Abstract
The cell is known to be the most basic unit of life. However, this basic unit of life is dependent on the proper function of many intracellular organelles to thrive. One specific organelle that has vast implications on the overall health of the cell and cellular viability is the mitochondrion. These cellular power plants generate the energy currency necessary for cells to maintain homeostasis and function properly. Additionally, when mitochondria become dysfunctional, they can orchestrate the cell to undergo cell-death. Therefore, it is important to understand what insults can lead to mitochondrial dysfunction in order to promote cell health and increase cellular viability. After years of research, is has become increasingly accepted that age has a negative effect on mitochondrial bioenergetics. In support of this, we have found decreased mitochondrial bioenergetics with increased age in Sprague-Dawley rats. Within this same study we found a 200 to 600% increase in ROS production in old rats compared to young rats. Additionally, the extent of mitochondrial dysfunction and ROS production seems to be spatially defined affecting the spinal cord to a greater extent than certain regions of the brain. These tissue specific differences in mitochondrial function may be the reason why certain regions of the Central Nervous System, CNS, are disproportionately affected by aging and may play a pivotal role in specific age-related neurodegenerative diseases like Amyotrophic Lateral Sclerosis, ALS.
Keywords: Aging, Amyotrophic Lateral Sclerosis (ALS), Brain, Mitochondria, Mitochondrial Permeability Transition Pore (mPTP), Respiration, Spinal Cord
Introduction
Mitochondria are now accepted as an integral component of the most basic unit of life: the cell. The study of mitochondria and their bioenergetics has opened new findings in a broad area of interests ranging from genetics to cell biology. At the most well understood level, mitochondria are essential for defining the metabolic profile and level of activity of a cell. As the energy power plant of the cell, they produce the metabolic currency used by the cell, specifically Adenosine Triphosphate, ATP (Chance and Williams 1955; Chance and Williams 1955; Chance and Williams 1955; Chance and Williams 1955; Chance, Williams et al. 1955). This ATP is then used by the cell to carry out cell specific functions as well as maintain cellular homeostasis. However, their importance expands even further than metabolism, specifically being implicated as playing a pivotal role in cell death.
Well aligned with the analogy of a manmade power plant, when mitochondria are functioning properly there is not much attention given to them. However, similar to a power plant, when mitochondria become dysfunctional, grave occurrences can ensue. Dysfunctional mitochondria are now known to play an integral part in various cell death pathways. Additionally, mitochondria play an important role in buffering cytosolic Ca2+. In an electrogenic fashion, mitochondria can take up Ca2+ ions, removing them from the cytosol, thereby decreasing the activation of various detrimental Ca2+ mediated pathways. As the mitochondria buffer this cytosolic Ca2+, their membrane potential will decrease as a consequence. With high enough Ca2+ levels and the corresponding decrease in membrane potential, the mitochondrial permeability transition pore can be opened leading to the initiation of cell death.
Through this Ca2+ mediated mitochondrial cell death mechanism and others, mitochondria have been implicated in various age related neurodegenerative diseases such as Amyotrophic Lateral Sclerosis, ALS (Brown, Geddes et al. 2004). Therefore, scientists have turned their interests to finding novel therapeutic approaches in order to target mitochondrial function with the hopes of avoiding the onset and/or progression of diseases where mitochondrial dysfunction is present and amendable. However, as scientists discovered approaches that were able to target mitochondrial function, a debate ensued as to whether mitochondria are a homogenous population across various tissue types.
With the investigation of mitochondrial targeting therapeutic approaches for various disease states, unexpected insights into the nature of mitochondria have surfaced. Mitochondrial function does not seem to be consistent across all tissue types or even within different regions of a specific cell type, specifically synaptic versus non-synaptic mitochondria (Brown, Sullivan et al. 2006). Additionally, it is also thought that aging further increases these differences (Brown, Geddes et al. 2004). Therefore, to date, the field is beginning to probe the idea that mitochondria may not all be alike and may react to insults, such as aging or toxins, differentially. Within this article, we will review these differences and highlight new data supporting this hypothesis from our laboratory.
