Abstract
The paper focuses on the biology of stress and resilience and their biomarkers in humans from the system science perspective. A stressor pushes the physiological system away from its baseline state towards a lower utility state. The physiological system may return towards the original state in one attractor basin but may be shifted to a state in another, lower utility attractor basin. While some physiological changes induced by stressors may benefit health, there is often a chronic wear and tear cost due to implementing changes to enable the return of the system to its baseline state and maintain itself in the high utility baseline attractor basin following repeated perturbations. This cost, also called allostatic load, is the utility reduction associated with both a change in state and with alterations in the attractor basin that affect system responses following future perturbations. This added cost can increase the time course of the return to baseline or the likelihood of moving into a different attractor basin following a perturbation. Opposite to this is the system’s resilience which influences its ability to return to the high utility attractor basin following a perturbation by increasing the likelihood and/or speed of returning to the baseline state following a stressor. This review paper is a qualitative systematic review; it covers areas most relevant for moving the stress and resilience field forward from a more quantitative and neuroscientific perspective.
Keywords: psychological stress, systems science, allostatic load, resilience
1. Introduction
Psychological stress is common in our society. A recent survey indicated that 25% of Americans reported high stress and 50% identified a major stressful event during the previous year [1]. Chronic psychological stress increases risk of health problems and contributes to cardiovascular problems [2, 3], neurologic and psychiatric diseases such as epilepsy [4], Parkinson's disease [5], multiple sclerosis [6], eating disorders, addictions [7], post-traumatic stress disorder (PTSD), and sleep difficulties. Therefore, it is important to develop evidence-based methods that minimize stress impact. A fuller understanding of stress physiology and psychology can be achieved by approaching this topic from different angles. This work offers a review of stress physiology and psychology from a systems science perspective.
Systems science is a methodology used to understand complex systems from organizational, structural, and dynamic perspectives [8]. From a systems science viewpoint, stress often corresponds to a state away from optimal in a dynamical system where the optimal location represents a high utility attractor. An attractor basin in a dynamical system corresponds to the conceptual space of locations in which the system resides over time. The state of stress results from a perturbation arising from the internal or external environment (stressor). This stressor could result in the system returning to the baseline optimal attractor or moving into a lower utility attractor basin. The attractor basin is the region of space that shares the same attractor and the whole space may have multiple attractors (Figure 1).
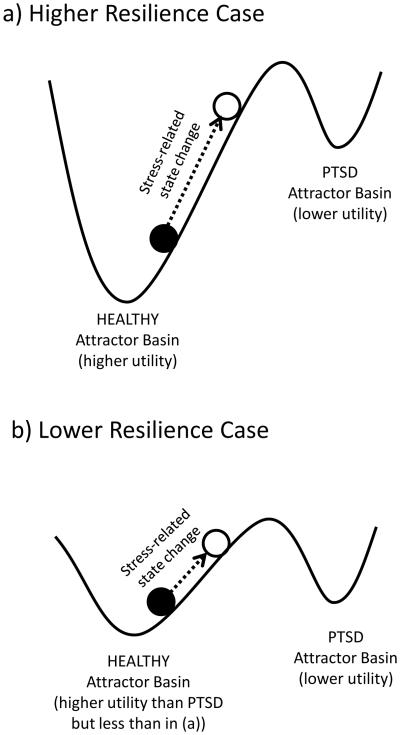
Figure 1. Attractor basins, utility, and resilience.
Hypothetical example of space of possible human physiological states with two attractor basins, one being a healthy higher utility condition and one a lower utility condition state of PTSD (in this figure, higher utility is downward). The attractor basins can tolerate movement of the hypothetical person (solid circle) in the horizontal direction from an external stressor without leaving its basin of attraction. However, with sufficient movement from a stressor, one may go from a higher utility healthy condition basin to a lower utility PTSD basin. The healthy condition in b has lower resilience than in a, with less stress required to shift it to the lower utility basin.
The attractor in the human system is not a fixed point attractor given the multidimensional nature and, almost inherent, within-subject temporal variability of the physiological measures of state. The noise present in the measurement of the many variables constituting the human system implies the observed human system is stochastic; thus, the attractors are very difficult to describe. In addition, given the varying time frames over which the components of the human physiological system change, the terms state and variable describing more immediate changes and the terms trait or parameter describing longer time frame changes represent an artificial separation of the various physiological measures that have different units and widely distributed half-lives. Whatever the attractor, even if the system returns to the baseline high utility attractor, there is often some underlying cost. This cost to the system is a change in the underlying physiology that may: 1) decrease the rate of return to the high utility attractor or 2) decrease the likelihood of returning to the optimal attractor following a future stressor perturbation because the size of the attractor basin is smaller or the attractor has moved closer to a boundary with a non-optimal attractor basin. The movement of the dynamical system into a different attractor basin could also be due to a single severe stressor potentially via a dynamical system catastrophe, for example, development of PTSD following a single event (Figure 2).
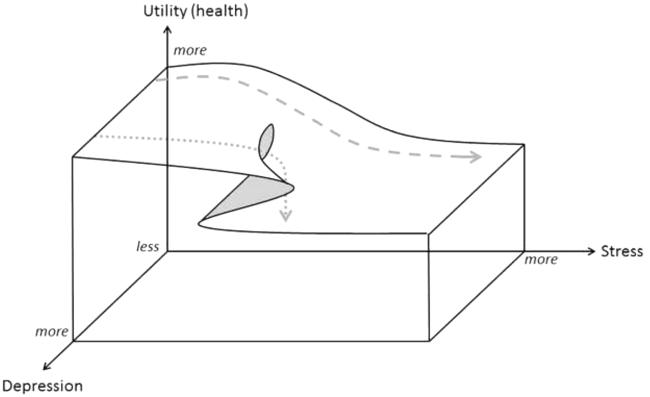
Figure 2. Cusp catastrophe.
An example of a cusp catastrophe where the state space of human physiology has a complex 3-dimensional shape, with no pictorial representation of attractors, and there may be an abrupt state change. In this example, as stress increases at higher levels of depression there may be a sudden drop in location to a new state, PTSD (marked by a dotted line). Here, utility is up rather than down as in Figure 1.
Besides negative effects, the stressor can also induce beneficial changes leaving the system more resilient to future perturbations, i.e., cause the opposite of 1) and 2) above. The term resilience includes several conceptual aspects. Resilience refers to how effectively and quickly the system returns to baseline [9]. This includes whether the human dynamical system avoids moving to a lower utility disease state following a stressor [10]. A related term is stability which refers to how well the system can maintain its current high utility condition without being pushed away.
Although a stressor may cause a short-term decrease in some measure of utility, sometimes it results in longer-term utility increase. In the case of humans, this is related to learning as discussed below. The human dynamical system may experience some low-stress environmental perturbation that results in a relatively immediate gain in reward or utility, e.g., obtaining food when hungry or some longer-term gain in utility, e.g., the brain acquiring a better understanding of the environment. There is an apparent inverted u-shaped effect of stress on longer-term utility, such that occasional small amounts of stress may improve both short- and long-term utility but experiencing no stress or large amounts of stress may have negative long-term effects on the organism. Though the term “human” will be used, most of this discussion applies to other animals and to systems in general.
