Abstract
Falls in patients with Parkinson’s disease (PD) are a major and levodopa-unresponsive source of morbidity. We previously described an animal model of falls resulting from impairments in attentional-motor interactions. Reproducing the multisystem dopaminergic-cholinergic cell loss in patients with a history for falls, partial loss of striatal dopamine innervation interacted with loss of forebrain cholinergic neurons to generate falls that was hypothesized to reflect impairments in the attentional control of gait and balance and the sequencing of complex movements [1]. As clinical evidence also indicates that basal ganglia dopamine (DA) loss per se is associated with severe discoordination and thus a greater risk for falls, here we demonstrate that relatively extensive striatal DA loss, in contrast to the lack of effects of smaller, dorsal striatal DA losses and sham lesions, increased falls and slips and caused slowing while traversing dynamic surfaces. Falls in large DA rats were associated specifically with spontaneous or slip-triggered stoppages of forward movement. Collectively, the evidence suggests that low motivation or vigor for movement in general, and for initiating corrective movements in particular, are major sources for falls in rats with large DA losses. Falls are a result of complex cognitive-motor interactions, and rats with large DA losses model the impact of a propensity for freezing of gait when traversing dynamic surfaces.
1. Introduction
About two thirds of patients with PD experience a fall in a given year [2,3]. These falls are a primary reason that PD patients are hospitalized or admitted to nursing homes [4]. While the importance of levodopa-unresponsive cognitive impairments and gait abnormalities has been recognized, the role of dopaminergic and non-dopaminergic circuits and how they interact to cause levodopa-unresponsive falls remains poorly understood, posing a critical barrier to the development of therapies for these disabling symptoms [5].
We previously reported that rats with dual cortical cholinergic and dorsal striatal dopaminergic deafferentation exhibited a relatively high rate of falls when tested on a new behavioral instrument designed to assess gait, balance and complex movement control [1]. Such rats with cortical cholinergic and dorsal striatal DA losses (termed “dual” or DL rats in Ref. 1) model clinical evidence indicating that cholinergic loss and the associated impairments in attentional control contribute critically to impairments in gait control and thus increase the propensity for falls in PD patients [6-9]. Our prior findings are consistent with the hypothesis that after such dual cholinergic-dopaminergic lesions, attentional resources can no longer be recruited to compensate for diminished striatal control of complex movements. Thus, loss of cortical cholinergic inputs “unmasks” the impact of relatively small, dorsal striatal DA losses. This interpretation is also consistent with a neuronal circuitry model that describes interactions between cortical attentional control mechanisms and the striatal selection and sequencing of movements (see Fig. 2 in Ref. 10; see also Refs. 11,12).
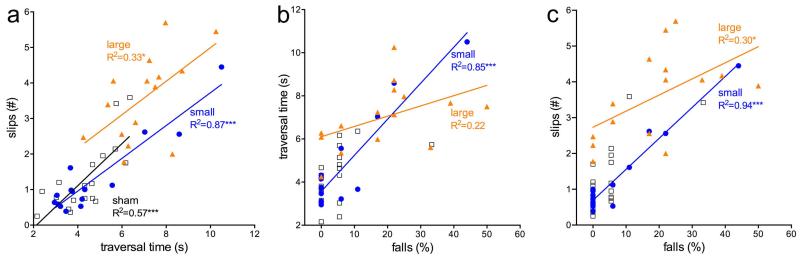
Fig. 2.
Correlations between traversal time, slips, and falls recorded for rotating rod performance. Slower traversal time was associated with more slips in all three groups of rats (a). Likewise, slips and falls were correlated in all three groups (c). Traversal time and falls were not correlated in large DA rats (b), suggesting that in these animals, relatively high rates of falls resulted from additional risk factors. Note that because of the low fall rates in shams, this correlation, albeit significant, is not shown. It is also worth noting that the lesion score of the small DA rat that exhibited the highest fall rate (b,c) was below the average for this group.
Given the multitude of potential cognitive and sensory-motor causes for falls in PD, falls form a complex neuro-behavioral construct. In addition to falls that originate from the combined attentional-motoric deficits described above, falls may result from relatively severe impairments in primarily motoric functions (e.g., postural control, balance and movement selection), and from freezing of gait (FoG). Such falls may be especially likely when traversing dynamic surfaces [3,13-16]. In these situations, compensatory mechanisms to limit the degree of gait disruption and limb discoordination or to disengage from the freezing response may be insufficient and often are deployed too late to prevent falls [17]. Furthermore, FoG-associated falls are more closely related to the severity of the disease [18,19] and perhaps to relatively extensive dopamine losses in the basal ganglia [20-22]. The present experiment was designed to determine whether more extensive striatal dopamine losses cause FoG-associated falls in our animal model. For these experiments we again employed the Michigan Complex Motor Control Task (MCMCT; Ref. 1), including assessing the effects of a doorframe distractor known in patients to trigger FoG [23]. The pattern of data for small DA lesions replicates our previous results whereas relatively large striatal DA loss slowed the traversal of dynamic surfaces and increased the number of movement stoppages, slips and falls.
2. Materials and Methods
2.1 Animals and animal housing
Adult Sprague Dawley rats (Harlan) between 3 and 6 months of age and weighing between 350 and 450 g at the beginning of the experiment were individually housed in opaque single standard cages (27.70 cm × 20.30 cm) in a temperature- and humidity-controlled environment (23 C, 45%) under a 12:12 hour light/dark schedule. Food (Rodent Chow; Harlan Teklad) and water were available ad libitum. All procedures were conducted in adherence with the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, protocols approved by the University Committee on Use and Care of Animals at the University of Michigan, and in laboratories accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
2.2 Number of animals and experimental timeline
This experiment employed N=48 rats. The rats underwent a battery of beam traversal conditions, using the Michigan Complex Motor Control Test (MCMCT; Ref. 1), before and after receiving lesions (see Table 1). The rats were assigned to one of three groups according to fall rates from the rotating rod as part of the pre-surgery sequence. These groups were then randomly designed to receive either small 6-OHDA (n=15; ‘small DA’), large 6-OHDA (n=15; ‘large DA’), or sham (n=18) lesions. In both sequences the rats were tested between 4 and 6 days per week. Both sequences were completed within 16 days. Following 6-OHDA infusion or sham surgeries, the rats recovered for 21 days in their home cages. Immediately after completion of behavioral testing the animals were perfused for histological analyses of the lesions.
