Abstract
Background and Objectives:
The tumoral biomarkers have a rather well known effect upon the tumor control period of several types of malignant lesions. The aim of this study was to evaluate the impact of immunohistochemical (IHC) markers of Ki-67 and p53 on the long-term outcome of growth hormone (GH)-secreting pituitary adenomas treated surgically.
Materials and Methods:
We conducted and followed a cohort of 47 consecutive patients with GH-secreting pituitary adenomas referred to our department during a 4 year period for trans-sphenoidal microsurgical adenomectomy. The expression levels of Ki-67 and p53 were determined by IHC study of the tissue samples. Periodical pituitary magnetic resonance imaging (MRI), visual field studies and hormonal evaluations (GH and insulin-like growth factor-1 [IGF-1]) performed during the follow-up period were the outcome measures.
Results:
The level of Ki-67 expression was higher among patients with postoperative residual tumor (3.5 vs. 1.7%) and those with a hormonal recurrence (4.3 vs. 1.6%). The p53 expression level was remarkably higher in patients with radiological recurrence (18 vs. 6.3%). Patients with invasive features (i.e. cavernous sinus and suprasellar invasion) had significantly higher p53 and Ki-67 values and higher IGF-1 levels during the follow-up period. The patients younger than 30 years of age and those with mixed GH-prolactin secreting adenomas had significantly higher hormonal remission and lower radiological recurrence rates.
Conclusion:
Each of the biomarkers, Ki-67 and p53, along with patient's age and mixed GH-prolactin secretion showed a kind of correlation with each of aspects of the clinical, hormonal and radiologic outcome of GH-secreting pituitary adenomas in this series.
Keywords: Acromegaly, growth hormone, immunohistochemistry, Ki-67, p53, pituitary adenoma
Introduction
Pituitary adenoma accounts for 90% of intra-sellar pathologies and 10-15% of all intracranial neoplasms and is usually a benign tumor.[1] The most common hormone-secreting adenomas are prolactinomas, followed by growth hormone (GH) secreting and mixed GH and prolactin-producing adenomas, respectively.[2] The incidence of acromegaly is 5 cases/million/year and the prevalence is 60 cases/million.[3] The diagnosis is often preceded by around 10 years of active, but unrecognized disease.[4]
Many of the GH-secreting adenomas are larger than 1 cm at the time of diagnosis and may have mass effect on the adjacent structures or even invade them.[5,6] Due to wide fluctuations of the serum GH levels, it is neither a sensitive nor a specific test for diagnosis of acromegaly. For diagnostic purposes, the oral glucose tolerance test (OGTT) seems to be a more reliable method by revealing a diminished or even reversed effect of increasing serum glucose on GH values.[7] Some of the recent studies suggest that the even OGTT has a limited diagnostic value in patients with biochemically active acromegaly who have only mildly increased GH output.[8,9,10]
The insulin-like growth factor-1 (IGF-1) or somatomedin C, which is synthesized in the liver, is the main mediator of GH for exerting its biologic effects. The raised serum level of IGF-1 is widely accepted as the first-line screening test for evaluation of patients suspected to have acromegaly.[3] It is an ideal screening test because it has a long half-life of 18-20 h and the serum levels remain stable throughout the day. The IGF-I level is affected by age and gender, decreasing by approximately 14% per decade during adult life.[4]
Surgery is often the primary therapeutic option for acromegalic patients, although it may not be curative for some of them.[11] There are several studies carried out so far to find the major determinants of treatment outcome in patients with GH-secreting pituitary adenoma. Tumor size, invasion to the surrounding structures and the serum GH level before and just after the surgery have been proposed as possible outcome predicting factors. Despite these predictive factors, it is rather common for the patients with similar acromegalic characteristics to have different clinical and hormonal outcomes. This is the reason many investigators search for other clinical or paraclinical markers that influence the long-term behavior of GH-secreting adenomas.