2. The human physiologic system: brain structure and network (Figure 3)
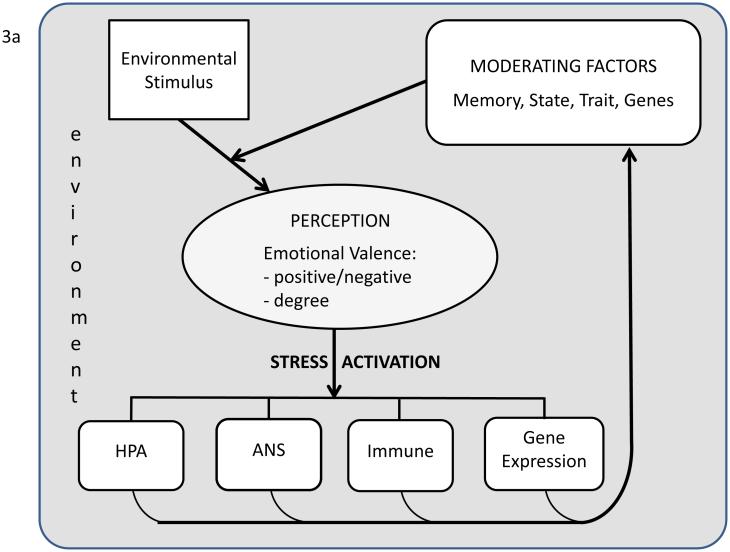
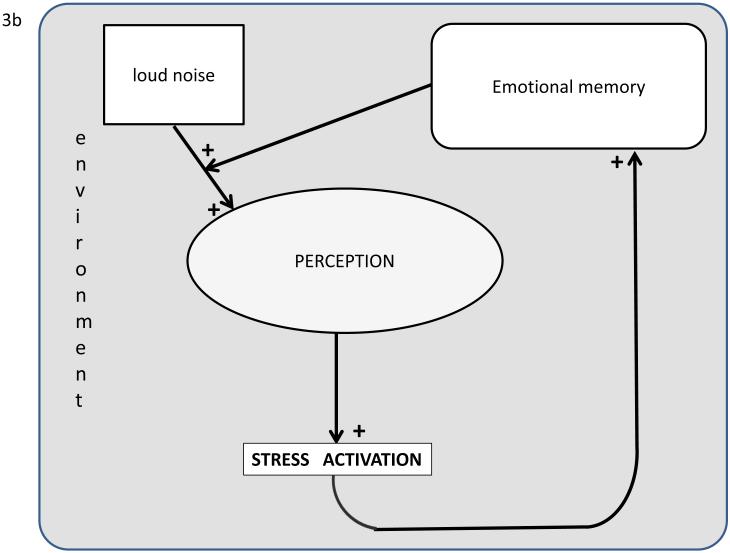
Figure 3. a). Interpretation of stressors: brain processing and communication.
The brain’s perception of the emotional valence of an external event as a stressor is dependent on the current environment and modulated by previous experiences (memory), current physiological state, traits (e.g., neuroticism), and genotype. The brain generates outputs to the autonomic nervous systems (ANS), the hypothalamo-pituitary-adrenal (HPA) axis, the immune system, gene expression and epigenetics (overall increasing time duration of stress activation components from left to right. These responses directly affect the body but also feedback to the brain. Learning includes assessment of risks and rewards and it can be clinical designed to reduce reactivity, e.g., allergy therapy or mindfulness meditation. b). Example of self-reinforcing stress response system that is pathological if in a non-threatening environment. Normally, while stress activation from a loud non-threatening noise may initially activate a stress response, response to repeated loud noise will be attenuated through negative feedback (e.g., habituation). In PTSD emotional memories and the stress activation itself may contribute to an auto-reinforcing positive feedback loop. As mentioned in the text and Figure 2, this PTSD attractor basin may be entered secondary to a single severe negative event via a catastrophic dynamical systems event. This pathological transition is more likely in those with predispositions, e.g., neurotransmitter alterations such as depression.
A human is a dynamical system composed of subsystems that help maximize utility of the organism. Utility may be defined: 1) from a purely biological perspective such as immediate reproductive success or obtaining food, or 2) from a more complex, perhaps hedonic or longer-term perspective such as longer-term reproductive success, obtaining more resources, gaining group support or enjoying an amusement park ride. Longer-term utility could extend beyond the lifespan, e.g., survival of the related social unit or the entire species (see section 9 for more information about utility). The organism is maintained by many critical systems and subsystems, such as cardiovascular and renal, but this paper focuses on the brain dynamical system and its communication links with the body via autonomic nervous system (ANS), hypothalamo-pituitary-adrenal (HPA) axis and neuroimmune system. The limbic system is involved in psychological aspects of stress, including neocortex activation by emotional states and memories of events associated with emotional valences. Older and more caudal brain parts including the brainstem and spinal cord are generally not critical for the following discussion with some exceptions including ANS components. The sympathetic portion of the ANS involving central catecholaminergic systems is particularly important for communicating the brain perception of stress to the whole body by causing changes such as increased blood pressure and heart rate. The hypothalamus is an important communication link secreting neurohormones, e.g., adrenocorticotrophic hormone (ACTH). Given this background, the most commonly discussed physiologic responses to a stressor involve the HPA axis, the locus coeruleus-norepinephrine-sympathetic nervous system pathway, the parasympathetic system, the immune system, and gene expression and alterations including epigenetic changes.
The two-way communication between the major effector systems (ANS, HPA, and immune) and the brain exist in part to ensure the stress-related systems provide feedback for learning and help avoid over-reactivity. The communication system between the immune system and the brain constitutes an entire field itself, psychoneuroimmunology [11]. The immune system - brain communication is significantly mediated by cytokines. All these two-way communication systems directly impact the brain via its receptors for norepinephrine, ACTH, cortisol, and cytokines, with prefrontal cortex, hippocampus, and amygdala being most prominent [12]. Feedback is often inhibitory and is not perfect. Occasional errors in this two-way communication system may arise. For example, a major increase in heart rate in an exercising older adult with atherosclerosis might be accompanied by an attempt to decrease the heart rate, but this decrease may be insufficient to prevent a myocardial infarction and even a sudden death [13]. Additionally, the awareness of stress may itself be a stressor; however, this type of stress is distinct from experiencing external environmental stressors. Stress awareness may be commonly related to the “recall” or association of particular environmental inputs with prior stress.
3. Stressor
A stressor is an environmental event that significantly perturbs the entire human dynamical system away from the optimal attractor resulting in a state of lower utility. The stressor may move the physiological system to a different attractor basin, move the system state closer to the edge between its current attractor basin and another attractor basin of the physiological system (“precariousness”), or slow the rate at which the system returns to the optimal attractor. The movement of the system is not dependent solely on objective measures of the stressor but also on the individuals’ traits of distress proneness and their perceptions of the stressor. If the perturbation is perceived to impact an organism negatively or associated with obvious threats (hunger/visualization of aggressor), there is an immediate effect to reduce the likelihood of a negative stressor impact. For example, seeing a bear with her cub while hiking will generate physiological changes important for action (elevated heart rate and blood pressure) and increased attention to environmental stimuli, thus improving encoding of the situation for future recollection. These perturbations increase likelihood of survival over the short-term but if maintained long-term may have deleterious effects. For example, a transient increase in blood pressure is tolerable and may be helpful, but a chronic increase in blood pressure is not high utility. Stress doses that are not high enough to cause significant health problems such as disease or death from a state change may produce higher average utility within the basin by altering the shape of the basin or by moving to a different, higher utility basin. In an athletics example, both short-term stress at an Olympic competition and longer-term stress from high effort athletic activity over a training period may improve athletic performance. However, excessive or repeated perturbations may have a cost to the underlying system that outweighs the benefit.
Stressors may include external environment perturbations such as extreme heat or icy roads while driving. Stressors may also include internal environment perturbations such as infections or elevated glucose. Stressors may be predominantly psychological and mediated by brain perception and future expectancy. Stressors are not necessarily physical changes in the environment but may involve loss of a significant relationship, financial stress, negative neighborhood characteristics, or social threats including discrimination [14-17].
For most of this discussion, the stressor referring to perturbations under tight physiological control will be omitted. Information signals from these perturbations such as alterations in serum sodium do not need to reach the brain level to be regulated. Homeostasis refers to the dynamic control of these state variables maintained within a narrow window for humans to successfully function. The dynamical system representing the whole person is regularly exposed to more heterogeneous stressors than serum sodium changes, including potential stressors that are anticipated. Allostasis has been used to describe “actively maintaining homeostasis” [18], but the practicality of this distinction from homeostasis is uncertain [19].
Some stressors represent state perturbations to which the person may respond without any obvious long-term negative ramifications. Some stressors, in part related to their chronicity, may have negative long-term ramifications. The perturbation may induce changes in several systems. For example, as time passes from the previous meal, a human’s stomach is growling and blood sugar is getting lower; the brain senses hunger and mobilizes to address the perturbation stressor. Part of the response to a stressor will be mediated directly by the internal environment without requiring any mediation by the brain, e.g., hunger causing the release of hormones to break down glycogen. Part of the response is directly mediated by the brain responsible for planning how to interact with the external environment, e.g., walking into the kitchen to get food. The perturbation may induce changes in physiological parameters, e.g., DNA transcription or epigenetic modifications to alter neurotransmitter receptor sensitivity. Responding to these stress perturbations may induce some cost to the system. This cost may involve the movement of the system into another basin of attraction or an increase in the probability that the system will move into another basin following future perturbations.
