-8 and MCP-1) in response to TCR stimulation (Fig. 8B). Interestingly, the production of IL-4, IL-5, IL-8, and MCP-1 was significantly reduced at a concentration of NU7441 that exerted no inhibitory effect on CD4+T-cell proliferation. Most prominently, IL-4 production was almost completely blocked at a concentration of the drug (0.5 μM) that minimally affected TCR-stimulated T-cell proliferation (less than 15%).
The effect of DNA-PK inhibition on cytokine production was not indiscriminate. Indeed, as shown in Figure 8C, NU7441 treatment did not affect the production of IL-2, IL-12(p40) and IP-10, with a minimal effect on IFN-γ at its highest concentration. These findings show that DNA-PK may have a specific role in allergen-mediated inflammation, partly through its control CD4+T-cell function, without affecting their proliferation.
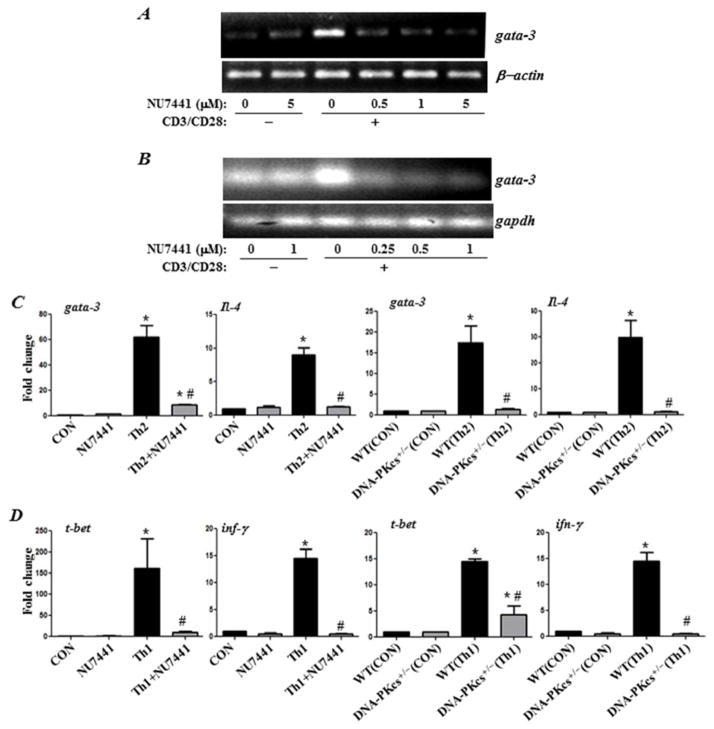
DNA-PK inhibition severely blocks CD3/CD28-induced gata-3 expression in naïve mouse or human CD4+T cells and prevents in vitro differentiation of mouse Th1 and Th2 cells under respective Th1- and Th2-skewing conditions
The transcription factor GATA-3 is considered the master regulator for the development of Th2 cells 16 as it binds and promotes the activation of the Il4/Il5/Il13 cytokine locus. Interestingly, GATA-3 expression was shown to be highly dependent on p50 NF-κB 8. As shown by us, DNA-PK regulates NF-κB-dependent genes by phosphorylating p50 NF-κB 4. We thus speculated that DNA-PK may be critical for the expression of GATA-3. Indeed, as shown in Figure 9A and B, DNA-PK function was critical for the efficient expression of gata-3 upon CD3/CD28 stimulation of mouse or human CD4+T cells. It is noteworthy that a complete inhibition of gata-3 expression was achieved with the lowest concentration (0.25 μM) of NU7441 tested, which caused no inhibition of T cell proliferation. These results may explain the modulatory effect of DNA-PK inhibition on IL-4, IL-5, and IL-13 production and the overall protective effect against the manifestation asthma traits in our animal model. We next examined whether DNA-PK inhibition differentially affects mouse CD4+ T cell differentiation into Th2 and Th1 cells when grown under respective Th2 and Th1-skewing conditions. The results show that DNA-PK inhibition, pharmacologically or by gene heterozygosity, completely blocked the differentiation of CD4+ T cells into Th2 cells as assessed by the expression of gata-3 and IL-4 mRNA (Fig. 9C) as well as IL-5 and IL-13 (data not shown). Surprisingly, however, DNA-PK inhibition was equally efficient in reducing Th1 cell differentiation as assessed by the expression of t-bet and IFN-γ suggesting a broader role for the kinase in T cell function and differentiation.
Figure 9. DNA-PK inhibition blocks CD3/CD28-induced gata-3 expression in naïve mouse or human CD4+T cells and prevents in vitro differentiation of mouse Th1 and Th2 cells under respective Th1- and Th2-skewing conditions.