The Ki-67 antigen is a protein present in the nuclei of cells in the G1, S, and G2 phases of the cell cycle as well as in mitosis. It is not expressed in quiescent or resting cells in the G0 phase, in which many proteins involved in proliferation are degraded.[12,13] The presence of Ki-67 antigen is measured by a monoclonal antibody called MIB-1. The MIB-1 immunoreactive nuclear index, also known as the MIB-1 index or the Ki-67 labeling index (LI), is expressed as a percentage of Ki-67 antigen positive nuclei among total nuclei.[12]
The p53 protein is the product of TP53 tumor suppressor gene. Although it is well-established that TP53 mutations are uncommon in pituitary tumors, the dysregulation and expression of the protein during tumor genesis, and its utility in the interpretation of the pathology specimens as a marker of aggressiveness, have been widely debated.[14]
The role of immunohistochemical (IHC) markers in recognition of “atypical” pituitary adenomas remains yet as a controversy. The World Health Organization (WHO), 2004, classification of endocrine tumors defines four prognostic features as the variants increasing the likelihood of aggressive behavior for pituitary adenomas and defines these adenomas as “atypical”; an increased mitotic index, invasive tumor growth, Ki-67 ≥ 3% and extensive nuclear p53 positivity.[15,16,17]
This controversy is mainly due to; (a) the relative unidentified impact of any of these features, to predict tumor behavior, (b) the scarcity of case series with long-term follow-up and (c) the lack of studies specifying each hormonal subtype of pituitary adenoma.
The aim of this research protocol was to evaluate the impact of p53 and Ki-67 protein labeling indices, along with other clinical and paraclinical parameters of patients with GH-secreting pituitary adenomas upon the long-term hormonal and radiologic outcome of these patients.[18,19,20]
Materials and Methods
The study did not alter the standard therapeutic and follow-up protocols of acromegalic patients in our department and thus all patients were informed and accepted to participate.
In a cohort of 47 cases, we enrolled all patients with acromegaly in whom, the diagnosis of GH-secreting pituitary adenoma was confirmed and were referred to the neurosurgery department of a single center, from January 2004 to March 2008 and operated trans-sphenoidally. The preoperative hormonal evaluations analyzed by a neuro-endocrinology study group (LM and vaginal opening), and a single surgeon (KA) did all trans-sphenoidal microsurgical adenomectomies (TSSA).
Our diagnostic criteria for acromegaly mentioned in our previous communication[1] was GH level > 10 10 mU/L after OGTT with 75 g glucose, and the level of IGF-1 was also determined and adjusted to its age range and gender according to the reference table for each patient.
The cases with elevated serum prolactin levels beyond the so-called stalk effect were considered to have mixed type of hormonal secretion and confirmed with immunostaining for prolactin secretion in their tumor specimens.
The exclusion criteria of the study were: Huge suprasellar extension mandating transcranial or combined surgical approaches, history of previous surgery for pituitary adenoma or other pathologies in the region of the pituitary fossa, history of brain radiation therapy for any reason, patients with a history of surgery or radiotherapy for nasal or nasopharyngeal tumors and patients refusing surgical treatment.
All patients underwent brain MRI with and without contrast enhancement and visual acuity and field (V/A, V/F) evaluation, before surgery. Preoperative MRI studies were reviewed by the neuroradiology unit of the hospital. The tumors were classified according to their size into microadenomas (<10 mm) and macroadenomas (≥10 mm), and lesions were also classified for the presence of invasive features to the clivus, sphenoid sinus, cavernous sinus or extension beyond the level of foramen Monroe. We did not consider possible invasion of the tumor to the dura as an independent feature of invasion of the tumor.[21,22,23,24,25]
All patients underwent TSSA through transnasal transseptal microsurgical approach with the assistance of an otolaryngologist ear, nose, throat (ENT). The follow-up visits were arranged at 6 weeks and 3 months after the operation and then every 6 months. The following evaluations were carried out for all these 47 patients in the postoperative follow-up period:
Endocrinology assay in the first night after surgery to rule out treatment failure
Daily evaluation for possible complications, e.g. CSF leakage, diabetes insipidus
V/A, V/F evaluations and contrast-enhanced brain MRI, 6 weeks after surgery
Brain MRI, evaluation of V/A, V/F, ENT visit (if necessary) and measurement of hormone levels at 3, 6, 12 and18 months following surgery
The surgical specimens were fixed in formalin and processed with paraffin. Selected blocks were serially cut and stained with Hematoxylin and Eosin staining. Tissue micro-array analysis of the pathologic samples using IHC for hypophyseal hormones, mutant p53 protein and Ki-67 antigen was also performed.
