Abstract
Hydroxycarbamide therapy has been associated with significant oscillations in peripheral blood counts from myeloid, lymphoid and erythroid lineages in patients with polycythaemia vera and chronic myeloid leukaemia. We retrospectively evaluated serial blood counts over an 8-year period from 44 adult patients with sickle cell disease receiving hydroxycarbamide. Platelet counts, leucocyte counts, haemoglobin values and reticulocyte counts, apportioned by hydroxycarbamide status, were analysed using a Lomb-Scargle periodogram algorithm. Significant periodicities were present in one or more counts in 38 patients receiving hydroxycarbamide for a mean duration of 4.81 years. Platelet and leucocyte counts oscillated in 56.8% and 52.3% of patients, respectively. These oscillations generally became detectable within days of initiating therapy. During hydroxycarbamide therapy, the predominant periods of oscillation were 27±1 days for platelet counts and 15±1 days for leucocyte counts. Despite an absolute decrease in leucocyte and platelet counts during hydroxycarbamide treatment, the amplitudes between nadirs and zeniths remained similar regardless of exposure. Our observations appear consistent with previously proposed models of cyclic haematopoiesis, and document that hydroxycarbamide-induced oscillations in blood counts are innocuous phenomena not limited to myeloproliferative disorders as described previously. We speculate the known cell cycle inhibitory properties of hydroxycarbamide may accentuate otherwise latent constitutive oscillatory haematopoiesis.
Keywords: sickle cell disease, oscillatory haematopoiesis, hydroxycarbamide, complete peripheral blood count, periodicity
Introduction
Hydroxycarbamide belongs to a class of compounds called hydroxamic acids, whose members share a common ability to bind metals. The primary cytotoxic effect of hydroxycarbamide as an inhibitor of ribonucleotide reductase occurs due to its binding of the enzyme's two iron molecules, with subsequent inactivation of a critical tyrosyl radical leading to arrest of de novo DNA synthesis (Yarbro, 1992). In sickle cell disease (SCD) this cytotoxic effect is utilized clinically to selectively reduce the production of red cells containing a high level of sickle haemoglobin (HbS), which tend to arise from rapidly dividing precursors, while favouring the production of red cells containing a high fetal haemoglobin level, which arise from progenitors that divide less rapidly through incompletely understood mechanisms of stress erythropoiesis (Platt, 2008). The primary tools utilized to monitor the clinical effect and toxicity of hydroxycarbamide are peripheral blood counts, in which several predictable effects can be seen beginning within days of initiating therapy: a significant reduction in the leucocyte count and the platelet count, and an increase in the mean cell volume (MCV) of the red blood cells. The blood counts, particularly the absolute neutrophil count (ANC) and platelet count, must be monitored closely during the course of therapy in order to ensure that excessive myelosuppression does not occur. During the process of titration to a maximum tolerated dose as part of the standard of care for SCD patients, temporary dosage interruptions due to low peripheral blood counts often occur at some point in the course of their treatment (Platt, 2008).
Oscillatory behaviour in peripheral blood counts generally involves a regular pattern of rises and falls over time in the values of one or more mature circulating blood elements, in contrast to the variability inherent to serial measurements of blood counts. These oscillations have been observed outside of hydroxycarbamide treatment in a wide variety of patient cohorts including chronic myeloid leukaemia (CML) (Morley et al, 1967; Vodopick et al, 1972; Mehta & Agarwal, 1980; Malhotra & Salam, 1991), cyclic neutropenia (Dale et al, 2002), polycythaemia vera (PV) (Morley, 1969) and even as a feature of normal haematopoiesis (Morley et al, 1970; Moser et al, 2008). Oscillations in association with hydroxycarbamide therapy have also been reported in several retrospective case series totalling 7 patients with CML (Kennedy, 1970; Bennett & Grunwald, 2001), and 20 patients with PV (Tefferi et al, 2000; Steensma et al, 2001; Tauscher et al, 2010; Burthem & Chaudhry, 2008). Though the subject of numerous case reports, it has not been until recently that a statistical method of distinguishing these oscillation patterns has been applied to blood count data (Bennett & Grunwald, 2001; Fortin & Mackey, 1999; Tauscher et al, 2010). Using this methodology, it was observed in patients with CML receiving hydroxycarbamide that neutrophil, monocyte, platelet, haemoglobin, and reticulocyte counts all demonstrated oscillations in a range of periods from 30–80 days (Fortin & Mackey, 1999; Bennett & Grunwald, 2001). This observation was also made in patients with PV receiving hydroxycarbamide in platelet and leucocyte counts, with their oscillations exhibiting a much narrower range of periods from 28–30 days (Tauscher et al, 2010; Bennett & Grunwald, 2001; Tefferi et al, 2000; Steensma et al, 2001; Burthem & Chaudhry, 2008). Oscillatory blood counts among these cohorts were noted to range far outside of normal values before reaching their absolute nadir or zenith. Amplification of the oscillatory behaviour, delineated by an increase in the waves’ amplitude, frequency or a combination of the two, exhibited a close temporal association following hydroxycarbamide dosage changes. The combination of these phenomena often meant that treating physicians were spurred to numerous unnecessary, potentially counterproductive, therapeutic interventions and hydroxycarbamide dosing alterations in response to peripheral blood count monitoring.
