Abstract
Objective
To examine the extent to which patients recall the contents of therapy from one session to the next and to determine whether recall is associated with treatment outcome.
Method
Thirty inter-episode individuals with bipolar disorder and comorbid insomnia (ages 21-62 years, 56.7% female, 56.7% Caucasian) participated in an RCT of psychotherapies. Patients received either Cognitive Behavioral Therapy for Insomnia (CBTI-BP; n = 17) or Psychoeducation (PE; n = 13). At the beginning of each weekly session, patients freely recalled as many therapy points (i.e., distinct ideas, principles, and experiences) as they could from their previous session. After each session, therapists recorded a list of all therapy points delivered. Treatment outcome was measured via the Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), Patient-Reported Outcome Measurement Info System—Sleep (PROMIS-Sleep), and Quality of Life—Sleep (QOL-Sleep), administered at pre and post treatment, and treatment evaluation questions administered at post treatment.
Results
Patients recalled 19.6% to 36.9% of therapy points listed by therapists. Raw numbers of therapy points recalled were positively correlated with reductions in ISI and gains in QOL-Sleep, and with most treatment evaluation questions. Percentages of therapy points recalled were positively correlated with gains in QOL-Sleep, but with no other sleep outcome measures or any of the treatment evaluation questions. Patients in CBTI-BP recalled more therapy points than those in PE, but did not differ in the percentages of points recalled.
Conclusions
Memory for therapy is poor. The amount of content recalled is positively associated with treatment outcome. Enhancing memory for therapy might play a key role in improving treatment outcome.
Keywords: bipolar disorder, cognitive-behavioral therapy, memory, recall
Memory for medical advice is staggeringly poor. Cancer patients recall 23% to 33% of treatment recommendations (Bober, Hoke, Duda, & Tung, 2007; Jansen et al., 2008). Patients with osteoporosis recall as little as 31% of treatment information (Pickney & Arnason, 2005), and among chronic pain patients, recall is as low as 30% (Lewkovich & Haneline, 2005). Recall is particularly poor for advice regarding health-related behavior change (Flocke & Stange, 2004; Kravitz et al.,1993). These findings are important because poor memory for the content of a doctor's visit has adverse effects on treatment adherence (e.g., Bober et al., 2007; Jansen et al., 2008), leading to the incorrect or incomplete carrying out of recommendations (Vermeire, Hearnshaw, Van Royen, & Denekens, 2001).
Although the aforementioned studies have focused on memory for physician recommendations following clinic visits for non-mental health related issues, relatively little is known about the extent to which patients remember the contents of psychotherapy sessions. To the authors’ best knowledge, only one study has examined patient memory for therapy. Chambers (1991) reported that patients with insomnia forget one third of the instructions given during behavioral therapy for insomnia, and for some types of recommendations, recall is as low as 13%. As patient recall in the Chambers (1991) study was measured within the months following treatment, shorter-term memory for therapy has yet to be examined. As such, the first aim of the present study was to establish the extent to which patients recall the contents of therapy from one weekly session to the next.
As already highlighted, there is evidence that poor memory for a doctor's visit can have adverse effects on treatment adherence (Bober et al., 2007; Jansen et al., 2008), but the relation between memory for therapy and treatment outcome is yet to be established. On the one hand, baseline memory impairment appears to be a predictor of poor cognitive-behavioral therapy outcome among cocaine-dependent patients (Aharanovic, Nunes, & Hasin, 2003) and individuals with posttraumatic stress disorder (Wild & Gur, 2008). On the other hand, Chambers (1991) found no significant association between recommendations recalled and treatment outcome among patients with insomnia receiving behavioral therapy. However, recall and outcome were measured between 2 to 18 months post treatment. The extent to which memory for therapy during treatment plays a role in treatment outcome remains a critical gap. As such, our second aim was to establish the relation between recall rates and treatment outcome, extending the earlier work pioneered by Chambers (1991). Specifically, we examined the association between patient recall (measured across eight weekly therapy sessions) and associated treatment outcome measured within a week following treatment.
The present study examines patient memory for therapy using data from a recent trial of individuals with bipolar disorder and comorbid insomnia. Patients in the present study were randomly allocated to one of two non-pharmacological treatment groups: cognitive behavioral therapy for insomnia (CBTI-BP) or psychoeducation (PE). CBTI-BP is a bipolar-disorder-specific modification of cognitive behavioral therapy for insomnia that integrates elements from cognitive behavioral therapy for insomnia (Morin, Bootzin, Buysse, Edinger, Espie, & Lichstein, 2006), interpersonal and social rhythm therapy (Frank, 2005), and motivational interviewing (Miller & Rollnick, 2002). PE is a treatment in which patients receive general information about sleep and bipolar disorder but no plan for change is discussed. Patients receiving PE are first introduced to a model in which sleep, stress, diet, health, exercise, and mood are all inter-related and have reciprocal effects. They are then provided with information about the etiology, prodromes, and symptoms of bipolar disorder, as well as didactic information about stress management, relaxation, and breathing techniques. Throughout the PE protocol, patients are asked for a detailed description of their experience with each topic covered, but are not encouraged to implement or practice strategies for behavior change. Although memory for medical advice is poor across various patient populations receiving differing forms of treatment, to the best of our knowledge, no study has examined whether memory for therapy varies based on treatment type. As such, an additional objective of the present study was to examine whether patient recall varies based on treatment received (i.e., CBTI-BP vs. PE)—and possible links to differential outcome.
To summarize, the present study examined memory for therapy and associated treatment outcome among a sample of individuals with bipolar disorder receiving one of two psychotherapies for insomnia. First, we established the extent to which patients recall the contents of therapy (Aim 1) and determined whether patient recall is associated with treatment outcome (Aim 2). We then examined whether patient recall differs based on the type of treatment received (Aim 3), and whether the relation between patient recall and corresponding treatment outcome varies based on treatment type (i.e., CBTI-BP versus PE; Aim 4). Given the evidence that patients remember about a third or less of physician recommendations following non-mental health related clinic visits, we expected that patients would remember less than a third of therapy contents. We also hypothesized that poorer recall would be associated with worse self-reported ratings of sleep and evaluations of treatment. Given the evidence that memory for medical advice is poor across various treatment types, we predicted that there would be no differences in patient recall rates or associations between patient recall and treatment outcome between the two treatment groups.
Method
Participants
Thirty individuals diagnosed with bipolar disorder (ages 21-62) participated in an NIMH-funded randomized control trial of psychological treatments for sleep disturbance were observed for the purpose of this study. All participants were recruited from the greater Alameda County to reflect population demographics. The University of California, Berkeley, Committee for the Protection of Human Subjects approved the study. All participants provided written informed consent and were financially compensated.