We defined the endocrinological remission of the patients as; having basal GH level < 2.5 ng/mL, GH level < 1 ng/mL after OGTT and a normal IGF-1 value for related age and gender. We assessed the surgical outcome from; endocrinological, radiological and clinical points of view. Recurrences were divided into those with elevated hormone levels, but without significant radiologic findings and the ones with both hormonal recurrence and radiological evident tumor regrowth.
An antigen retrieval method was used in all instances, and positive and negative controls were carried out and reacted appropriately. Labeling index was defined as the proportion of labeled cells to the total number of cells analyzed in a field consisting of 1000 cells, in the area where the density of labeled cells was the highest.[24,25,26,27,28,29,30] The Ki-67 and p53-labeled nuclei were evaluated in the tumoral areas where these markers were predominantly expressed. Only nuclei with a strongly positive label were counted. The same tissue block was utilized for all IHC staining. The same viewer manually counted all p53 and MIB-1 IHC studies with 1000 cells counted for each analysis using an ocular grid. The results of the analysis of Ki-67 and p53 LIs were expressed as the percentage of tumor cells with positive nuclei.
Statistical analysis
The analysis of the results was performed using SPSS software (version 18, SPSS Inc. Released 2009. PASW Statistics for Windows, Chicago). Individual patient data were analyzed using univariate and multivariate survival methods. The association between MIB-1 and P53-positive rate, and the preoperative findings and outcome measurements were evaluated using Chi-square and Fisher exact tests. The P < 0.05 indicated statistical significance. For univariate analysis, a Kaplan–Meier survival curve was used to display cumulative hormone control interval. Each prognostic factor was analyzed using the log-rank test stratified by study.
Results
In this study, 47 patients (21 male and 26 female) with the diagnosis of GH-secreting pituitary adenoma underwent TSSA. The mean age of the patients was 40.8 years (range: 17-73 years). The mean duration of symptoms before diagnosis was 18.7 months (range: 6-73 months). The mean follow-up duration was 5.2 years (range: 3-8.5 years).
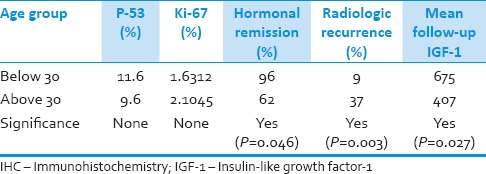
There was no significant difference in the level of Ki-67 between males and females, but the level of p53 was significantly higher in females (P = 0.001). The patients were classified arbitrarily into the age groups of younger and older than 30 years old. Although Ki-67 and p53 levels were not significantly different between the two age, ranges and those who were older than 30 at the time of surgery, had a significantly worse outcome (the mean follow-up duration had no significant difference between the two age groups) [Table 1]. The preoperative duration of symptoms did not show any significant correlation with other preoperative findings and outcome parameters.
Table 1.
Depicts outcome measures and IHC marker levels according to the age group of the patients. There have been cases in whom the changes in control imaging were not accompanied by clinical/laboratory recurrences
Among 43 patients (91.3%) the chief complaint was typical clinical acromegalic features while the other 4 (8.7%) were seeking medical care mainly for symptoms such as blurred vision and headache However, the patients primary complaint did not show any significant relationship with other clinical and radiologic characteristics and did not affect the outcome parameters.
About 20 patients (42.5%) had bitemporal hemianopia, 11 (23.4%) had unilateral hemianopia, and 16 patients (34%) had normal visual field exams. The severity of visual field involvement did not show significant correlation with other characteristics of the patients and had no influence on the clinical, hormonal and radiologic outcomes.
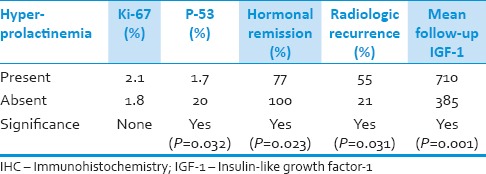
In the present study, 15 (31.9%) of the patients had a mixed type of GH and prolactin-secreting tumors. These patients had a statistically lower mean age at presentation than those without hyper-prolactinemia (with mean ages of 35.03 vs. 46 years, respectively, P = 0.04). The Ki-67 levels did not show any significant difference between the groups, but p53 expression was significantly lower in patients with hyper-prolactinemia (P = 0.032). The presence of a mixed type of GH and prolactin-secreting tumor improved the hormonal remission rate of acromegaly and decreased the chance of radiologic recurrence after surgery. The mean follow-up duration had no significant difference between the two groups [Table 2].