Though the stressor has some objective qualities, it can be difficult to quantify because physiological stress effects are highly dependent on the subjective perception. Quantifying an individual’s stressors has been attempted [20]. Some examples of stressors include events that have novelty, unpredictability, (any information-rich input beyond the brain processing ability), threat to one's ego, or sense of loss of control (NUTS) [21]. Short-term laboratory experimental stressors are related to these NUTS concepts including the Trier Social Stress Test, [22], the Montreal Imaging Stress Task [23], titrated Stroop color-word interference task [24], physical (e.g., putting a hand in ice water) [25], or perceptual stressors (e.g., the disturbing pictures of the International Affective Picture Scale [26]). Stress responses can also be conditioned [27] allowing for comparison between humans and other animals. It is more challenging to study long-term stressors experimentally but occasional misfortunes such as wars and other disasters have generated informative epidemiological data, e.g., the World Trade Center disaster. Stressors may involve awareness of a stressor, even if it is erroneous, e.g., misperception of an environmental change. Relevant examples include erroneous stress associations with ordinary loud sounds that have developed from explosion-related PTSD or a pheochromocytoma producing a surge of catecholamines perceived as a stress state because of diaphoresis and a fast heart rate.
In general, frequent perturbations into a stressed state away from the high utility attractor have a cost to the system. The cost of going to the refrigerator when feeling hungry is low. However, a related perturbation, the blood sugar increase and the need to secrete insulin due to overeating high-sugar items may eventually cause long-term negative effects. If repeated enough, it may diminish the human’s ability to stay in a positive functional attractor, and the lack of responsiveness to insulin at the cellular level (i.e., insulin resistance) may cause adult type 2 diabetes. This common stress-related change has resulted in a common diabetes measure, glycosylated hemoglobin HgbA1c, frequently used as a chronic stress biomarker. In humans, allostatic load is the cost to the system due to repeatedly returning to baseline, i.e., the costs of executing the physiological changes and the potential costs of making the changes in architecture of the basins of attraction (their size, depth, etc.) following a stressor as well as the eventual impacts of the architecture change. Allostasis has been used to describe the dynamical control over these variable perturbations for maintaining a functional state. Though there is some controversy over whether allostasis is truly different from homeostasis [19], the term allostatic load has been used as a conceptual measure of the physiological cost due to chronic stressors [28] and be will be used in this paper. Attempts to define a metric of allostatic load for experimental use are discussed below.
4. Measurement of stress
The term stress describes a state of physiologic and behavioral responses to a stressor with the brain being the critical interpreter of what is stressful. Though inconsistently used, the stressed state in humans for the purposes of this discussion is linked to dynamical physiological change. The stressed state also involves the conscious and unconscious stressor interpretation by the brain including the conscious perception of the stressors and the perception of the physiologic response generated by the stressor [29-31]. Stressors result in changes in state variables and parameters and have been measured using various biomarkers.
There are many objective ways to measure human stress responses other than commonly used self-rated scales. As previously noted, physiologic responses to stress include activation of the HPA axis, activation of the locus coeruleus-norepinephrine-sympathetic nervous system pathway, the parasympathetic system, immune system, and genes [29, 31-35]. Importantly, the timing of these changes is variable. When measured as state variables, they may or may not shed light on the dynamical nature of the physiologic system, resilience, or allostatic load. Dynamical aspects of stress and resilience may be estimated with repeated measurements over longer periods during daily routines or following a known experimental stressor.
4.1. Peripheral biomarkers
Each biological assessment has a sampling time window. For example, a peripheral blood draw to assess cortisol reflects cumulative changes over minutes, cortisol overnight urine collection measure reflects cumulative changes over hours, and a hair sample may reflect cumulative changes over months.
HPA axis activity biomarkers include glucocorticoids: free cortisol (or corticosterone in experimental animals), ACTH, and corticotropin releasing hormone [36, 37]. In addition to acute stressor-induced changes in these biomarkers, there are alterations in diurnal fluctuations with chronic stress, e.g., in cortisol awakening response [38, 39]. Dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) act to counter-regulate cortisol [40]. DHEA is used as a stress marker by itself [41] or as a ratio to cortisol and has been affected by depression [42]. Mineralocorticoids may also be stress biomarkers [43].
Several autonomic activity measures are associated with acute or chronic stress including blood pressure, electrodermal response, skin temperature, respiratory rate, heart rate and heart rate variability (HRV) [44]. A variety of HRV measures in the time and frequency domains have been evaluated [45, 46]. While HRV may look at dynamical changes over long periods, e.g., 24 hours or more, longer-term HRV requires more sophisticated data processing to correct for exercise and unrelated to stress activities modifying the heart rate.
Many measures correlated with stress have been treated as relatively static measures. There are alterations in immunologic function including cytokines; gene and epigenetic modifications involving telomere changes; and metabolic activity fluctuations resulting in generation of reactive oxygen and nitrogen species damaging to cellular structures [29, 33, 35, 47-49] .
There are other biomarkers not directly related to the currently discussed physiological stress pathways. To assess stress responses researchers have used measures of muscle activity e.g., using electromyographic activity for biofeedback in treatment of muscle contraction and other types of headaches. Biofeedback has been used on many physiological measures with only few (peripheral temperature and electrodermal activity) being closely related to ANS activation [50]. Additionally, as many have casually observed, stress alters voice characteristics [51] and posture in a chair [52]. Other biomarkers are listed below under allostatic load.
4.2. Brain changes
4.2.1. Cognition
Cognitive function including memory is significantly altered by stress in humans and non-human animals [53-55]. Cognitive decline associated with stress (and the closely related construct depression) may affect speed, attention, and executive function [54, 56]. Prefrontal cortical dysfunction is particularly impacted by stress [57]. This pathological relationship becomes more evident with age [58], and highly stressed elders such as dementia caregivers may be particularly at risk [48].
4.2.2. Structural brain changes
Stress-related states such as PTSD and fear conditioning are linked to decreased hippocampal size, decline in prefrontal cortex, increased size of portions of the amygdala, and decreased inhibition of the amygdala and related brain regions by the frontal lobes [55, 57, 59, 60]. The brain changes are at least partially mediated by cortisol with increased cortisol related to smaller hippocampi [61]. The time course of structural change is much longer than the half-life of cortisol; cortisol elevation needs to be sustained to cause longer-lasting brain changes. Smaller hippocampi are common among people with PTSD or trauma exposure [62, 63] and they also are linked to increased risk for PTSD development [64] so the causative relationship is uncertain. Further, PTSD sufferers are at higher risk of dementia [65] and those with smaller hippocampi have increased the risk of dementia [66]. Therefore, defining the causative aspects of these relationships is critical and can affect other important health concerns. From the perspective of beneficial effects, research shows increased hippocampal volume and improved verbal declarative memory in PTSD patients after using a selective serotonin reuptake inhibitor (SSRI) antidepressant for 9-12 months [67]. This is likely related to SSRI-related neurogenesis increase [68].
4.2.3. Physiological brain changes: EEG, event-related potential, fMRI
EEG stress-related changes, particularly frontal asymmetries [69, 70], and alterations in event-related potentials [71] have been noted, but these changes have not been consistent, in part due to lack of distinction between state and trait markers and limitations in signal processing [72]. Chronic psychological stress impairs sleep and the resultant sleep deprivation may impact EEG. PET and fMRI detect brain activation changes due to experimental stressors [73-75].
4.2.4. Genetic changes in brain
There are different functional gene classes that underlie the diverse effects of glucocorticoids on brain function, e.g., energy metabolism, signal transduction, neuronal structure, and neurotransmitter catabolism [32]. Stress effects on telomeres have been mentioned but assessments of human telomeres are generally performed on peripheral blood limiting their direct brain association.