In the present study, we report on a cohort of 44 patients diagnosed with sickle haemoglobinopathies who have serial records available of peripheral blood count monitoring prior to and during hydroxycarbamide therapy. This cohort’s complete peripheral blood count data was analysed for its periodic content in order to determine the prevalence and characteristics of oscillatory haematopoiesis among patients without a primary myeloproliferative disorder subject to chronic hydroxycarbamide therapy.
Materials and Methods
Medical and laboratory records that were screened for inclusion in the present cohort were originally obtained as part of the National Heart Lung and Blood Institute’s natural history study of sickle cell disease (ClinicalTrials.gov identifier NCT00081523). All patients gave informed consent for participation in this natural history study, which included the use of their study data for additional post-hoc analyses. The present study utilized a retrospective repeated measures design, with each patient having defined intervals with and without hydroxycarbamide exposure to serve as controls. Complete peripheral blood counts (CBC) with white blood cell differential counts, reticulocyte counts and hydroxycarbamide prescribing records were obtained retrospectively utilizing the National Institutes of Health Clinical Center’s Biomedical Translational Research Information System (BTRIS) (Cimino & Ayres, 2010). These records provide a chronological series of CBC data for each individual, matched to their NIH Clinical Center pharmacy records, containing dosages of hydroxycarbamide prescriptions filled by the subject.
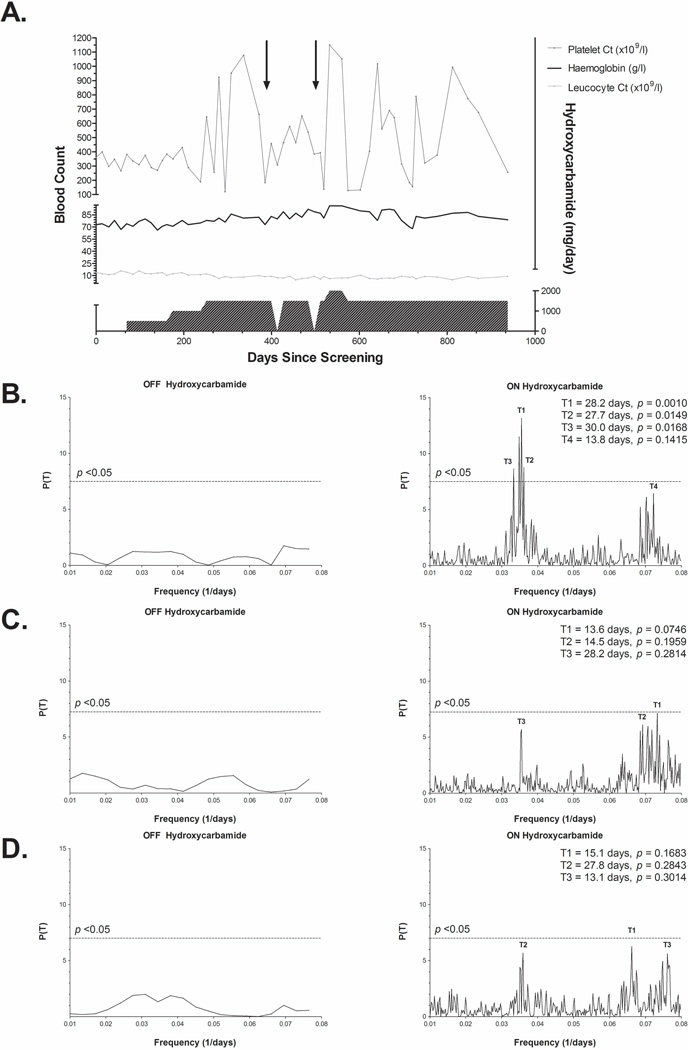
Spectral analysis was performed to obtain significant periodicities of the time series CBC data using the Lomb-Scargle periodogram implemented by Glynn et al (2006). Originally developed within the field of astrophysics to address problems inherent with Fourier power spectral analysis of unevenly spaced time series data sets, the Lomb-Scargle periodogram is derived based on a least squares fitting of sinusoidal curves to the data of interest (Lomb, 1976). It has been used extensively to discover periodic signals distinct from random fluctuations and signal noise in a wide variety of biological systems (Van Dongen et al, 1999, 2001; Ruf, 1999; Yang et al, 2011). The ordinate of the periodogram, P(T) (i.e. the normalized power spectral density), is the likelihood of the data representing a regularly-oscillating sinusoidal wave with a period of T (1/frequency). The associated p-value for an observed peak in the Lomb-Scargle periodogram can subsequently be calculated to test the null hypothesis that the peak’s associated periodicity is due to random fluctuations, with significant peaks indicating a high probability that the signal is truly oscillatory (Scargle, 1982). An example of this analysis performed on a representative patient from the cohort can be seen in Figure 1.