Table 1. MCMCT testing sequence.
| Pre-Surgery Sequence | |||||
|---|---|---|---|---|---|
| Day | Trial type | Rotating (10 rpm) | Incline (degrees) |
Distractor | Number of trials |
| 1 | plank shaping | 0 | 2 | ||
| plank | 0 | 3 | |||
| 2 | rod shaping | 0 | 2 | ||
| rod | 0 | 3 | |||
| 3 | plank | 25 | 3 | ||
| rod | 25 | 3 | |||
| 4 | rod | cc | 0 | 3 | |
| rod | cc | 25 | 3 | ||
| 5 | rod | cc-cw-cc-cw | 0 | 4 | |
| rod | cc-cw | 0 | doorframe | 2 | |
| 6 | rod | cc-cw-cc-cw | 25 | 4 | |
| rod | cc-cw | 0 | doorframe | 2 | |
| 7 | plank | 40 | 3 | ||
| rod | 40 | 3 | |||
| 8 | rod | cc | 40 | 3 | |
| rod | cc-cw | 0 | doorframe | 2 | |
| 9 | rod | cc-cw-cc-cw | 40 | 4 | |
| rod | cc-cw | 0 | doorframe | 2 | |
| Post-Surgery Sequence | |||||
| 1 | plank | 0 | 3 | ||
| rod | 0 | 3 | |||
| 2 | plank | 40 | 3 | ||
| rod | 40 | 3 | |||
| 3 | rod | cc-cw-cc-cw-cc-cw | 0 | 6 | |
| 4 | rod rod |
cc-cw-cc-cw-cc-cw | 25 | 6 | |
| 5 | rod rod |
cc-cw-cc-cw-cc-cw | 40 | 6 | |
| 6 | rod | cc-cw | 0 | 2 | |
| rod | cc-cw | 0 | doorframe | 2 | |
| 7 | rod | cc-cw | 0 | 2 | |
| rod | cc-cw | 0 | doorframe | 2 | |
| 8 | rod | cc-cw | 0 | 2 | |
| rod | cc-cw | 0 | doorframe | 2 | |
cc, cw: counterclockwise, clockwise
2.3 Surgeries
Bilateral lesions of striatal dopaminergic afferents were achieved by infusion of 6-OHDA. The rats were injected with desipramine hydrochloride (10 mg/kg; i.p., Sigma-Aldrich) 30 minutes prior to infusion of 6-OHDA for protection of noradrenergic neurons [24]. Surgeries were performed under aseptic conditions. The rats were first placed in vaporization chambers (SurgiVetIsotec 4 Anesthesia Vaporizer) and anesthetized with 4-5% isoflurane (delivered at 0.6 L/min O2). The animals were mounted to a stereotaxic instrument (David Kopf Instruments). Isoflurane was delivered at 1-3% for the duration of the procedure. The temperatures of the animals were maintained at 37° C using Deltaphase isothermal pads (Braintree Scientific). Rats received an intraperitoneal injection of an antibiotic (amikacin; 100 mg/kg). The eyes were protected and lubricated with opthalamic ointment. 1.0 ml/100g of 0.9% NaCl was administered (s.c.) to prevent hypovolemia and hemodynamic instability during prolonged surgeries. The animals received analgesic (buprenorphine: 0.01 mg/kg; s.c. injection) during surgery and once daily beginning the day after surgery.
6-OHDA (Sigma-Aldrich; 4.0 μg/2 μL/infusion) was dissolved in 0.9% NaCl with 0.1% ascorbic acid and infused bilaterally into either two (‘small’ lesion group) or four (‘large’ lesion group) sites of the caudate/putamen complex. 6-OHDA was delivered using a 30 gauge needle attached to a 2-μL Hamilton syringe. Both groups received infusions using the following two targets: (AP +1.8 and +0.6 mm; ML ±2.6 and 3.0 mm; DV −4.5 and −4.8 mm). For large DA lesions, two additional infusions per hemisphere were conducted AP 0.0 and −0.6 mm; ML ±3.4 and 4.0 mm; DV −5.0 mm). The needle was left in position for 5-10 min to foster absorption of the 6-OHDA. For sham-lesions, animals received the same volume per injection site of 0.9% NaCl with 0.1% ascorbic acid only. Non-absorbable sutures were used to close the incision and a topical antibiotic (Neosporin) was applied to the wound.
2.4 Michigan Complex Motor Control Test (MCMCT)
The MCMCT consisted of a runway with interchangeable surfaces (plank or square rod) on which rats were required to traverse to their home cages. The apparatus was made of a U-shaped central rib with a start platform on one end and a cradle for the home cages on the other. The distance between the center of the start platform and the home cage was 2 m. The beam surfaces were 1.8 m in length. For this experiment, a flat plank 13.3 cm wide and a 2.54 cm2 square-shaped rod were used as traversal surfaces. The ends of the beam were held in sockets that allowed the rod surface to be rotated by a gear motor (10 RPM) coupled to one end of the beam element. A pulse frequency modulator allowed adjustment of the rotational speed. The lower central section of the U frame was held in a support saddle that allowed the upper section to pivot and incline the beam elements. This allowed the traversal beams to be adjusted to any upward angle up to 45°. Hand clamps secured the rig at the intended angle. The start platform was hinged and adjusted to accommodate the incline angles. The upper ends of the U-shaped frame also supported a rectangular frame for securing a safety net (0.7 × 0.2 m) section of a badminton net (generic), placed 20 cm below the beam element. The net frame also served as a mounting point for the various cameras, mirrors, and doorframe distractor element. The doorframe distractor, consisting of a 46.0 cm × 39.4 cm foam core with a door frame-shaped cutout of 20.0 cm × 10.0 cm, was placed at the 100 cm mark of the rod with the side jambs 3.5 cm from the rod surface on either side and the top border of the doorframe cutout 11.0 cm above the rod surface. The size of the cutout provided adequate space for the rats to pass underneath the doorframe without the need to adjust their postures.
Four cameras (KT&C; model KPCS190SH Black/White Bullet Camera with 1/3” SONY Super HAD CCD; resolution 600 TVL; scanning frequency 15.734 kHz (H), 59.94 Hz (V); pixels 795 (H) × 596 (V), power DC12 V (±10%), maximal 90 mA) with rotatable bases were fastened to the outer support frame of the outer side of the apparatus by hand clamps. The cameras were aligned in parallel, clamped ~50 cm apart, and adjusted so that the entire length of the beam (including the start platform and edge of the home cage) was filmed. The images were relayed to four grids by a splitter (Panasonic; WJ-420 Quad Unit; 120 V/60 Hz) displayed on a monitor (Panasonic CT-51390). The monitor was able to display the images from all four cameras simultaneously and also zoom on images captured from each individual camera. The traversals were recorded using a DVD-R (DR430; Toshiba) and Verbatim DataLifePlus DVRs, and the recordings were converted to MPEG-4 Part 14 (mp4) using Handbrake software (free open source; version 0.9.8). Two rectangular-shaped mirrors (78.0 × 15.5 cm) separated by ~20 cm were fastened by hand clamps to the side of the apparatus opposite of the cameras to allow for visualization of the animals’ movements on the side of the apparatus opposite to the cameras. All trials were recorded for further detailed analyses of performance measures.