Aging and Mitochondria
One specific research field that has become very interested in mitochondrial bioenergetics is that of Aging. One of the scientists who lead progress in this area was Denham Harman. In the 1960s, he proposed mitochondria to be important organelles that contributed to the progression of cellular aging. His work lead to the Mitochondrial Free Radical Theory of Aging (MFRTA) (Harman 1955; Harman 1956; Harman 1972; Brody, Harman et al. 1975; Ames 1992; Ames 1993). Although some specifics regarding the MFRTA are heavily debated, there are specifics that have yet to be disproven. Within MFRTA, Harman acknowledged that through the normal function of the electron transport chain, reactive electrons are released. These electrons are then able to react with free oxygen, generating reactive oxygen species, which can thereby react with nearby proteins, nucleic acids, etc. leadings to metabolic mediated oxidative stress. Over time, the oxidative insult perpetuates and mitochondrial and cellular damage can occur. After many years, this damage begins to accumulate leading to decreased bioenergetic efficiency, increased electron slippage/release and decreased ATP production. In cell types with low bioenergetics profiles, this change in bioenergetics is not a large concern. However, in cells that have a high energy demand such as neurons, decreased ATP production can lead to serious detrimental effects which can result in severe functional changes due to disruption of cellular homeostasis.
Although debated, data supporting MFRTA has been published for many years. Specifically, age-associated impairments in mitochondrial oxidative phosphorylation (OXPHOS) due to oxidative damage has been shown in multiple tissues including the brain, muscle, liver and heart (Hansford 1978; Paradies and Ruggiero 1991; Paradies, Ruggiero et al. 1991; Shigenaga, Hagen et al. 1994; Gibson, Sheu et al. 1998). However, the proteins damaged seem to be very specific and not necessarily the result of a complete destruction to all mitochondrial structures. Therefore, mitochondrial proteins are, seemingly, more vulnerable to age-related metabolic mediated oxidative stress. Specifically, mitochondria isolated from various rodent tissues have shown decreased mitochondrial electron transport chain enzymatic activity with age, specifically within complexes I and IV, however complexes II and III seemingly remain unaffected (Navarro and Boveris 2004; Navarro and Boveris 2007). Based on data obtained from our laboratory, it has also become apparent that age related mitochondrial dysfunction may be the result of a regional specific dysregulation in Ca2+ buffering by the mitochondria (Brown, Geddes et al. 2004). In this specific study, mitochondria isolated from aged rat cortex and hippocampus showed increased ROS production and decreased Ca2+ buffering capacity compared to young rats however this was not observed in mitochondria isolated from the cerebellum of these animals. This study also found that aged brain mitochondria were able to undergo mitochondrial permeability at a lower Ca2+ threshold than young animals with the exception of cerebellar mitochondria where no difference was measured.
It is thought that as oxidative damage and the resulting mitochondrial dysfunction increases with age, mitochondria will become more vulnerable to insults by environmental toxins, etc. leading to an increase in mitochondrial mediated cell death. This rational is supported by the increased prevalence of neurodegenerative diseases in the aged population (Moreira, Zhu et al. 2010). Additionally, these age-related changes in mitochondrial bioenergetics do not seem to occur equally in all regions of the central nervous system. Certain regions within the central nervous system experience increased mitochondrial dysfunction, increased ROS concentrations and decreased Ca2+ buffering capacity making these tissue types more vulnerable to the initiation of mitochondrial mediated cell death pathways.
Mitochondrial differences in the brain versus spinal cord
Back in the early 2000s, our laboratory and others were investigating Cyclosporin A for the treatment of injuries to the central nervous system, CNS. At the time, Cyclosporin A was a known immunosuppressant however it also had a secondary target within the mitochondria. Specifically, it was found to bind a protein within the mitochondria called Cyclophilin D, thereby inhibiting the mitochondrial permeability transition. In the model of brain injury, this drug provided a means of limiting part of the excitotoxic cell death cascade that occurs following injury. However, within these studies it was noticed that the therapeutic effect of Cyclosporin A was lost when it was moved from brain injury to a model of spinal cord injury. This work provided evidence that mitochondria in one tissue type differs from mitochondria within another tissue (Brown, Geddes et al. 2004). Upon further research, it became apparent that these differences could also occur in tissues that were histologically similar. For instance, the brain and the spinal cord are one continuous structure, thereby sharing similar cell types. However, it has been shown that mitochondrial from the brain have different bioenergetics than those in the spinal cord. Later it became apparent that even mitochondria within one area of a cell can have a different energetic profiles compared to mitochondria within another area of cell. Specifically, our work has shown that neuronal mitochondria located in the synapse of neurons have different bioenergetics, calcium buffering capacities and respond differently to mitochondrial targeting drugs compared to mitochondria found within non-synaptic pools (Yamamoto, Rossi et al. 1999; Brown, Sullivan et al. 2004; Sullivan, Rabchevsky et al. 2004; Hall, Sullivan et al. 2005; Brown, Sullivan et al. 2006; Singh, Sullivan et al. 2006; Naga, Sullivan et al. 2007; Patel, Sullivan et al. 2009). This data further supports the theory that mitochondrial bioenergetics may be location specific.