Table 2.
Outcome measures and IHC marker levels according to the presence or absence of hyper-prolactinemia in their laboratory assay
Hypopituitarism was detected in 10 patients (21%). When compared to the other patients, the preoperative GH level was higher in patients with hypopituitarism (P = 0.044). While the Ki-67 levels did not show any significant difference between the two groups, the p53 levels were significantly higher in patients presenting with hypopituitarism (P = 0.012). There was no significant difference in the mean age and prevalence of hyper-prolactinemia between those with and without hypopituitarism.
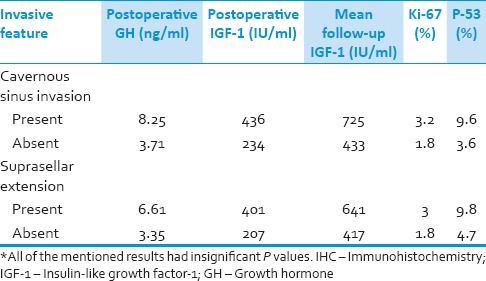
In patients, harboring adenomas with suprasellar and/or cavernous sinus invasion, the mean first postoperative GH level and the mean IGF-1 levels measured during the follow-up period was significantly higher in comparison with the patients without such extensions [Table 3]. The mean values of Ki-67 and p53 based on the presence or absence of suprasellar and/or cavernous sinus invasions are also mentioned in Table 3. There was no significant difference in Ki-67 expression between patients with micro and macroadenomas, but the p53 levels were higher in the macroadenoma group (mean p53 of 7.2% in the macroadenoma patients vs. 0.14% among the microadenoma group).
Table 3.
The postoperative hormonal values and IHC marker levels according to presence or absence of radiological invasive features*
The patients with tumor remnants evident in early MRI after surgery had significantly higher postoperative GH values in contrast to patients without remnant (P = 0.009).
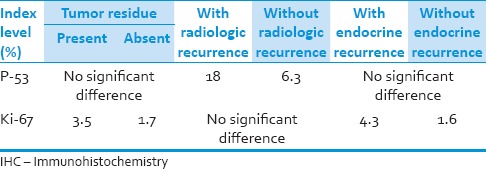
The level of Ki-67 expression had a significant relationship with hormonal recurrence rate and presence of tumor residue after surgery. The mutant p53 level correlated somehow with radiologically evident recurrence rate of adenomas even though non-significant [Table 4]. Notably, each of the mutant p53 and Ki-67 expression levels could independently predict some aspects of the outcome in our patients.
Table 4.
The results of the comparison of IHC marker levels between the groups with favorable or unfavorable outcomes
The Kaplan–Meier survival curves showed the relation between hormone control interval of the patients and the Ki-67 and p53 expression rate.
Discussion
The goal of the present study was to evaluate the impact of the IHC markers of Ki-67 and p53 expression level on the long-term outcome of GH producing pituitary adenomas and to elucidate whether there is any correlation between the other variables evaluated and the outcomes. Even though some of these correlations have been mentioned previously and separately in some reports, but we have tried to collect all the possible data in our cases and compare the results with those other reports briefly to leave a benchmark for future young investigators.
In a numbers of the previous studies, it has been shown that Ki-67 level had a significant relationship with gender, and some of them reported higher expression levels in males while others reported higher levels among female patients.[30,31,32] In the current study, we did not observe any significant difference in Ki-67 level between males and females, but the p53 level was significantly higher in the male group of patients. Such a correlation between p53 and gender has not been reported previously and to our knowledge, this is the first study measuring the level of p53 in GH-producing pituitary adenomas.
While p53 and Ki-67 levels did not show any significant relationship with the age groups (below and above 30 years), it is shown that the age older than 30 years was an independent predictor of poor hormonal and radiologic outcome.[22,23]
In patients with hyper-prolactinemia (mixed adenomas), the mean age of presentation was about one decade lower than in those without hyper-prolactinemia.[33] The p53 expression rate was remarkably lower in patients with mixed adenoma (P = 0.032), and the presence of this feature was associated with higher postoperative hormonal remission and lower endocrinological recurrence rates. This indicates that the hyper-prolactinemia may be correlated with the most favorable hormonal outcome observed in, the lower age group in this series. We would like to suggest that this could be due to the earlier diagnosis of such group of the tumors, possibly due to the presence of the clinical manifestations of hyper-prolactinemia.