Figure 1. Peripheral blood count monitoring and spectral analysis of a 26-year-old man with sickle cell anaemia.
(A) Representative platelet count, leucocyte count and haemoglobin tracings are shown plotted against time and hydroxycarbamide daily prescribed dosages for the approximate 6-year time span from which data was available. Precipitous shifts in platelet count, in some cases to values >1000 × 109/l, can be seen to coincide closely with hydroxycarbamide dosage alterations. On two occasions, hydroxycarbamide was held briefly due to low absolute neutrophil counts, during which time the platelet count oscillations appeared to be dampened (arrows). Lomb-Scargle periodograms for platelet count (B), leucocyte count (C), and haemoglobin (D) are shown for time periods off (left) and on (right) hydroxycarbamide, with the p <0.05 threshold of significance highlighted by the dotted lines. Select period peaks are labelled on each plot with their associated p-value.
Thresholds for determining the number of pre-toxic and toxic adverse drug events were obtained from the US Food and Drug Administration (FDA) hydroxycarbamide monitoring guidelines (http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016295s041s042lbl.pdf [Accessed May 4, 2013]; Platt, 2008). These guidelines recommend CBC monitoring every 2 weeks during escalation to a maximally tolerated dose. “Pre-toxic ranges” were defined as a platelet count <95 ×109 /l or an ANC <2.5×109/l, while “toxic ranges” were defined as a platelet count <80 ×109/l or an ANC <2 ×109/l. When counts are in the pre-toxic range the guidelines suggest maintaining the same hydroxycarbamide dosage, and when the counts fall to the toxic range a temporary discontinuation of hydroxycarbamide is required until those counts recover. The number of events was divided by the duration of therapy for each patient to arrive at an average event rate for each of these ranges.
While the “off hydroxycarbamide” intervals included a mixture of both the initial baseline period prior to initiating the drug as well as short periods where the drug was held for toxicity or illness, the latter intervals generally lasted less than 7 days and represented a minority of the total “off” time for each individual (Table I). Because of the greater potential for confounding factors in these brief periods, for the purposes of periodogram analysis the “off hydroxycarbamide” period was taken as the interval prior to receiving the first dose of hydroxycarbamide, confirmed by the NIH Clinical Center’s pharmacy records.
Table I.
Clinical demographics for the cohort
| Variable | Total cohort (n = 44) |
|---|---|
| Age at end of surveillance (years) | |
| --Mean (range) | 39.4 (21–80) |
| Male sex [n (%)] | 17 (39) |
| Disease phenotype [n (%)] | |
| --Haemoglobin SS | 41 (93.2) |
| --Haemoglobin SC | 2 (4.5) |
| --Sβ+ thalassemia | 1 (2.3) |
| Mean duration of follow up per patient (months) | |
| --Off hydroxycarbamide (± SD, range) | 21.9 (27.5, 1.05–138) |
| --On hydroxycarbamide (± SD, range) | 57.7 (25.4, 8.51–87.7) |
| Average sampling rate (weeks) | |
| --Off hydroxycarbamide (± SD) | 4.7 ± 4.5 |
| --On hydroxycarbamide (± SD) | 2.8 ± 1.6 |
| Average hydroxycarbamide dosage [n (%)] | |
| --≤1000 mg/day | 14 (31.8) |
| --1000–1500 mg/day | 15 (34.1) |
| --1500–2500 mg/day | 15 (34.1) |
SD, standard deviation.
Median zenith counts were characterized by the value at the 75th percentile of each blood count measure for each patient, while median nadir counts were characterized by the value at the 25th percentile. The median oscillatory amplitude for each of the blood count species was represented by the interquartile range, calculated as the difference between the 75th and 25th percentile values. Means, standard deviations, quartiles and event rates were all calculated using standard statistical and database management toolkits in Microsoft Excel (Microsoft Corporation, Redmond, WA). Statistical comparisons between groups were made using the Cochran-Armitage test for trend and student’s t-test as appropriate. P-values < 0.05 were considered significant. All histograms and figures were generated using Graphpad Prism 5.0 statistical packages (GraphPad, La Jolla, CA).