4.3. Allostatic load
The underlying biological definition of allostatic load is very broad since the physiological system represents a highly multidimensional state space with many parameters. Potential examples of underlying load include the cost of gene transcription, metabolic activity, and alteration in cell receptor sensitivity. Frequent DNA processing may produce changes in telomere length.
Allostatic load was originally developed as a composite marker of chronic stress-related disequilibrium generated from a number of physiological measures. The originally described allostatic load score was a composite of 10 measures (systolic and diastolic blood pressure; waist-hip ratio; ratio of total cholesterol to high density lipoproteins; high density lipoprotein cholesterol; glycosylated hemoglobin; overnight 12-hour urinary cortisol, epinephrine and norepinephrine; and DHEA-S [76]. The score obtained by summing the ten measures (0 if normal, 1 if 75th percentile or worse) was associated with mortality. Related composite allostatic load measures have been correlated to childhood poverty [77] and measures of work exhaustion [78]. The latter study added several measures (tissue necrosis factor-alpha, C-reactive protein, fibrinogen, and D-dimer) and other measures have also been added, e.g., pro-coagulant activity. Despite the widespread interest in allostatic load, the optimum measure has not been defined; the measures currently used are based on non-experimental approaches (e.g., simple availability and a priori rationales). As a result there is much variety in the definition of a composite measure [79], but there needs to be improvement in its definition to advance the field of biomarkers for chronic psychological stress. This could potentially result from better analytic techniques.
Allostatic load measures have highly variable time frames. Some may change relatively quickly, e.g., fibrinogen, some are integrated over some time period (e.g., 12-hour urinary cortisol), and some change much more slowly or are integrated over longer time frames (e.g., waist-hip ratio or HgbA1c). Most physiological parameters are not only stress indicators but also change with other biorhythms, e.g., circadian or prandial.
Another rationale for allostatic load as a composite measure of stress effects is that different people likely have different subsystems affected by stress. Some people experiencing high stress develop headaches, while others develop gastrointestinal or other disorders. The particular organ systems affected by stress is an interaction between these systems and the brain. The individual reactions to stress are dependent on an individual’s genes, learning and environment. Thus, it is likely that different people have different patterns of alteration in stress-related biomarkers or allostatic load component measures that may potentially be discerned by better analytic techniques, e.g., structural equation modeling or machine learning. It may ultimately be important to understand the individual relationships, but at this state of the research it may be helpful to have a combined measure.
4.4. Stress and disease
Acute stress may have some metabolic, immunologic and cognitive benefits. For example, alterations in system properties may produce a higher transient utility, decrease the likelihood that a stressor will move the state of the system away from an optimal attractor (robustness), or increase the size of an attractor basin (see hormesis below). A helpful example is the immune system which learns to react to foreign substances when exposed to non-virulent ones that do not result in death. If the immune system is not exposed to sufficient foreign substances, the result could be over-reactivity to foreign substances or allergies [80]. However, as stated in the introduction, more often impairments in health and a broad range of diseases are produced by chronic psychological stress.
Chronic stress may cause cognitive decline, adverse effects in the hippocampus, and contribute to neurodegenerative diseases either directly or through stress mediators including allostatic load [3, 18, 81-83]. The negative effect of psychological stress on cognitive function may be greater with aging [58, 84, 85]. Stressors including anesthesia, drugs, depression may be more likely to result in a state of impaired cognitive function with increased age. Cognitive reserve, a measure of how well the brain works [86], may be one aspect of resilience to the effects of stress on cognition.
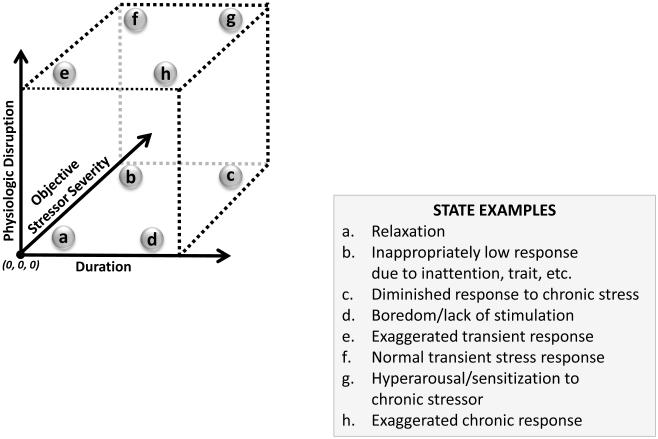
5. Dynamics of stress system - time course of stress-induced physiological changes: state/trait and variables/parameters (Figure 4)
Figure 4. A three-dimensional model of stress-related states.
A rough schematic of three dimensions related to stress. Physiological activation can be low or high and sustained for a short or long period of time. The response can be to a stressor that is relatively low from an objective or population perspective or relatively high. Normal function usually goes from relaxation state (a) to short duration high physiological activation when exposed to a stressor (f). If the stressor response is too sustained or occurs too frequently, there is some cost to the system.
Stress can cause a perturbation of state but the associated changes to physiological measures occur at varying time scales. The time courses of marker changes in psychology are sometimes grouped into fairly mobile, shorter-term changes reflecting the person’s current state and longer-term, more stable changes reflecting traits. Standard measures of psychological stress aspects, such as anxiety, are often measured by a widely used inventory, e.g. the State-Trait Anxiety Inventory [87]. However, even relatively stable traits, such as the personality trait neuroticism, often considered stable over a lifespan, can be malleable thus limiting the clear distinction between state and trait. Systems science uses terms analogous to state and trait: variables reflecting current state measures and parameters reflecting more stable attributes of the system. The change in parameters may decrease the likelihood of the system staying in the optimal attractor basin in the face of typical environmental fluctuations, but the distinction from variables is simply the time scale and thus is somewhat artificial. This section is focused on the varying time courses of physiological makers which are only moderately correlated with commonly used self-rated markers. All biomarker measurements, including common physiological measurements (e.g., cortisol) and many anatomic and experimental physiological measurements (e.g., hippocampal size or neuronal receptor sensitivity) change over time, but the time courses differ.
The sympathetic branch of the ANS is the quickest to respond. Stress response can be measured by heart rate, blood pressure, electrodermal activity, or catecholamine release [88]. Epinephrine and norepinephrine release occur in seconds. The two-minute half-life of epinephrine highlights the generally short time course of this response. This ANS response is presumably geared to short-acting flight-or-fight changes such as metabolic needs, blood flow, and non-specific alerting of the brain [89], with norepinephrine projecting throughout the brain contributing to both phasic and tonic alertness [90, 91]. HPA activity has a slower time course and is activated by threats and negative consequences even when only anticipated. Cortisol has effects throughout the body and is impacted by many factors other than stress. Cortisol also directly affects the brain via cortisol receptors present in the pituitary, cerebellum, hypothalamus paraventricular nucleus and in neocortex. The cortisol peak onset occurs 15-30 minutes after a stressor [73, 92].
Stressor effects on the immune system have a long-time course, and effects on learning and DNA have even a longer-time frame and are important for sustained stress effects. Some personality traits have been linked to specific genotypes, e.g., single nucleotide polymorphisms. For example, a specific genotype (5HTTLPR) relevant for stress affects serotonin transport and has been related to stress reactivity [93] and the personality trait of neuroticism. Particularly relevant for our discussion involving time courses in human stress are the brain network changes altering perception of the stressfulness of an environmental stimulus; this may be related to sudden awareness (consciousness) of the stressor or of the induced physiological state change. A system that reacts differently if consciousness is achieved and responds based on conscious perceptions and concepts, such as the perception of causality, is inherently biased.
There are different approaches to measure stress and resilience dynamically. One can measure the magnitude of the change at some time point following a stressor, e.g., the cortisol increase from baseline to 15 minutes after an experimental stressor. One can incorporate a more sophisticated temporal measure estimating the area under the curve or half-life of a biomarker stress response if enough assessments are available. Another measure is the time it takes to return to baseline following an experimental stressor, e.g., fMRI changes 2 hours after a stressor [74]. In the event one does not use an experimental stressor, one can observe response following a significant environmental stressor, as in epidemiological studies related to war injuries or catastrophes. If enough measurements over sufficient number of days are available it is possible to calculate the variability of the physiological system. This variability of the system relates to stress responses but other variables (e.g. age) enter as well. For example, aging is associated with increased variability of measures of performance, and this variability can serve as a marker for insipient dementia among elders [94].