According to Jaffrain et al.,[34] the Ki-67 level is higher in larger adenomas while in the study of Pinto and Bronstein,[35] the Ki-67 expression is independent from the size of the adenoma. Ferreira et al.,[36] found a kind of relationship between p53 and the volume of nonfunctional pituitary adenomas, but it was not detected for Ki-67.[17,19,20]
In this study, the Ki-67 level did not show a significant difference between micro and macroadenomas, but p53 expression was higher among patients with macroadenoma. The p53 expression rate was also significantly higher in patients with hypopituitarism. Therefore, one may conclude that the higher p53 expression level is correlated with larger adenomas and more mass effect on the surrounding structures compatible with higher rates of hypopitutarism.
Paek et al.[37] and Nakabayashi et al.,[38] reported a correlation between Ki-67 and the recurrence of pituitary adenomas after surgery, while several other studies did not find such a correlation.[39,40,41] The results of the present study show a significant relationship between Ki-67 and higher postoperative hormonal recurrence rate.
According to Ozer et al.,[39] tumors with multiple recurrences harbor higher level of p53. In our study, the p53 expression rate was 3 times higher in adenomas with radiologic recurrence than those without radiologic recurrences were. To our knowledge, this is the first study investigating the impact of P53 upon the recurrence of functional pituitary adenomas.
Some reports indicate a relation between invasiveness of pituitary adenoma and higher Ki-67 levels. According to Thapar et al.,[17] Ki-67 with a cutoff value of 3% has 73% sensitivity and 97% specificity in distinguishing invasive adenomas from noninvasive tumors. Suliman et al.,[21] offered a 3.5% cutoff value for distinguishing tumor invasiveness. In the study of Fusco et al.,[27] adenomas with cavernous sinus invasion had a higher Ki-67 expression. They concluded that Ki-67 level should be measured in all candidates for surgery of pituitary adenoma. Our study shows higher levels of the mentioned IHC markers among those with radiological invasive features [Table 3]. This is in contrary with other studies stressing upon Ki-67 expression level being unable to predict the invasive nature of pituitary adenomas. In a study done by Yarman et al.,[41] the invasive behavior of nonfunctional pituitary adenomas did not show any correlation with different levels of expression of p53 and Ki-67. Botelho et al.[18] proposed C-erb-B2 as a better predictor of invasiveness of pituitary adenomas. Based on the previous studies, Müller et al.[42] has concluded that none of the known IHC markers being capable of making distinction between invasive and noninvasive adenomas.
According to the consensus of neuropathologists, reported in 2004 WHO classification of tumors, adenomas with higher levels of p53 and Ki-67 expression levels are considered as “atypical adenomas”.[34,43,44,45,46] However, this classification was largely based on the expert opinion, and the limited number of studies on nonfunctional adenomas and could not be generalized into all types of pituitary adenomas. The results of this study along with the other upcoming researchers may provide further evidence for this classification.
Limitations
The major limitation of this and similar other studies is the small sample size that could be overcome by larger multicenteric prospective cohorts. Not all the cases operated by the group could undergo full screening either regarding IHC study of their specimens or in the follow-up evaluations. The impact of Ki-67 upon the outcome of surgery for acromegalic patients has been shown previously, and we have reconfirmed this issue in our series. The next major shortcoming is that several other potentially important biomarkers of tumor growth, which might denote the invasive nature of the tumors, were not included, because of limitation of resources.