CD4+T cells isolated from spleens of WT mice (A) or PBMCs collected from healthy donors (B) were stimulated with antibodies to CD3/CD28 for 24 h in the absence or presence of different concentrations of NU7441. Isolated RNA was reverse-transcribed and the resulting cDNA was subjected to conventional PCR with primer sets specific to human or mouse gata-3. CD4+ T cells isolated from spleens of OVA-sensitized WT or DNA-PKcs+/− were skewed towards a Th1 with IL-12 and anti-IL-4 antibodies or towards a Th2 phenotype with IL-4 and anti-IL-12 antibodies. Subsets of WT CD4+T cells were skewed towards Th1 and Th2 in presence of 1μM NU7441. Isolated RNA was reverse-transcribed and the resulting cDNA was subjected to real time PCR with primer sets specific to mouse gata-3 or Il-4 (C) or with primer sets specific to t-bet or Ifn-γ (D). *, difference from non-stimulated cells; #, difference from Th2 (C) or Th1 (D) skewed cells; p <0.01.
DISCUSSION
Asthma manifestations require the involvement of many cell types including immune, inflammatory and structural cells, as well as a large number of mediators. The role of endothelial cells in different stages of asthma is undoubtedly very important as they regulate the trafficking and transmigration of inflammatory cells including eosinophils, lymphocytes, monocytes, and neutrophils 17. VCAM-1 expression is critical for the recruitment of eosinophils and other inflammatory and immune cells to the lung 17. In the current study, we confirmed that DNA-PK plays an active role in inflammatory diseases. The effect of the DNA-PK inhibitor on VCAM-1 expression was clearly evident in the lungs of OVA-treated mice and in cultured endothelial cells. The reducing effect of DNA-PK inhibition on the expression of cytokines (e.g., IL-5 and eotaxin) that are essential for the differentiation, recruitment, and survival of eosinophils in OVA-exposed lungs may be an important cause of the observed marked reduction in eosinophilia. Interestingly, a single administration of the kinase inhibitor was sufficient to achieve such protection even after repeated challenges with OVA. These results suggest that DNA-PK inhibition may reduce already established disease. Given that drugs can exert non-specific effects, we demonstrated the specificity of the connection between DNA-PK and the inflammatory response by using a genetic approach.
We reexamined the connection between DNA-PK and asthma using inhaled delivery of HDM, a model that more closely resembles human asthma. Such model involves mucosal sensitization within the lungs with a true allergen to induce Th2-driven inflammation 14. DNA-PK inhibition, pharmacologically or by gene heterozygosity, provided an excellent protection against many of the asthma-like traits observed in this model. It is noteworthy that a single injection of the DNA-PK inhibitor was sufficient to significantly reduce OVA or HDM-induced AHR. The fact that eosinophilia and IL-4 were markedly affected by DNA-PK inhibition suggests a potential reduction of IgE. However, the levels of allergen-specific IgE were minimal in HDM-exposed WT mice. This is consistent with the study by De Alba et al. 18 where they reported a requirement for an adjuvant (e.g. Alum) to induce detectable levels of HDM-specific IgE in mice. Accordingly, it is difficult to establish whether DNA-PK inhibition would reduce production of the immunoglobulin.
The DNA-PK complex is critical for NHEJ, which, in turn, is necessary for V(D)J recombination, a process that assures normal B and T cell differentiation 2. The gene deletion of DNA-PKcs 15, Ku70 19, or Ku80 20 causes a deficiency in NHEJ, and mice harboring the homozygous deletion of one of these genes exhibit major defects in T and B cell populations, thus developing a SCID phenotype. Interestingly, DNA-PKcs heterozygosity, which reduces the expression of the kinase by approximately 50% (Fig. 1D and Fig. 4A)13 and cellular enzymatic activity by a similar level, does not cause this defect. These mice have T and B cell populations that are comparable to those in WT, as shown in Fig. 7 and originally reported by Gao et al. 15. These findings demonstrate that DNA-PKcs heterozygosity is sufficient for NHEJ and the V(D)J recombination necessary to produce normal T and B cell populations. The remarkable aspect of our findings is that the ability of DNA-PK to play a key role in inflammation may be independent of its role in T and B cell development. This conclusion is supported by our finding that even repeated administration of the kinase inhibitor did not cause a major reduction in T or B cell populations. Our results, however, suggest an important role for the kinase in T and B cell function. Adoptive transfer of Th2-skewed CD4+ T cells was sufficient to reverse Th2 cytokine production but only partially that of OVA-specific IgE in DNA-PKcs+/− mice. The role of DNA-PK may not be restricted to immune or inflammatory cells. Indeed, the adoptive transfer of activated T cells did not reverse AHR in DNA-PKcs+/− mice, which suggests an important role for DNA-PK in cells that participate in AHR manifestation such as SMC.
