Abstract
Breast density, where collagen I is the dominant component, is a significant breast cancer risk factor. Cell surface integrins interact with collagen, activate focal adhesion kinase (FAK), and downstream cell signals associated with xenobiotics (AhR, ARNT) and hypoxia (HIF-1α, ARNT). We examined if mammary cells cultured in high density (HD) or low density (LD) collagen gels affected xenobiotic or hypoxic responses. ARNT production was significantly reduced by HD culture and in response to a FAK inhibitor. Consistent with a decrease in ARNT, AhR and HIF-1α reporter activation and VEGF production was lower in HD compared to LD. However, P450 production was enhanced in HD and induced by AhR and HIF-1α agonists, possibly in response to increased NF-kB activaton. Thus, collagen density differentially regulates downstream cell signals of AhR and HIF-1α by modulating the activity of FAK, the release of NF-kB transcriptional factors, and the levels of ARNT.
Keywords: Collagen, hypoxia, xenobiotics, breast density, focal adhesion kinase (FAK)
1. Introduction
Breast cancer is a worldwide clinical problem amounting to approximately 1.38 million diagnoses and 450,000 deaths each year (Ferlay et al. 2010). Various risk factors have been identified in the development of breast cancer including increasing age, high breast density, nulliparity, obesity, hormone replacement therapy, alcohol consumption, early age of menarche, late age of menopause, and radiation exposure (Dumitrescu and Cotarla 2005). Of these factors, high breast density has been indicated to be one of the greatest independent risk factors across various breast cancer subtypes (McCormack and dos Santos Silva 2006, Phipps et al. 2012). Histological examination of dense and normal breast tissue has revealed that collagen is a primary component of dense breast tissue (Guo et al. 2001). The increased presence of type I collagen has also been clinically linked to metastatic tumors via genetic based analyses of tumor biopsies (Ramaswamy et al. 2003), suggesting that cellular responses to collagen may be linked to tumorigenesis.
Collagen is an extracellular matrix (ECM) protein known to interact with cell surface integrins in mammary gland development and tumor formation (Keely 2011). The protein is an established component of normal breast architecture and the dominant component of dense breast tissue, a significant breast cancer risk factor (Guo et al. 2001, McCormack and dos Santos Silva 2006, Phipps et al. 2012). We have previously shown that increased stromal collagen in mouse mammary tissue significantly increases tumor formation and metastases (Provenzano et al. 2008b). Moreover, mammary cells cultured in stiff collagen matrices exhibit mechanosignaling events that regulate gene expression and subsequent cellular differentiation and proliferation (Schedin and Keely 2011).
Signaling through focal adhesion kinase (FAK) is a significant signaling pathway by which cells respond to dense collagen matrices(Provenzano et al. 2009). This tyrosine kinase localizes at contact points where cell surface integrins interact with components of the ECM, and plays a critical role in the downstream processes of cell spreading, adhesion, motility, survival and cell cycle progression (Golubovskaya and Cance 2010). FAK is also implicated in breast tumorigenesis, particularly in mouse models where tissue-specific knock-out of FAK in the mammary gland significantly diminishes tumor formation and the development of cancerous hyperplasias (Lahlou et al. 2007, Provenzano et al. 2008a, Pylayeva et al. 2009). Microarray analyses of the benign tumors arising in FAK knock-out mammary glands identified several genes that had previously been associated with a metastasis signature (Wang et al. 2002, Provenzano et al. 2008a). Among mRNAs decreased in tumors lacking FAK, we identified AhR, HIF-1α and ARNT for further investigation as possible transcriptional regulators of breast cancer progression.
Hypoxia inducible transcription factors (HIF-1α, HIF-1β) dimerize and activate downstream genes in promoting aerobic glycolysis and tumorigenesis (Curran and Keely 2013, Morandi and Chiarugi 2014). Overexpression of HIF-1α has been identified in primary breast cancers and murine models where increased production of vascular endothelial growth factor (VEGF) is also identified (Kimbro and Simons 2006, Stein et al. 2009, Curran and Keely 2013). HIF-1β, which is a dimer partner to HIF-1α, is also known as ARNT (aryl hydrocarbon receptor nuclear translocator) and a dimer partner to the aryl hydrocarbon receptor (AhR) in xenobiotic metabolism. Xenobiotic ligands in the cytoplasm bind AhR which induces the release of AhR from a multiprotein complex and allows the receptor to translocate to the nucleus, dimerize with ARNT and activate phase enzymes involved in the efflux of the chemical/ligand (Chen et al. 2012b). In breast cancer, dysregulation of AhR and particular phase I enzymes have been associated with increased tumorigenesis (Dialyna et al. 2001, Goode et al. 2013).
AhR is mostly commonly known for ligand induced activation in response to polycyclic aromatic hydrocarbons such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Additionally, AhR is known to bind to the endogenous ligand, kynurenine, which is up-regulated in breast cancer (Mezrich et al. 2010, Lyon et al. 2011, Tang et al. 2014). Certain drugs used in the therapeutic treatment of breast cancer in human studies or murine models include microtubule-interfering agents, tamoxifen, doxorubicin, VEGF antagonist SU5416, and tranilast, which are all also known AhR ligands (Overmoyer et al. 2007, Vrzal et al. 2008, DuSell et al. 2010, Prud'homme et al. 2010, Volkova et al. 2011, Mezrich et al. 2012). Both AhR and HIF-1α regulate genes involved in the assembly and maintenance of the ECM (Kung et al. 2009, Gilkes et al. 2014). These transcription factors are also known to exhibit possible crosstalk via the shared dimer component ARNT (Chan et al. 1999). In the current study, we investigated whether changes in the collagen matrix alter the responses of normal mammary gland cells to the ARNT-coupled pathways involving AhR and HIF-1α.
2. Material and methods
2.1 Cell lines and cell culture
The MCF-10A cell line was used as representative models of normal breast epithelia (Debnath et al. 2003). The MCF-10A cell line was obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in stiff (3 mg/ml) or compliant (1 mg/ml) type I collagen gels as previously described . In brief, rat tail collagen (Corning, 354249, Bedford, MA) was mixed thoroughly with a neutralization solution (1:2) containing 100 mM Hepes and 2x PBS (pH 7.4) and placed on ice for no more than 10 min. High density (3 mg/ml) or low density (1 mg/ml) collagen in neutralized solutions were added to cells suspended in complete media. Complete media contained 50% DMEM with high glucose (Gibco Life Technologies, Grand Island, NY), 50% F-12 Nutrient mix (Gibco), 5% horse serum (Gibco), 10 μg/ml bovine insulin (Cell Applications, Inc., San Diego, CA), 0.5 mg/ml hydrocortisone (Sigma-Aldrich, St. Louis, MO), and 20 ng/ml epidermal growth factor (EMD Millipore/Calbiochem, Darmstadt, Germany). Cells and media were thoroughly mixed with neutralized collagen. A total volume of 1.5 ml cells/media/collagen was aliquotted to one well of a 6-well sterile non-tissue culture treated plate (BD Falcon™, Franklin Lakes, NJ) and incubated at room temperature for 10 min. The plate was transferred to a 5% CO2, 37°C incubator for 2 hours. The gels were then released from the plate by gently sliding a 200 μl pipet tip around the outer edge of the gel and 2 ml of complete media was added to the gel prior to returning the plate to a 5% CO2, 37°C incubator. In some experiments, cells were treated with 100 μM deferoxamine mesylate salt (DFOM, Sigma), 100 μM tranilast, 10 μM FAK ATP inhibitor PF-562271 (Selleckchem, Houston, TX ) or 10 μM Src kinase inhibitor PP2 (Calbiochem/Millipore, Billerica, MA).