Results
The baseline laboratory and clinical characteristics of the cohort are shown in Table I. Significant oscillatory patterns were found in the blood counts of 86.4% of the patients studied using Lomb-Scargle spectral analysis, which provides a statistical confidence level that such patterns are nonrandom when applied to unevenly sampled time series data sets (Fortin & Mackey, 1999; Glynn et al, 2006; Lomb, 1976; Scargle, 1982; Tauscher et al, 2010; Bennett & Grunwald, 2001). There was a differential propensity across the haematological lineages for oscillations, which appeared to parallel the mature elements’ circulating life span (Table II). The majority of patients manifested oscillations in either platelet (56.8%) or leucocyte counts (52.3%), while the absolute lymphocyte count oscillated in only 25% of the cohort during hydroxycarbamide treatment. These significant oscillations were detected more frequently among patients with greater exposure to hydroxycarbamide, though significant periods of oscillation were detected in some patients at baseline as well (Supplement Table 1S).
Table II.
Prevalence of oscillatory behaviour within the cohort by hydroxycarbamide status
| Variable | Total cohort (n = 44) | |
|---|---|---|
| Significant periodicity present in blood count [n (%)]† |
Off hydroxycarbamide |
On hydroxycarbamide |
| Any lineage | 19 (43.2) | 38 (86.4) |
| --Platelet count | 14 (31.8) | 25 (56.8) |
| --Leucocyte count | 7 (15.9) | 23 (52.3) |
| --Haemoglobin | 7 (15.9) | 19 (43.2) |
| --Red blood cell count | 5 (11.4) | 20 (45.5) |
| --Absolute reticulocyte count (ARC) | 8 (18.2) | 14 (31.8) |
| --Absolute neutrophil count (ANC) | 6 (13.6) | 21 (47.7) |
| --Absolute lymphocyte count (ALC) | 3 (6.8) | 11 (25) |
| --Absolute monocyte count (AMC) | 1 (2.3) | 20 (45.5) |
At least one peak within Lomb-Scargle periodogram associated with p <0.05.
Interestingly, the prevalence of significant platelet count oscillations was highly dependent upon hydroxycarbamide dose: only 33.3% of patients with an average hydroxycarbamide dose <1200 mg/day had detectable oscillations. This increased to 46.7% with an average dose between 1200–1600 mg/day and 92.9% with an average dose >1600 mg/day; p = 0.0013, Cochran-Armitage test for trend (Figure 2A). In contrast, the prevalence of significant neutrophil count oscillations appeared to be dependent upon the duration of hydroxycarbamide therapy: only 14.3% of patients with hydroxycarbamide treatment duration <40 months had detectable oscillations, while the prevalence increased to 62.5% for a duration between 40 – 75 months and 64.3% for a duration >75 months; p = 0.0081, Cochran-Armitage test for trend (Figure 2B). The relationship of drug exposure to oscillatory induction is probably complex, with many factors contributing to the observed effects of increasing dosage level or duration of therapy for each haematopoietic lineage.
Figure 2. Dose and duration effects of hydroxycarbamide treatment on oscillatory behaviour.
An increased prevalence of significant oscillations was seen across all measured blood counts in patients with greater exposure to hydroxycarbamide, as measured by either an average daily dosage received (A) or by duration of therapy (B). Platelet counts (A) and absolute neutrophil counts (B) are shown to illustrate the complex interplay seen between the daily dosage levels and the duration of therapy in contributing to this increased induction into oscillations. The p-values for Cochran-Armitage test for trend are shown for each group.
The blood counts from many patients in the cohort showed multiple distinct periods of oscillation. The population distribution of all significant periods of oscillation for platelet and leucocyte counts showed that the most prevalent periods across the cohort were 27±1 days for platelets and 15±1 days for leucocytes (Figure 3). Less prevalent peaks were observed for platelets at 15±1 days and 45±1 days, and for leucocytes at 30±1 days and 78±1 days. Each of these periods noted within our cohort overlap with values previously described in the literature for both haematological lineages (Bennett & Grunwald, 2001; Fortin & Mackey, 1999; Tefferi et al, 2000; Steensma et al, 2001; Tauscher et al, 2010; Burthem & Chaudhry, 2008). At the cohort level, hydroxycarbamide appeared to have a consolidating effect upon the distribution of detectable periods for oscillating platelet- and leucocyte-producing bone marrow populations, leading to fewer dominant periods while receiving the drug (Figure 3).
Figure 3. Distribution of oscillatory peaks in all patients.
Histogram of significant periodicities present in platelet counts (A) and leucocyte counts (B) for the cohort delineated by hydroxycarbamide status. Prevalent periods within the cohort distribution are labelled on each plot. All data points included represent distinct Lomb-Scargle periodogram peaks with an associated p-value <0.05.