Participants were assessed via in-person interview to meet DSM-IV-TR (American Psychological Association, 2000) diagnostic criteria for bipolar disorder, type I, as well as subjective complaints of insomnia, defined as having self-reported difficulties falling asleep, staying asleep, and/or problems with un-refreshing/non-restorative sleep with associated daytime complaints for at least 3 days per week within the past month (Edinger et al., 2004; American Academy of Sleep Medicine, 2005; American Psychiatric Association, 2000). At study entry, participants were required to be inter-episode, determined by a score of 12 or less on the Inventory of Depressive Symptomatology-Clinician and a score of 7 or less on the Young Mania Rating Scale-Clinician (Rush et al., 2003; Chengappa et al., 2003; Thompson et al., 2005) at pre-treatment.
Participants were excluded for the presence of an active and progressive physical illness (e.g., congestive heart failure, dementia, or multiple sclerosis) directly related to the onset and course of insomnia, the presence of sleep apnea (apnea/hypopnea index > 15), restless legs syndrome or periodic limb movements during sleep (PLMS with arousal > 15 per hour), circadian-based sleep disorders (e.g., delayed or advanced sleep phase syndrome), alcohol or drug abuse within the past 3 months, current posttraumatic stress disorder, current suicidal risk (assessed by item 18 on the IDS-SR), and the use of medications known to alter sleep (e.g., steroids, theophylline, propranolol). Participants were not excluded for the use of mood stabilizing and other psychotropic medications, but were required to be on a stable regimen of medications (i.e., no changes in the dosage or frequency of medication use for at least four consecutive weeks) prior to starting treatment. The use of medications remained as a naturalistic variable in this study to maximize the generalizability of the findings.
Procedure
Patients were randomly allocated to receive either CBTI-BP or PE. Both treatment groups consisted of weekly one-on-one sessions lasting 50 minutes each for 8 consecutive weeks. Both conditions were matched for the number and quality of handouts. Therapists were upper-level doctoral students receiving clinical training at the University of California, Berkeley. Further information regarding treatment rationale, content, and fidelity is detailed in Harvey et al. (under review).
At the beginning of each session (starting with the second session), participants were handed a sheet of paper with the following instructions on top: Take a moment to think back to your last session. Please write down all distinct points, principles, and/or experiences that you think were important to remember and/or implement as part of your treatment. Make sure to recall as many distinct ‘take-home’ messages as possible. Each participant then wrote down as many points as they could remember in the remaining blank section of the paper. During each session, participants in each treatment group were encouraged to take notes on what they learned during sessions. Immediately following each therapy session, the therapist filled out a form with the following instructions on top: Please take a moment to write down all therapy points that were delivered during this session. Therapy point definition: the points, principles, or experiences that we want individual patients to remember and/or implement. These should be relevant to the specific patient (e.g., if the patient doesn't smoke, we would not consider information about sleep and smoking to be relevant to the patient), as well as relevant to treatment (e.g., we would not expect patients to report standard assessments of mood, a nonspecific review of the sleep diary, agenda setting, eliciting/giving feedback, or session scheduling). Instructions to rehearse learned principles were considered to be a form of homework, and were not included as examples of therapy points. For instance, the instruction, “Please complete this sleep diary each day” was considered to be a form of homework, while information as to how one would go about completing a sleep diary, or why a sleep diary might be useful, was considered to be an example of a therapy point.
Patient recall was then measured via a scoring system designed for this study given the lack of a psychometrically validated alternative. Based on the finding by Blanchard et al. (1995) that changing from the most liberal to the most conservative scoring rule results in a change in diagnosis of PTSD from 44% to 29%, two forms of scoring rules were devised: Easy Scoring, which consisted of a relatively lenient grading system, and Hard Scoring, which consisted of a rather conservative grading system. See Figure 1.
Figure 1.
Recall Scoring Rubric.
Patient recall data were collected at the beginning of sessions 2 through 8, and corresponding therapy point lists were collected immediately following sessions 1 through 7. See Figure 2 for sample patient recall data.
Figure 2.
Sample Recall Data
Patient recall for a given session was then measured in two ways for each scoring rubric: [1] the raw number of therapy points freely recalled (# Points Recalled); and [2] the percentage of therapy points freely recalled based on the corresponding therapist's list (% Points Recalled). Thus, given the two scoring rubrics (Easy vs. Hard Scoring) and two types of recall measured (# Points Recalled and % Points Recalled), we derived four recall variables: # Points Recalled (Easy), # Points Recalled (Hard), % Points Recalled (Easy), and % Points Recalled (Hard).
Therapy points recalled by patients that were not on the corresponding therapists’ list were not counted towards or against their recall scores. Therapy points recalled by patients that were not on therapists’ lists were regarded as “extraneous therapy points.” Hence, a given extraneous point could have been a false response by the patient, or a correct response that therapists failed to record on the therapist's list. As such, extraneous points were not counted toward or against any of the patients’ recall scores.
Homework was assigned at the end of each session. This included assignments such as completing a sleep diary, carrying out behavioral experiments, and filling out activity logs. Immediately following each session, therapists recorded their response to the question, To what extent did your patient complete the homework assignments this past week? on a scale from 0% (did not complete) to 100% (fully completed).
Measures
Participants were assessed for bipolar severity using the following measures at baseline:
Young Mania Rating Scale (YMRS)
The YMRS is an 11-item measure used to assess the severity of manic symptoms, with each item rated on a five-point scale. It has been shown to have good reliability and validity (Young et al., 1978).
Inventory of Depressive Symptomatology, Clinician Rating (IDS-C)
The IDS-C is a widely-used 30-item instrument assessing depressive symptoms, with each item rated on a four-point scale. The measure has demonstrated good reliability and validity (Rush et al., 1996).
Treatment outcome was measured via the following outcome measures at pre and post treatment:
Insomnia Severity Index (ISI)
The ISI is a brief 7-item assessment of nighttime variables (difficulties falling asleep, staying asleep, early morning awakenings) and daytime variables (satisfaction with sleep, degree of impairment with daytime functioning, noticeability of impairments, distress or concern with sleep). The ISI has adequate internal consistency (Cronbach's alpha = 0.91) and temporal stability (r = 0.80), and has been validated against diary and polysomnographic measures of sleep (Bastien, Vallieres, & Morin, 2001).
Pittsburgh Sleep Quality Index (PSQI)
The PSQI measures sleep quality based on self-reported ratings of 18 questions across seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. Scoring of the answers is based on a 0 to 3 scale, whereby 3 reflects the negative extreme on the Likert Scale. The PSQI has sufficient internal consistency and a reliability coefficient (Cronbach's alpha = 0.83; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989).