2.5 Measures of MCMCT performance and video analysis
MCMCT performance was quantified by counting traversal time, slips, and falls from trials with rods. For trials using the plank, only traversal time was measured. For each trial, measurement began after the rat achieved an upright and stable body posture and began forward movement. Behaviors were not quantified near the end of the beam (within 5 cm from the end and while the rat climbed into the home cage).
Traversal time was defined as the latency to traverse the beam. Timing started when the rat began forward movement and ended when the hind limbs crossed the 160 cm mark. For trials ending with a fall, traversal time was prorated by multiplying the ratio of the distance of a full traversal to the distance where the hind limbs lost contact with the rod during the fall. A slip was scored when during traversal any of the rats’ paws lost contact with the surface of the rod and extended below the lower horizontal border of the rod. Slips were counted for each paw, regardless of the extent of each slip. After a slip, the paw that slipped needed to be repositioned securely on the rod before another slip was counted from the same paw. When a fall occurred, two slips were counted (one for each hindpaw) in addition to any slips that occurred before the fall.
A fall was scored in the following circumstances: when both of the rats’ hind limbs lost contact with the rod, causing a rat to fall onto the netting below the rod or hang from the rod by its front paws, when a rat ceased forward movement and clung to the rod while it rotated (thus rotating upside down with it), or when an animal ceased forward movement and sat perpendicularly on the rod for greater than 2s while attempting but failing to resume forward movement. When a fall occurred, the trial was ended and the rat was placed back into its home cage for at least 60 s before starting the next trial. Falls were not counted if a rat fell from the starting platform or stepped off the platform and fell before achieving an upright, stable posture on the rod. If a rat refused to initiate forward movement or could not establish an upright posture on the rod, the experimenter assisted the rat in beginning traversal. The fall rate was determined by dividing the total number of falls by the total number of trials for each traversal condition. A maximum of one fall per trial was scored. Thus, the fall rate for each rat was the percentage of trials in which the rat fell (considered across multiple runs).
2.6 Histological analyses and scoring of lesions
After completing the post lesion MCMCT battery, animals were deeply anesthetized and transcardially perfused at a rate of 50 ml/min with 0.1M phosphate buffer solution for 2 minutes followed by perfusion with 4% paraformaldehyde in 0.4M Na-phosphate solution and 15% picric acid (pH 7.4) for 9 minutes. Brains were rapidly removed and postfixed for 2-6 h at 4° C and then rinsed in 0.1M phosphate buffer solution and stored in 30% sucrose solution and allowed to sink. Coronal sections (40 micrometers) were sliced using a freezing microtome (CM 2000R; Leica) and stored in antifreeze solution. Sections were then stained for the immunohistochemical visualization of striatal tyrosine hydroxylase (TH) sections. For immunostaining, an orbital shaker was used throughout incubation and rinse periods. The sections were mounted onto gelatin-coated glass slides and allowed to dry completely. After mounting and coverslipping, slides were imaged using a Leica DM400B digital microscope.
TH immunostaining was performed using a Vectastain Elite ABC kit (PK-6100; Vector Laboratories; rabbit IgG) and a primary antibody (ab112; Abcam; polyclonal rabbit anti-TH). Sections were first rinsed in 0.1M PBS three times for 10 minutes each before a 30 min incubation in 0.3% hydrogen peroxide-0.1M phosphate buffer solution. Sections were then rinsed with 0.3% Triton X-0.1M phosphate buffer solution (3×3 min) and incubated in a 1.5% normal goat serum (blocking serum) with 0.3% Triton X for 1 h. The sections were then rinsed with 0.3% Triton X-0.1M PBS (3×5 min) before incubation in primary antibody (rabbit anti-TH made in goat; 1:1000) overnight at 4 C. After ~24 h, the sections were rinsed with 0.3% Triton X-0.1M PBS (3×3 min) and incubated in the secondary antibody (biotinylated rabbit anti-goat; 1:200; supplied in the Vectastain Elite ABC kit) for 30 min. Three 3-minute rinses were then done in 0.3% Triton X-0.1M PBS before incubating the tissue in the avidin-biotin complex (1:25) (no Triton) for 30 min. The sections were then rinsed in 0.1M phosphate buffer solution (3×3 min) and developed using a Vector Laboratories DAB Peroxidase Substrate Kit (SK-4100) until they reached a desired color (~1-3 min). Sections were then rinsed in 0.1M PBS three times for 3 minutes each before being mounted on gelatin-coated slides and allowed to dry overnight. The following day, slides were dehydrated in an ascending alcohol series (70%, 90%, and 100% ethanol) and defatted in xylene before coverslipping.
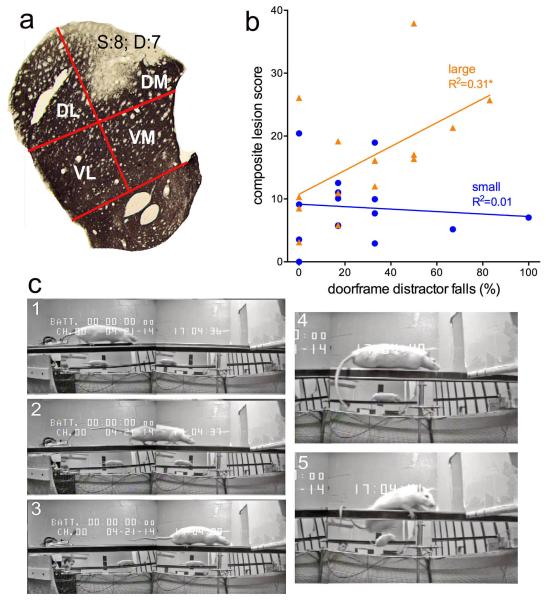
Sections were imaged using a Leica DM400B digital microscope. SPOT 5.1 software (Spot Imaging Solutions) was used to capture images. Lesions were scored on three sections (AP +1.8, +0.6 and −0.2 mm). Using Paint software (Microsoft Corp.), lines were drawn to separate the dorsal striatum (caudate-putamen) into four approximately equal subsections (dorsomedial, DM; dorsolateral, DL; ventromedial, VM, ventrolateral, VL; see Fig. 4a). Each section was then rated using a scale from 0 to 10 for both size and the degree of TH per quadrant. A rating of 10 for size corresponded to a lesion covering 100% of the quadrant. A rating of 10 for degree corresponded to complete TH clearance within the lesion area per quadrant (that is, no areas of residual TH staining; see Fig. 4a). The two scores were multiplied by quadrant; thus, a lesion score of 100 for a quadrant indicated a lesion that covered an entire quadrant without any residual TH stain in that quadrant. Lesions scores per quadrant and hemisphere were used for the analysis of relationships between lesions and performance. An overall composite lesion score was determined for each quadrant by averaging the lesion scores of both hemisphere and all 3 AP sections (1, 2, and 3). The composite lesion score was used for the analysis of relationships between lesions and performance.