2.2 Reporter assay
The luciferase plasmids containing the hypoxia response element (HRE) or xenobiotic response element (XRE) were kind gifts from Dr. Christopher A. Bradfield, University of Wisconsin-Madison and the Renilla plasmid was a kind gift from Dr. Michele A. Wozniak, University of Pennsylvania. Cells (5×105/5ml) were plated in a 60mm tissue culture dish (BD Falcon™, Franklin Lakes, NJ) for 24 hours and transfected with 2 μg of luciferase plasmid, 1 μg of Renilla plasmid and 12 μl of Lipofectamine® 2000 Transfection Reagent (Invitrogen Life Technologies, Carlsbad, CA). After transfecting 24 hours, cells were lifted and cultured in collagen gels +/−100 μM deferoxamine mesylate salt (Sigma) or 200 μM tranilast (Sigma) for an additional 24 hours. Gels were rinsed with PBS, placed in 250 μl Glo Lysis Buffer (Promega, Madison, WI) and repeatedly passed through an 18 gauge needle. Cell lysates were centrifuged (15,800xg, 1m) and the aqueous solution was aliquotted in duplicate to a 96-well Microfluor® white flat bottom plate (Thermo Electron Corporation, Milford, MA). Luminescence was assessed via the Dual-Glo® Luciferase Assay System (Promega). Plates were read on a Fluoroskan Ascent® FL plate reader. Activity in each sample was assessed and duplicates were averaged in each experiment.
2.3 Immunoblots
Cells cultured in collagen gels were washed in PBS and lysed in 200 μl 2X RIPA buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 2 mM Na2EDTA, 2% NP-40, 0.5% deoxycholic acid, 2 mM NaF, 1 mM Na3VO4). The lysate was repeatedly passed through an 18 gauge needle and centrifuged (15,800xg, 10 min, 4°C) to separate the collagen from the cell lysate. The aqueous solution was saved and mixed with 10X sample buffer (100 mM Tris [pH 8], 7.5 mM EDTA, 100 mM dithiothreitol, 10% sodium dodecyl sulfate, 30% glycerol, 1% bromophenol blue) for a 1X sample buffer final concentration. Protein samples were boiled (8 min), loaded onto an SDS-PAGE gel, transferred to a 0.45 mm Immobilion-P polyvinylidene difluoride membranes (Millipore). Blots were cut for multiple detection and incubated with antibodies raised against FAK (pY577) (Life Technologies, 44614G, Grand Island, NY, 1:3000), FAK(pY397) (Life Technologies, 44624G, 1:3000), total FAK (Millipore, 05-537, 1:3000), CYP4B1 (Santa Cruz, sc-134896, 1:200), CYP1A1 (Santa Cruz, sc-9828, 1:200), or GAPDH (Santa Cruz, sc-25778, 1:200). Blots were washed and subsequently incubated with HRP-conjugated secondary antibodies. Bound secondary antibody was visualized following incubation of the membrane with Super Signal West chemiluminescent HRP substrate (Thermo Scientific Pierce) and using an Epichemi II darkroom UVP equipped with a 12-bit cooled CCD camera. Luminescence was quantified and evaluated via the application of ImageJ software (National Institutes of Health).
2.4 ELISA
Anti–VEGF mAbs (clone 26503; 1:300; R&D Systems) in 0.1 M sodium carbonate buffer, pH 9.6, were coated onto 96-well enzyme immunoassay/radioimmunoassay plates (Costar, Corning, NY). Blocking buffer containing 1% BSA (Sigma Chemical) and 0.5% Tween 20 (Fischer Scientific, Pittsburgh, PA) in PBS was added to wells for 2 h. Serial dilutions of VEGF standard (293-VE; R&D Systems) and cell-free supernatants were aliquoted and incubated at 4°C overnight. VEGF was detected with biotinylated VEGF Abs (1:1000; R&D Systems) and subsequent exposure to streptavidin HRP-40 (Fitzgerald Industries International, Concord, MA). A colorimetric HRP substrate tetramethylbenzidine (Biofx Laboratories, Owings Mills, MD) was used to evaluate captured HRP activity, and the enzymatic reaction was stopped with 0.18 M sulfuric acid. OD was determined on an ELX800 Universal Microplate Reader (BioTek Instruments, Winooski, VT). Absorbance was quantified at 450 nM, using 600 nM as a reference wavelength. VEGF concentrations were calculated by interpolation from a standard curve, and all determinations were performed in triplicate.
3. Results
3.1 HRE and XRE reporter responses to density and agonists
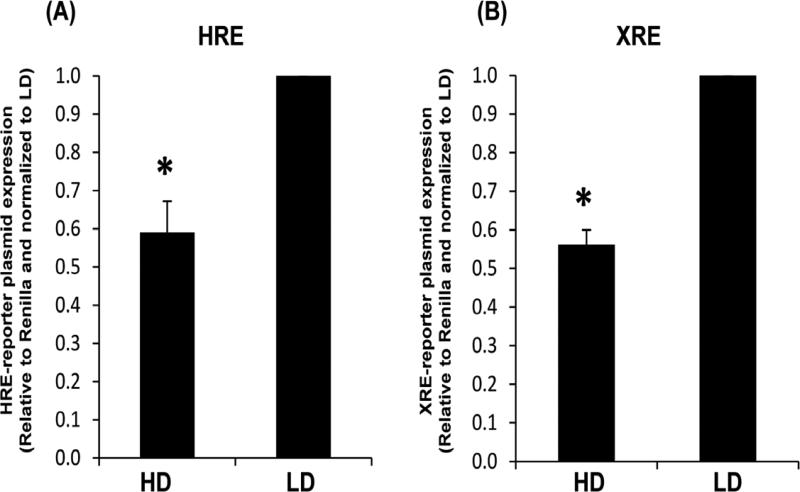
To initially assess the relationship of collagen and the xenobiotic or hypoxic response, cells were cultured in collagen gels and examined for transcriptional activation. Specifically, normal human breast epithelial cells (MCF10A) cultured in high and low density collagen gels were assessed for reporter activation of the xenobiotic response element (XRE) or the hypoxic response element (HRE). As shown in Figure 1, transcriptional activation of both hypoxic and xenobiotic response elements are significantly less in high density (HD, stiff) collagen gels compared to low density (LD, compliant) collagen gels.