PROMIS-Sleep Disturbance (PROMIS-SD)
PROMIS-Sleep Disturbance (PROMIS-SD) was developed as part of the NIH Roadmap initiative designed to improve patient report outcomes using state-of-the-art psychometric methods. The short form (8 items) is scored 1 ‘not at all’ to 5 ‘very much’ and has established reliability and validity (convergent and construct). The sleep-related impairment item bank focuses on self-reported perceptions of alertness, sleepiness, and tiredness during usual waking hours, and the perceived functional impairments during wakefulness associated with sleep problems or impaired alertness. The sleep-related impairment short form is generic rather than disease-specific. It assesses sleep-related impairment over the past seven days, and is a validated measure (Buysse et al., 2010; Yu, Buysse, Germain, & Moul, 2011)
Quality of Life in Bipolar Disorder – Sleep (QOL-Sleep)
The QOL-Sleep is one of 12 domains measured via the QOL, which has established reliability and validity (Michalak, Murray, & CREST.BD, 2010). The QOL-sleep domain evaluates sleep and sleep-related daytime functioning over the past week. Specifically, patients are asked to think over the past 7 days and rate the extent to which they have woken up feeling refreshed, had no problems getting out of bed, had about the right amount of sleep for him/her, and kept a routine sleep-wake schedule. Patients rate their answers on a scale from strongly disagree (1) to strongly agree (5).
Participants were also asked to fill out a treatment evaluation questionnaire at post treatment that included the following questions: Do you consider that you still suffer from sleep problems? (1=Not at all; 9=Very Much); Do you think that you would need additional treatment for sleep problems? (1=Not at all; 9=Very Much); How much improvement has occurred in your sleep problems since the beginning of treatment? (0% - 100%).
Data Analysis
Inter-rater reliability between two independent raters scoring 32 therapy sessions was established for both Easy and Hard Scoring rubrics (r = 0.82, p<.001 and r = 0.92, p<.001, respectively). Disagreements were resolved via discussion. Mean recall data across sessions were analyzed for each participant. Participants with fewer than 4 out of 7 sessions worth of data for a given measure were excluded from analyses. Patient recall rates and correlations between recall and treatment outcome were measured first across the collective sample, then separately for each treatment condition (i.e., CBTI-BP vs. PE). To control for multiple comparisons, a Bonferroni-adjusted alpha rate was utilized (0.05 divided by the total number of comparisons).
Results
Patient Characteristics
A total of N = 32 participants were recruited and randomized for this study. One participant in the CBTI-BP group dropped out of the study after completing 1 out of 7 sessions worth of recall data. One participant in the PE group dropped out of the study after completing 3 out of 7 sessions worth of recall data. The remaining N = 30 participants completed treatment and were thus included in the analyses for the present study. The number of non-completers (N = 2) was too small to test for significant differences between completers and non-completers. Patient characteristics by treatment type are presented in Table 1. The CBTI-BP group had significantly more years of education than the PE group. No other significant group differences were observed.
Table 1.
Participant Characteristics
| CBTI-BP (n = 17) | PE (n = 13) | Test Statistic | p | |
|---|---|---|---|---|
| Gender, n (% Female) | 10 (58.8) | 7 (53.8) | x2 = .07 | .785 |
| Race | ||||
| White, n (%) | 11 (64.7) | 6 (46.2) | ||
| Black, n (%) | 3 (17.6) | 2 (15.4) | ||
| Asian, n (%) | 0 | 3 (23.1) | ||
| Not Specified, n (%) | 2 (11.8) | 0 | ||
| Other, n (%) | 0 | 1 (7.7) | ||
| Native American, n (%) | 0 | 1 (7.7) | ||
| Multi-Racial, n (%) | 1 (5.9) | 0 | ||
| x2 = 9.30 | .157 | |||
| Ethnicity | ||||
| Non-Hispanic, n (%) | 16 (94.1) | 11 (84.6) | ||
| Hispanic, n (%) | 1 (5.9) | 2 (15.4) | ||
| x2 = .38 | .739 | |||
| Age (years) | 39.47 (13.47) | 36.92 (9.87) | t(28) = .57 | .571 |
| Education (years) | 16.47 (3.02) | 13.64 (2.94) | t(24) = 2.39 | .025* |
| YMRS (Pre) | 2.82 (2.98) | 5.12 (3.74) | t(28) = −1.87 | .072 |
| IDS-C (Pre) | 10.29 (8.04) | 13.00 (7.66) | t(28) = −.93 | .359 |
Note. Mean (SD) presented unless otherwise noted. YMRS = Young Mania Rating Scale; IDS-C = Inventory of Depressive Symptomatology – Clinician Rated.
p<.05
Means of Sleep Outcome Measures and Treatment Evaluation Questions
Means and standard deviations of sleep outcome measures and treatment evaluation questions by treatment type are presented in Table 2. There were no baseline differences in sleep outcome measures between CBTI-BP and PE groups. At post treatment, the CBTI-BP group had better sleep on two of the four sleep outcome measures (ISI and PROMIS-Sleep), and with all treatment evaluation questions. To decrease the variance associated with baseline differences in years of education, one-way between-subjects analyses of covariance (ANCOVA) were also conducted with years of education as a covariate. All results were maintained when controlling for years of education.
Table 2.
Means of Sleep Outcome Measures and Treatment Evaluation Questions
| CBTI-BP (n = 17) | PE (n = 13) | t | df | p | |
|---|---|---|---|---|---|
| Sleep Outcome (Pre) | |||||
| ISI | 18.41 (4.17) | 19.54 (4.50) | −.71 | 28 | .484 |
| PSQI | 10.94 (3.13) | 11.92 (3.17) | −.85 | 28 | .405 |
| PROMIS-Sleep | 24.47 (6.49) | 26.92 (5.60) | −1.86 | 28 | .287 |
| QOL-Sleep | 7.64 (2.66) | 10.27 (3.64) | −1.94 | 20 | .066 |
| Sleep Outcome (Post) | |||||
| ISI | 7.44 (5.72) | 13.92 (5.91) | −2.99 | 27 | .006** |
| PSQI | 6.29 (3.95) | 8.77 (3.14) | −1.85 | 28 | .074 |
| PROMIS-Sleep | 18.00 (7.37) | 23.62 (5.88) | −2.25 | 28 | .033* |
| QOL-Sleep | 12.53 (4.12) | 11.00 (4.14) | .98 | 26 | .336 |
| Treatment Evaluation (Post) | |||||
| Still suffer from sleep problems? | 4.33 (2.74) | 6.85 (2.11) | −2.68 | 26 | .013** |
| Need additional treatment for sleep? | 2.87 (2.26) | 6.38 (2.36) | −4.02 | 26 | <.001*** |
| % Improvement in sleep? | 71.33 (22.95) | 35.00 (30.00) | 3.63 | 26 | .001*** |
| % Homework Compliance | 82.08 (17.02) | 79.95 (29.53) | .25 | 28 | .112 |
Note: Mean (SD) presented. ISI = Insomnia Severity Index, PSQI = Pittsburgh Sleep Quality Index, PROMIS-Sleep = Patient-Reported Outcomes Measurement Info System – Sleep, QOL-Sleep = Quality of Life – Sleep.