In general, the slowly changing traits or parameters are potentially harder if not impossible to measure empirically. Given the variable time frame of the biomarkers, assessment by many repeated measurements over a prolonged period may provide a better representation of the dynamical stress system response to psychological stress than single time-point assessments. This is especially true because each biomarker already captures the physiological system over some cumulative time window. The many physiological measurements needed over a prolonged time can be obtained over days or weeks using continuous recording in a lab or repeated assessments using ecological momentary assessment [95, 96]. Looking at reactivity to an experimental laboratory stressor may also provide good markers of the dynamic nature of the physiological system related to stress. Epidemiological studies can use data acquired following population exposure to a common stressor. Figure 4 offers a schematic representation of the conditions related to shorter- and longer-term stressors and physiological responses. There are many systems science methodologies that could be used to analyze the multidimensional nature of stress physiology including system dynamics modeling, agent-based modeling, network analysis, discrete event analysis, Markov modeling, and control systems engineering [8].
6. Resilience
As discussed in the introduction, the term resilience has been used in different ways. Resilience affects how effectively and quickly the system returns to a high utility attractor basin [9]. Despite the neuroscientific interest in resilience [10, 97], its definitions remain variable. Resilience or robustness is the capacity of the system to return to a high utility attractor following perturbation, the system’s ability to avoid shifting to another attractor basin presented in this paper as a dysfunctional or diseased condition, or moving more quickly to its optimal location within its original attractor basin (Figure 1). Specific examples of resilience from a systems perspective include: 1) the distance of a location in one attractor basin to the boundary of an adjacent basin of inferior utility, i.e., greater resilience means the attractor is further away from boundaries with low utility neighboring regions; and 2) the strength of the vector field in the basin, where resilience might mean more rapid return to the attractor, so a repeat of a state perturbation before full return will make leaving the basin less likely. From a biological perspective, resilience may refer simply to the ability of a person to cope with a significant external stressor or insult. Related terms include: stability or resistance, indicating the difficulty moving a system away from its baseline "optimal' region; precariousness suggesting system proximity to some threshold of moving into another attractor basin, and latitude related to the maximum amount of change the system undergoes before losing its ability to remain within its high utility attractor basin. The resilience of a dynamical system to maintain itself within a functional high utility attractor basin is very important to the long-term health of the system. Resilience is not simply the opposite of allostatic load. Allostatic load is a measure of physiological system parameters that may impact resilience but it also has other effects on long-term health or disease risk.
It is known that many human stressors are best remediated by significant behavior change affecting stressor exposure (e.g., ingesting less glucose if pre-diabetic or decreasing work hours in a stressful job if hypertensive); some stressors in humans are related to the perception of the stressor more than the stressor itself. For example, someone with PTSD is in a pathological lower utility attractor that could relate to the brain misperceiving the environment in a way harmful to the person's health (e.g., a truck backfire causing a veteran to engage in recollections and emotions associated with war).
Resilience to psychological stress is evident when some people avoid significant psychopathology, such as PTSD and depression when exposed to a stressor [10]. In the World Trade Center disaster resilience, measured by a likelihood of developing PTSD, was related to age (older did better), gender (males did better), social support (more did better), self-esteem (higher did better) and lifetime history of depression (worse with a positive history), but was not related to education [98].
Some amount of stress in the environment may be useful for maximizing the system's ability to respond to future stressors. Humans living with no stressors may lose the ability to respond to future stressors. From the brain perspective, some amount of stress is useful for maximizing learning and maintaining cognitive function. Systems that learn to cope with some amount of stress may be less affected by future stressors. Hormesis refers to a biphasic response to a stressor, “a process in which exposure to a low dose of … environmental factor that is damaging at higher doses induces an adaptive beneficial effect on the cell or organism" [99]. This adaptation could be to environmental stressors such as cold and exercise [100]. A stressor can cause the system to be non-optimal for a short time but still result in returning to baseline. While there may be some allostatic load cost, the stressor may induce changes in system physiological parameters that strengthen the future ability to return to its greater utility locations, i.e., increase resilience. This low level of stress exposure occurs in some clinical treatments, e.g., allergy therapy and exposure therapy in PTSD. In some sense such exposures to a low-level stressor is a way to exercise the resilience aspects of the system. In general, repeated external stimuli elicit less of a physiological response because of habituation that can be measured by fMRI, event-related potentials or electrodermal response [101, 102]. However, in some cases repeated external stressors result in the excessive response, as in PTSD (e.g., hyperarousal to loud noises) and become self-reinforced rather than extinguished.
This decreased efficiency and ability of the human dynamical physiological system to stay in or get back to a functionally positive attractor basin is the negative effect of chronic stress or allostatic load. Changing the parameters of the human system to bring the system back to the optimal state or high utility attractor often entails a cost to the basic human constituents but the changes can be used to simply indicate previous stress exposure. This could be DNA modification, receptor sensitivity changes, or changes to blood vessels from high blood pressure. Another example of changes to the underlying system is aging, which can make a person more likely to exist in a non-optimal state or attractor basin. It could be that the attractor basin becomes smaller or less steep. The change of the state space attractor basin that decreases the system’s ability to stay in its higher utility states without moving to lower utility states in its current attractor basin or to a lower average utility attractor basin represents the chronic stress effect or allostatic load. These changes over time can be defined mathematically. The suboptimal attractor basins do not become necessarily larger; rather, the high utility attractors become smaller with shallower sides. Thus, the time required for return to the baseline state tends to increase.
From a probabilistic perspective, the resilience of the system could be considered the probability that an environmental perturbation results in returning to the high utility attractor basin, as opposed to ending up in an attractor basin with lower utility. The capacity of a system to stay in a high utility attractor basin could be defined stochastically: the likelihood that following a particular perturbation the person returns to the high utility attractor basin. The capacity to stay in this high utility attractor basin is especially relevant when, following a stressor, the state may be closer to the basin boundary and be more likely to shift to a non-optimal attractor basin should another stressor manifest. Even without changing the specific attractor basin but simply the shape of the basin, resilience could be defined based on the probabilistically weighted average utility in a single attractor basin following expected stressors.
PTSD is a useful example of state space and attractors since some of the physiologic responses may initially have been an adaptive response during specific time and environment but when they persist in other environments, the result is moving to a lower utility attractor where the abnormal response is self-reinforcing. A high stress physiological state may be high utility during a war but if that state persists after returning home it can be lower utility. The transition to PTSD is not reversed immediately as soon as causes are reversed or disappear. Reversal might require going all the way back to an earlier state in a system which induces the possibility for a cusp catastrophe (Figure 2).
7. Environment and its perception
In addition to knowing the physiological state of the person, one should also know the state of their environment because certain physiological measures may be a reaction to the environment. It must be reiterated that although some environmental stressors have a direct effect on stress responses, e.g., extreme cold, stress responses are significantly related to the person’s perception of the stressor. The perception of the environment (Figure 3) is affected by a person's prior experiences through attention and memory. Many environmental stressors are stressful because of the way they are perceived and processed. A person focused on an important phone call may not realize it’s hailing outside because of their attention on call. As a result, one may not be worrying about whether the car was left outside the garage. Attention refers to systems in the brain that allow some information to be processed more than other information [103]. Memory is a broad term with many subsystems loosely divided into declarative and non-declarative memory [104]. Emotional memory has critical brain hubs not relevant for other types of memory. The amygdala rather than the hippocampus is critical for registering the emotional valence of an event [105]. Beta-blockers that block aspects of the ANS can have an impact on emotional memory without any impact on episodic memory [105, 106]. The memory-induced changes in neural connectivity that result from gene expression and protein synthesis require hours to days. A person with a memory of a previous environmental stressor will perceive the perturbation differently from the person with no prior associations to it. For example, a physically abused wife might associate the noise of her husband returning home with the physical abuse that often follows. The sound of an opening door will have different neural associations to her than her non-abused neighbor.