In our in vitro studies with human CD4+T cells, DNA-PK inhibition at concentrations of NU7441 that exerted no effect on cell proliferation (0.25 μM) markedly and, in some instances, severely reduced a number of cytokines and chemokines, such as IL-4, IL-5, IL-8, IL-13, and MCP-1. Conversely, treatments using concentrations that severely reduced CD4+ T-cell proliferation had no effect on IL-12. The significant effect of NU441 on IFN-γ was observed only at the highest concentration (5 μM) tested and may stem from the actual decrease in cell proliferation. Overall, the effect on IFN-γ is consistent with the effect of DNA-PK inhibition, either by drugs or gene heterozygosity, on cytokine production in OVA-challenged mice. The lack of an effect of DNA-PK inhibition on IL-12 in TCR-stimulated CD4+T cells is in sharp contrast to the decrease observed in the animal model. Although speculative, the decrease of IL-12 in the animal model may be associated with the severe reduction in lymphocytes in the lungs of treated mice, and DNA-PK enzymatic activity may not be necessary for IL-12 production. Despite these results attained using CD4+ T cells from healthy human subjects, our experiments using mouse CD4+ T cells showed that DNA-PK inhibition, pharmacologically or by gene heterozygosity, completely reduced expression of factors necessary for Th2 and Th1 differentiation of cells. The reduction in IFN-γ in Th1-skewed CD4+ T cells may not be surprising given our observation that DNA-PK inhibition completely blocked production of the cytokine in the HDM model. However, these results are in contrast with those attained using human CD4+T cells (Fig. 9). We believe that this difference may be because human cells express more than 50 times the level of the kinase observed in rodent cells 21. This result is very intriguing given that although IFN-γ expression is affected by DNA-PK inhibition in mouse cells, it is not affected in human CD4+T cells; it appears that specificity of DNA-PK to the Th2 response may be a human trait. When we consider the results of the in vivo model, for instance, DNA-PK inhibition, pharmacologically or by gene heterozygosity, reduced but did not completely abolish IL-5 production suggesting that indeed Th2 cells were generated upon OVA exposure. Accordingly, a great deal of work is required to reconcile such discrepancy.
In CD4+T cells, DNA-PK appeared to be critical for GATA-3 expression upon TCR stimulation. This requirement was observed in both human and mouse cells. GATA-3 is a major regulator of the Il4/Il5/Il13 cytokine locus 16. The inhibition of the expression of this transcription factor may explain the modulatory effect of DNA-PK inhibition of IL-4, IL-5, and IL-13 production and the protective effect against the manifestation of asthma traits in the animal model. Interestingly, GATA-3 expression is highly dependent on p50 NF-κB 8 and the efficient function of p50 NF-κB, in turn, is mediated its phosphorylation by DNA-PK 4. Accordingly, it is highly likely that the modulation of GATA-3 gene expression by DNA-PK inhibition is associated with a blockade in p50 NF-κB phosphorylation. This notion is supported by the fact that the effects of p50 NF-κB gene deletion on OVA challenge-induced asthma is rather similar to that achieved upon DNA-PK inhibition 8. Indeed, while IL-4, IL-5, IL-13, eotaxin, and MCP-1 were markedly reduced in OVA-challenged p50 NF-κB−/− mice, IL-2, IFN-γ and IP-10 were not 6, 7. It is important to acknowledge that phosphorylation of p50 NF-κ B by DNA-PK may not be the sole reason for the connection between the kinase and the expression of Th2 cytokines. Other factors may contribute to the protective effect of DNA-PK inhibition. The expression of t-bet is not regulated by p50 NF-κB as its expression is not altered by p50 NF-κB gene deletion as reported by Das et al. 8. Accordingly, it is quite possible that DNA-PK is involved in additional signaling processes beside that of NF-κB. Clearly, a great deal of experimentation is required to fully understand the mechanism by which DNA-PK influences allergic inflammation.
Overall, the present study only begins to unravel a novel facet of DNA-PK for its participation in the complex process of allergic inflammation and AHR. Our findings suggest that the kinase may represent a novel therapeutic target for the treatment of asthma.
Clinical Implications.
Our results suggest DNA-PK as a novel determinant of asthma and that its potential as a therapeutic target for the treatment of the disease merits further examination.
Acknowledgments
Sources of funding: This work was supported, in part, by grant HL072889 from the NIH and funds from the Louisiana Cancer Research Center (New Orleans, LA) to A.H. Boulares, by the Egyptian Cultural and Educational Bureau (Egypt), by P20GM103501 from the NIH to A. S. Naura (overall PI: Dr. A Ochoa), and by postdoctoral fellowship 11POST8000008 to J. Ju and predoctoral fellowship 14PRE19630012 to K Pyakurel from the American Heart Association.