Fig. 4.
Illustration of method used to quantify striatal DA lesions (a) and correlation between composite lesion scores and falls in the presence of the doorframe distractor (b). As detailed in methods, for each hemisphere, the size (S) and degree (D) of TH-immunoreactivity loss was quantified in four striatal quadrants (DL, dorsolateral; DM, dorsomedial; VL, ventrolateral; VM, ventromedial). Estimation of the ‘degree’ of loss reflected the homogeneity of loss within a quadrant; in the example in a, about a third of the DM quadrant showed residual TH-immunoreactivity, yielding a D score of 7 (out of 10; see Methods for the calculation of one composite score per animal). Composite lesion scores from large DA, but not small DA lesions, significantly correlated with the number of falls in the presence of the doorframe (b). c: Rats with a large DA lesion traversing the rotating rod (frames 1-3; 36-30 s), freezing forward movement when reaching the doorframe (4, 40th s), causing a fall (5). Importantly, this rat did not fall during the four pre-surgery exposures to the doorframe.
2.7 Statistical Analyses
Within-subjects repeated measures ANOVAs were used to compare performance measures (percentage of falls, traversal time, and slips) between the lesion groups (sham, small, and large DA) in separate pre and post surgery analyses. Different within-subjects factors were used to assess performance on different traversal conditions. The within-subjects factor incline (0°, 25°, or 40°) was used for assessment of performance with the plank (pre and post surgery), stationary rod (pre and post surgery), and rotating rod (pre surgery). In trials in which the direction of the rotating rod was alternated, rotation direction (clockwise or counterclockwise) was also used as a within-subjects factor. The within-subjects factors rotation direction and day (1, 2, 3, or 4) were used to evaluate falls with the door frame distractor. In addition, the effect of the doorframe distractor on falls was assessed by comparison with falls on analogous non distractor trials (as described in Results). Post hoc analyses for within-subjects comparisons were performed using the t test and Least Significant Difference (LSD) test. Statistical analyses were performed using the SPSS for Windows (version 17.0: SPSS). Assumptions underlying the statistical model were assessed. In cases of violation of the sphericity assumption, Huyhn–Feldt-corrected F values, along with uncorrected degrees of freedom, are given. Alpha was set at 0.05. Exact p values are reported as recommended previously [25]. Effect sizes (Cohen’s d) are given for major comparisons, in part to guide the interpretation of results from separate analysis of the effects of lesions on dependent measures of performance. Repeated measures ANOVAs were used to compare lesion scores between the striatal regions in each lesion group and quadrants (DM, DL, VM, and VL). Post hoc analyses comparisons were performed using the LSD test. Pearson product-moment correlation coefficients were computed to indicate relationships between lesion scores and MCMCT performance measures. The standard error of mean (SEM) was indicated for all means reported.
3. Results
3.1 Pre-lesion performance
Prior to receiving lesions, a total 48 rats were tested on a 9-day MCMCT test sequence (Table 1). This sequence was designed to offer persistent cognitive-motor challenge by progressively increasing the demands of the traversal conditions [1]. Following the test sequence, the rats were assigned to 3 lesion groups (sham, small or large striatal 6-OHDA lesion) balanced according to fall rates from the rotating rods. Because performances did not differ between these groups, the following description of pre-surgery performance will focus on main measures and the effects of main task conditions.
As previously observed, inclined plank traversal time decreased compared with horizontal plank traversal (F(2,90)=13.99, P<0.001; 0° incline: 4.83±0.20 s, 25°: 3.75±0.15 s, 40°: 3.77±0.22 s; 0°vs. 40°: d=0.73). Likewise, on the stationary rod, falls were highest at the 0° incline (F(2,90)=4.05, P=0.021; 0° incline: 2.72±1.35% falls, no falls at 25° or 40° inclines; d=0.42 for both). Measures of performance did not differ between groups of animals (all F<2.97, all P>0.12).
Trials assessing rotating rod traversal began on the 4th day of the pre-surgery sequence. There were fewer falls at the 25° incline than the 0° incline, and no difference in falls between the 0° and 40° inclines (F(2,90)=6.33, P=0.003; 0° incline: 13.21±2.63% falls, 25°: 1.36±0.99% falls; 40°: 7.28±2.96% falls; 25° less falls than 0°, p<0.001; d=0.87). Rotating rod traversal time was slowest at the 40° incline (F(2,90)=48.60, P<0.001; 0° incline: 4.26±0.24 s, 25°: 4.11±0.17 s: 40°: 6.82±0.40 s; 40° slower than 0° and 25°, p<0.001 for both; 0°vs. 40°: d=1.08). Once again, measures did not differ between the groups (all F<1.78, all P>0.18).
On the 5th, 6th, and 9th day of pre-surgery testing the direction of the rod was reversed to the clockwise (cw) direction and alternated with trials using the familiar cc direction. While traversal time did not differ between the two rotation directions (F(1,45)=0.03, P=0.86), more falls occurred on the less familiar cw trials (F(1,45)=6.25, P=0.016; cc: 6.31±1.69% falls; cw: 11.85±2.14% falls; d=0.42) but neither traversal time nor falls differed between groups (both F<2.52, both P>0.07).
The doorframe distractor was presented twice on four separate test days (8 distractor trials total). The doorframe distractor increased falls (F(1,45)=6.94, P=0.012; d=0.58) in all rats (distractor × group: F(2,45)=0.06, P=0.95). Doorframe distractor-associated falls decreased on later test days relative to early days (main effect of day, F(3,135)=18.01, P<0.001; Day 1: 53.13±5.45% falls, Day 2: 23.33±4.46% falls, Day 3: 13.33±4.40% falls, Day 4: 13.33±3.07% falls; pairwise comparisons: Days 2, 3, and 4 less than Day 1 (all P<0.001 for all, all d≥0.85), Day 4 less than Day 2 (P=0.011, d=0.47)). There were no interactions between group, day, or rotation direction (all F≤1.37, all P≥0.23).