High reactivity to negative events produces physiological changes [107]. In fact, negative reactions to events are more predictive of emotional well-being than the event itself [108]. Reactivity to stress can be examined though neuroticism, one of the five factors in the widely used five-factor personality inventory [109]. Neuroticism has genetic, neurobiological, and environmental contributions [85, 110, 111]. High neuroticism contributes to many health disorders [112] and relates to increased age-related cognitive change and clinical Alzheimer’s disease in longitudinal studies [113-115]. The cognitive deficits related to distress proneness are not specific and most consistently included frontal-executive function and perceptual speed [113, 115], not dissimilar to cognitive changes associated with affective disorders such as PTSD and depression [116, 117]. Neuroticism with its negative effects on cognition is a modifiable risk factor [118] with a potentially large impact on population health [119].
The internal physical components of the human are part of the brain environment, considered the internal environment in contrast to the external environment located outside the physical body. The brain has partial awareness of the internal (interoception) and external (exteroception) environment. Interoception and exteroception may produce brain and other physiological changes without awareness, but humans can become aware of their internal states such as anxiety or stress. Interoception may be taught as awareness and control over internal organs (e.g., learning to modulate one's blood pressure through biofeedback or mind-body practices).
As previously mentioned, the effect of an environmental stressor on health may be modified by how the brain perceives the environment. This perception can be altered by higher level concepts beyond attention and memory as highlighted by the concept of hope. From a health perspective, optimists fare better than pessimists [120] and those with higher religious involvement and spirituality do better than those with lower involvement [121]. The beneficial placebo response, i.e., the improvements in physiological measures or perceptions of health following administration of a treatment without any direct biological affect, can be elicited by merely telling someone that a treatment may work (even if there is no directly active components in the treatment) [122, 123]. It is likely that some mechanisms of placebo or expectancy effects overlap with some of the mechanisms underlying perception of stress [124]. The major stress hormone cortisol can be altered by experimental manipulation of expectancy in placebo effect studies [125, 126].
8. Stress and resiliency biomarker changes with treatment
There are physiological and genetic markers associated with improved resilience to stress-induced physiological changes [117, 127, 128], and there are also psychological tools to increase resilience, or the ability to tolerate stress perturbations without decreasing utility. Exposure therapy has been used to reduce the person’s reactivity to stressors, e.g., an allergen or an environmental stimulus precipitating PTSD symptoms. Mind-body techniques and biofeedback provide cognitive strategies to decrease emotionally-activated responses, avoid unnecessary negative internal associations (i.e. sense of stress) to current events, and to maximize capacity to return to a positive state attractor following a stressor.
A key facet of many mind-body therapies is mindfulness, attending to the present moment in a non-judgmental way. With several ways to measure mindfulness, the judging and negative appraisal of thoughts, emotions, and behavior factor may be particularly important for stress management. The mindfulness-non-judgmental score, i.e., being aware of the environment without attaching an emotional tag [129], is diminished by the chronic stress in dementia caregivers and in veterans with post-traumatic stress disorder [85, 130].
Mind-body studies have suggested biomarker changes related to mindfulness or mindfulness training partially overlap with the allostatic load biomarkers but in the opposite direction. These include telomerase [131], immune function [132, 133], cognitive function [133, 134], catecholamines [135], HRV [136], cortisol [133, 137-139], EEG [140], structural MRI [141, 142] and fMRI [143]. Meditation alters physiological responses to an experimental stressor [144]. However, the preferred or composite biomarkers relating to benefits of mind-body medicine have not been identified.
9. Utility
Utility is essentially the same as success of the organism (e.g., life, procreation or, in the case of humans, earning money). Long-term health is an important focus of the utility definition concerning stress-related impact on human health. While utility is the benefit to the person (or genes), the benefit also depends on the environment, i.e., the specific calculation of utility varies with the environment and the time course over which it is calculated. During war, utility is more immediate, perhaps simply surviving to the next day with a very high discount for future situations. Therefore, utility of a response to a stressor depends on the environment and on a person's degree of discounting future events. Thus, the calculation of utility in different environments will be dependent on the rewards and penalties in the current environment and on the time duration and differential weighting used for calculating the utility.
10. Conclusions
This paper has described human stress physiology and psychology from the systems science perspective. Specifically we focused on environmental perturbation stressors that produce significant long-term changes in the human dynamical system. Acute stressors usually do not produce long-term negative effects although a significantly powerful acute stressor may push the brain dynamical system into a new, functional attractor basin with lower utility. In general, chronic psychological stress produces changes in the system, such as a slower response to a future stressor or a higher potential for moving to a new lower utility attractor basin. If a human is exposed to a “tolerable” dose of a stressor that results in return to the original high utility attractor basin, the outcome may be improved resilience. From a systems science perspective, behavioral and physiological measurements attempting to capture the degree of stress of a system should incorporate the dynamics of the physiological stress response system as well as some measures of the environmental stressors and their perception. Understanding stress will require all of the interacting components from Figure 3 to be measured and described, at least partially. In general, the systems dynamics of stress physiology has much less temporal empirical data to inform the model than, for example, meteorological data because of the difficulty acquiring the human data. Nevertheless, analyzing dynamical data will be important to better understand stress physiology since the timing and strength of feedback loops likely contributes to disorders of stress and resilience to stress. In addition to measuring stress responses over time, it may be useful to repeat administration of experimental stressors to understand self-reinforcing loops. These systems science concepts and better measurement techniques will lead to better understanding of the stress system that ultimately can be used to improve the resilience of the human system and thereby improve long-term health.
Highlights.
Stress physiology was reviewed from a systems science perspective.
Stressors push biological systems from baseline towards lower utility states.
The system change is based on objective attributes and perceptions of the stressor.
Allostatic load is utility reduction due to stress-related state changes.
Resilience affects ability to return to high utility state following perturbations.
Acknowledgements
Funded in part by a grant from the National Institutes of Health (AT005121). We acknowledge Martin Zwick, PhD for reviewing an earlier version of the paper, Elena Goodrich for help with figures, and discussions about some topics with Helané Wahbeh, ND, MCR and other colleagues.