Fig. 1. HRE and XRE reporter plasmid expression in MCF10A cells cultured in high density (HD) or low density (LD) collagen gels.
MCF10A breast epithelial cells were transfected with HRE (A) or XRE (B) firefly luciferase reporter plasmids for 24 h. Transfected cells were transferred to HD or LD collagen gels for an additional 24 h. Reporter plasmid expression levels are displayed relative to the Renilla luciferase controls and normalized to LD, +/− SEM, N=6, *p<0.004 vs respective LD control.
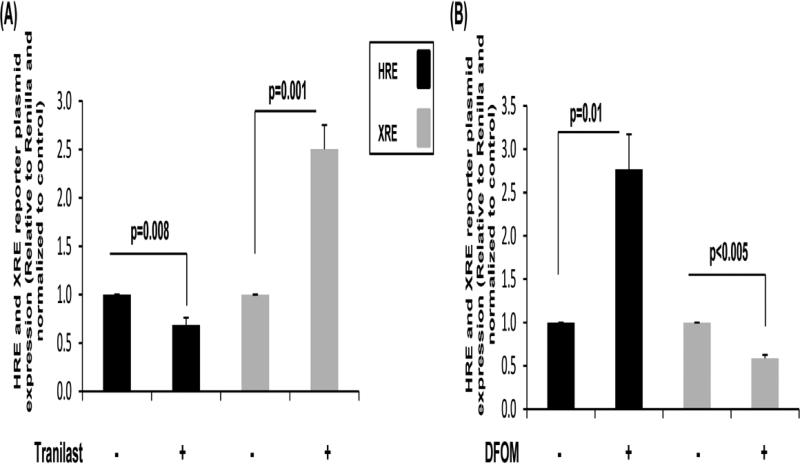
To further explore the relationship between HRE and XRE responses in collagen, cells were cultured in the presence or absence of the hypoxia-inducing reagent deferoxamine mesylate salt (DFOM) or the AhR agonist, tranilast. In Figure 2A, tranilast significantly decreased the HRE response but increased the XRE response in MCF10A cells. In contrast, DFOM resulted in an inverse response in the MCF10A cells by increasing the HRE response and decreasing the XRE response (Figure 2B). These results suggest that the XRE or HRE genes activated may also exhibit a similar crosstalk pattern.
Fig. 2. HRE and XRE reporter plasmid expression in cells cultured in low density collagen gels in the presence or absence of an agonist.
MCF10A breast epithelial cells were transfected with HRE or XRE firefly luciferase reporter plasmids for 24 h. Transfected cells were transferred to low density collagen gels +/− 100 μM tranilast (A) or 100 μM deferoxamine mesylate salt (DFOM) (B) for 24 h. Reporter plasmid expression levels are displayed relative to the Renilla luciferase controls and normalized to the vehicle control, +/−SEM, N>4.
3.2 VEGF production in response to density and HRE/XRE agonists
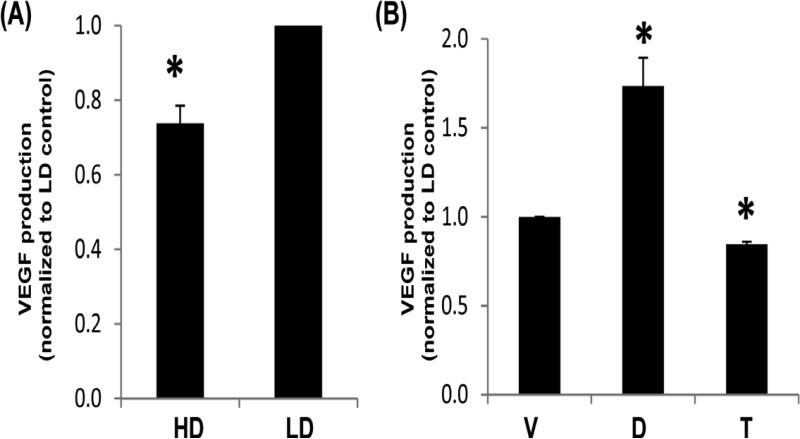
In assessing XRE and HRE responses, we next examined cells cultured in collagen (stiff or compliant) for regulation of VEGF, which is up-regulated by HRE activation (Saponaro et al. 2013). VEGF-A mRNA is effectively decreased by collagen density as detailed in our microarray of normal murine mammary epithelial cells (NMuMG) (Provenzano et al. 2009), and is a known factor in breast cancer progression (Curran and Keely 2013). Examination of the media from cells revealed that production of VEGF from MCF10A cells was decreased in HD compared to LD collagen gels (Figure 3A). MCF10A cells cultured in LD collagen retained the ability to respond to ligands, as DFOM increased VEGF production (Figure 3B). Conversely, the AhR ligand tranilast decreased VEGF production (Figure 3B). Thus, production of VEGF matches the findings at the reporter level for the HRE response.
Fig. 3. VEGF production from MCF10A cells in response to density or ligands known to activate HRE and XRE.
Cells were cultured in high density (HD) or low density (LD) collagen gels for 24 h (A) or cultured in LD collagen gels +/− vehicle control, 100 μM DFOM or tranilast for 24 h (B). Supernatants were assessed for the presence of VEGF via ELISA. Data displayed represent the mean concentration normalized to LD control, +/− SEM, N>4 *p<0.01 vs respective LD control.
3.3 P450 production in response to density and HRE/XRE agonists
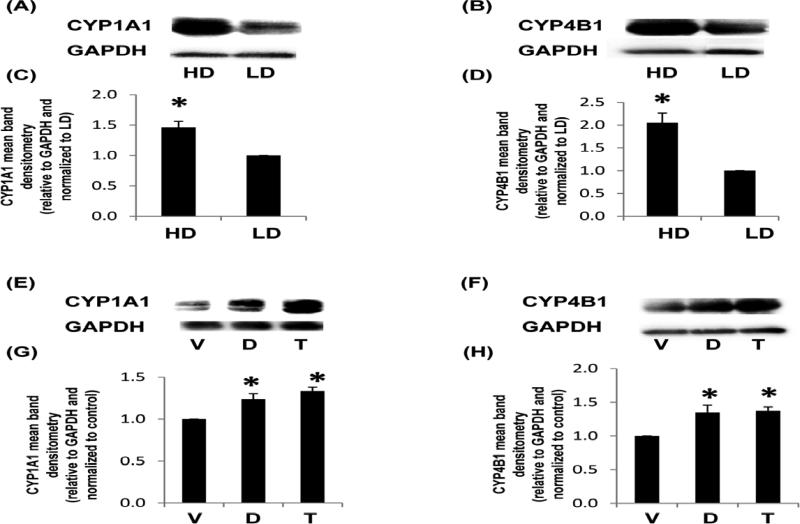
In assessing the downstream activity of the XRE response, we examined one of the most characterized P450 enzymes, CYP1A1. This extra-hepatic phase I drug metabolizing enzyme primarily functions as a mono-oxygenase in the metabolism of endogenous (hormones, inflammatory mediators) and exogenous (dietary metabolites, carcinogens) substrates (Badal and Delgoda 2014). CYP1A1 is also reduced or genetically altered in breast cancer (Hafeez et al. 2012, Wang and Wang 2013) which may yield insight into studies that detail CYP1A1 function in both tumor prevention and induction (Androutsopoulos et al. 2009). In addition, AhR ligand-induced expression of CYP1A1 is enhanced by cell culture of rat mammary epithelial cells atop collagen (Larsen et al. 2004). In Figure 4A and 4C, we show that in the absence of an AhR ligand, MCF10A cells produced CYP1A1 when cultured in collagen gels. In contrast to the regulation of XRE in our reporter assay (Figure 1), CYP1A1 expression was higher in HD compared to LD.