As expected owing to the recommended monitoring guidelines, peripheral blood count sampling was more frequent while patients were taking hydroxycarbamide than when not taking the drug; p = 0.0085, 2-tailed paired student’s t-test (Table I). Throughout the cohort the expected myelosuppressive drug effects of hydroxycarbamide were observed, including a decrease in the leucocyte count, ANC, reticulocyte count and platelet count, as well as a variable increase in haemoglobin level (Figure 4). Despite the significant baseline decrease, the oscillatory amplitudes were essentially unchanged from the patients’ pre-hydroxycarbamide state, resulting in lower nadirs while receiving the drug (Supplement Table 1S).
Figure 4. Complete blood count behaviour in all patients.
The line plots show the effect of hydroxycarbamide treatment on all measured blood count parameters with respect to the mean or baseline values (solid) and the median oscillatory amplitudes (open). While nearly all blood counts except haemoglobin and RBC counts experienced a significant decrease in their baseline once hydroxycarbamide was introduced, the amplitudes regularly created by these oscillations did not differ significantly from the patients’ pretreatment variability. Error bars represent one standard deviation from the mean in each group. Before and after groups were compared using a paired student’s t-test, with significant results shown on the plots using the convention * = p <0.01; ** = p <0.001; *** = p <0.0001.
Despite these oscillations to lower absolute counts, peripheral platelet and neutrophil counts rarely reached toxic ranges that required temporary discontinuation of hydroxycarbamide. Myelotoxicity was most often indicated by a low ANC, occurring approximately 2.5 times per patient-year on average, and less often by a low platelet count, approximately once every 10 patient-years on average. This pattern also holds true for pre-toxic events, with the event rates being approximately doubled (Supplement Table 1S).
Discussion
To our knowledge, this study provides the first documented observation of oscillatory behaviour in peripheral blood counts during hydroxycarbamide therapy in patients unaffected by a myeloproliferative disease. Because of the previous apparent association of blood count oscillations with myeloproliferative disease states, numerous theories attributed the mechanism of these oscillations to malignant or pre-malignant specific pathobiology. While there is some evidence that even in normal individuals there may be a continuous stable oscillation of every haematopoietic cell lineage (Morley et al, 1970, 1967; von Schulthess & Gessner, 1986; Moser et al, 2008), the myeloproliferative disease-centric theories postulate that clonal bone marrow progenitor cells present in myeloproliferative diseases may be abnormally sensitive to lineage-specific growth signals, hydroxycarbamide therapy, or a combination of factors (Tefferi et al, 2000; Steensma et al, 2001; Santillán et al, 2000; Zent et al, 1999). These neoplastic alterations in signalling pathways are thought to manifest as oscillations apparent in macroscopic measures, such as peripheral blood counts. Since the time of these models’ publication, such oscillatory behaviour has been debated, primarily owing to two main factors: the absence of any obvious genetic or environmental abnormalities in the bone marrow niche to affect the development of the progenitor stem cell pool and the inability of previous statistical modelling to accurately distinguish periodic oscillations from signal noise (Maughan et al, 1973; Mackey, 1978; Dale et al, 1973, 2002). Given our current findings, we believe a more fundamental mechanism may contribute to this phenomenon, such that even heterogeneous marrow populations may be susceptible to an oscillogenic drug effect of hydroxycarbamide.
The regulation of haematopoiesis, although incompletely understood at present, is probably mediated in part through negative feedback controls. Such negative feedback-mediated systems have a tendency to fluctuate around an established set point in mathematical modelling (see Figure 5 inset), due to the inherent time delay required for the system to monitor the input of interest and subsequently respond via regulatory signals (Haurie et al, 1998; von Schulthess & Gessner, 1986; Santillán et al, 2000; Dunn, 1983). Several studies have expanded upon this negative feedback model with the addition of a dampening factor related to the mature blood elements’ circulating half-life (TE) and the time required for these elements to transit the marrow space (TM), to model the functions of both the obligate time delay inherent to cellular proliferation as well as the size of the marrow’s reserve capacity (C) of partially differentiated precursor stem cells able to respond to the aforementioned proliferative signals (Figure 5) (Morley et al, 1970; Dunn, 1983; Schmitz et al, 1990). This combined model predicts that when a measured blood element has a long circulating half-life in comparison to the time required for the marrow to generate mature circulating elements, the system will be sufficiently dampened so that oscillatory behaviour is suppressed not only at the system’s functioning baseline, but in response to sub-maximal perturbations in circulating mature blood elements or marrow precursors as well. Our own cohort appears to reflect this dampening effect: shorter lived platelet, reticulocyte and neutrophil lineages demonstrated the highest prevalence of significant oscillations at baseline; while longer lived lymphocyte, monocyte and erythrocyte lineages had the lowest prevalence.
Figure 5. Model of oscillatory haematopoiesis.