From our current understanding, it seems as if there is convincing evidence supporting the theory that mitochondria within the spinal cord may have significant differences in metabolic profiles than mitochondria within the brain. Work from our laboratory and others have found that mitochondria within the spinal cord produce more reactive oxygen species and have increased oxidative damage compared to age-matched brain mitochondria. Additionally, in normal, non-aged mitochondria obtained from 3 month old rats, spinal cord mitochondria have decreased maximum NADH linked mitochondrial respiration. However, in this study no difference in FADH2/succinate mediated respiration was demonstrated (Sullivan, Rabchevsky et al. 2004). Additionally, spinal cord mitochondria have a lower calcium mediated threshold for the formation of the mitochondrial permeability transition pore, which is likely related to increased expression of cyclophilin D compared to brain mitochondria. Because of these differences, drugs that inhibit the formation of the mitochondrial permeability transition pore such as Cyclosporin A require significantly higher concentrations in order to provide the same degree of neuroprotection in the spinal cord that is seen in the brain. Although these findings were interesting, they lead to more questions. Specifically, does age influence the differences seen between mitochondria from the brain versus the spinal cord?
Age related differences between brain and spinal cord mitochondria
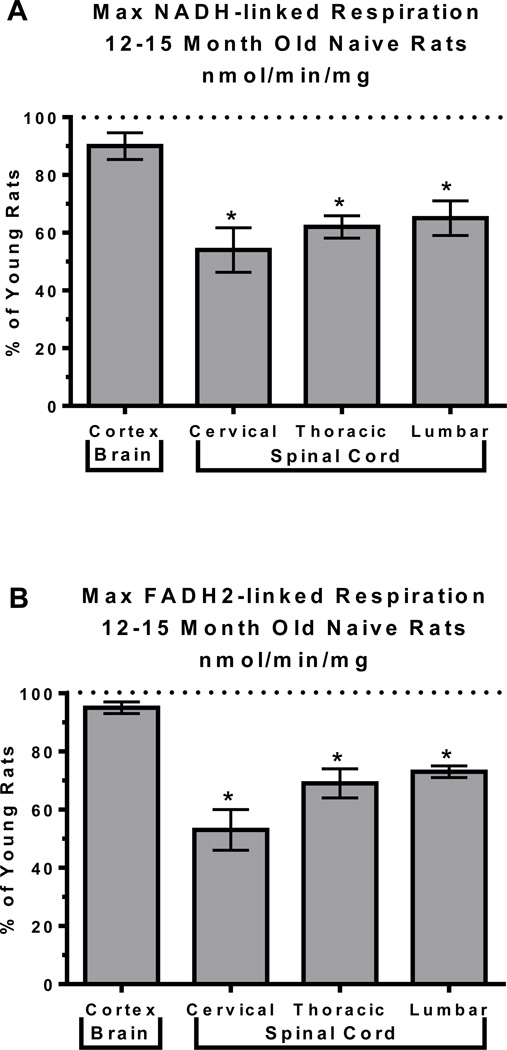
We hypothesized that since age and location of mitochondria both have individual effects on mitochondrial bioenergetics, then when combined together an even further deviation in mitochondrial function will occur. To test this hypothesis we compared mitochondrial bioenergetics within the brain to those within the spinal cord in rats at different ages, specifically 3 month compared to 12–15 month old male Sprague-Dawley rats (9 per age group, tissue from 3 animals pooled for mitochondrial assessments). Within this study we looked at differences in the total mitochondrial population within the cortex of the brain and cervical, thoracic and lumbar regions of the spinal cord using techniques as described in (Brown, Geddes et al. 2004; Brown, Sullivan et al. 2004; Sullivan, Rabchevsky et al. 2004; Brown, Sullivan et al. 2006). Analysis of the NADH-linked maximum respiration data using a 2way ANOVA demonstrated a significant age X region interaction (F=4.955, p = 0.012) as well as significant effects of age (F=72.69, p < 0.0001) and region (F=39.46, p < 0.0001). FADH2-linked maximum respiration demonstrated significant effects of age (F=31.30, p < 0.0001) and region (F=29.68, p < 0.0001) with no significant age X region interaction (F=3.238, p = 0.050). To further establish age