Fig. 4. CYP1A1 and CYP4B1 production from MCF10A cells in response to density or ligands known to activate HRE and XRE.
Cells were cultured in low density (LD) or high density collagen gels for 24 h and assessed for P450 enzyme production (A-D) or cultured in LD collagen gels +/− vehicle control (V), 100 μM DFOM (D) or tranilast (T) for 24 h (E-H). Representative immunoblots are displayed (A,B,E,F) and the mean band densitometry relative to the GAPDH and normalized to the control are charted (C,D,G,H) +/− SEM, N>4 *p<0.05 vs respective control.
To further assess the XRE response, mammary epithelial cells were examined for CYP4B1. This P450 enzyme has been identified at the mRNA level in normal and tumor tissue samples (Iscan et al. 2001) as wells in the MCF10A cell line (Satih et al. 2010). In addition, we have previously identified that the expression of CYP4B1 is regulated by density and FAK in our microarray of normal murine mammary epithelial cells (NMuMG) (Provenzano et al. 2009). In Figure 4B and 4D, we show that density increased the expression of CYP4B1, indicating that cell signals associated with collagen ligation or mechanical stress alter P450 enzyme production. Lastly, in Figures 4E-H, we show that both DFOM and tranilast enhanced CYP1A1 and CYP4B1 expression in the MCF10A cell line. Thus, unlike VEGF production and the HRE response (Figures 1-3), the regulation of P450 enzymes does not match the XRE response, suggesting that in non-tumorigenic cells, additional cell signals other than the AhR:ARNT pathway, may also play role in the regulation of P450 expression as has been previously explored (Guigal et al. 2000, Mastyugin et al. 2004, Zordoky and El-Kadi 2009, Villard et al. 2011).
3.4 FAK activation in dense collagen
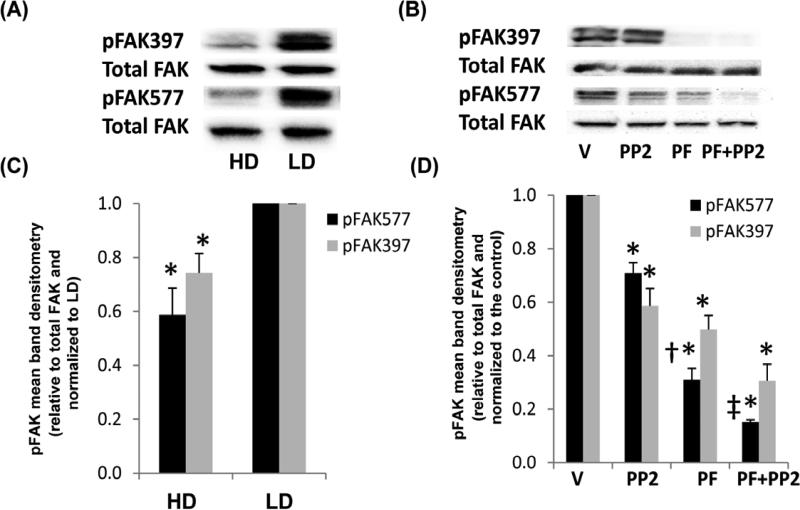
To examine the mechanisms of P450 and VEGF expression in collagen, downstream signals associated with cell adhesion to collagen were assessed. Integrin binding to collagen induces cells signals via Src-family kinases, the recruitment FAK, and the subsequent activation of Rho family GTPases that regulate the downstream effects of cell contractility, integrin clustering, and mechanical stress (Keely 2011). Consequently, the activity of FAK may have a functional role in XRE and HRE responses which are also altered by collagen density. In Figure 5A and 5C, we show that phosphorylation of FAK is less in HD compared to LD collagen, suggesting that decreased FAK activation may be responsible for the altered activation of XRE and HRE cell signals. In Figure 5B and 5D, we demonstrate that the FAK ATP inhibitor PF-562271 significantly reduced phosphorylation at tyrosine 577, an indicator of kinase activity, and at the autophosphorylation site pY397. The Src kinase inhibitor (PP2) exhibited a modest effect on the phosphorylation of FAK (pY577 or pY397) alone. In combination with PF-562271, PP2 exhibited a synergistic response in reducing pY577 activity but not pY397 compared to controls.
Fig. 5. FAK phosphorylation in response to density, Src or FAK inhibitors.
MCF10A cells were cultured in HD or LD collagen gels +/− vehicle control (V), 10 μM PF-562271 FAK ATP inhibitor (PF) or src inhibitor PP2 for 24 h. Cells were lysed and examined for pFAK397 or pFAK577 compared to total FAK by immunoblot (A,B) and densitometry (C,D). Data are displayed as the mean relative to total FAK and normalized to the control. N=5, +/− SEM, *p<0.02 vs respective LD control (C). N=3, +/− SEM, *p<0.02 vs respective control, †p<0.04 vs PP2, ‡p<0.03 vs PF (D).