p<.05
p<.01
p<.001
Patient Recall
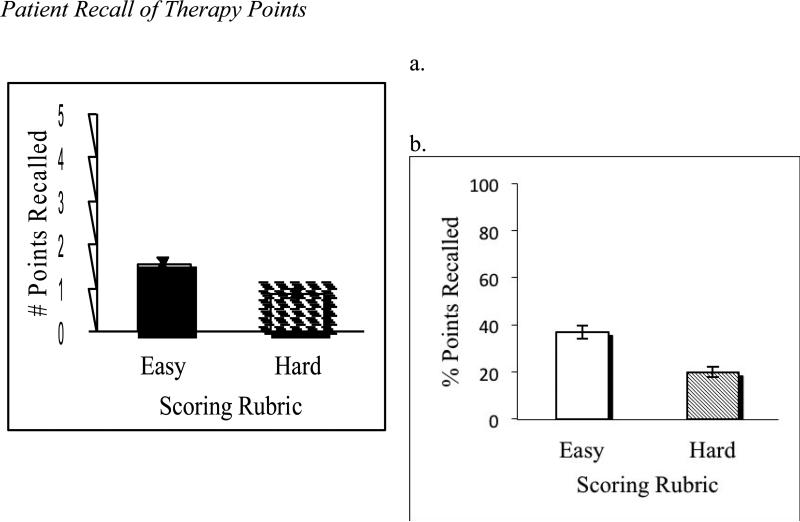
Participants recalled, on average, 1.54 (SD = 0.74) therapy points listed by therapists per session when evaluated via Easy Scoring rules, and 0.87 (SD = 0.57) therapy points when rated via Hard Scoring rules (see Figure 3a). In terms of percent points recalled, patients freely recalled an average of 36.9% (SD = 2.9%) of points listed by therapists from one week to the next when evaluated via Easy Scoring rules, and 19.6% (SD = 2.2%) of therapy points listed by therapists when rated via Hard Scoring rules (see Figure 3b).
Figure 3.
Patient Recall of Therapy Points
The mean number of therapy points listed by therapists immediately following a given session (for both Easy and Hard scoring) was 4.59 (SD = 1.88). An incidental finding was that the number of therapy points delivered was positively correlated with the raw number of points recalled using the Easy Scoring rules (r = .71; p < .001) and Hard Scoring rules (r = .57; p =.001), but not with the percentage of points recalled (based on therapists’ lists) using the Easy Scoring rules (r = −.34; p = .068) or Hard Scoring rules (r = −.09; p = .636).
Mean homework compliance rates across sessions were not correlated with any of the recall measures, # Points Recalled (Easy): r = .27, p = .145; # Points Recalled (Hard): r = .23, p = .214; % Points Recalled (Easy): r = .30, p = .103; % Points Recalled (Hard): r = .32, p = .090, or with the mean number of therapy points delivered across sessions: r = .09, p = .651.
Baseline measures of mood and insomnia severity ratings were not correlated with any of the recall measures, YMRS: # Points Recalled (Easy): r = −.34, p = .065; # Points Recalled (Hard): r = −.40, p = .027; % Points Recalled (Easy): r = −.13, p = .495; % Points Recalled (Hard): r = −.23, p = .223; IDS-C: # Points Recalled (Easy): r = −.18, p = .330; # Points Recalled (Hard): r = −.21, p = .275; % Points Recalled (Easy): r = −.05, p = .811; % Points Recalled (Hard): r = −.16, p = .414; ISI: # Points Recalled (Easy): r = −.18, p = .348; # Points Recalled (Hard): r = −.03, p = .894; % Points Recalled (Easy): r = .30, p = .107; % Points Recalled (Hard): r = .35, p = .062, given a Bonferroni-adjusted alpha-level of .013.
A one-way within subjects ANOVA was conducted to compare the effect of session number on patient recall. There were no significant effects of the session number on any of the four recall measures examined, # Points Recalled (Easy): Wilks' Lambda = .67, F(6,9) = .73, p = .638; # Points Recalled (Hard): Wilks' Lambda = .56, F(6,9) = 1.16, p = .402; % Points Recalled (Easy): Wilks' Lambda = .71, F(6,9) = .61, p = .720; % Points Recalled (Hard): Wilks' Lambda = .57, F(6,9) = .41, p = .407. These findings suggest that mean recall does not vary significantly from one session to the next. An additional one-way within subjects ANOVA was conducted to compare the effect of session number on number of therapy points delivered. There was no significant effect of the session number on number of therapy points delivered, Wilks' Lambda = .61, F(6,9) = .95, p = .508, indicating that the number of therapy points also do not vary significantly from one session to the next.
Patient Recall vs. Sleep Outcome
Correlations between patient recall and sleep outcome are presented in Table 3.
Table 3.
Correlations between Patient Recall and Sleep Outcome Measures
| Measure | ISI | PSQI | PROMIS-Sleep | QOL-Sleep |
|---|---|---|---|---|
| # Points Recalled (Easy) | .50* | .26 | .37 | −.69* |
| # Points Recalled (Hard) | .57* | .34 | .41 | −.69* |
| % Points Recalled (Easy) | .16 | .19 | .19 | −.26 |
| % Points Recalled (Hard) | .38 | .33 | .23 | −.53* |
Note. ISI = Insomnia Severity Index, PSQI = Pittsburgh Sleep Quality Index, PROMIS-Sleep = Patient-Reported Outcomes Measurement Info System – Sleep, QOL-Sleep = Quality of Life - Sleep. Recall rates are means across all sessions for each participant. Sleep outcome measures are pre-post treatment deviation scores. Bonferroni-adjusted significance is indicated by
p<.013.
Easy Scoring
Mean number of therapy points recalled was significantly correlated with two out of the four sleep outcome measures, namely the ISI and QOL-Sleep, given a Bonferroni-adjusted alpha-level of .013. Significant correlations were in the hypothesized direction (i.e., # points recalled was positively associated reduced insomnia severity ratings via the ISI, and improved sleep quality ratings via the QOL-Sleep over the course of treatment). Although the number of points recalled was not significantly correlated with PROMIS-Sleep, a marginally significant association was observed, also in the hypothesized direction. Percentage of points recalled was not significantly correlated with any of the sleep outcome measures.
Hard Scoring
Number of points recalled was positively correlated with reductions in insomnia severity ratings measured via the ISI, and improved sleep quality ratings measured via the QOL Sleep. When examining the percentage of points recalled versus sleep outcome measures, marginally significant effects were observed in two out of the four sleep outcome measures – namely, the ISI and QOL Sleep, also in the hypothesized directions.