We would like to thank Dr F. Alt (Harvard University, Boston MA) for providing us with the DNA-PK+/− mice and Dr. C. Espinoza (LSU Health Sciences Center, New Orleans, LA) for providing us with human tissues. We would also like to thank Mrs. Constance Porretta from the LSUHSC Comprehensive Alcohol Research Center Core (CARC) for her assistance and data analysis with the FACS assays.
Abbreviations
- DNA-PK
DNA-dependent protein kinase
- SCID
severe combined immunodeficiency disease
- VCAM-1
vascular cell adhesion molecule-1
- HUVECs
Human umbilical vein endothelial cells
- LSMCs
lung smooth muscle cells
- BALF
bronchoalveolar lavage fluids
- IHC
immunohistochemistry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meek K, Dang V, Lees-Miller SP. DNA-PK: the means to justify the ends? Adv Immunol. 2008;99:33–58. doi: 10.1016/S0065-2776(08)00602-0. [DOI] [PubMed] [Google Scholar]
- 2.Alt FW, Zhang Y, Meng FL, Guo C, Schwer B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell. 2013;152:417–29. doi: 10.1016/j.cell.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodbine L, Neal JA, Sasi NK, Shimada M, Deem K, Coleman H, et al. PRKDC mutations in a SCID patient with profound neurological abnormalities. J Clin Invest. 2013;123:2969–80. doi: 10.1172/JCI67349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ju J, Naura AS, Errami Y, Zerfaoui M, Kim H, Kim JG, et al. Phosphorylation of p50 NF-kappaB at a single serine residue by DNA-dependent protein kinase is critical for VCAM-1 expression upon TNF treatment. J Biol Chem. 2010;285:41152–60. doi: 10.1074/jbc.M110.158352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd CM, Saglani S. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol. 2013;131:1267–74. doi: 10.1016/j.jaci.2013.02.016. quiz 75. [DOI] [PubMed] [Google Scholar]
- 6.Wright JG, Christman JW. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med. 2003;2:211–9. doi: 10.1007/BF03256650. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739–50. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 9.Naura AS, Hans CP, Zerfaoui M, You D, Cormier SA, Oumouna M, et al. Post-allergen challenge inhibition of poly(ADP-ribose) polymerase harbors therapeutic potential for treatment of allergic airway inflammation. Clin Exp Allergy. 2008;38:839–46. doi: 10.1111/j.1365-2222.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Thomas HD, Batey MA, Cowell IG, Richardson CJ, Griffin RJ, et al. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006;66:5354–62. doi: 10.1158/0008-5472.CAN-05-4275. [DOI] [PubMed] [Google Scholar]
- 11.Naura AS, Kim H, Ju J, Rodriguez PC, Jordan J, Catling AD, et al. Minocycline blocks asthma-associated inflammation in part by interfering with the T Cell receptor-NF-kB-GATA-3-IL-4 axis without a prominent effect on PARP. J Biol Chem. 2013;288:1458–68. doi: 10.1074/jbc.M112.419580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez CP, Morrow K, Lopez-Barcons LA, Zabaleta J, Sierra R, Velasco C, et al. Pegylated arginase I: a potential therapeutic approach in T-ALL. Blood. 2010;115:5214–21. doi: 10.1182/blood-2009-12-258822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurimasa A, Ouyang H, Dong LJ, Wang S, Li X, Cordon-Cardo C, et al. Catalytic subunit of DNA-dependent protein kinase: impact on lymphocyte development and tumorigenesis. Proc Natl Acad Sci U S A. 1999;96:1403–8. doi: 10.1073/pnas.96.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson CS, Birrell MA. Moving towards a new generation of animal models for asthma and COPD with improved clinical relevance. Pharmacol Ther. 2011;130:93–105. doi: 10.1016/j.pharmthera.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver DT, Alt FW. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity. 1998;9:367–76. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- 16.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 17.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 18.De Alba J, Raemdonck K, Dekkak A, Collins M, Wong S, Nials AT, et al. HDM induces direct airway inflammation in vivo: implications for future disease therapy? Eur Respir J. 2009 doi: 10.1183/09031936.00022908. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Seidl KJ, Rathbun GA, Zhu C, Manis JP, van der Stoep N, et al. Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity. 1997;7:653–65. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- 20.Nussenzweig A, Chen C, da Costa Soares V, Sanchez M, Sokol K, Nussenzweig MC, et al. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. 1996;382:551–5. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 21.Neal JA, Dang V, Douglas P, Wold MS, Lees-Miller SP, Meek K. Inhibition of homologous recombination by DNA-dependent protein kinase requires kinase activity, is titratable, and is modulated by autophosphorylation. Mol Cell Biol. 2011;31:1719–33. doi: 10.1128/MCB.01298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]