dependent differences, unpaired t-tests were performed for each region in young vs aged animals. We determined aged rats had a 20 to 50% decrease in NADH mediated respiration within all regions of the spinal cord compared to the young cohort (p < 0.05). Aged animals also showed a significant decrease in succinate/FADH2 mediated respiration within the various regions of the spinal cord (p < 0.05), which was not demonstrated in the brain for either NADH or FADH2 mediated respiration (FIGURE 1A and 1B and TABLE 1). These findings are very interesting because significant NADH mediated inhibition of cortical mitochondria has been documented with age in the past however this study did not find the same conclusions (Brown, Geddes et al. 2004). The differences observed however may be due to inherent strain differences and/or the ages of animals examined in these two studies. We did, however, find a significant decrease in both NADH and FADH2 mediated respiration in all the tested areas of the spinal cord in the aged animals compared to young. With the results found in young animals, mitochondria within the spinal cord seem to have decreased bioenergetics, compared to brain mitochondria, which continues to decrease with age. This decrease in bioenergetics has the potential of making these areas more vulnerable to insults due to decreased energetic reserves, deceased ATP production and decreased capacity for ROS, which would increase the probability of a transition to cell death and therefore, increase the possibility of neurodegeneration.
FIGURE 1. Increased age in rats lead to decreases in NADH and FADH2 mediated respiration.
Total mitochondria were isolated from young (3 month) and aged (12–15 months) male Sprague-Dawley rats (9 per age group, tissue from 3 animals pooled for final n=3 per group) and oxygen consumption assessed in a sealed, stirred, thermostated chamber equipped with a Clark-type electrode and expressed as nmol of oxygen consumed per min per mg of mitochondrial protein. Maximum NADH-linked (A) and FADH2-linked (B) respiration data were then analyzed using 2way ANOVAs following by unpaired t-tests when warranted to assess age effects on mitochondrial bioenergetics. Data are expressed as % of respiration measured in young animals, *p < 0.05.
TABLE 1. Mitochondrial Mediated Respiration.
Mean (nmol oxygen/min/mg) ± SD
| State III | State IV | State V | State Vsuc | |
|---|---|---|---|---|
| Young Cortex | 94.64 ± 4.61 | 16.09 ± 1.42 | 114.09 ± 5.50 | 125.46 ± 1.77 |
| Young Cervical | 70.67 ± 7.72 | 10.73 ± 1.88 | 94.44 ± 13.19 | 100.16 ± 14.02 |
| Young Thoracic | 42.32 ± 3.99 | 7.75 ± 1.06 | 81.97 ± 2.80 | 85.60 ± 4.36 |
| Young Lumbar | 48.52 ± 11.80 | 7.96 ± 0.70 | 72.24 ± 11.29 | 77.79 ± 5.74 |
| Aged Cortex | 85.29 ± 14.47 | 17.87 ± 5.67 | 102.98 ± 4.72 | 118.01 ± 11.43 |