3.5 VEGF and P450 production in response to FAK inhibition
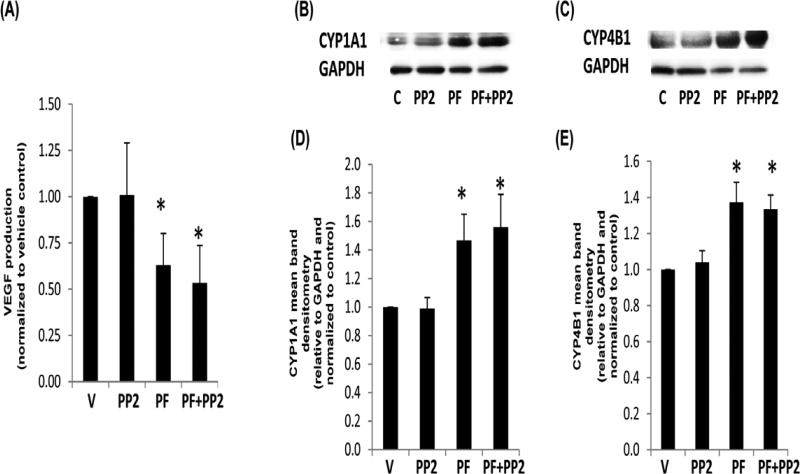
To examine if FAK inhibition also affects genes activated by AhR and HIF-1α, we examined the production of VEGF and the P450 enzymes. As shown in Figure 6A, the production of VEGF decreased in response to FAK inhibition. However, in Figure 6B-E, P450 enzyme production is shown to increase in response to FAK inhibition. These data suggest that FAK activation disparately affects XRE and HRE cell signals that respectively lead to P450 and VEGF production. These findings additionally correlate with reduced FAK activity and VEGF production but higher P450 expression identified in HD culture.
Fig. 6. VEGF and P450 enzyme production in response to FAK or src inhibitors.
MCF10A cells were cultured in LD collagen gels +/− vehicle control (V), 10 μM PF-562271 FAK ATP inhibitor (PF) or src inhibitor PP2 for 24 h. Cell supernatant was harvested and assessed for VEGF via ELISA (A). Cells were also lysed and examined for CYP1A1 or CYP4B1 by immunoblot (B,C) and densitometry (D,E). VEGF data are displayed as the mean normalized to the vehicle control, N=6, +/− SEM, *p<0.003 vs control (A). P450 data are displayed as the mean relative to GAPDH and normalized to the control. N>4, +/− SEM, *p<0.05 vs respective control (D,E).
3.6 ARNT production in response to density and FAK inhibition
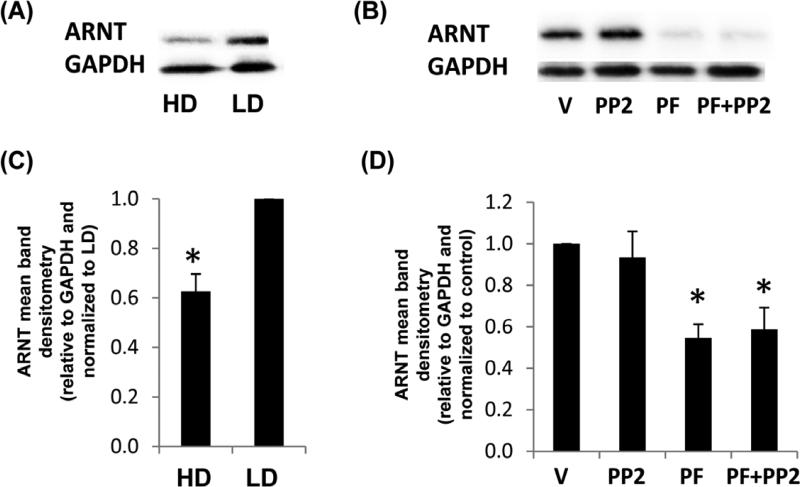
In understanding the nature of FAK inhibition on the XRE and HRE pathways, we examined the common dimer between AhR and HIF-1α, ARNT. ARNT was significantly reduced by the FAK ATP inhibitor, but not by PP2 (Figure 7). The expression level of ARNT was also significantly reduced in HD compared to LD. Although studies have indicated that ARNT may exhibit splice variants in certain estrogen-negative tumor cell lines (MDA-MB-231 and MDA-MB-435) (Qin et al. 2001), we did not significantly identify any additional bands for ARNT by immunoblot in the MCF10A cell line (data not shown).
Fig 7. ARNT production in response to density, Src or FAK inhibitors.
MCF10A cells were cultured in HD or LD collagen gels +/− vehicle control (V), 10 μM PF-562271 FAK ATP inhibitor (PF) or src inhibitor PP2 for 24 h. Cells were lysed and examined for ARNT production compared to GAPDH by immunoblot (A,B) and densitometry (C,D). Data are displayed as the mean relative to GAPDH and normalized to the control. N=4, +/− SEM, *p<0.02 vs LD control (C). N=5, +/− SEM, *p<0.01 vs vehicle control (D).
3.7 IκB-α production in response to density and FAK inhibition
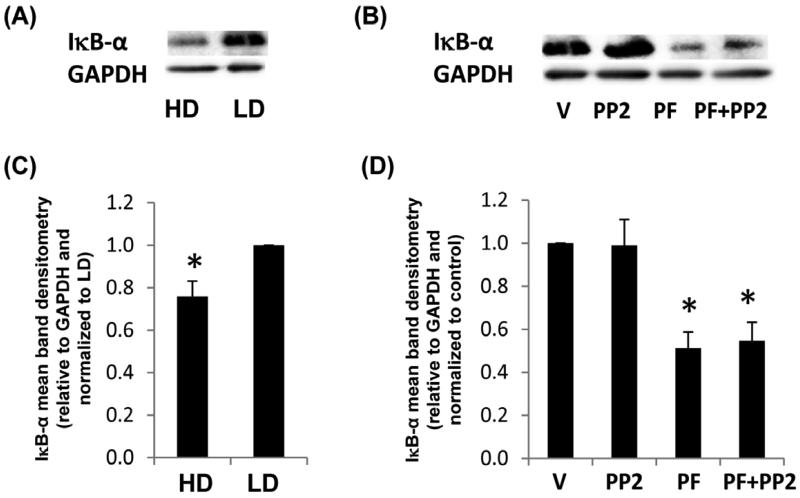
Because the production of P450 enzymes have been linked to the activity of NF-κB (Zordoky and El-Kadi 2009), we investigated the expression level of the regulatory protein, inhibitor of NF-κB (IκB)-α. Cell signals leading to NF-κB via the canonical pathway induce the phosphorylation of IκB-α and targeted proteasome degradation of IκB-α, which yields the release of NF-κB dimer components such as p50 and RelA (Neumann and Naumann 2007). In Figure 8, we show that like ARNT, production of IκB-α was also significantly reduced in HD and in response to the FAK ATP inhibitor but not PP2. Thus, the inactivation of FAK may induce activation of NF-κB cell signals.
Fig. 8. IκB-α production in response to density, Src or FAK inhibitors.
MCF10A cells were cultured in HD or LD collagen gels +/− vehicle control (V), 10 μM PF-562271 FAK ATP inhibitor (PF) or src inhibitor PP2 for 24h. Cells were lysed and examined for IκB-α production compared to GAPDH by immunoblot (A,B) and densitometry (C,D). Data are displayed as the mean relative to GAPDH and normalized to the control. N=5, +/− SEM, *p<0.03 vs LD control (C). N=5, +/− SEM, *p<0.006 vs vehicle control (D).