A potential mechanism for the induction of oscillatory behaviour due to the activity of hydroxycarbamide within haematopoietic negative feedback loops, using thrombopoiesis as a model. (Bennett & Grunwald, 2001; Kaushansky, 2005; Wolber & Jelkmann, 2002) Peripheral and central regulatory loops governing thrombopoiesis are shown above, along with the presumed block in proliferating cells during their S-phase created by hydroxycarbamide’s mechanism of action (A). Below, a model negative feedback system paralleling the biologicalsystem is shown (B), along with the theoretical normal response over time for a monitored parameter within this system, illustrated by the solid line (inset). The resulting effect on the monitored parameter due to the increased time delay created by hydroxycarbamide within this system is illustrated by the overlaid dotted line (Morley et al, 1970).
CFU-Meg, megakaryocyte colony-forming units
This model could be further expanded to explain our observations with hydroxycarbamide therapy. When daily pulsatile doses of hydroxycarbamide are added to such a system, its mechanism of action predictably creates a point of restricted S-phase entry into the cell cycle that may act to partially synchronize the rapidly dividing precursor cells upon metabolic clearance of the drug, which has a 3- to 4-h half-life (Yarbro, 1992). Such cell cycle synchronization effects are commonly exploited experimentally in tissue culture and yeast cells (Day et al, 2004; Davis et al, 2001). As a consequence of this cell cycle synchronization, oscillations would become more phase coherent and thus more easily detectable apart from random fluctuations in macroscopic measures, such as peripheral blood counts (Schmitz et al, 1990). Furthermore, this restriction point would be expected to cause an increase in the marrow transit time required to respond to growth signals that would lead to a decreased dampening effect upon the system according to Morley’s coefficient (Figure 5). This effect appears consistent within our cohort and individual data as well: the prevalence of oscillatory behaviour across all peripheral blood counts increased with more prolonged and intensive exposure to hydroxycarbamide, which could represent an incremental accumulation of these perturbations within the haematopoietic feedback systems. The fact that oscillations are more readily detected with greater dose-intensity of hydroxycarbamide suggests a dose-response relationship between the drug and oscillations of peripheral blood counts.
These observations have direct relevance to clinical practice, as this study provides evidence that oscillatory behaviour can occur commonly in patients with no predisposition to haematological malignancy. Rather, this phenomenon may represent the consequence of a drug effect upon negative feedback loops governing the regulation of haematopoiesis and as such, in any patient group receiving cell cycle-specific myelosuppressive agents, such a result may be anticipated. Within the scope of hydroxycarbamide maintenance therapy in SCD, while the shifts between nadirs and zeniths in these patients’ blood counts may appear precipitous in the short term, our data indicates that, in general, this pattern does not pose a danger of myelotoxicity to the patient, and recovery to normal ranges occurs in many cases without intervention. As noted in previous cases, it appears that stable and consistent hydroxycarbamide dosing leads to dampening of the oscillatory behaviour over time, while frequent dosage changes often lead to amplification of the oscillations and even more far ranging nadirs and zeniths (Kennedy, 1970; Bennett & Grunwald, 2001; Tefferi et al, 2000; Steensma et al, 2001; Tauscher et al, 2010). Importantly, our results indicate that oscillatory haematopoiesis is not a phenomenon limited to malignant or pre-malignant haematological disorders, a finding that should prompt further investigation of its significance and prevalence in other patient populations receiving cell cycle-specific inhibitors.
Supplementary Material
Acknowledgments
JHB and GJK designed the research study, analysed the data and wrote the manuscript; CPM, JML, SA, MJ, JGT, and GJK acquired the data for the study; JML, XT, and CW assisted in the analysis and interpretation of the data. All authors reviewed and approved the manuscript.
This research was supported by the Division of Intramural Research of the National Heart, Lung and Blood Institute (1 ZIA HL006014-03). Fellowship funding of JHB was provided by the Clinical Research Training Program, a public-private partnership supported jointly by the National Institutes of Health (NIH) and Pfizer Inc. (via a grant to the Foundation for NIH from Pfizer Inc.). The authors thank Mary K. Hall for expert protocol management; protocol coordinators James Nichols, Catherine Seamon, Marlene Peters-Lawrence, and Darlene Allen for assistance in patient recruitment and data collection; and Cindy Clark, NIH Library Writing Center, for manuscript editing assistance.
Footnotes
There are no conflicts of interest for all the authors.
