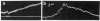Abstract
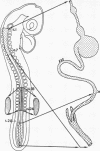
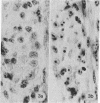
The levels of the neural axis from which parasympathetic and orthosympathetic neurons and adrenomedullary cells are derived under normal developmental conditions were determined in avian embryos by a biological labeling technique. The technique is based on nuclear differences between two species of birds, the chick and the quail. In quail interphase nuclei a part of the chromatin is condensed in large heterochromatic masses associated with nucleolus, while in the chick, DNA is evenly dispersed in the nucleoplasm. These characteristics provide a stable nuclear marker that can be used to study cell migrations and differentiation in chimeric embryos resulting from the association of quail and chick tissues. Isotopic and heterotopic transplantations of quail neural primordium into chick before the outset of neural crest cell migration show that the autonomic ortho- and parasympathetic neuroblasts are not determined to differentiate into cholinergic or adrenergic neurons when they begin to migrate. The neurotransmitter synthesized by crest autonomic neuroblasts depends on the microenvironment in which crest cells become localized at the term of their migration. The splanchnic mesoderm induces presumptive adrenergic cells to become fully differentiated cholinergic neurons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew A. The origin of intramural ganglia. IV. The origin of enteric ganglia: a critical review and discussion of the present state of the problem. J Anat. 1971 Jan;108(Pt 1):169–184. [PMC free article] [PubMed] [Google Scholar]
- Cohen A. M. Factors directing the expression of sympathetic nerve traits in cells of neural crest origin. J Exp Zool. 1972 Feb;179(2):167–182. doi: 10.1002/jez.1401790204. [DOI] [PubMed] [Google Scholar]
- DIAMANT B. Further observations on the effect of anoxia on histamine release from guinea-pig and rat lung tissue in vitro. Acta Physiol Scand. 1962 Sep;56:1–16. doi: 10.1111/j.1748-1716.1962.tb02477.x. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973 Aug;30(1):31–48. [PubMed] [Google Scholar]
- Le Douarin N. A biological cell labeling technique and its use in expermental embryology. Dev Biol. 1973 Jan;30(1):217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- Norr S. C. In vitro analysis of sympathetic neuron differentiation from chick neural crest cells. Dev Biol. 1973 Sep;34(1):16–38. doi: 10.1016/0012-1606(73)90336-9. [DOI] [PubMed] [Google Scholar]
- Teillet M. A., Le Douarin N. Détermination par la méthode des greffes hétérospécifiques d'ébauches neurales de caille sur l'embryon de poulet, du niveau du névraxe dont dérivent les cellules médullo-surrénaliennes. Arch Anat Microsc Morphol Exp. 1974 Jan;63(1):51–62. [PubMed] [Google Scholar]
- Weston J. A. The migration and differentiation of neural crest cells. Adv Morphog. 1970;8:41–114. doi: 10.1016/b978-0-12-028608-9.50006-5. [DOI] [PubMed] [Google Scholar]