The number of therapy points delivered was correlated with the ISI (r = .39; p = .037) and QOL-Sleep (r = −.54; p = .009), but not significantly correlated with the PSQI (r = .17; p = . 371) or PROMIS-Sleep (r = .18; p = .338). To provide a window into whether the relationship between therapy points delivered and treatment outcome is mediated by the number of points recalled, we conducted a meditational analysis following the procedures of Baron and Kenny (1986). Mediated effects were divided by the corresponding standard error to calculate z-scores (See MacKinnon & Dwyer, 1993). The # Points Recalled (Easy) significantly mediated the relationship between therapy points delivered and treatment outcome (ISI, z = 1.85; p = .033; QOL Sleep, z = 3.23; p < .001). Similarly, the # Points Recalled (Hard) significantly mediated the relationship between therapy points delivered and treatment outcome (ISI, z = 2.13; p = .017; QOL Sleep, z = 2.31, p = .017).
Patient Recall vs. Treatment Evaluation Measures
Correlations between patient recall and treatment evaluation measures are summarized in Table 4.
Table 4.
Correlations between Patient Recall and Treatment Evaluation Measures
| Measure | Still suffer from sleep problems? | Need additional treatment for sleep? | % Improvement in sleep? |
|---|---|---|---|
| # Points Recalled (Easy) | −.71* | −.56* | .42 |
| # Points Recalled (Hard) | −.64* | −.66* | .44 |
| % Points Recalled (Easy) | −.14 | .13 | −.08 |
| % Points Recalled (Hard) | −.31 | −.24 | .12 |
Note. Recall rates are means across all sessions for each participant. Treatment evaluation measures were administered at post treatment. Bonferroni-adjusted significance is indicated by
p<.013.
Easy Scoring
Mean number of therapy points recalled was significantly correlated with two out of the three treatment evaluation questions administered at post treatment, specifically, the extent to which patients rated that they still suffer from sleep problems, and the extent to which patients rated that they still need additional treatment for sleep. As hypothesized, the number of points recalled was correlated with lower evaluations of the extent to which patients rated that they still suffer from sleep problems, as well as lower evaluations of the extent to which patients rated that they still need additional treatment for sleep. There was a marginally significant positive association between the number of points recalled and the extent to which patients evaluated the percent improvement in their sleep over the course of treatment. Percentage of points recalled was not significantly correlated with any of the treatment evaluation questions.
Hard Scoring
A greater number of points recalled was negatively correlated with the extent to which patients rated that they still suffer from sleep problems, as well as the extent to which they rated that they still need additional treatment for their sleep problems at post treatment. A marginally significant association was found between the number of points recalled and the extent to which patients rated the percent improvement in their sleep over the course of treatment. No significant correlations were observed between percentage of points recalled and treatment evaluation conditions.
The number of therapy points delivered was correlated with all three of the treatment evaluation questions, including the extent to which patients rated the extent to which they still suffer from sleep problems (r = −.61; p = .001), the extent to which they rated that they need additional treatment for sleep (r = −.62; p < .001), and the % improvement in sleep (r = .50; p = . 007).
Patient Recall Based on Treatment Type
The mean number of therapy points delivered by therapists in CBTI-BP was 5.79 (SD = 1.42) and 3.02 (SD = 1.05) in the PE group. Participants in the CBTI-BP group received significantly more therapy points than participants in the PE group, t(28) = 5.89, p = .006.
Mean differences in patient recall rates by treatment type are presented in Table 5. Patients in the CBTI-BP group recalled significantly more therapy points than those in the PE group, for both Easy and Hard scoring conditions. There were no group differences in the percentage of points recalled. To decrease the variance associated with baseline differences in years of education, one-way between-subjects analyses of covariance (ANCOVA) were also conducted with years of education as a covariate. The results were maintained when controlling for years of education, # Points Recalled (Easy): CBTI-BP Adjusted M = 2.05, PE Adjusted M = 1.01, F(1,23) = 15.93, p = .001; # Points Recalled (Hard): CBTI-BP Adjusted M = 1.22, PE Adjusted M = .47, F(1,23) = 12.71, p = .002; % Points Recalled (Easy): CBTI-BP Adjusted M = .33, PE Adjusted M = .42, F(1,23) = 2.25, p = .148; % Points Recalled (Hard): CBTI-BP Adjusted M = .20, PE Adjusted M = .19, F(1,23) = .007, p = .936.
Table 5.
Patient Recall by Treatment Type
| Measure | CBTI-BP | PE | t (df) | p |
|---|---|---|---|---|
| # Points Recalled (Easy) | 1.86 (.77) | 1.14 (.47) | 2.96 (28) | .006* |
| # Points Recalled (Hard) | 1.12 (.57) | .54 (.37) | 3.19 (28) | .003* |
| % Points Recalled (Easy) | 31.8 (11.2) | 43.6 (18.9) | −2.00 (18) | .060 |
| % Points Recalled (Hard) | 19.0 (10.1) | 20.3 (14.1) | −.29 (28) | .775 |
Note. Mean (SD) recall scores are presented. Recall rates are means across all sessions for each participant. Bonferroni-adjusted significance is indicated by
p<.013.
Mean homework completion rates across all sessions are presented in Table 2. Mean homework completion rates did not differ between CBTI-BP and PE groups, even when controlling for years of education, CBTI-BP Adjusted M = 82.52, PE Adjusted M = 76.31, F(1,23) = .142, p = .710.
Correlations of Patient Recall vs. Sleep Outcome Measures by Treatment Type
Correlations of patient recall vs. sleep outcome measures by treatment type are presented in Table 6. Significance tests employing Fisher's r-to-z transformations were used to examine differences in Pearson's r correlations between CBTI-BP and PE groups. The CBTI-BP group had a stronger correlation between the percentage of points recalled (Hard) and pre-to-post PROMIS-Sleep deviation (r = .76) scores compared to PE (r = −.08). No other group differences in recall vs. sleep outcome correlations were observed.
Table 6.
Correlations between Patient Recall and Sleep Outcome Measures by Treatment Type
| Measure | ISI | PSQI | PROMIS-Sleep | QOL-Sleep |
|---|---|---|---|---|
| # Pts Recalled (Easy) | ||||
| CBTI-BP | .33 | .06 | .52 | −.67 |
| PE | .49 | .48 | .09 | −.63 |
| z | −.43 | −1.12 | 1.17 | −.14 |
| # Pts Recalled (Hard) | ||||
| CBTI-BP | .45 | .14 | .70* | −.66 |
| PE | .45 | .54 | −.03 | −.60 |
| z | 0 | −1.12 | 2.17 | −.20 |
| % Pts Recalled (Easy) | ||||
| CBTI-BP | .52 | .02 | .67* | −.54 |
| PE | .31 | .56 | .11 | −.55 |
| z | .57 | −1.48 | 1.69 | .03 |
| % Pts Recalled (Hard) | ||||
| CBTI-BP | .58 | .16 | .76* | −.62 |
| PE | .31 | .55 | −.08 | −.58 |
| z | .76 | −1.10 | 2.60* | −.13 |
Note. ISI = Insomnia Severity Index, PSQI = Pittsburgh Sleep Quality Index, PROMIS-Sleep = Patient-Reported Outcomes Measurement Info System – Sleep, QOL-Sleep = Quality of Life – Sleep. Recall rates are means across all sessions for each participant. Sleep outcome measures are pre-post treatment deviation scores. Fisher's r-to-z transformations are presented. Bonferroni-adjusted significance is indicated by
p<.013.