References
- Bennett M, Grunwald AJ. Hydroxyurea and periodicity in myeloproliferative disease. European Journal of Haematology. 2001;66:317–323. doi: 10.1034/j.1600-0609.2001.066005317.x. [DOI] [PubMed] [Google Scholar]
- Burthem J, Chaudhry MS. Hydroxycarbamide associated platelet count oscillations in a patient with polycythaemia vera. A case report and review of the literature. Platelets. 2008;19:234–235. doi: 10.1080/09537100701882053. [DOI] [PubMed] [Google Scholar]
- Cimino JJ, Ayres EJ. The clinical research data repository of the US National Institutes of Health. Studies in Health Technology and Informatics. 2010;160:1299–1303. [PMC free article] [PubMed] [Google Scholar]
- Dale DC, Alling DW, Wolff SM. Application of Time Series Analysis to Serial Blood Neutrophil Counts in Normal Individuals and Patients Receiving Cyclophosphamide. British Journal of Haematology. 1973;24:57–64. doi: 10.1111/j.1365-2141.1973.tb05727.x. [DOI] [PubMed] [Google Scholar]
- Dale DC, Bolyard AA, Aprikyan A. Cyclic neutropenia. Seminars in Hematology. 2002;39:89–94. doi: 10.1053/shem.2002.31917. [DOI] [PubMed] [Google Scholar]
- Davis PK, Ho A, Dowdy SF. Biological methods for cell-cycle synchronization of mammalian cells. BioTechniques. 2001;30:1322–1326. 1328, 1330–1331. doi: 10.2144/01306rv01. [DOI] [PubMed] [Google Scholar]
- Day A, Schneider C, Schneider BL. Yeast cell synchronization. Methods in molecular biology (Clifton, N.J.) 2004;241:55–76. doi: 10.1385/1-59259-646-0:55. [DOI] [PubMed] [Google Scholar]
- Dunn CD. Cyclic hematopoiesis: the biomathematics. Experimental Hematology. 1983;11:779–791. [PubMed] [Google Scholar]
- FDA. Princeton, NJ: Bristol Myers Squibb Company; 2012. [Accessed May 4, 2013]. Droxia (hydroxyurea) capsules [package insert] Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016295s041s042lbl.pdf. [Google Scholar]
- Fortin P, Mackey MC. Periodic chronic myelogenous leukaemia: spectral analysis of blood cell counts and aetiological implications. British Journal of Haematology. 1999;104:336–345. doi: 10.1046/j.1365-2141.1999.01168.x. [DOI] [PubMed] [Google Scholar]
- Glynn EF, Chen J, Mushegian AR. Detecting Periodic Patterns in Unevenly Spaced Gene Expression Time Series Using Lomb–Scargle Periodograms. Bioinformatics. 2006;22:310–316. doi: 10.1093/bioinformatics/bti789. [DOI] [PubMed] [Google Scholar]
- Haurie C, Dale DC, Mackey MC. Cyclical neutropenia and other periodic hematological disorders: a review of mechanisms and mathematical models. Blood. 1998;92:2629–2640. [PubMed] [Google Scholar]
- Kaushansky K. The molecular mechanisms that control thrombopoiesis. The Journal of Clinical Investigation. 2005;115:3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BJ. Cyclic leukocyte oscillations in chronic myelogenous leukemia during hydroxyurea therapy. Blood. 1970;35:751–760. [PubMed] [Google Scholar]
- Lomb NR. Least-squares frequency analysis of unequally spaced data. Astrophysics and Space Science. 1976;39:447–462. [Google Scholar]
- Mackey MC. Unified hypothesis for the origin of aplastic anemia and periodic hematopoiesis. Blood. 1978;51:941–956. [PubMed] [Google Scholar]
- Malhotra OP, Salam SR. Cyclic oscillations of leucocyte counts in chronic myeloid leukaemia. Postgraduate Medical Journal. 1991;67:87–89. doi: 10.1136/pgmj.67.783.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan WZ, Bishop CR, Pryor TA, Athens JW. The Question of Cycling of the Blood Neutrophil Concentrations and Pitfalls in the Statistical Analysis of Sampled Data. Blood. 1973;41:85–91. [PubMed] [Google Scholar]
- Mehta BC, Agarwal MB. Cyclic oscillations in leukocyte count in chronic myeloid leukemia. Acta Haematologica. 1980;63:68–70. doi: 10.1159/000207373. [DOI] [PubMed] [Google Scholar]
- Morley A. Blood-cell cycles in polycythaemia vera. Australasian Annals of Medicine. 1969;18:124–126. doi: 10.1111/imj.1969.18.2.124. [DOI] [PubMed] [Google Scholar]
- Morley A, King-Smith EA, Stohlman FJ. Symposium on Hemopoietic Cellular Proliferation. St. Elizabeth’s Hospital Centennial, 1869–1969. Edited by Frederick Stohlman, Jr. Boston, MA: Grune & Stratton; 1970. Oscillatory Nature of Hemopoiesis; pp. 3–14. [Google Scholar]
- Morley AA, Baikie AG, Galton DA. Cyclic leucocytosis as evidence for retention of normal homoeostatic control in chronic granulocytic leukaemia. Lancet. 1967;2:1320–1323. doi: 10.1016/s0140-6736(67)90910-5. [DOI] [PubMed] [Google Scholar]