3.2 Large striatal dopamine loss slows traversal and increases slips and falls
3.2.1 Plank
Rats with large DA lesions required more time to traverse the plank than shams and rats with small DA lesions (F(2,45)=5.70, P=0.006; shams: 4.41±0.22 s; small DA: 5.18±0.55 s; large DA: 6.40±0.53 s; large DA slower than shams, d=1.24, and small DA, p<0.04 for both). In contrast to the pre-surgery tests, inclined plank traversal required more time (F(1,45)=9.64, P=0.003; 0° incline: 4.87±0.28 s, 40°: 5.73±0.31 s; d=0.40) but this effect did not differ significantly between groups (incline × group: F(2,45)=2.28, P=0.11).
3.2.2 Stationary Rod
Rats with large DA lesions also required more time to traverse the stationary rod when compared to the other two groups (F(2,45)=24.78, P<0.001; shams: 5.30±0.35 s, small DA: 5.36±0.54 s; large DA: 9.95±0.70 s; large DA slower than shams, d=2.13, and small DA, P<0.001 for both). Moreover, large DA rats committed more slips (F(2,45)=11.54, P<0.001; shams:1.20±0.37 slips; small DA: 1.03±0.27; large DA: 3.47±0.50; large DA more slips than shams, d=1.27, and small DA, P<0.001 for both). However, fall rates on the stationary rod did not differ between the groups (F(2,45)=0.47, P=0.63).
For both slips and traversal time, there were significant main effects of incline (F>53.15, P<0.001 for both) and interactions between the effects of group and incline (F>4.97, P<0.011 for both). Although inspection of the data suggested that these interactions reflected greater slowing and more slips in rats with large DA lesions when traversing the rod at 40° incline, post hoc analyses indicated significantly greater effects of incline in all groups and for both measures (all F>7.94, all P<0.012) and thus failed to locate the source for these interactions.
3.2.3 Rotating Rod
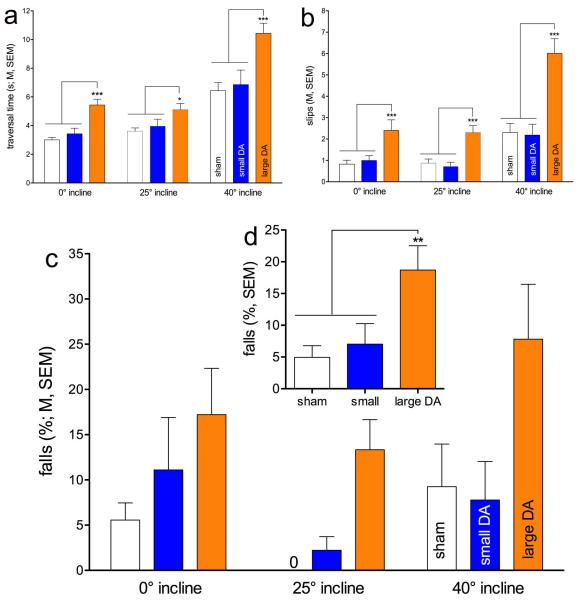
Rotating rod traversal, at 0°, 25° and 40° inclines, were assessed in combination with alternating rotation directions on days 3-5 (Table 1). As detailed below, compared with shams and small DA lesions, large DA losses increased falls and slips and slowed traversal performance. As we previously observed that traversing rotating rods most robustly revealed the impact of cholinergic-dopaminergic losses [1], results based on rotating rod traversals are described in detail (see Figure 1 and Table 2 for statistical results, including effect sizes).
Fig. 1.
Rotating rod performance by rats with sham, small DA and large DA lesions. Rotating rod performance was assessed with the rod placed at at 0°, 25° or 40° incline (see Table 1). Compared with sham and small DA lesions, rats with large DA lesions required more time to traverse the rod (a) and they committed more slips (b) and falls (c; the insert d depicts the main effect of group on falls; see Table 2 for a summary of the statistical results). The effects of incline and group interacted with respect to traversal time and slips, although post hoc one-way ANOVAs on the effects of group within each incline condition failed to locate the source of this interaction. In general, large DA rats required more time and committed more slips than small DA and sham-operated rats. (Symbols for this and following figures: *,**,***, P<0.05, 0.01, 0.001.)
Table 2. Postsurgery rotating rod performance.
| traversal time | slips | falls | |
|---|---|---|---|
| Group (G) | F(2,45)=11.28, P<0.001 sham vs. large: d=1.93 small vs. large: d=1.32 |
F(2,45)=22.15, P<0.001 sham vs. large: d=2.05 small vs. large: d=1.95 |
F(2,45)=6.15, P=0.004 sham vs. large: d=1.16 small vs. large: d=0.85 (Figure 1d) |
| Incline (I) | F(2,90)=99.45, P<0.001 0° vs. 40°: d=1.44 25° vs. 40°: d=1.34 |
F(2,90)=49.85, P<0.001 0° vs. 40°: d=0.98 25° vs. 40°: d=1.02 |
F(2,90)=5.75, P=0.004 0° vs. 25°: d=0.43 25° vs. 40°: d=0.55 |
| Rotation Direction (R) |
n.s. | F(1,45)=30.24, P<0.001 d=0.59 |
n.s. |
| GxI | F(4,90)=3.44, P=0.012 sham vs. large (all inclines): d≥0.66 small vs. large (all inclines) : d≥1.12 (Figure 1a) |
F(4,90)=5.97, P<0.001 sham vs. large (all inclines): d≥1.37 small vs. large (all inclines) : d≥1.23 (Figure 1b) |
n.s. (Figure 1c) |
| GxR | n.s. | n.s. | n.s. |
| GxRxI | n.s. | n.s. | n.s |
Compared to both shams and small DA rats, large DA rats required more time to traverse the rotating rod and they slipped and fell more frequently. Furthermore, there were main effects of incline, reflecting that traversing the rotating rod at the steepest incline further slowed performance and augmented slip and fall rates. The performance of rats with large DA lesions was more severely affected by incline than all other animals, however, the interactions between the effects of incline and group reached significance only for traversal time and slips and not for falls, reflecting the considerable variability of fall rates across inclines (post hoc one-way ANOVAs on the effects of incline on falls in all three groups: all P>0.08).
3.2.4 Microbehavioral correlates of falls
We investigated the rats’ microbehavior during rotating rod traversal in order to identify behavioral characteristics and risk factors associated with the higher fall rates in large DA rats. We observed that large DA rats more frequently stopped forward movement and paused. To analyze these pauses of movement we quantified all such stops that lasted ≥ 250 ms, during runs on the rotating rod set at 0° incline. Overall, large DA rats exhibited a greater frequency of stops (t(1,29)=15.15, P=0.001, d=1.42; small DA: 0.55±0.10, large DA: 1.09±0.09 stops/run). Furthermore, we were interested in identifying potential triggers for these movement pauses. Stoppages either had no obvious trigger or were triggered by slips; the frequency of both category of stoppages again was higher in large DA rats (both t(1,29)>5.20, both P<0.007, both d>0.83). Thus, large DA rats stopped forward movement more frequently than small DA rats.