Correlations of Patient Recall vs. Treatment Evaluation Measures by Treatment Type
Correlations of patient recall vs. treatment evaluation measures by treatment type are depicted in Table 7. Fisher's r-to-z transformations were used to explore differences in Pearson's r correlations between CBTI-BP and PE groups. No significant group differences were observed.
Table 7.
Correlations between Patient Recall and Treatment Evaluation by Treatment Type
| Measure | Still suffer from sleep problems? | Need additional treatment for sleep? | % Improvement in sleep? |
|---|---|---|---|
| # Pts Recalled (Easy) | |||
| CBTI-BP | −.68* | −.51 | .24 |
| PE | −.52 | −.24 | .23 |
| z | −.59 | −.74 | .02 |
| # Pts Recalled (Hard) | |||
| CBTI-BP | −.59 | −.67* | .30 |
| PE | −.42 | −.30 | .14 |
| z | −.54 | −1.17 | .39 |
| % Pts Recalled (Easy) | |||
| CBTI-BP | −.59 | −.28 | .40 |
| PE | −.24 | −.06 | .07 |
| z | −1.01 | −.53 | .83 |
| % Pts Recalled (Hard) | |||
| CBTI-BP | −.52 | −.59 | .41 |
| PE | −.25 | −.13 | .03 |
| z | −.75 | −1.28 | .95 |
Note. Recall rates are means across all sessions for each participant. Treatment evaluation measures were administered at post treatment. Fisher's r-to-z transformations are presented. Bonferroni-adjusted significance is indicated by
p<.013.
Discussion
The first aim of the present study was to determine the extent to which patients with bipolar disorder and co-morbid insomnia can accurately recall the contents of therapy from one weekly session to the next. Patients recalled, on average, 0.87 to 1.54 therapy points per session, depending on whether a conservative or lenient scoring rubric was used. The mean of number of therapy points delivered was 4.59 per given session (for both scoring systems used). Compared to the 2.27 recommendations recalled by patients and 3.83 recommendations delivered in the Chambers’ (1991) examination of memory for behavioral treatment of insomnia, patients in the present study recalled fewer therapy points, while more points were delivered by therapists.
When examining the percentage of points recalled, patients recalled between 19.6% and 36.9% of points listed by therapists, measured via conservative and lenient scoring rubrics, respectively. In other words, patients freely recalled roughly a third or less of therapy contents from one weekly session to the next. This finding is congruent with the related literature on memory for non-mental health related treatment recommendations, which report recall rates between 19% and 33% within the months following a clinic visit (Bober et al., 2007; Jansen et al., 2008; Pickney & Arnason, 2005; Lewkovich & Haneline, 2005, Chambers, 1991).
The second aim was to examine whether patient recall is correlated with treatment outcome. In our sample of individuals with bipolar disorder, the raw number of points recalled was significantly correlated with two out of the four sleep outcome measures. Specifically, higher numbers of therapy points recalled were correlated with greater reductions on the ISI (a measure of insomnia severity) and greater gains on the QOL-Sleep (a measure of sleep quality). The remaining sleep outcome variables (PSQI and PROMIS-Sleep) were not significantly correlated with the number of points recalled, though moderate effect sizes in the hypothesized direction were observed (i.e., greater reductions in the PSQI and PROMIS-Sleep were associated with higher numbers of therapy points recalled). While all four sleep outcome measures assess for nighttime sleep quality as well as sleep-related daytime functioning, the ISI and QOL-Sleep place a greater emphasis on sleep-related daytime impairment compared to the PSQI and PROMIS-Sleep. Given that the sleep interventions targeted both daytime and nighttime sleep factors, it is possible that the ISI and QOL-Sleep were more sensitive to the gains in treatment, and thus more strongly correlated with patient recall than the PSQI and PROMIS-Sleep. The number of therapy points recalled was also correlated with positive treatment evaluation scores. Specifically, a higher number of therapy points recalled was associated with lower ratings of the extent to which patients evaluated that they still suffer from sleep problems, and the extent to which patients rated that they still needed additional treatment for their sleep at post treatment. Such findings are consistent with the existing medical literature which posits that poor memory for the content of a doctor's visit can lead to lower treatment adherence (e.g., Bober et al., 2007; Jansen et al., 2008), which can in turn lead to worse treatment outcome (Vermeire et al., 2001). When examining percentage of points recalled, however, no significant relation between patient recall and treatment outcome was observed. It appears that the quantity of points recalled, not the percentage of points recalled based on corresponding therapists’ lists, is associated with treatment outcome.
The third and fourth aims were to explore differences in recall rates and associated relationships with treatment outcome based on two types of treatment. Participants receiving CBTI-BP recalled a significantly higher number of therapy points than those receiving PE. However, there were no significant differences in the percentage of points recalled between groups. This can be explained by the fact that significantly more therapy points were delivered in the CBTI-BP group compared to the PE group. Participants in the CBTI-BP group received a larger dose of therapy points, and also recalled a proportionately larger amount of therapy points than those in the PE group. While participants in the CBTI-BP group had significantly more years of education than those in PE at baseline, these group differences in recall were maintained when controlling for years of education. Intriguingly, no pattern of group differences in the correlations between patient recall and treatment outcome were observed.
There are several potential explanations for the group differences in number of points recalled. One is that the quality of therapy points delivered may have been inconsistent across treatment conditions. Specifically, therapy points in PE involved broad and general facts about bipolar disorder and sleep, while points in CBTI-BP involved individualized information relevant to improving sleep among individuals with bipolar disorder. It is possible that the more personalized information characteristic of CBTI-BP was more memorable. Indeed, Gutchess, Kensinger, Yoon, and Schacter (2007) demonstrated that self-referenced information is more easily remembered than information that is not personalized. However, future research is needed to demonstrate whether such self-reference effects influence patient recall in the context of therapy. Given that CBTI-BP involved explicit plans for change, whereas PE did not, it also seems possible that different types of therapy points were delivered in each of the treatment conditions. Specifically, therapy points in the CBTI-BP condition likely involved a combination of skills and strategies to implement, alongside didactic information about bipolar disorder and sleep, while therapy points in the PE condition likely involved strictly didactic information about bipolar disorder and sleep. Future research is necessary to examine whether qualitative differences in the types of therapy points delivered can account for group differences in patient recall and subsequent outcome. Another potential explanation for these group differences is the fact that significantly more therapy points were delivered by therapists in the CBTI-BP group compared to the PE group. As mentioned earlier, if it is the case that the number of therapy points delivered serves as one of the driving mechanisms behind patient recall and associated treatment outcome, such group differences could be accounted for by the difference in sheer quantity of points delivered across treatment conditions. Future studies could benefit from controlling for the nature of therapy, particularly the quality and quantity of therapy points delivered in a given session while examining patient memory for therapy.