4. Discussion
High breast density is a significant risk factor in the development of breast cancer, and is linked to an abundance of type I collagen (Guo et al. 2001, McCormack and dos Santos Silva 2006, Phipps et al. 2012). Collagen has an essential role in the activation of FAK in human mammary epithelial cells (Provenzano et al. 2009). Moreover, AhR, HIF-1α and ARNT are linked to mammary tumor progression and the expression of FAK (Provenzano et al. 2008a). In the current study, we find that high density collagen matrices diminish XRE and HRE activity of normal mammary cells.(Figure 1). We also found a possible interplay between XRE and HRE responses where agonists to XRE alter the HRE response and vice versa (Figure 2). These data suggest that drug and cell metabolism may be altered in dense breast tissue.
Furthermore, VEGF production is regulated by XRE and HRE responses, density, and FAK activity (Figures 3 and 6). VEGF has been previously linked to FAK and growth factor activation in endothelial cells (Eliceiri et al. 2002, Chen et al. 2012a). VEGF identified by ELISA includes VEGFA165 and VEGFA121 which are linked to VEGF receptors (VEGFR)-1 and VEGFR2. These ligands and receptors are also chemotherapeutic targets of current interest (Rapisarda and Melillo 2012). Thus, molecular reagents that may alter this pathway, such as AhR agonists or FAK inhibitors, may possibly deter the angiogenic response in the mammary epithelium. The lower expression of VEGF in HD collagen suggests that breast density alone is not sufficient to induce VEGF-mediated angiogenesis and that other extracellular or intrinsic factors associated with the tumor are required.
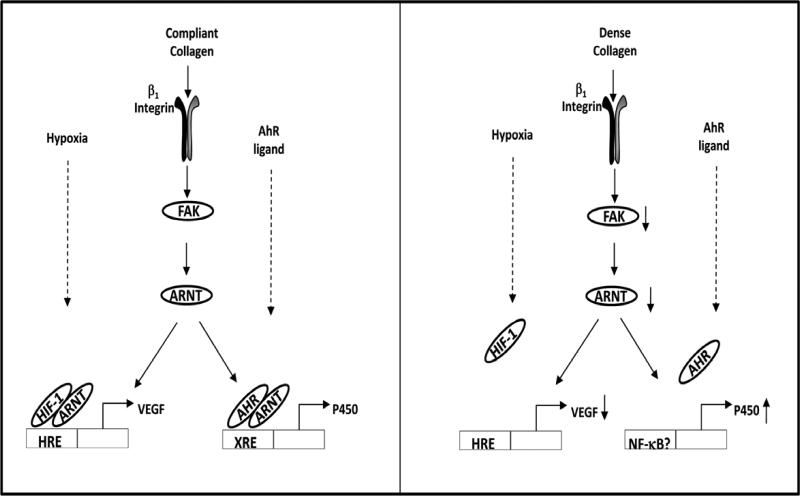
In addition, we make the novel observation that ARNT levels are regulated by FAK activity, as pharmacological inhibition of FAK significantly diminished ARNT levels (Figure 9). Reduced expression of ARNT (Figure 7) may explain the lower XRE and HRE reporter responses (Figure 1) and the consequential decreases in VEGF production (Figure 3) in HD versus LD. Surprisingly, we observed a decrease in FAK activation in HD collagen relative to LD collagen at 24 h (Figure 5). This is in contrast to the findings of ourselves and others that FAK phosphorylation is increased for mammary epithelial cells that are in HD/stiff matrices compared to more compliant matrices (Paszek et al. 2005, Provenzano et al. 2009). These differences likely reflect the fact that FAK phosphorylation in the previous investigation was evaluated after 7 days in 3D culture (Provenzano et al. 2009). We previously observed that FAK can be activated under compliant conditions when evaluated for short time frames (Modzelewska et al. 2006). One explanation is that cells actively contract a compliant/LD matrix for the first 4 days of 3D culture, during which time there may be high FAK activity. Once the cells reach isometric equilibrium, FAK activity may be down-regulated in LD collagen.
Fig. 9. Collagen density modulates HRE and XRE responses.
Mammary cells cultured in compliant collagen are able to effectively contract and pull on the surrounding extracellular matrix (ECM) within 24 h. At this time point, FAK, HRE and XRE cell signaling is active. Mammary cells cultured in dense collagen are unable to effectively contract the gel which alters cell signaling by reducing the expression levels of active FAK, ARNT, and VEGF. The increase of P450 enzymes in dense collagen suggests alternative cell signaling which may involve the activation of NF-κB.
Promoter activity of a purified XRE is identified in cells cultured in collagen matrices, consistent with previous findings that increased activation of the XRE signals occurs in cells suspended in semisolid media containing 1.68% methylcellulose (Sadek and Allen-Hoffmann 1994, Hao et al. 2012) or cultures within ultralow attachment plates (Sengupta et al. 2014). Despite the decrease in XRE activity in HD matrices, the expression of CYP1B1 and CYP4B1, which are regulated in part by XRE elements, are significantly increased in HD matrices. Somewhat paradoxically, inhibition of FAK also significantly enhances the expression enzymes. We also identified increased P450 expression in response to both XRE and HRE agonists (Figure 4), which supports the potential activation of the AhR pathway but also suggests that additional cell signals may modulate the response (Guigal et al. 2000, Mastyugin et al. 2004, Zordoky and El-Kadi 2009, Villard et al. 2011). This is the first report to identify increased P450 enzyme expression in association with dense collagen and the reduced activation of FAK.
The idea that P450 expression increases in the absence of ARNT is complex (Figures 6 and 7). The P450 genes are also regulated by NF-κB, HIF-1, and SRF (serum response factor) (Guigal et al. 2000, Mastyugin et al. 2004, Zordoky and El-Kadi 2009, Villard et al. 2011). Moreover, HIF-1α, ARNT, and AhR expression are regulated by NF-κB (Gorlach and Bonello 2008, van Uden et al. 2011, Vogel et al. 2014) as well as the downstream genes (P450s and VEGF) (Tabruyn and Griffioen 2008, Zordoky and El-Kadi 2009). Previous study of Hepa-1c1 cells in 1.68% methylcellulose demonstrated that increased CYP1A1 was dependent on AhR expression (Sadek and Allen-Hoffmann 1994). AhR is also known to functionally cooperate with RelA in binding to NF-κB elements in MCF10A cells (Kim et al. 2000). The degradation of IκB-α involves the release of RelA (Neumann and Naumann 2007) and the expression of RelA has also been linked to CYP1A1 in the MCF-7 breast cancer cell line (Hollingshead et al. 2008). We found that IκB- α was decreased downstream of FAK by a HD matrix. Thus, the increased expression of P450 enzymes in high density may be associated with possible crosstalk between AhR and NF-κB cell signals.