3.2.5 Correlations
As would be expected given that there were main effects of group on all three measures of rotating rod performance, traversal time, slips and falls are correlated measures and, with one exception, this correlation persists in rats with lesions (Fig. 2). In rats with large DA lesions, traversal time and falls were not significantly correlated, suggesting that in contrast to small DA and sham rats, traversing relatively slowly across the rotating rod was not a sole risk factor for falls. (Note that Fig. 2b,c does not indicate the correlations between falls and the other two measures for sham-operated rats. Even though these correlations nominally were highly significant (p<0.001 for both), the absence of falls in many sham-rats and the compression of data toward zero falls limits the validity of the statistical result).
3.2.6 Doorframe-distractor-induced falls
The doorframe distractor was presented on test days 6, 7, and 8, twice per test day, with the rod rotated in the regular (cc) direction during the first trial and the reverse direction (cw) during the second trial. Similar to pre-surgery effects, the doorframe increased falls (F(1,45)=22.10, P<0.001; no doorframe (0° incline, rotating rod trials on test day 3): 11.27±2.52% falls; doorframe: 26.56±3.36% falls, d=0.73). The rate of falls did not change across the 3 test days (F(2,90)=2.65, P=0.08). Striatal DA loss did not affect falls and the effects of group, and distractor did not interact significantly (all F≤1.93, p≥0.16 for all).
3.2.7 Correlations between lesion scores and performance
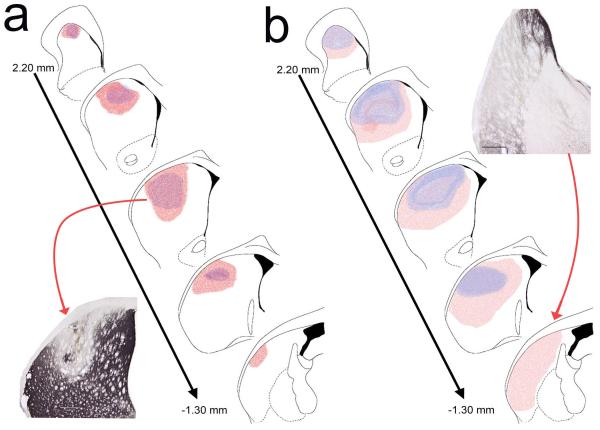
Figure 3 depicts the smallest and largest DA depletions in rats with small DA and large DA lesions. In the small DA group, the average lesions extended along the AP axis from AP +2.0 to AP +0.20 mm (1.8 mm). The large DA lesions occupied a larger space along the AP axis, ranging on average from AP +2.0 to AP −1.0 mm (3 mm). The analysis of extensions into the lateral and ventral portions of the striatum was based on quadrant-based scores (Fig. 4a).
Fig. 3.
Schematic representation of the extent of small (a) and large (b) DA lesions. As detailed in Methods, for small DA lesions 6-OHDA was infused into two sites per hemisphere and for large DA lesions into 4 sites. For each group, the smallest (blue) and the largest (pink) lesion are shown. Note that large DA lesions also extended further into the posterior lateral and ventral striatum (see Results for quantitative analyses).
As would be expected, the lesion scores (see Methods) from large DA rats indicated larger lesions than in small DA rats (F(1,28)=8.19, P=0.008, d=1.05; small: 8.67±1.55, large: 16.44±2.30). Moreover, for both groups, the lesions primarily depleted the dorsal quadrants of the striatum (see Fig. 4a; main effect of quadrant: F(3,84)=36.67, P<0.001; DM and DL versus VM and VL: all P<0.001, all d>1.06).
The average lesion scores for the dorsal quadrants (DM and DL) were averaged across the AP sections and hemispheres to generate a composite lesion score for each rat. For both small and large DA groups, correlations between composite lesion score and falls, slips, or traversal time (overall or at any individual incline) on the rotating rod trials (without the doorframe distractor) remained insignificant (all P>0.23). However, Iarge DA lesion scores correlated significantly with falls that occurred in the presence of the doorframe distractors (large DA: R2=0.31, P=0.03; small DA: R2=0.01, P=0.74; Fig. 4b). Inspection of doorframe-associated falls in large DA rats indicated that, while approaching the door, rats’ forward movement came to a halt and lowered their bodies onto the rotating rod (Fig. 4c). Falls triggered by the doorframe in large DA rats were characterized by relatively early stoppage of movement and immobility (see Fig. 4a), occurring 30.21±3.88 cm in front of the doorframe. Moreover, immobility lasted typically until the fall occurred, for 3.79±0.85 s. Falls resulting from such freezing episdoes were ‘slow and controlled’ with the trunk remaining close to the beam.
4. Discussion
The present findings indicate that striatal DA losses that extended into the dorsolateral and ventrolateral caudate nucleus, relative to sham-lesions or DA losses restricted largely to the dorsomedial caudate, increased falls from the rotating rod, slowed traversal on all surfaces tested (plank and stationary and rotating rods) and caused more slips from the stationary and rotating rods. Relatively small dorsomedial DA losses, on the other hand, and as shown previously [1], did not affect MCMCT performance, perhaps because striatal dopamine tone was maintained [26]. Reversing the direction of rotation of the rod to the less familiar direction and introducing a doorframe distractor in later trials did not reveal additional fall propensity in rats with large DA losses, suggesting that more complex movement requirements and processing of distractors were not affected by the DA lesions. Importantly, the large DA rats exhibited more spontaneous and slip-triggered stoppages of forward movement (‘freezing episodes’) compared to small DA rats on the rotating rods and, as elaborated below, doorframe-induced falls in large DA rats were preceded by relatively longer freezing episodes than in rats tested previously with small DA lesions combined with cortical cholinergic loss [1]. Below, we will primarily discuss the hypothesis that falls on the MCMCT caused by large DA lesions model falls in PD patients that result primarily from low vigor for movement and the propensity to freeze and we will compare this model with the cholinergic-dopaminergic dual lesion model described in our prior report (in Ref. 1).
Our results suggest that the elevated rate of falls in large DA rats were closely associated with slow gait speed and impairments in initiating corrective steps to recover balance and continue forward movements or, in more general terms, the propensity of large DA rats to freeze during forward movement. Large DA rats exhibited a relatively high frequency of stoppages of forward movement, with no obvious trigger or following stepping errors. We hypothesize that the higher rate of falls resulting from large striatal DA losses reflect primarily a relatively low level of vigor for movement and for initiating movement recovery after slips. This behavior may be considered a model of freezing of gait (FoG).