Conclusion
The results of this study suggest that the tissue levels of the Ki-67 and p53 biomarkers and mixed GH-prolactin secretion by the tumor seem to influence some aspects of the outcome measures in patients with GH producing pituitary adenomas. We recommend that a complete set of clinical, radiologic and paraclinical data may predict the outcome of these cases more precisely than the few known parameters which are currently used.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Nasseri K, Mills JR. Epidemiology of primary brain tumors in the Middle Eastern population in California, USA 2001-2005. Cancer Detect Prev. 2009;32:363–71. doi: 10.1016/j.canep.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn K, Erhardt F, Fahlbusch R, Pickardt CR, Werder KV, Scriba PC. Recurrent goiter, hyperthyroidism, galactorrhea and amenorrhea due to a thyrotropin and prolactin-producing pituitary tumor. J Clin Endocrinol Metab. 1976;43:137–43. doi: 10.1210/jcem-43-1-137. [DOI] [PubMed] [Google Scholar]
- 3.Lugo G, Pena L, Cordido F. Clinical manifestations and diagnosis of acromegaly. Int J Endocrinol 2012. 2012 doi: 10.1155/2012/540398. 540398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3189–202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeGroot L. 4th ed. New York, Philadelphia: W.B. Saunders; 2001. Endocrionology; pp. 167–377. [Google Scholar]
- 6.Bengtsson BA, Edén S, Ernest I, Odén A, Sjögren B. Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Med Scand. 1988;223:327–35. doi: 10.1111/j.0954-6820.1988.tb15881.x. [DOI] [PubMed] [Google Scholar]
- 7.Barkan AL, Beitins IZ, Kelch RP. Plasma insulin-like growth factor-I/somatomedin-C in acromegaly: Correlation with the degree of growth hormone hypersecretion. J Clin Endocrinol Metab. 1988;67:69–73. doi: 10.1210/jcem-67-1-69. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro-Oliveira A, Jr, Faje AT, Barkan AL. Limited utility of oral glucose tolerance test in biochemically active acromegaly. Eur J Endocrinol. 2011;164:17–22. doi: 10.1530/EJE-10-0744. [DOI] [PubMed] [Google Scholar]
- 9.Bidlingmaier M, Freda PU. Measurement of human growth hormone by immunoassays: Current status, unsolved problems and clinical consequences. Growth Horm IGF Res. 2010;20:19–25. doi: 10.1016/j.ghir.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subbarayan SK, Fleseriu M, Gordon MB, Brzana JA, Kennedy L, Faiman C, et al. Serum IGF-1 in the diagnosis of acromegaly and the profile of patients with elevated IGF-1 but normal glucose-suppressed growth hormone. Endocr Pract. 2012;18:817–25. doi: 10.4158/EP11324.OR. [DOI] [PubMed] [Google Scholar]
- 11.Swearingen B, Barker FG, 2nd, Katznelson L, Biller BM, Grinspoon S, Klibanski A, et al. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998;83:3419–26. doi: 10.1210/jcem.83.10.5222. [DOI] [PubMed] [Google Scholar]
- 12.Prevedello DM, Jagannathan J, Jane JA, Jr, Lopes MB, Laws ER., Jr Relevance of high Ki-67 in pituitary adenomas. Case report and review of the literature. Neurosurg Focus. 2005;19:E11. doi: 10.3171/foc.2005.19.5.12. [DOI] [PubMed] [Google Scholar]
- 13.Guillaud P, du Manoir S, Seigneurin D. Quantification and topographical description of Ki-67 antibody labelling during the cell cycle of normal fibroblastic (MRC-5) and mammary tumour cell lines (MCF-7) Anal Cell Pathol. 1989;1:25–39. [PubMed] [Google Scholar]
- 14.Madsen H, Borges TM, Knox AJ, Michaelis KA, Xu M, Lillehei KO, et al. Giant pituitary adenomas: Pathologic-radiographic correlations and lack of role for p53 and MIB-1 labeling. Am J Surg Pathol. 2011;35:1204–13. doi: 10.1097/PAS.0b013e31821e8c96. [DOI] [PubMed] [Google Scholar]
- 15.Buckley N, Bates AS, Broome JC, Strange RC, Perrett CW, Burke CW, et al. p53 Protein accumulates in Cushings adenomas and invasive non-functional adenomas. J Clin Endocrinol Metab. 1994;79:1513–6. doi: 10.1210/jcem.79.5.7962351. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber S, Saeger W, Lüdecke DK. Proliferation markers in different types of clinically non-secreting pituitary adenomas. Pituitary. 1999;1:213–20. doi: 10.1023/a:1009933820856. [DOI] [PubMed] [Google Scholar]