Given that patient recall for therapy appears to be low and is associated with worse treatment outcome, it is likely that patients could benefit from strategies that target the enhancement of patient memory for therapy (Harvey et al., 2014). For instance, therapists’ use of attention-recruiting techniques – demonstrated to be effective in enhancing the encoding process of a memory (e.g., Carney & Levin, 2002) – appears to be a promising strategy for increasing the memorability of the contents of a session. It is also well established that self-generated information is more memorable than presented information (e.g., DeWinstanley & Bjork, 2004). Thus, helping patients to generate their own list of therapy points in a given session might be another avenue by which patient memory for sessions could be improved. As reviewed by Johnson and Johnson (2009), there are numerous studies demonstrating how cooperative learning – which involves two or more individuals working collaboratively to solve problems, explore new information, or answer questions – can be more effective in promoting individuals’ long-term retention of the learned material compared to traditional learning settings (e.g., a student listening to a teacher give a lecture). As such, strengthening the collaborative nature of the patient-therapist relationship might be another plausible pathway for which patient memory for therapy contents can be improved. While such strategies have been documented to be effective in promoting memory in the cognitive and education psychology literatures, future research is needed to establish whether such memory-supporting techniques are effective in a therapeutic context.
In addressing the issue of improving patient memory for therapy, careful attention needs to be given to the underlying mechanisms that might be contributing to poor memory for therapy, above and beyond memory difficulties that are experienced by the patients (e.g., the verbal and declarative episodic memory impairments characteristic of bipolar disorder; see Malloy-Diniz et al., 2009 and Martinez-Aran et al., 2004). An incidental finding arising from the present study was that the number of therapy points delivered was highly correlated with the raw number of points recalled, two of the four sleep outcome measures (ISI and QOL-Sleep), and most of the treatment evaluation questions. Specifically, greater numbers of therapy points delivered was associated with more therapy points recalled, and greater improvements in sleep and evaluations of treatment. Furthermore, we found that the number of points recalled mediated the relationship between the number of therapy points delivered and treatment outcome. While causal relationships have yet to be established, this finding raises the possibility that increasing the number of points delivered in a given session might play a role in improving memory for therapy and subsequent treatment outcome. Future research is needed to establish whether specific kinds of therapy points are more easily recalled than others, and whether qualitative differences in therapy points recalled differentially influence outcome. Additionally, it is possible that specific patient behaviors, such as taking notes during sessions, could play a role in the extent to which patients recalled the contents of therapy (Harvey et al., 2014). In the present study, patients in both conditions were encouraged to take notes, but note taking was not quantified. Measuring patient note taking and its impact on subsequent memory for therapy is an important direction for future research.
There are several limitations. First, a causal relationship between patient recall and treatment outcome needs to be established in future research. Also, there is a need to replicate these findings with a treatment that does not involve improving sleep, given that improving sleep can also improve memory (Walker, 2008). However, in the present study patient recall did not vary significantly from one session to the next, while it is likely that sleep improved gradually over the course of the treatment. Indeed, Edinger and Sampson (2003) demonstrated that significant improvements in sleep can be made after only two sessions of CBT-I. Hence, if sleep were driving patient recall, we would expect recall rates to improve steadily across therapy sessions. Nonetheless, future research should also employ manipulations of patient recall (via techniques implemented by therapists) to examine whether bolstering patient recall causally affects changes in treatment outcome. Second, in the present study, therapists were not asked to record therapy points as the session unfolded due to concerns that the increased cognitive load of such a task would interfere with the progress of the therapy. While we cannot rule out the possibility that a therapist did not remember all therapy points, we took a number of precautions to try to ensure accuracy of therapist recall. Specifically, the therapists recorded the therapy points immediately following the session, the treatment manual specified which therapy points to deliver each session, and therapists were encouraged to refer to the treatment manual to serve as a memory cue while recording therapy points. Third, participants were not given advance notice that they would be given a recall task at the end of each session. However, we assume that patients learned after one or two sessions that the recall task was a standard procedure. The role of expectations of completing a recall task on subsequent recall performance should be addressed in future studies. Fourth, given the effects of circadian phase on memory (Valentinuzzi, Menna-Barreto, & Xavier, 2004), future research should control for time of day during which therapy sessions are held.
In sum, patient memory for therapy is strikingly poor, and appears to be associated with treatment outcome. There is also preliminary evidence that memory for therapy depends on the type of treatment offered, as well as the quality and quantity of therapy points delivered. Future work is needed to examine the mechanisms contributing to poor memory for therapy, and to develop techniques by which therapists can help bolster patient recall of therapy contents to thereby improve treatment outcome. Together, the present findings may well be relevant transdiagnostically given that memory impairment is a feature of many psychiatric conditions including major depression (Behnken et al., 2010), bipolar disorder (Martino, Igoa, Marengo, Scarpola, & Strejilevich, 2011), schizophrenia (Varga, Magnusson, Flekkoy, David, & Opjordsmoen, 2007), posttraumatic stress disorder (Isaac, Cushway, & Jones, 2006), and other anxiety disorders (Airaksinen, Larsson, & Forsell, 2005).
Acknowledgments
This project was supported by National Institute of Mental Health Grant R34MH080958. We are grateful to Kerrie Hein, Dr. Kate Kaplan, Dr. Adriane Soehner, and Dr. Joseph Williams for helpful input on the topic of this paper.
Footnotes
Public Impact Statement: This study highlights the importance of taking into consideration the role of patients’ memory for therapy contents while delivering therapy. Improving patients’ memory for therapy might be a pathway to improving outcome.