5. Conclusions
In summary, the microenvironment has been identified as a pivotal factor in the progression of cancer. We have shown that dense breast tissue and cell signals associated with FAK significantly alter the XRE and HRE responses in the normal mammary epithelial cell line, MCF10A. Tumorigenic cells that often exhibit mutations in the XRE or HRE pathways are likely influenced by dense collagen in the microenvironment. Because breast tumors have been characterized to exhibit hypoxia (Curran and Keely 2013) and are known to secrete endogenous XRE ligands (Lyon et al. 2011, Tang et al. 2014), normal epithelia cell signals around the tumor may be modified as described herein. The potential for FAK to regulate XRE and HRE cell signals yields insight into the differential responses identified in suspension cultures compared to normal tissue culture. Breast density is an identified risk factor in breast cancer. Elucidation of differences in cell signaling may enhance perspectives on the nature of this risk. Consequentially, understanding the functions of FAK and matrix density in cell and drug metabolism merits further study.
Highlights.
Collagen density regulates xenobiotic and hypoxic response of mammary epithelial cells
► We examined if collagen density affected hypoxic or xenobiotic responses
► ARNT and VEGF but not P450 production decreased in dense collagen
► FAK inhibition lowered ARNT and VEGF production but not P450 enzyme production
► Increased P450 production in dense collagen may be linked to NF-κB activation
► Collagen density influences hypoxic and xenobiotic metabolism
Acknowledgements
This work was supported by N.I.H. grants CA142833 and CA114462 to P.J.K. and T32-007015 to C.S.C.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ARNT
AhR nuclear translocator
- DFOM
deferoxamine mesylate salt
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- HD
high density
- HIF
hypoxia inducible transcription factor
- HRE
hypoxia response element
- LD
low density
- VEGF
vascular endothelial growth factor
- XRE
xenobiotic response element
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests:
The authors have no conflicts of interest to declare
References
- Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC cancer. 2009;9:187. doi: 10.1186/1471-2407-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badal S, Delgoda R. Role of the modulation of CYP1A1 expression and activity in chemoprevention. Journal of applied toxicology : JAT. 2014;34:743–753. doi: 10.1002/jat.2968. [DOI] [PubMed] [Google Scholar]
- Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. The Journal of biological chemistry. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD. VEGF-induced vascular permeability is mediated by FAK. Developmental cell. 2012a;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tang Y, Guo C, Wang J, Boral D, Nie D. Nuclear receptors in the multidrug resistance through the regulation of drug-metabolizing enzymes and drug transporters. Biochemical pharmacology. 2012b;83:1112–1126. doi: 10.1016/j.bcp.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran CS, Keely PJ. Breast tumor and stromal cell responses to TGF-beta and hypoxia in matrix deposition. Matrix biology : journal of the International Society for Matrix Biology. 2013;32:95–105. doi: 10.1016/j.matbio.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Dialyna IA, Arvanitis DA, Spandidos DA. Genetic polymorphisms and transcriptional pattern analysis of CYP1A1, AhR, GSTM1, GSTP1 and GSTT1 genes in breast cancer. International journal of molecular medicine. 2001;8:79–87. doi: 10.3892/ijmm.8.1.79. [DOI] [PubMed] [Google Scholar]
- Dumitrescu RG, Cotarla I. Understanding breast cancer risk -- where do we stand in 2005? Journal of cellular and molecular medicine. 2005;9:208–221. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuSell CD, Nelson ER, Wittmann BM, Fretz JA, Kazmin D, Thomas RS, Pike JW, McDonnell DP. Regulation of aryl hydrocarbon receptor function by selective estrogen receptor modulators. Molecular endocrinology. 2010;24:33–46. doi: 10.1210/me.2009-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. The Journal of cell biology. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. Journal international du cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nature reviews. Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Cance W. Focal adhesion kinase and p53 signal transduction pathways in cancer. Frontiers in bioscience. 2010;15:901–912. doi: 10.2741/3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode GD, Ballard BR, Manning HC, Freeman ML, Kang Y, Eltom SE. Knockdown of aberrantly upregulated aryl hydrocarbon receptor reduces tumor growth and metastasis of MDA-MB-231 human breast cancer cell line. International journal of cancer. Journal international du cancer. 2013 doi: 10.1002/ijc.28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Bonello S. The cross-talk between NF-kappaB and HIF-1: further evidence for a significant liaison. The Biochemical journal. 2008;412:e17–19. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- Guigal N, Seree E, Bourgarel-Rey V, Barra Y. Induction of CYP1A1 by serum independent of AhR pathway. Biochemical and biophysical research communications. 2000;267:572–576. doi: 10.1006/bbrc.1999.1959. [DOI] [PubMed] [Google Scholar]
- Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:243–248. [PubMed] [Google Scholar]
- Hafeez S, Ahmed A, Rashid AZ, Kayani MA. Down-regulation of CYP1A1 expression in breast cancer. Asian Pacific journal of cancer prevention : APJCP. 2012;13:1757–1760. doi: 10.7314/apjcp.2012.13.5.1757. [DOI] [PubMed] [Google Scholar]
- Hao N, Lee KL, Furness SG, Bosdotter C, Poellinger L, Whitelaw ML. Xenobiotics and loss of cell adhesion drive distinct transcriptional outcomes by aryl hydrocarbon receptor signaling. Molecular pharmacology. 2012;82:1082–1093. doi: 10.1124/mol.112.078873. [DOI] [PubMed] [Google Scholar]
- Hollingshead BD, Beischlag TV, Dinatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer research. 2008;68:3609–3617. doi: 10.1158/0008-5472.CAN-07-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscan M, Klaavuniemi T, Coban T, Kapucuoglu N, Pelkonen O, Raunio H. The expression of cytochrome P450 enzymes in human breast tumours and normal breast tissue. Breast cancer research and treatment. 2001;70:47–54. doi: 10.1023/a:1012526406741. [DOI] [PubMed] [Google Scholar]
- Keely PJ. Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion. Journal of mammary gland biology and neoplasia. 2011;16:205–219. doi: 10.1007/s10911-011-9226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocrine-related cancer. 2006;13:739–749. doi: 10.1677/erc.1.00728. [DOI] [PubMed] [Google Scholar]
- Kung T, Murphy KA, White LA. The aryl hydrocarbon receptor (AhR) pathway as a regulatory pathway for cell adhesion and matrix metabolism. Biochemical pharmacology. 2009;77:536–546. doi: 10.1016/j.bcp.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, Muller WJ. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MC, Brake PB, Pollenz RS, Jefcoate CR. Linked expression of Ah receptor, ARNT, CYP1A1, and CYP1B1 in rat mammary epithelia, in vitro, is each substantially elevated by specific extracellular matrix interactions that precede branching morphogenesis. Toxicological sciences : an official journal of the Society of Toxicology. 2004;82:46–61. doi: 10.1093/toxsci/kfh242. [DOI] [PubMed] [Google Scholar]