In PD patients, FoG is associated with the severity of the disease (see Introduction) and advanced stages of PD are characterized by larger and more complete depletion of striatal DA (see also Ref. 27). As DA losses extend through the striatum, low motivation or vigor for movement manifests as a general symptom of PD [11,28,29]. In large DA rats assessed on the MCMCT, the failure to initiate corrective movements after spontaneous or slip-triggered stoppages may model this deficit, with movement stoppage being considered an extreme expression such low vigor for movement.
Comparisons between the effects of small and large DA lesions may provide only limited new insights into the roles of dopaminergic innervation of striatal subdivisions, in part because our experiment did not include a test of the effects of relatively small DA losses in the more posterior-lateral striatum. Relatively small dorsomedial DA losses alone did not affect MCMCT performance in neither this nor our prior study [1]. As large DA losses extended into more lateral and posterior, sensorimotor regions of the striatum (Fig. 3), they may have increasingly disrupted the habitual or automatic components of rod traversal [30-33]. In large DA rats, such loss of automatic execution of complex motor sequences may have converged with impaired goal-directed performance [32] resulting from dorsomedial DA depletion, to generate movement stoppages, slips, and eventually falls. It is difficult to determine whether slow or ‘low-vigor traversal of the rod, and freezing episodes, are expressions of such combined impairments in habitual and goal-directed movement control, or whether they represent dissociable contributions to deficits in gait and balance seen in large DA rats.
Large DA losses produced impairments in MCMCT performance that, at first glance, mirror those seen in rats with small DA losses when combined with loss of cortical cholinergic afferents (DL rats in Ref. 1). However, there are important differences that suggest differential cognitive-behavioral mechanisms causing falls in the two models. First, across comparable conditions, DL rats fell more than twice as frequently as large DA rats. Second, changing rod rotation to the less familiar clockwise direction caused more falls in DL rats but not in DA rats. Third, doorframe-triggered immobility occurred early and was relatively lasting (see Results). To compare the latter observations with doorframe-associated falls in DL rats, we analyzed 28 such falls in DL rats (data taken from our prior study). Compared with DL rats, large DA rats stopped forward movement much earlier into the run and at a point further away from the doorframe (t(1,51)=6.82, P=0.012, 30.21±3.88 cm (DA) versus 19.11±2.11 cm (DL)). Furthermore, this period of immobility lasted longer in DA rats (t(1,51)=4.20, P=0.046; for 3.79±0.85 s (DA) versus 2.13±0.20 s). Together, these additional observations are consistent with the view that large DA rats fall because of their propensity for freezing forward movement, while DL rats model falls resulting from impairments in attentional-motor interactions (as discussed in detail in Ref. 1).
FoG is both a complex clinical phenomenon and a complex behavioral construct that involves intricate interactions between cognitive and sensory-motor mechanisms and cues [34-37]. The varying clinical features of FoG and related motor arrest phenomena in PD likely reflect marked striatal dopaminergic denervation and varying pathologies in other components of the complex systems controlling gait [34]. Consistent with our observations in large DA rats, FoG tends to occur in patients with more advanced PD, more widespread striatal dopaminergic denervation, and is most common in the “off state” when serum, and presumably striatal, dopamine levels are at a nadir. FoG in PD is associated also with the presence of extra-striatal pathologies, specifically neocortical cholinergic denervation and amyloid deposition [38]. Therefore, large DA rats may not be considered a complete model that reproduces falls associated primarily with deficient motor behavior in PD. Rather, both large DA and DL rats model falls resulting from different aspects of impaired striatal cognitive-motor interactions. These impaired interactions produce a propensity for freezing behavior in large DA rats that causes falls when required to traverse dynamic (e.g., rotating) surfaces. In contrast, DL rats fall because basal forebrain cholinergic cell loss removes the benefits of attention for performing complex movements in the presence of small DA losses in the striatum.
Quadrupeds modeling bipeds
One potential limitation of modeling and interpreting falling behavior on the MCMCT concerns the obvious locomotion differences between rats and humans. Rats are quadruped, and compared to biped humans, possess a lower center of gravity. This raises the question as to whether falls while traversing rotating rods, and the demonstration of falls in an animal model, bears relevance for understanding falls in humans. Our prior evidence indicated that in lesioned rats (DL rats in Ref. 1), as in humans, slow gait speed, insufficient recovery movements following slips, abnormal traversing posture, reduced step frequency, and attentional impairments are risk factor for falls [see Ref. 4 for review]. Here, we additionally determined that frequent stoppages of forward movement (freezing episodes) were associated with the increased fall risk in large DA rats, thus potentially modeling the well-established link between FoG in human patients and falls [39]. Certainly and obviously, the topography of slip-triggered compensatory responses to regain posture, balance and forward movement differs drastically between quadrupeds and bipeds; however, falls in rats are hypothesized to be due to the impaired behavioral-cognitive processes that also cause falls in patients. Just as quadrupeds model so many other aspects of human behavior and cognition, despite their dramatically different behavioral repertoire, they model falls arising from common cognitive motor risk factors.
5. Conclusion
Together with our prior findings [1], two at least partly different animal models of falls in PD have emerged. DL rats model the impairments in cognitive-motor functions that result from losses in cortical cholinergic as well as basal ganglia circuitry. In contrast, the increased rate of falls caused by large DA lesions may model the impact of a propensity for FoG that is associated with more advanced disease states. Given that cholinergic losses in PD occur early in PD [7] and that such loss is more clearly associated with fall status in patients (Ref. 8; see also Introduction), DL rats arguably represent a more comprehensive model for research on PD-related falls and putative treatments. The present findings, however, underscore the heterogeneity of pathologies underlying falls in PD and suggest that it may be of interest to assess cortical cholinergic deafferentation in interaction with larger striatal DA losses, to integrate the propensity for freezing behavior and attentional deficits in an even more complete animal model of falls in PD.
Highlights.