- Moser M, Frühwirth M, Kenner T. The symphony of life. Importance, interaction, and visualization of biological rhythms. IEEE Engineering in Medicine and Biology Magazine: The Quarterly Magazine of the Engineering in Medicine & Biology Society. 2008;27:29–37. doi: 10.1109/MEMB.2007.907365. [DOI] [PubMed] [Google Scholar]
- Platt OS. Hydroxyurea for the treatment of sickle cell anemia. The New England Journal of Medicine. 2008;358:1362–1369. doi: 10.1056/NEJMct0708272. [DOI] [PubMed] [Google Scholar]
- Ruf T. The Lomb-Scargle Periodogram in Biological Rhythm Research: Analysis of Incomplete and Unequally Spaced Time-Series. Biological Rhythm Research. 1999;30:178–201. [Google Scholar]
- Santillán M, Mahaffy JM, Bélair J, Mackey MC. Regulation of platelet production: the normal response to perturbation and cyclical platelet disease. Journal of Theoretical Biology. 2000;206:585–603. doi: 10.1006/jtbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- Scargle JD. Studies in astronomical time series analysis. II - Statistical aspects of spectral analysis of unevenly spaced data. The Astrophysical Journal. 1982;263:835–853. [Google Scholar]
- Schmitz S, Loeffler M, Jones JB, Lange RD, Wichmann HE. Synchrony of bone marrow proliferation and maturation as the origin of cyclic haemopoiesis. Cell Proliferation. 1990;23:425–442. doi: 10.1111/j.1365-2184.1990.tb01135.x. [DOI] [PubMed] [Google Scholar]
- Steensma DP, Harrison CN, Tefferi A. Hydroxyurea-associated platelet count oscillations in polycythemia vera: a report of four new cases and a review. Leukemia & Lymphoma. 2001;42:1243–1253. doi: 10.3109/10428190109097749. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Siegel F, Petrides PE. Hydroxyurea induced oscillations in twelve patients with polycythemia vera. Haematologica. 2010;95:1227–1229. doi: 10.3324/haematol.2010.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Elliott MA, Kao PC, Yoon S, El-Hemaidi I, Pearson TC. Hydroxyurea-induced marked oscillations of platelet counts in patients with polycythemia vera. Blood. 2000;96:1582–1584. [PubMed] [Google Scholar]
- Van Dongen HP, Olofsen E, VanHartevelt JH, Kruyt EW. Searching for biological rhythms: peak detection in the periodogram of unequally spaced data. Journal of biological rhythms. 1999;14:617–620. doi: 10.1177/074873099129000984. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Ruf T, Olofsen E, VanHartevelt JH, Kruyt EW. Analysis of problematic time series with the Lomb-Scargle Method, a reply to ‘emphasizing difficulties in the detection of rhythms with Lomb-Scargle periodograms’. Biological rhythm research. 2001;32:347–354. doi: 10.1076/brhm.32.3.347.1348. [DOI] [PubMed] [Google Scholar]
- Vodopick H, Rupp EM, Edwards CL, Goswitz FA, Beauchamp JJ. Spontaneous cyclic leukocytosis and thrombocytosis in chronic granulocytic leukemia. The New England Journal of Medicine. 1972;286:284–290. doi: 10.1056/NEJM197202102860603. [DOI] [PubMed] [Google Scholar]
- Von Schulthess GK, Gessner U. Oscillating platelet counts in healthy individuals: experimental investigation and quantitative evaluation of thrombocytopoietic feedback control. Scandinavian Journal of Haematology. 1986;36:473–479. doi: 10.1111/j.1600-0609.1986.tb02283.x. [DOI] [PubMed] [Google Scholar]
- Wolber E-M, Jelkmann W. Thrombopoietin: the novel hepatic hormone. News in Physiological Sciences: An International Journal of Physiology Produced Jointly by the International Union of Physiological Sciences and the American Physiological Society. 2002;17:6–10. doi: 10.1152/physiologyonline.2002.17.1.6. [DOI] [PubMed] [Google Scholar]
- Yang R, Zhang C, Su Z. LSPR: an integrated periodicity detection algorithm for unevenly sampled temporal microarray data. Bioinformatics (Oxford, England) 2011;27:1023–1025. doi: 10.1093/bioinformatics/btr041. [DOI] [PubMed] [Google Scholar]
- Yarbro JW. Mechanism of action of hydroxyurea. Seminars in Oncology. 1992;19:1–10. [PubMed] [Google Scholar]
- Zent CS, Ratajczak J, Ratajczak MZ, Anastasi J, Hoffman PC, Gewirtz AM. Relationship between megakaryocyte mass and serum thrombopoietin levels as revealed by a case of cyclic amegakaryocytic thrombocytopenic purpura. British Journal of Haematology. 1999;105:452–458. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.