Abbreviations
- ACTH
adrenocorticotrophic hormone
- ANS
autonomic nervous system
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- EEG
electroencephalogram
- fMRI
functional magnetic resonance imaging
- HgbA1c
glycosylated hemoglobin A1c
- HPA axis
hypothalamo-pituitary-adrenal axis
- HRV
heart rate variability
- PET
positron emission tomography
- PTSD
post-traumatic stress disorder
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].NPR. Foundation RWJ. Health HSoP The Burden of Stress in America. 2014 http://www.rwjf.org/content/dam/farm/reports/surveys_and_polls/2014/rwjf414295;
- [2].Bairey Merz CN, Dwyer J, Nordstrom CK, Walton KG, Salerno JW, Schneider RH. Psychosocial stress and cardiovascular disease: pathophysiological links. Behav Med. 2002;27:141–7. doi: 10.1080/08964280209596039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lupien SL, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nature Reviews Neuroscience. 2009 doi: 10.1038/nrn2639. Advance Online. [DOI] [PubMed] [Google Scholar]
- [4].Novakova B, Harris PR, Ponnusamy A, Reuber M. The role of stress as a trigger for epileptic seizures: a narrative review of evidence from human and animal studies. Epilepsia. 2013;54:1866–76. doi: 10.1111/epi.12377. [DOI] [PubMed] [Google Scholar]
- [5].Hemmerle AM, Herman JP, Seroogy KB. Stress, depression and Parkinson's disease. Exp Neurol. 2012;233:79–86. doi: 10.1016/j.expneurol.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burns MN, Nawacki E, Kwasny MJ, Pelletier D, Mohr DC. Do positive or negative stressful events predict the development of new brain lesions in people with multiple sclerosis? Psychol Med. 2014;44:349–59. doi: 10.1017/S0033291713000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cleck JN, Blendy JA. Making a bad thing worse: adverse effects of stress on drug addiction. J Clin Invest. 2008;118:454–61. doi: 10.1172/JCI33946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mobus GE, Kalton MC. Principles of Systems Science. Springer; New York: 2015. [Google Scholar]
- [9].Holling CS. Resilience and Stability of Ecological Systems. Annu Rev Ecol Syst. 1973;4:1–23. [Google Scholar]
- [10].Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ader R. Psychoneuroimmunology. 4th Elsevier Academic Press; San Diego: 2007. [Google Scholar]
- [12].McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- [13].Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis. 2008;50:404–19. doi: 10.1016/j.pcad.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johns LE, Aiello AE, Cheng C, Galea S, Koenen KC, Uddin M. Neighborhood social cohesion and posttraumatic stress disorder in a community-based sample: findings from the Detroit Neighborhood Health Study. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1899–906. doi: 10.1007/s00127-012-0506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kemeny ME. Psychobiological responses to social threat: evolution of a psychological model in psychoneuroimmunology. Brain Behav Immun. 2009;23:1–9. doi: 10.1016/j.bbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [16].Young M, Schieman S. When hard times take a toll: the distressing consequences of economic hardship and life events within the family-work interface. J Health Soc Behav. 2012;53:84–98. doi: 10.1177/0022146511419204. [DOI] [PubMed] [Google Scholar]
- [17].Harrell JP, Hall S, Taliaferro J. Physiological responses to racism and discrimination: an assessment of the evidence. Am J Public Health. 2003;93:243–8. doi: 10.2105/ajph.93.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- [19].Day TA. Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1195–200. doi: 10.1016/j.pnpbp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- [20].Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychol Bull. 2006;132:477–95. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lupien S. Well Stressed: how you can manage stress before it turns toxic. Wiley; Mississauga, Ontario: 2012. [Google Scholar]
- [22].Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- [23].Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. 2005;30:319–25. [PMC free article] [PubMed] [Google Scholar]
- [24].Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–35. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lovallo W. The cold pressor test and autonomic function: a review and integration. Psychophysiology. 1975;12:268–82. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- [26].Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. University of Florida, Center for Research in Psychophysiology; Gainesville: 1999. [Google Scholar]
- [27].Berretta S. Cortico-amygdala circuits: role in the conditioned stress response. Stress. 2005;8:221–32. doi: 10.1080/10253890500489395. [DOI] [PubMed] [Google Scholar]
- [28].McEwen BS, Lasley EN. Allostatic load: when protection gives way to damage. Adv Mind Body Med. 2003;19:28–33. [PubMed] [Google Scholar]
- [29].McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Research. 2000;886:172–89. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- [30].McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- [31].Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Datson NA, Morsink MC, Meijer OC, de Kloet ER. Central corticosteroid actions: Search for gene targets. Eur J Pharmacol. 2008;583:272–89. doi: 10.1016/j.ejphar.2007.11.070. [DOI] [PubMed] [Google Scholar]
- [33].Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD. Accelerated telomere shortening in response to life stress. PNAS. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bernston GG, Caciollo JT, Quigley KS. Autonomic determinism. the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–87. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- [35].Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and Psychosomatic Medicine: Back to the Future. Psychosom Med. 2002;64:15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- [36].Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. vii-viii. [DOI] [PubMed] [Google Scholar]
- [37].Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–79. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- [38].Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med. 2002;65:92–9. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- [39].Wahbeh H, Kishiyama S, Zajdel D, Oken B. Salivary cortisol awakening response in mild Alzheimer's disease, caregivers, and non-caregivers. Alzheimer's Disease & Related Disorders. 2008;22:181–3. doi: 10.1097/WAD.0b013e31815a9dff. [DOI] [PubMed] [Google Scholar]
- [40].Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Noto Y, Sato T, Kudo M, Kurata K, Hirota K. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg. 2005;101:1873–6. doi: 10.1213/01.ANE.0000184196.60838.8D. [DOI] [PubMed] [Google Scholar]
- [42].Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am J Psychiatry. 2002;159:1237–9. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]
- [43].de Kloet ER. From vasotocin to stress and cognition. Eur J Pharmacol. 2010;626:18–26. doi: 10.1016/j.ejphar.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [44].Cacioppo JT, Tassinary LG, Bernston GG. 3rd Cambridge University Press; New York: 2007. p. 898. [Google Scholar]
- [45].Pumprla J, Howorka K, Groves D, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. International Journal of Cardiology. 2002;84:1–14. doi: 10.1016/s0167-5273(02)00057-8. M C. [DOI] [PubMed] [Google Scholar]
- [46].Mukherjee S, Yadav R, Yung I, Zajdel D. Sensitivity to Mental Effort and Test-Retest Reliability of Heart Rate Variability Measures in Healthy Seniors. Clinical Neurophysiology. 2011;122:2059–66. doi: 10.1016/j.clinph.2011.02.032. B.S. O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- [48].Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. International Psychogeriatrics. 2014;26:725–47. doi: 10.1017/S1041610214000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tomiyama AJ, O'Donovan A, Lin J, Puterman E, Lazaro A, Chan J, et al. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav. 2012;106:40–5. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schwartz MS, Andrasik F. Biofeedback: A Practitioner's Guide. 3rd New York: Guilford Press: 2003. p. 930. [Google Scholar]
- [51].Dietrich M, Verdolini Abbott K. Vocal function in introverts and extraverts during a psychological stress reactivity protocol. J Speech Lang Hear Res. 2012;55:973–87. doi: 10.1044/1092-4388(2011/10-0344). [DOI] [PubMed] [Google Scholar]
- [52].Arnrich B, Setz C, La Marca R, Troster G, Ehlert U. What does your chair know about your stress level? IEEE Trans Inf Technol Biomed. 2010;14:207–14. doi: 10.1109/TITB.2009.2035498. [DOI] [PubMed] [Google Scholar]
- [53].McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–16. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- [54].Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- [55].Bremner JD. Does stress damage the brain? Biological Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- [56].Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stawski RS, Sliwinski MJ, Smyth JM. Stress-related cognitive interference predicts cognitive function in old age. Psychology and Aging. 2006;21:535–44. doi: 10.1037/0882-7974.21.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- [60].Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–6. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- [61].Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- [62].Childress JE, McDowell EJ, Dalai VV, Bogale SR, Ramamurthy C, Jawaid A, et al. Hippocampal volumes in patients with chronic combat-related posttraumatic stress disorder: a systematic review. J Neuropsychiatry Clin Neurosci. 2013;25:12–25. doi: 10.1176/appi.neuropsych.12010003. [DOI] [PubMed] [Google Scholar]
- [63].Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–8. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- [64].Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–13. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kaye J, Swihart T, Howieson D, Dame A, Moore M, Karnos T, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly destined to develop dementia. Neurology. 1997;48:1297–134. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- [67].Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011;16:738–50. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Perez-Edgar K, Kujawa A, Nelson SK, Cole C, Zapp DJ. The relation between electroencephalogram asymmetry and attention biases to threat at baseline and under stress. Brain Cogn. 2013;82:337–43. doi: 10.1016/j.bandc.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: a twin study. Biol Psychol. 2006;71:289–95. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ceballos NA, Giuliano RJ, Wicha NY, Graham R. Acute stress and event-related potential correlates of attention to alcohol images in social drinkers. J Stud Alcohol Drugs. 2012;73:761–71. doi: 10.15288/jsad.2012.73.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Davidson RJ. What does the prefrontal cortex "do" in affect: perspectives on frontal EEG asymmetry research. Biol Psychol. 2004;67:219–33. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- [73].Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–71. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- [74].Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, et al. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci. 2013;7:313. doi: 10.3389/fnhum.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–40. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- [76].Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences. 2001;98:4770–5. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences. 2009;106:6545–9. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bellingrath S, Weigl T, Kudielka BM. Chronic work stress and exhaustion is associated with higher alostatic load in female school teachers. Stress. 2009;12:37–48. doi: 10.1080/10253890802042041. [DOI] [PubMed] [Google Scholar]