- 17.Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER., Jr p53 expression in pituitary adenomas and carcinomas: Correlation with invasiveness and tumor growth fractions. Neurosurgery. 1996;38:765–70. [PubMed] [Google Scholar]
- 18.Botelho CH, Magalhães AV, Mello PA, Schmitt FC, Casulari LA. Expression of p53, Ki-67 and c-erb B2 in growth hormone-and/or prolactin-secreting pituitary adenomas. Arq Neuropsiquiatr. 2006;64:60–6. doi: 10.1590/s0004-282x2006000100013. [DOI] [PubMed] [Google Scholar]
- 19.Hentschel SJ, McCutcheon lE, Moore W, Durity FA. P53 and MIB-1 immunohistochemistry as predictors of the clinical behavior of nonfunctioning pituitary adenomas. Can J Neurol Sci. 2003;30:215–9. doi: 10.1017/s0317167100002614. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira MC, Marroni CP, Pizarro CB, Pereira-Lima JF, Barbosa-Coutinho LM, Ferreira NP. Expression of p53 protein in pituitary adenomas. Braz J Med Biol Res. 2002;35:561–5. doi: 10.1590/s0100-879x2002000500008. [DOI] [PubMed] [Google Scholar]
- 21.Suliman M, Royds J, Cullen D, Timperley W, Powell T, Battersby R, et al. Mdm2 and the p53 pathway in human pituitary adenomas. Clin Endocrinol (Oxf) 2001;54:317–25. doi: 10.1046/j.1365-2265.2001.01195.x. [DOI] [PubMed] [Google Scholar]
- 22.Dubois S, Guyétant S, Menei P, Rodien P, Illouz F, Vielle B, et al. Relevance of Ki-67 and prognostic factors for recurrence/progression of gonadotropic adenomas after first surgery. Eur J Endocrinol. 2007;157:141–7. doi: 10.1530/EJE-07-0099. [DOI] [PubMed] [Google Scholar]
- 23.Pizarro CB, Oliveira MC, Coutinho LB, Ferreira NP. Measurement of Ki-67 antigen in 159 pituitary adenomas using the MIB-1 monoclonal antibody. Braz J Med Biol Res. 2004;37:235–43. doi: 10.1590/s0100-879x2004000200011. [DOI] [PubMed] [Google Scholar]
- 24.Scheithauer BW, Gaffey TA, Lloyd RV, Sebo TJ, Kovacs KT, Horvath E, et al. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery. 2006;59:341–53. doi: 10.1227/01.NEU.0000223437.51435.6E. [DOI] [PubMed] [Google Scholar]
- 25.Turner HE, Nagy Z, Gatter KC, Esiri MM, Wass JA, Harris AL. Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: Relationship to tumour behaviour. Br J Cancer. 2000;82:1441–5. doi: 10.1054/bjoc.1999.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gejman R, Swearingen B, Hedley-Whyte ET. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. 2008;39:758–66. doi: 10.1016/j.humpath.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Fusco A, Zatelli MC, Bianchi A, Cimino V, Tilaro L, Veltri F, et al. Prognostic significance of the Ki-67 labeling index in growth hormone-secreting pituitary adenomas. J Clin Endocrinol Metab. 2008;93:2746–50. doi: 10.1210/jc.2008-0126. [DOI] [PubMed] [Google Scholar]
- 28.Knosp E, Kitz K, Perneczky A. Proliferation activity in pituitary adenomas: Measurement by monoclonal antibody Ki-67. Neurosurgery. 1989;25:927–30. [PubMed] [Google Scholar]
- 29.Hsu DW, Hakim F, Biller BM, de la Monte S, Zervas NT, Klibanski A, et al. Significance of proliferating cell nuclear antigen index in predicting pituitary adenoma recurrence. J Neurosurg. 1993;78:753–61. doi: 10.3171/jns.1993.78.5.0753. [DOI] [PubMed] [Google Scholar]
- 30.Wolfsberger S, Wunderer J, Zachenhofer I, Czech T, Böcher-Schwarz HG, Hainfellner J, et al. Expression of cell proliferation markers in pituitary adenomas – correlation and clinical relevance of MIB-1 and anti-topoisomerase-IIalpha. Acta Neurochir (Wien) 2004;146:831–9. doi: 10.1007/s00701-004-0298-0. [DOI] [PubMed] [Google Scholar]
- 31.Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J. Sex-related difference in the growth of prolactinomas: A clinical and proliferation marker study. J Clin Endocrinol Metab. 1997;82:2102–7. doi: 10.1210/jcem.82.7.4088. [DOI] [PubMed] [Google Scholar]