References
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug and Alcohol Dependence. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. doi:10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. doi:10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine . International classification of sleep disorders (ICSD): Diagnostic and coding manual. 2nd ed. Author; Westchester, IL: 2005. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th Edition. American Psychiatric Association; Washington, D.C.: 2000. Text revision. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator distinction in social psychological research: Conceptual, Strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. doi:10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. doi:10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Behnken A, Schöning S, Gerss J, Konrad C, de Jong-Meyer R, Arolt V. Persistent non-verbal memory impairment in remitted major depression - caused by encoding deficits? Journal of Affective Disorders. 2010;122:144–148. doi: 10.1016/j.jad.2009.07.010. doi:10.1016/j.jad.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Forneris CA, Loos W, Jaccard J. Effects of varying scoring rules of the clinician-administered PTSD scale (CAPS) for the diagnosis of post-traumatic stress disorder in motor vehicle accident victims. Behavioral Research and Therapy. 1995;33:471–475. doi: 10.1016/0005-7967(94)00064-q. doi:10.1016/0005-7967(94)00064-Q. [DOI] [PubMed] [Google Scholar]
- Bober SL, Hoke LA, Duda RB, Tung NM. Recommendation recall and satisfaction after attending breast/ovarian cancer risk counseling. Journal of Genetic Counseling. 2007;16:755–762. doi: 10.1007/s10897-007-9109-0. doi:10.1007/s10897-007-9109-0. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. doi:10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Moul DE, Germain A, Yu L, Stover AM, Dodds NE, Pilkonis PA. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–92. doi: 10.1093/sleep/33.6.781. doi:10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RN, Levin JR. Pictorial illustrations still improve students’ learning from text. Educational psychology review. 2002;14:5–26. doi:10.1023/a:1013176309260. [Google Scholar]
- Chambers MJ. Patient recall of recommendations in the behavioural treatment of insomnia. Sleep Research. 1991;20:222. [Google Scholar]
- Chengappa KNR, Kupfer DJ, Frank E, Houck PR, Grochocinski VJ, Cluss PA, Stapf DA. Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. American Journal of Psychiatry. 2003;60:1636–1642. doi: 10.1176/appi.ajp.160.9.1636. doi:10.1176/appi.ajp.160.9.1636. [DOI] [PubMed] [Google Scholar]
- DeWinstanley PA, Bjork EL. Processing strategies and the generation effect: Implications for making a better reader. Memory & Cognition. 2004;32:945–955. doi: 10.3758/bf03196872. doi:10.3758/bf03196872. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Stepanski EJ. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. doi:10.1016/s0513-5117(08)70324-7. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep: Journal of Sleep and Sleep Disorders Research. 2003;26:177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- Flocke SA, Stange KC. Direct observation and patient recall of health behavior advice. Preventive Medicine. 2004;38:343–349. doi: 10.1016/j.ypmed.2003.11.004. doi:10.1016/j.ypmed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Frank E. Treating bipolar disorder: A clinician's guide to interpersonal and social rhythm therapy. Guilford Press; 2007. [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, Schacter DL. Ageing and the self-reference effect in memory. Memory. 2007;15:822–837. doi: 10.1080/09658210701701394. doi:10.1080/09658210701701394. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Lee J, Williams J, Hollon SD, Walker MP, Thompson MA, Smith R. Improving outcome of psychosocial treatments by enhancing memory and learning. Perspectives on Psychological Science. 2014;9:161–179. doi: 10.1177/1745691614521781. doi:10.1177/1745691614521781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Soehner AM, Kaplan KA, Hein K, Lee JY, Kanady J, Buysse DJ. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: A pilot randomized controlled trial. doi: 10.1037/a0038655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Journal of Psychiatric Research. 2006;40:47–58. doi: 10.1016/j.cpr.2005.12.004. doi:10.1016/j.cpr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Jansen J, Butow PN, Van Weert JCM, Van Dulmen S, Devine RJ, Heeren TJ, Tattersall MHN. Does age really matter? Recall of information presented to newly referred patients with cancer. Journal of Clinical Oncology. 2008;26:5450–5457. doi: 10.1200/JCO.2007.15.2322. doi:10.1200/jco.2007.15.2322. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Johnson RT. An educational psychology success story: Social interdependence theory and cooperative learning. Educational Researcher. 2009;38:365–379. doi:10.3102/0013189x09339057. [Google Scholar]
- Kravitz RL, Hays RD, Sherbourne CD, DiMatteo MR, Rogers WH, Ordway L, Greefield S. Recall of recommendations and adherence to advice among patients with chronic medical conditions. Archives of Internal Medicine. 1993;153:1869–1878. doi:10.1001/archinte.153.16.1869. [PubMed] [Google Scholar]
- Lewkovich GN, Haneline MT. Patient recall of the mechanics of cervical spine manipulation. Journal of Manipulative and Physiological Therapeutics. 2005;28:708–712. doi: 10.1016/j.jmpt.2005.09.014. doi:10.1016/j.jmpt.2005.09.014. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation Review. 1993;17:144–158. doi:10.1177/0193841x9301700202. [Google Scholar]
- Martino DJ, Igoa A, Marengo E, Scarpola M, Strejilevich SA. Neurocognitive impairments and their relationship with psychosocial functioning in euthymic bipolar II disorder. The Journal of Nervous and Mental Disease. 2011;199:459–464. doi: 10.1097/NMD.0b013e3182214190. doi:10.1097/nmd.0b013e3182214190. [DOI] [PubMed] [Google Scholar]
- Michalak EE, Murray G, CREST.BD Development of the QoL.BD: a disorder-specific scale to assess quality of life in bipolar disorder. Bipolar Disorder. 2010;12:727–740. doi: 10.1111/j.1399-5618.2010.00865.x. doi:10.1111/j.1399-5618.2010.00865.x. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick SP. Motivational interviewing: Preparing people for change. The Guilford Press; 2002. [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Pickney CS, Arnason JA. Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporosis International. 2005;16:1156–1160. doi: 10.1007/s00198-004-1818-8. doi:10.1007/s00198-004-1818-8. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. doi:10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. doi:10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, Young AH. Neurocognitive impairment in euthymic patients with bipolar affective disorder. British Journal of Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. doi:10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Menna-Barreto L, Xavier GF. Effect of circadian phase on performance of rats in the morris water maze task. Journal of Biological Rhythms. 2004;19:312–324. doi: 10.1177/0748730404265688. doi:10.1177/0748730404265688. [DOI] [PubMed] [Google Scholar]
- Varga M, Magnusson A, Flekkoy K, David AS, Opjordsmoen S. Clinical and neuropsychological correlates of insight in schizophrenia and bipolar I disorder: does diagnosis matter? Comprehensive Psychiatry. 2007;48:583–591. doi: 10.1016/j.comppsych.2007.06.003. doi:10.1016/j.comppsych.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. Journal of clinical pharmacy and therapeutics. 2001;26:331–342. doi: 10.1046/j.1365-2710.2001.00363.x. doi:10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- Walker MP. Sleep-dependent memory processing. Harvard Review of Psychiatry. 2008;16:287–298. doi: 10.1080/10673220802432517. doi:10.1080/10673220802432517. [DOI] [PubMed] [Google Scholar]
- Wild J, Gur R. Verbal memory and treatment response in post-traumatic stress disorder. British Journal of Psychiatry. 2008;193:254–255. doi: 10.1192/bjp.bp.107.045922. doi:10.1192/bjp.bp.107.045922. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. doi:10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yu L, Buysse DJ, Moul DE, Germain A, Stover AM, Dodds NE, Johnston KL, Pilkonis PA. Development of short forms from the PROMIS® Sleep Disturbance and Sleep–Related Impairment Item Banks. Behavioral Sleep Medicine. 2011;10:6–24. doi: 10.1080/15402002.2012.636266. doi:10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]