Fall propensity was assessed in rats after dopamine lesions to the dorsal striatum
Large but not small dopamine losses robustly increased slips and falls
The extent of depletions correlated with falls caused by a doorframe distractor
Large dopamine loss increased spontaneous and slip-triggered stoppages of movement
Extensive dopamine loss in rats models freezing of gait in Parkinson’s Disease
Acknowledgements
Supported by NINDS Grant P50NS091856 (Morris K. Udall Center for Excellence in Parkinson’s Disease Research, University of Michigan). We thank Dr. Cathie Spino, Director, Statistical Analysis of Biomedical and Educational Research (SABER), University of Michigan, for assisting with the statistical analyses of results. Thanks also to Michael Hilden for his assistance in behavioral testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kucinski A, Paolone G, Bradshaw M, Albin RL, Sarter M. Modeling fall propensity in Parkinson’s disease: Deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation. J Neurosci. 2013;33:16522–39. doi: 10.1523/JNEUROSCI.2545-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson’s disease: Frequency, impact and identifying factors. J Neurol. 2005;252:1310–5. doi: 10.1007/s00415-005-0855-3. [DOI] [PubMed] [Google Scholar]
- [3].Wood BH. Incidence and prediction of falls in Parkinson’s disease: A prospective multidisciplinary study. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72:721–5. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grimbergen YA, Munneke M, Bloem BR. Falls in Parkinson’s disease. Current Opinion in Neurology. 2004;17:405–15. doi: 10.1097/01.wco.0000137530.68867.93. [DOI] [PubMed] [Google Scholar]
- [5].Langston JW. The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–6. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- [6].Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJ, Albin RL, Müller ML. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology. 2013;81:1611–6. doi: 10.1212/WNL.0b013e3182a9f558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011;221:564–73. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bohnen NI, Müller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–6. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Allcock LM, Rowan EN, Steen IN, Wesnes K, Kenny RA, Burn DJ. Impaired attention predicts falling in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:110–5. doi: 10.1016/j.parkreldis.2008.03.010. [DOI] [PubMed] [Google Scholar]
- [10].Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol. 2014;257:120–9. doi: 10.1016/j.expneurol.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mazzoni P, Hristova A, Krakauer JW. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–16. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim H, Lee D, Jung MW. Signals for previous goal choice persist in the dorsomedial, but not dorsolateral striatum of rats. J Neurosci. 2013;33:52–63. doi: 10.1523/JNEUROSCI.2422-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- [14].Michalowska M, Fiszer U, Krygowska-Wajs A, Owczarek K. Falls in Parkinson’s disease. Causes and impact on patients’ quality of life. Funct Neurol. 2005;20:163–8. [PubMed] [Google Scholar]
- [15].Cole MH, Silburn PA, Wood JM, Worringham CJ, Kerr GK. Falls in Parkinson’s disease: Kinematic evidence for impaired head and trunk control. Movement Disorders. 2010;25:2369–78. doi: 10.1002/mds.23292. [DOI] [PubMed] [Google Scholar]
- [16].Cole MH, Silburn PA, Wood JM, Kerr GK. Falls in Parkinson’s disease: Evidence for altered stepping strategies on compliant surfaces. Parkinsonism Relat Disord. 2011;17:610–6. doi: 10.1016/j.parkreldis.2011.05.019. [DOI] [PubMed] [Google Scholar]
- [17].Fasano A, Plotnik M, Bove F, Berardelli A. The neurobiology of falls. Neurol Sci. 2012;33:1215–23. doi: 10.1007/s10072-012-1126-6. [DOI] [PubMed] [Google Scholar]
- [18].Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, Meissner WG, Schelosky L, Tison F, Rascol O. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. 2014;71:884–90. doi: 10.1001/jamaneurol.2014.753. [DOI] [PubMed] [Google Scholar]
- [19].Lewis SJ, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:333–8. doi: 10.1016/j.parkreldis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- [20].Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res. 2008;185:391–8. doi: 10.1007/s00221-007-1161-3. [DOI] [PubMed] [Google Scholar]
- [21].Bohnen NI, Muller ML, Kuwabara H, Cham R, Constantine GM, Studenski SA. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J Rehabil Res Dev. 2009;46:1045–52. doi: 10.1682/jrrd.2009.03.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bartels AL, de Jong BM, Giladi N, Schaafsma JD, Maguire RP, Veenma L, Pruim J, Balash Y, Youdim MB, Leenders KL. Striatal dopa and glucose metabolism in PD patients with freezing of gait. Mov Disord. 2006;21:1326–32. doi: 10.1002/mds.20952. [DOI] [PubMed] [Google Scholar]
- [23].Cowie D, Limousin P, Peters A, Hariz M, Day BL. Doorway-provoked freezing of gait in Parkinson’s disease. Mov Disord. 2012;27:492–9. doi: 10.1002/mds.23990. [DOI] [PubMed] [Google Scholar]
- [24].Breese GR, Traylor TD. Depletion of brain noradrenaline and dopamine by 6-hydroxydopamine. Br J Pharmacol. 1971;42:88–99. doi: 10.1111/j.1476-5381.1971.tb07089.x. PMC1666995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Greenwald AG, Gonzalez R, Harris RJ, Guthrie D. Effect sizes and p values: What should be reported and what should be replicated? Psychophysiology. 1996;33:175–83. doi: 10.1111/j.1469-8986.1996.tb02121.x. [DOI] [PubMed] [Google Scholar]
- [26].Dreyer JK. Three mechanisms by which striatal denervation causes breakdown of dopamine signaling. J Neurosci. 2014;34:12444–56. doi: 10.1523/JNEUROSCI.1458-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: An evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–17. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- [28].Gepshtein S, Li X, Snider J, Plank M, Lee D, Poizner H. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26:645–57. doi: 10.1162/jocn_a_00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: Opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191:507–20. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- [30].Cousins MS, Salamone JD. Involvement of ventrolateral striatal dopamine in movement initiation and execution: A microdialysis and behavioral investigation. Neuroscience. 1996;70:849–59. doi: 10.1016/0306-4522(95)00407-6. [DOI] [PubMed] [Google Scholar]
- [31].Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–76. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- [32].Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: Implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Smith K, Graybiel A. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79:361–74. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. The Lancet Neurology. 2011;10:734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vercruysse S, Devos H, Munks L, Spildooren J, Vandenbossche J, Vandenberghe W, Nieuwboer A, Heremans E. Explaining freezing of gait in Parkinson’s disease: Motor and cognitive determinants. Mov Disord. 2012;27:1644–51. doi: 10.1002/mds.25183. [DOI] [PubMed] [Google Scholar]
- [36].Plotnik M, Giladi N, Hausdorff JM. Is freezing of gait in parkinson’s disease a result of multiple gait impairments? Implications for treatment. Parkinson’s Disease. 2012;2012:1–8. doi: 10.1155/2012/459321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Heremans E, Nieuwboer A, Vercruysse S. Freezing of gait in Parkinson’s disease: Where are we now? Curr Neurol Neurosci Rep. 2013;13:350. doi: 10.1007/s11910-013-0350-7. [DOI] [PubMed] [Google Scholar]
- [38].Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Constantine GM, Scott PJ, Albin RL, Müller ML. Extra-nigral pathological conditions are common in Parkinson’s disease with freezing of gait: An in vivo positron emission tomography study. Mov Disord. 2014;29:1118–24. doi: 10.1002/mds.25929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–84. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]