| Aged Cervical | 38.06 ± 8.52 | 9.34 ± 2.49 | 45.73 ± 9.69 | 52.44 ± 19.15 |
| Aged Thoracic | 26.38 ± 8.57 | 5.58 ± 1.82 | 45.40 ± 10.51 | 58.70 ± 13.67 |
| Aged Lumbar | 31.31 ± 4.32 | 5.44 ± 1.34 | 46.29 ± 7.20 | 56.10 ± 9.79 |
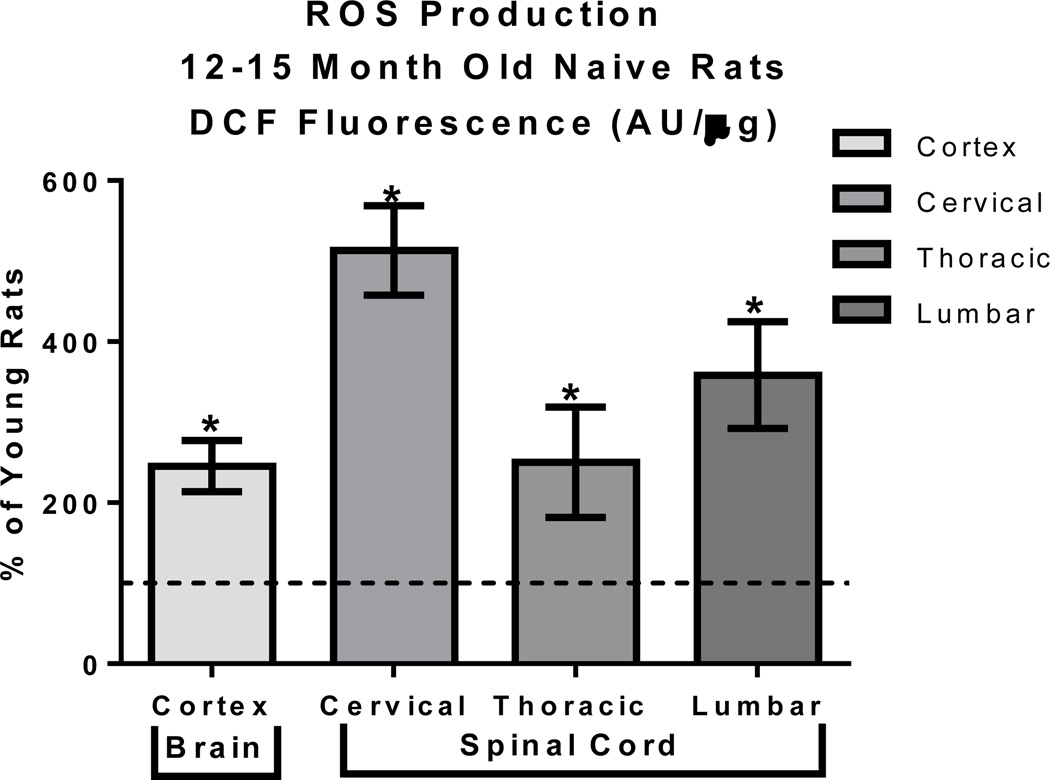
Mitochondrial mediated oxidative stress and mitochondrial dysfunction generally occur together however the causation between the two is debated, however MFRTA supports the theory that oxidative stress causes mitochondrial dysfunction. To further support this hypothesis that the deviations in mitochondrial respiration are the result of oxidative stress, we assessed mitochondrial ROS production as a function of age across CNS regions using methods previously described in (Brown, Sullivan et al. 2004; Brown, Sullivan et al. 2006). A 2way ANOVA of basal mitochondrial ROS production demonstrated a significant age X region interaction (F=12.81, p = 0.0002) as well as significant effects of age (F=183.6, p < 0.0001) and region (F=16.10, p < 0.0001). Unpaired t-tests revealed that mitochondria isolated from aged rats compared to young rats (12 months compared to 3 month old Sprague Dawley rats) had a significant (p < 0.01) increase in reactive oxygen species (ROS) production within all regions of the CNS, specifically the cortex and the cervical, thoracic and lumbar regions of the spinal cord (FIGURE 2 and TABLE 2). One factor that is ambiguous within this study is whether the high ROS production leads to decreased mitochondrial bioenergetics or the decreased bioenergetics leads to increased ROS production. However, what is known is that this age related increase in ROS can eventually lead to cellular dysfunction making aged animals more vulnerable to cellular insults. This increased vulnerability makes the animal more likely to experience cellular dysfunction and possibly cellular death within this region.
FIGURE 2. Mitochondrial ROS production increases with age.
Aged rats had a 200% to 600% increase in ROS production mitochondria isolated from the cortex and spinal cord compared to young rats. Total mitochondria were isolated from young (3 month) and aged (12–15 months) male Sprague-Dawley rats (9 per age group, tissue from 3 animals pooled for final n=3 per group) and ROS production assessed using the indicator DCF in the presence of respiratory substrates. Control wells included FCCP to induce minimum ROS production and oligomycin to induce maximum ROS production. Wells in which mitochondria were omitted were used for background subtraction. Raw data (DCF fluorescence AU/ug) were then analyzed using a 2way ANOVA following by unpaired t-tests when warranted to assess age effects on mitochondrial basal ROS production. Data are expressed as % of respiration demonstrated in young animals, *p < 0.05.
TABLE 2. Tissue Specific ROS Production.