- 32.Adams EF, Brockmeier S, Friedmann E, Roth M, Buchfelder M, Fahlbusch R. Clinical and biochemical characteristics of acromegalic patients harboring gsp-positive and gsp-negative pituitary tumors. Neurosurgery. 1993;33:198–203. doi: 10.1227/00006123-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Abbassioun K, Amirjamshidi M, Mehrazin A, Khalatbary I, Keynama M, Bokai H, et al. A prospective analysis of 151 cases of patients with acromegaly operated by one neurosurgeon: A follow-up of more than 23 years. Surg Neurol. 2006;66:26–31. doi: 10.1016/j.surneu.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 34.Jaffrain-Rea ML, Di Stefano D, Minniti G, Esposito V, Bultrini A, Ferretti E, et al. A critical reappraisal of MIB-1 labelling index significance in a large series of pituitary tumours: Secreting versus non-secreting adenomas. Endocr Relat Cancer. 2002;9:103–13. doi: 10.1677/erc.0.0090103. [DOI] [PubMed] [Google Scholar]
- 35.Pinto EM, Bronstein MD. Molecular aspects of pituitary tumorigenesis. Arq Bras Endocrinol Metabol. 2008;52:599–610. doi: 10.1590/s0004-27302008000400005. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira JE, de Mello PA, de Magalhães AV, Botelho CH, Naves LA, Nosé V, et al. Non-functioning pituitary adenomas: Clinical features and immunohistochemistry. Arq Neuropsiquiatr. 2005;63:1070–8. doi: 10.1590/s0004-282x2005000600029. [DOI] [PubMed] [Google Scholar]
- 37.Paek KI, Kim SH, Song SH, Choi SW, Koh HS, Youm JY, et al. Clinical significance of Ki-67 labeling index in pituitary macroadenoma. J Korean Med Sci. 2005;20:489–94. doi: 10.3346/jkms.2005.20.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakabayashi H, Sunada I, Hara M. Immunohistochemical analyses of cell cycle-related proteins, apoptosis, and proliferation in pituitary adenomas. J Histochem Cytochem. 2001;49:1193–4. doi: 10.1177/002215540104900916. [DOI] [PubMed] [Google Scholar]
- 39.Ozer E, Canda MS, Ulukus C, Guray M, Erbayraktar S. Expression of Bcl-2, Bax and p53 proteins in pituitary adenomas: An immunohistochemical study. Tumori. 2003;89:54–9. doi: 10.1177/030089160308900112. [DOI] [PubMed] [Google Scholar]
- 40.Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, et al. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: An analysis using the MIB-1 antibody. Neurosurgery. 1996;38:99–106. doi: 10.1097/00006123-199601000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Yarman S, Kurtulmus N, Canbolat A, Bayindir C, Bilgic B, Ince N. Expression of Ki-67, p53 and vascular endothelial growth factor (VEGF) concomitantly in growth hormone-secreting pituitary adenomas; which one has a role in tumor behavior? Neuro Endocrinol Lett. 2010;31:823–8. [PubMed] [Google Scholar]
- 42.Müller W, Saeger W, Wellhausen L, Derwahl KM, Hamacher C, Lüdecke DK. Markers of function and proliferation in non-invasive and invasive bi-and plurihormonal adenomas of patients with acromegaly: An immunohistochemical study. Pathol Res Pract. 1999;195:595–603. doi: 10.1016/S0344-0338(99)80124-1. [DOI] [PubMed] [Google Scholar]
- 43.Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G, et al. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: A clinical and immunohistochemical study. Clin Endocrinol (Oxf) 2006;65:536–43. doi: 10.1111/j.1365-2265.2006.02630.x. [DOI] [PubMed] [Google Scholar]
- 44.Righi A, Agati P, Sisto A, Frank G, Faustini-Fustini M, Agati R, et al. A classification tree approach for pituitary adenomas. Hum Pathol. 2012;43:1627–37. doi: 10.1016/j.humpath.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Lloyd RV, Kovacs K, Young WF, Farrel WE, Asa SL, Trouillas J, et al. Tumors of the pituitary. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. World Health Organization Classification of Tumors: Pathology and Genetics: Tumors of Endocrine Organs. Lyon: IARC; 2004. pp. 10–47. [Google Scholar]
- 46.Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007;156:203–16. doi: 10.1530/eje.1.02326. [DOI] [PubMed] [Google Scholar]