- [79].Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.10.002. doi:10.1016/neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [80].Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–91. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- [81].Lupien SJ, Nair NPV, Briere S, Maheu F, Tu MT, Lemay M, et al. Increased cortisol levels and impaired cognition in human aging: Implication for depression and dementia later in life. Reviews in the Neurosciences. 1999;10:117–39. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- [82].McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- [83].Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- [84].Lupien SJ, Fiocco A, Wan N, Maheu E, Lord C, Schramek T, et al. Stress hormones and human memory function across the lifespan. psychoneuroendocrinology. 2005;30:225–42. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- [85].Oken BS, Fonareva I, Wahbeh H. Stress-related cognitive dysfunction in dementia caregivers. Journal of Geriatric Psychiatry and Neurology. 2011;24:192–9. doi: 10.1177/0891988711422524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. [PubMed] [Google Scholar]
- [87].Spielberger C, Gorsuch R, Lushene R. The state trait anxiety inventory (STAI) test manual. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- [88].Andrews J, Ali N, Pruessner JC. Reflections on the interaction of psychogenic stress systems in humans: the stress coherence/compensation model. Psychoneuroendocrinology. 2013;38:947–61. doi: 10.1016/j.psyneuen.2013.02.010. [DOI] [PubMed] [Google Scholar]
- [89].Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- [90].Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: Physiological basis and measurement. Clinical Neurophysiology. 2006;117:1885–901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- [92].Engert V, Vogel S, Efanov SI, Duchesne A, Corbo V, Ali N, et al. Investigation into the cross-correlation of salivary cortisol and alpha-amylase responses to psychological stress. Psychoneuroendocrinology. 2011;36:1294–302. doi: 10.1016/j.psyneuen.2011.02.018. [DOI] [PubMed] [Google Scholar]
- [93].Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT Gene. Science. 2003;l301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- [94].Dodge HH, Mattek NC, Austin D, Hayes TL, Kaye JA. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology. 2012;78:1946–52. doi: 10.1212/WNL.0b013e318259e1de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Csikszentmihalyi M, Larson R. Validity and reliability of the Experience-Sampling Method. J Nerv Ment Dis. 1987;175:526–36. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- [96].Conner TS, Barrett LF. Trends in ambulatory self-report: the role of momentary experience in psychosomatic medicine. Psychosom Med. 2012;74:327–37. doi: 10.1097/PSY.0b013e3182546f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology. Brain. 2013;136:2510–26. doi: 10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Adams RE, Boscarino JA. Predictors of PTSD and delayed PTSD after disaster: the impact of exposure and psychosocial resources. J Nerv Ment Dis. 2006;194:485–93. doi: 10.1097/01.nmd.0000228503.95503.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–8. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- [101].Yamaguchi S, Hale LA, D'Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24:5356–63. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gati I, Ben-Shakhar G. Novelty and significance in orientation and habituation: a feature-matching approach. J Exp Psychol Gen. 1990;119:251–63. doi: 10.1037//0096-3445.119.3.251. [DOI] [PubMed] [Google Scholar]
- [103].Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–88. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hermans EJ, Battaglia FP, Atsak P, de Voogd LD, Fernandez G, Roozendaal B. How the amygdala affects emotional memory by altering brain network properties. Neurobiol Learn Mem. 2014;112:2–16. doi: 10.1016/j.nlm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- [106].Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–72. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- [107].Pieper S, Brosschot JF, van der Leeden R, Thayer JF. Cardiac effects of momentary assessed worry episodes and stressful events. Psychosom Med. 2007;69:901–9. doi: 10.1097/PSY.0b013e31815a9230. [DOI] [PubMed] [Google Scholar]
- [108].Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. Guilfor Press; New York, N.Y.: 1979. [Google Scholar]
- [109].Costa PT, McCrae RR. NEO Inventories: Professional manual. Psychological Assessment Resources, Inc; Lutz, FL: 2010. [Google Scholar]
- [110].Barlow DH, Ellard KK, Sauer-Zavala S, Bullis JR, Carl JR. The origins of neuroticism. Perspectives on Psychological Science. 2014;9:481–196. doi: 10.1177/1745691614544528. [DOI] [PubMed] [Google Scholar]
- [111].Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lahey BB. Public health significance of neuroticism. Am Psychol. 2009;64:241–56. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011;19:327–34. doi: 10.1097/JGP.0b013e31820119da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wilson RS, Evans DA, Bienias JL, Mendes De Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology. 2003;61:1479–85. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- [115].Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom Med. 2007;69:47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- [116].DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–96. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–95. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Oken B, Miller M, Goodrich E, Wahbeh H. Effects of mindfulness meditation on self-rated stress-related measures: improvements in neuroticism and ecological momentary assessment of stress. The Journal of Alernative and Complementary Medicine. 2014;20:A64. [Google Scholar]
- [119].Kremen WS, Lachman ME, Pruessner JC, Sliwinski M, Wilson RS. Mechanisms of age-related cognitive change and targets for intervention: social interactions and stress. J Gerontol A Biol Sci Med Sci. 2012;67:760–5. doi: 10.1093/gerona/gls125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: a meta-analytic review. Ann Behav Med. 2009;37:239–56. doi: 10.1007/s12160-009-9111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cotton S, Zebracki K, Rosenthal SL, Tsevat J, Drotar D. Religion/spirituality and adolescent health outcomes: a review. J Adolesc Health. 2006;38:472–80. doi: 10.1016/j.jadohealth.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [122].Oken B. Placebo effects: clinical aspects and neurobiology. Brain. 2008;131:2812–23. doi: 10.1093/brain/awn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta J-K. Neurobiological mechanisms of the placebo effect. The Journal of Neuroscience. 2005;25:10390–402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Oken B, Flegal K, Zajdel D, Kishiyama S, Haas M, Peters D. Expectancy effect: impact of pill administration on cognitive performance in healthy seniors. Journal of Clinical and Experimental Neuropsychology. 2008;30:7–17. doi: 10.1080/13803390701775428. [DOI] [PubMed] [Google Scholar]
- [125].Johansen O, Brox J, Flaten MA. Placebo and Nocebo responses, cortisol, and circulating beta-endorphin. Psychosom Med. 2003;65:786–90. doi: 10.1097/01.psy.0000082626.56217.cf. [DOI] [PubMed] [Google Scholar]
- [126].Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious Expectation and Unconscious Conditioning in Analgesic, Motor, and Hormonal Placebo/Nocebo Responses. J Neurosci. 2003;23:4315–23. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–9. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- [129].Baer RA, Smith GT, Hopkins J, Krietmeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- [130].Wahbeh H, Oken B, Lu M. Differences in veterans with and without posttraumatic stress disorder during relaxing and stressful condition. Annual Meeting American Psychosomatic Society. 2010;A61 2010. [Google Scholar]
- [131].Jacobs TL, Epel ES, Lin J, Blackburn EH. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.09.010. al. e. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [132].Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, et al. Alterations in Brain and Immune Function Produced by Mindfulness Meditation. Psychosom Med. 2003;65:564–70. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- [133].Tang YY, Ma Y, Wang J, Fan Y, Feng S. Short-term meditation training improves attention and self-regulation. PNAS. 2007;104:17152–6. doi: 10.1073/pnas.0707678104. ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Oken BS, Fonareva I, Haas M, Wahbeh H, Lane JB, Zajdel DP, et al. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. Journal of Alternative and Complementary Medicine. 2010;16:1031–8. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Jung Y-H, Kang D-H, Jang JH, Park HY, Byun MS, Kwon SJ, et al. The effects of mind-body training on stress reduction, positive affect, and plasma catecholamines. Neuroscience Letters. 2010;479:138–42. doi: 10.1016/j.neulet.2010.05.048. [DOI] [PubMed] [Google Scholar]
- [136].Tang YY, Ma Y, Fan Y, Feng H, Wang J, Feng S, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 2009;106:8865–70. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Matousek RH, Dobkin PL, Pruessner J. Cortisol as a marker for improvement in mindfulness-based stress reduction. Complement Ther Clin Pract. 2010;16:13–9. doi: 10.1016/j.ctcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [138].Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–81. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29:448–74. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- [140].Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, Davidson RJ. Mental training enhances attentional stability: neural and behavioral evidence. J Neurosci. 2009;29:13418–27. doi: 10.1523/JNEUROSCI.1614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci U S A. 2010;107:15649–52. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Weng HY, Fox AS, Shackman AJ, Stodola DE, Caldwell JZ, Olson MC, et al. Compassion training alters altruism and neural responses to suffering. Psychol Sci. 2013;24:1171–80. doi: 10.1177/0956797612469537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Mohan A, Sharma R, Bijlani RL. Effect of meditation on stress-induced changes in cognitive functions. J Altern Complement Med. 2011;17:207–12. doi: 10.1089/acm.2010.0142. [DOI] [PubMed] [Google Scholar]