Mean (DCF Flourescence AU/µg) ± SD
| Young Cortex | 95.88 ± 9.24 |
| Young Cervical | 121.95 ± 32.61 |
| Young Thoracic | 145.28 ± 39.95 |
| Young Lumbar | 144.95 ± 61.75 |
| Aged Cortex | 235.30 ± 45.52 |
| Aged Cervical | 625.79 ± 63.73 |
| Aged Thoracic | 293.04 ± 78.52 |
| Aged Lumbar | 485.65 ± 52.79 |
With increased production of ROS and decreased mitochondrial respiration, mitochondrial and cellular structures become more vulnerable to oxidative damage. As previously reported by our group, mitochondrial DNA, mtDNA, damage is significantly higher within the spinal cords compared to the brain (Sullivan, Rabchevsky et al. 2004). This increased damage to mtDNA can further perpetuate the mitochondrial dysfunction observed since protein structures made within the mitochondria have an increased chance of being mutated which can render mitochondrial proteins encoded by the mtDNA dysfunctional.
Summary
Well supported by the literature and the studies conducted in our lab, NADH and FADH2 mediated mitochondrial respiration decreases in the cortex and within the cervical, thoracic and lumbar regions of the spinal cord as age increases. In addition to changes in respiration, we have also observed increased mitochondrial ROS production with age in these same regions. Lastly, within the spinal cord of aged animals, it seems as in mtDNA damage is increased which could be a direct result of the increased mitochondrial ROS production. Although more studies may be needed to determine the exact age when the mitochondria become less bioenergetically efficient and to determine whether ROS or mitochondrial bioenergetics increase first with age leading to the other, MFRTA and the previously mentioned studies do give great insight into a potential therapeutic target that scientists could exploit in order to improve age related functional decline and thereby improving the quality of life for the older population.
Footnotes
Competing Interests: The authors have declared no existing competing interests.
References
- Ames BN, Shigenaga MK. Oxidants are a major contributor to aging. Annals of the New York Academy of Sciences. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H, Harman D, et al. Clinical, morphologic, and neurochemical aspects in the aging central nervous system. New York: Raven Press; 1975. [Google Scholar]
- Brown MR, Geddes JW, et al. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J Bioenerg Biomembr. 2004;36(4):401–406. doi: 10.1023/B:JOBB.0000041775.10388.23. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, et al. Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. J Neurosci Methods. 2004;137(2):299–303. doi: 10.1016/j.jneumeth.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, et al. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem. 2006;281(17):11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217(1):383–393. [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. II. Difference spectra. J Biol Chem. 1955;217(1):395–407. [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955;217(1):409–427. [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. IV. The respiratory chain. J Biol Chem. 1955;217(1):429–438. [PubMed] [Google Scholar]
- Chance B, Williams GR, et al. Respiratory enzymes in oxidative phosphorylation. V. A mechanism for oxidative phosphorylation. J Biol Chem. 1955;217(1):439–451. [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, et al. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105(8–9):855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, et al. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22(2):252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hansford RG. Lipid oxidation by heart mitochondria from young adult and senescent rats. Biochem J. 1978;170(2):285–295. doi: 10.1042/bj1700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging : a theory based on free radical and radiation chemistry. Berkeley, CA: University of California Radiation Laboratory; 1955. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20(4):145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Zhu X, et al. Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta. 2010;1802(1):212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naga KK, Sullivan PG, et al. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27(28):7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1244–R1249. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292(2):C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM. Effect of aging on the activity of the phosphate carrier and on the lipid composition in rat liver mitochondria. Arch Biochem Biophys. 1991;284(2):332–337. doi: 10.1016/0003-9861(91)90304-2. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, et al. The influence of hypothyroidism on the transport of phosphate and on the lipid composition in rat-liver mitochondria. Biochim Biophys Acta. 1991;1070(1):180–186. doi: 10.1016/0005-2736(91)90161-z. [DOI] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, et al. Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res. 2009;87(1):130–140. doi: 10.1002/jnr.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, et al. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, et al. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26(11):1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, et al. Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J Comp Neurol. 2004;474(4):524–534. doi: 10.1002/cne.20130. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Rossi S, et al. CSF and ECF glutamate concentrations in head injured patients. Acta Neurochir Suppl. 1999;75:17–19. doi: 10.1007/978-3-7091-6415-0_4. [DOI] [PubMed] [Google Scholar]