- Lyon DE, Walter JM, Starkweather AR, Schubert CM, McCain NL. Tryptophan degradation in women with breast cancer: a pilot study. BMC research notes. 2011;4:156. doi: 10.1186/1756-0500-4-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastyugin V, Mezentsev A, Zhang WX, Ashkar S, Dunn MW, Laniado-Schwartzman M. Promoter activity and regulation of the corneal CYP4B1 gene by hypoxia. Journal of cellular biochemistry. 2004;91:1218–1238. doi: 10.1002/jcb.20018. [DOI] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. Journal of immunology. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezrich JD, Nguyen LP, Kennedy G, Nukaya M, Fechner JH, Zhang X, Xing Y, Bradfield CA. SU5416, a VEGF receptor inhibitor and ligand of the AHR, represents a new alternative for immunomodulation. PloS one. 2012;7:e44547. doi: 10.1371/journal.pone.0044547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewska K, Newman LP, Desai R, Keely PJ. Ack1 mediates Cdc42-dependent cell migration and signaling to p130Cas. The Journal of biological chemistry. 2006;281:37527–37535. doi: 10.1074/jbc.M604342200. [DOI] [PubMed] [Google Scholar]
- Morandi A, Chiarugi P. Metabolic implication of tumor:stroma crosstalk in breast cancer. Journal of molecular medicine. 2014;92:117–126. doi: 10.1007/s00109-014-1124-7. [DOI] [PubMed] [Google Scholar]
- Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- Overmoyer B, Fu P, Hoppel C, Radivoyevitch T, Shenk R, Persons M, Silverman P, Robertson K, Ziats NP, Wasman JK, Abdul-Karim FW, Jesberger JA, Duerk J, Hartman P, Hanks S, Lewin J, Dowlati A, McCrae K, Ivy P, Remick SC. Inflammatory breast cancer as a model disease to study tumor angiogenesis: results of a phase IB trial of combination SU5416 and doxorubicin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5862–5868. doi: 10.1158/1078-0432.CCR-07-0688. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K, O'Meara ES, Li CI. Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Annals of epidemiology. 2012;22:340–348. doi: 10.1016/j.annepidem.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. The American journal of pathology. 2008a;173:1551–1565. doi: 10.2353/ajpath.2008.080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC medicine. 2008b;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme GJ, Glinka Y, Toulina A, Ace O, Subramaniam V, Jothy S. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PloS one. 2010;5:e13831. doi: 10.1371/journal.pone.0013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. The Journal of clinical investigation. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Wilson C, Blancher C, Taylor M, Safe S, Harris AL. Association of ARNT splice variants with estrogen receptor-negative breast cancer, poor induction of vascular endothelial growth factor under hypoxia, and poor prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:818–823. [PubMed] [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nature genetics. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- Rapisarda A, Melillo G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Advances in cancer research. 2012;114:237–267. doi: 10.1016/B978-0-12-386503-8.00006-5. [DOI] [PubMed] [Google Scholar]
- Sadek CM, Allen-Hoffmann BL. Suspension-mediated induction of Hepa 1c1c7 Cyp1a-1 expression is dependent on the Ah receptor signal transduction pathway. The Journal of biological chemistry. 1994;269:31505–31509. [PubMed] [Google Scholar]
- Saponaro C, Malfettone A, Ranieri G, Danza K, Simone G, Paradiso A, Mangia A. VEGF, HIF-1alpha expression and MVD as an angiogenic network in familial breast cancer. PloS one. 2013;8:e53070. doi: 10.1371/journal.pone.0053070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satih S, Chalabi N, Rabiau N, Bosviel R, Fontana L, Bignon YJ, Bernard-Gallon DJ. Gene expression profiling of breast cancer cell lines in response to soy isoflavones using a pangenomic microarray approach. Omics : a journal of integrative biology. 2010;14:231–238. doi: 10.1089/omi.2009.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harbor perspectives in biology. 2011;3:a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Johnson BP, Swanson SA, Stewart R, Bradfield CA, Thomson JA. Aggregate culture of human embryonic stem cell-derived hepatocytes in suspension are an improved in vitro model for drug metabolism and toxicity testing. Toxicological sciences : an official journal of the Society of Toxicology. 2014;140:236–245. doi: 10.1093/toxsci/kfu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T, Salomonis N, Nuyten DS, van de Vijver MJ, Gusterson BA. A mouse mammary gland involution mRNA signature identifies biological pathways potentially associated with breast cancer metastasis. Journal of mammary gland biology and neoplasia. 2009;14:99–116. doi: 10.1007/s10911-009-9120-1. [DOI] [PubMed] [Google Scholar]
- Tabruyn SP, Griffioen AW. NF-kappa B: a new player in angiostatic therapy. Angiogenesis. 2008;11:101–106. doi: 10.1007/s10456-008-9094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Lin CC, Spasojevic I, Iversen ES, Chi JT, Marks JR. A joint analysis of metabolomics and genetics of breast cancer. Breast cancer research : BCR. 2014;16:415. doi: 10.1186/s13058-014-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uden P, Kenneth NS, Webster R, Muller HA, Mudie S, Rocha S. Evolutionary conserved regulation of HIF-1beta by NF-kappaB. PLoS genetics. 2011;7:e1001285. doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard PH, Barlesi F, Armand M, Dao TM, Pascussi JM, Fouchier F, Champion S, Dufour C, Ginies C, Khalil A, Amiot MJ, Barra Y, Seree E. CYP1A1 induction in the colon by serum: involvement of the PPARalpha pathway and evidence for a new specific human PPREalpha site. PloS one. 2011;6:e14629. doi: 10.1371/journal.pone.0014629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WL, Wu D, Haarmann-Stemmann T, Hoffmann A, Denison MS. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. The Journal of biological chemistry. 2014;289:1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkova M, Palmeri M, Russell KS, Russell RR. Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovascular research. 2011;90:305–314. doi: 10.1093/cvr/cvr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrzal R, Daujat-Chavanieu M, Pascussi JM, Ulrichova J, Maurel P, Dvorak Z. Microtubules-interfering agents restrict aryl hydrocarbon receptor-mediated CYP1A2 induction in primary cultures of human hepatocytes via c-jun-N-terminal kinase and glucocorticoid receptor. European journal of pharmacology. 2008;581:244–254. doi: 10.1016/j.ejphar.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang WJ. Relationship between CYP1A1 polymorphisms and invasion and metastasis of breast cancer. Asian Pacific journal of tropical medicine. 2013;6:835–838. doi: 10.1016/S1995-7645(13)60148-0. [DOI] [PubMed] [Google Scholar]
- Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, Segall JE, Condeelis JS. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer research. 2002;62:6278–6288. [PubMed] [Google Scholar]
- Zordoky BN, El-Kadi AO. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Current drug metabolism. 2009;10:164–178. doi: 10.2174/138920009787522151. [DOI] [PubMed] [Google Scholar]